Abstract
Purpose
To determine local control according to clinical variables for patients with intermediate-risk rhabdomyosarcoma (RMS) treated on Children’s Oncology Group protocol D9803.
Patients and Methods
Of 702 patients enrolled, we analyzed 423 patients with central pathology–confirmed group III embryonal (n = 280) or alveolar (group III, n = 102; group I–II, n = 41) RMS. Median age was 5 years. Patients received 42 weeks of VAC (vincristine, dactinomycin, cyclophosphamide) or VAC alternating with VTC (T = topotecan). Local therapy with 50.4 Gy radiation therapy with or without delayed primary excision began at week 12 for group III patients. Patients with group I/II alveolar RMS received 36–41.4 Gy. Local failure (LF) was defined as local progression as a first event with or without concurrent regional or distant failure.
Results
At a median follow-up of 6.6 years, patients with clinical group I/II alveolar RMS had a 5-year event-free survival rate of 69% and LF of 10%. Among patients with group III RMS, 5-year event-free survival and LF rates were 70% and 19%, respectively. Local failure rates did not differ by histology, nodal status, or primary site, though there was a trend for increased LF for retroperitoneal (RP) tumors (P = .12). Tumors ≥5 cm were more likely to fail locally than tumors <5 cm (25% vs 10%, P = .0004). Almost all (98%) RP tumors were ≥5 cm, with no difference in LF by site when the analysis was restricted to tumors ≥5 cm (P = .86).
Conclusion
Local control was excellent for clinical group I/II alveolar RMS. Local failure constituted 63% of initial events in clinical group III patients and did not vary by histology or nodal status. The trend for higher LF in RP tumors was related to tumor size. There has been no clear change in local control over RMS studies, including IRS-III and IRS-IV. Novel approaches are warranted for larger tumors (≥5 cm).
Introduction
Although two-thirds of children with intermediate-risk rhabdomyosarcoma (RMS) will be cured, further improvement in this rate has been elusive (1). Relapse or progression of disease at the primary tumor site has been the dominant form of failure among these patients. Any effort to improve local control with more-aggressive therapy is tempered by the conflicting goal of minimizing late effects in survivors. It is imperative that we understand which tumors are most likely to fail locally so that we may risk-adapt interventions to improve the therapeutic ratio, curing more children while simultaneously minimizing the burden of long-term complications. In this study we seek to determine disease-specific risk factors by analyzing local failure according to histology, clinical group, primary site, nodal status, and tumor size.
Patients and Methods
Eligibility and systemic therapy
From 1999 to 2005, the Children’s Oncology Group (COG) conducted D9803, a phase 2 trial for patients with intermediate-risk RMS. The study design and overall results from COG D9803 have been published previously (2). Eligible patients included those with stage II and II clinical group III embryonal RMS, all nonmetastatic alveolar RMS, undifferentiated sarcoma or ectomesenchymoma, and patients aged <10 years with stage IV embryonal RMS. Six hundred seventeen eligible patients were randomized to 42 weeks of VAC (vincristine, dactinomycin, and cyclophosphamide) versus VAC alternating with vincristine, topotecan, and cyclophosphamide (VAC/VTC). The outcome for the VAC and VAC/VTC arms were similar, and both arms were combined for the purpose of this report. The present analysis of local control was further limited to patients enrolled in COG D9803 who had nonmetastatic, central pathology–confirmed alveolar or embryonal RMS. The pathologic classification for alveolar histology was based on central re-review of tumors (3). Patients were excluded for the following reasons: 70 undifferentiated sarcoma or mixed/not otherwise specified RMS, 44 clinical group IV embryonal RMS, 41 clinical group I/II patients reclassified as embryonal RMS after re-review, 23 alveolar RMS without re-review, 15 missing pathology or clinical group data, and 1 ineligible.
Local therapy
By definition, patients categorized as clinical group I and II had primary tumor resection before initiation of chemotherapy. These patients, all with alveolar or undifferentiated histology, received postoperative radiation therapy (RT) after week 12. All RT was delivered in 1.8-Gy daily fractions, with a total dose of 36 Gy for clinical group I node-negative (N0) patients or 41.4 Gy for patients with positive margins (clinical group 2) or nodal involvement (N1).
For clinical group III patients, local RT also began after week 12 except for patients with parameningeal RMS with intracranial extension, who received RT immediately. A dose of 50.4 Gy was used for definitive treatment of clinical group III tumors. By protocol design, patients with clinical group III tumors of the bladder dome, extremity, or trunk (including thorax, abdomen, and pelvis) were candidates for delayed primary excision (DPE) at week 12 if the primary tumor appeared resectable. Those who had complete resection with negative margins then received 36 Gy, whereas those with microscopic residual or biopsy confirmation of complete response received 41.4 Gy. Patients with gross residual disease after delayed surgery received 50.4 Gy postoperatively.
Megavoltage photon and/or electron beams were used, and brachytherapy was permitted in select cases. Proton therapy was not used. The target volume was the presurgical and prechemotherapy tumor volume plus a 1.5-to 2-cm margin. For patients receiving 50.4 Gy, a volume reduction was permitted to the original tumor volume plus 0.5 cm after 36 Gy if N0 or 41.4 Gy if N1.
The Quality Assurance Review Center performed a review of radiation treatment materials at the start of RT to maximize protocol compliance. Radiation oncology members of the COG Soft Tissue Sarcoma Committee then reviewed the materials for a final assessment of compliance. Doses within ±5% of the prescription dose were considered appropriate. A dose ±5–10% was considered a minor deviation, and doses <90% or >110% of the protocol dose were considered major deviations. The irradiated volume was considered appropriate if the pretreatment volume with a 1.5-cm margin was irradiated. A volume with a <1.5-cm margin was scored as a minor deviation; a volume that missed tumor was considered a major deviation.
Endpoints and statistical methods
Event-free survival (EFS) rates were estimated using the Kaplan-Meier method. Local failure was defined as progression or relapse at the primary tumor site as a first event, including those with concurrent local plus regional or distant failure. Time to local failure was defined from the date of protocol therapy initiation and was calculated using cumulative incidence curves that treated other nonlocal failures as competing risks. Cox proportional hazards regression modeling was used to estimate hazard ratios for risk factors for local failure. A P value of < .05 was considered statistically significant.
Results
Patient population
This analysis of local control was limited to the 423 patients, including 280 patients with clinical group III embryonal RMS, 102 with clinical group III alveolar RMS, and 41 with clinical group I/II alveolar RMS. Median follow-up was 6.6 years (range, 0.13–10.75 years).
Clinical group I–II alveolar RMS
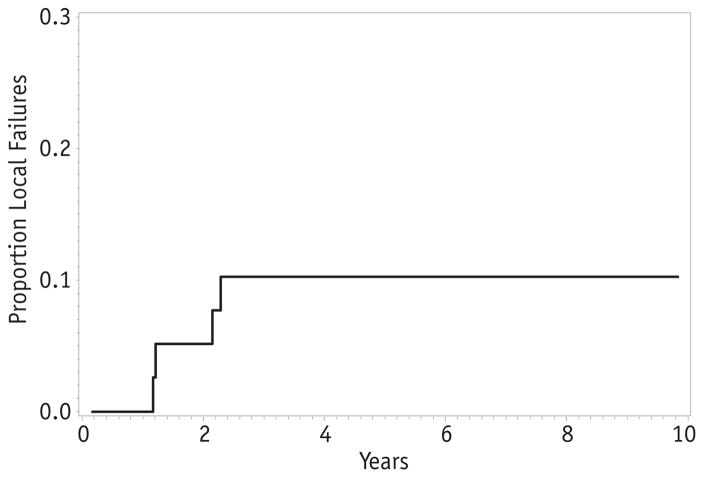
Forty-one patients had gross total resection of their tumors before initiation of chemotherapy (clinical group I or II). A wide range of primary sites were represented, including orbit (2), parameningeal (2), other head-and-neck (7), bladder (1), other genitourinary (5), extremity (15), and trunk (9). Fourteen patients had disease relapse, resulting in a 5-year EFS of 69% (95% confidence interval [CI] 52–81%). Four patients had local failure, for a 5-year local failure rate of 10% (Fig. 1). Thus, local failure accounted for less than one-third of relapses (4 of 14) in this subset of patients.
Fig. 1.
Local failure for patients with clinical group I–II alveolar rhabdomyosarcoma.
Clinical group III
Among the 382 patients with clinical group III disease, 171 patients (45%) had parameningeal tumors, and 73 (43%) of these had intracranial extension requiring immediate RT. The remaining primary sites were extremity (n = 54, 14%), retroperitoneal (n = 47, 12%), genitourinary, bladder/prostate (n = 45, 12%), and other (n = 65, 17%). Sixty-four (17%) underwent DPE before RT. Gross total resection was achieved in 53 patients, allowing for RT dose reduction to 36 Gy for negative margins or 41.4 Gy for microscopically positive margins. A thorough analysis of outcomes for patients undergoing DPE with reduced RT dosing has been reported elsewhere and showed similar local control compared with historic results in patients treated with definitive RT (4).
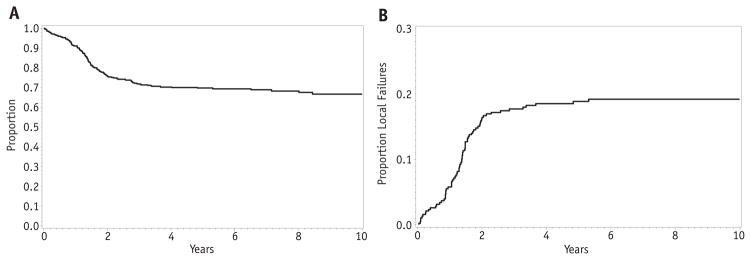
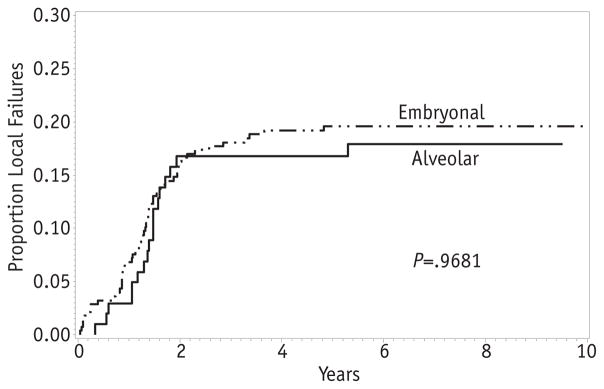
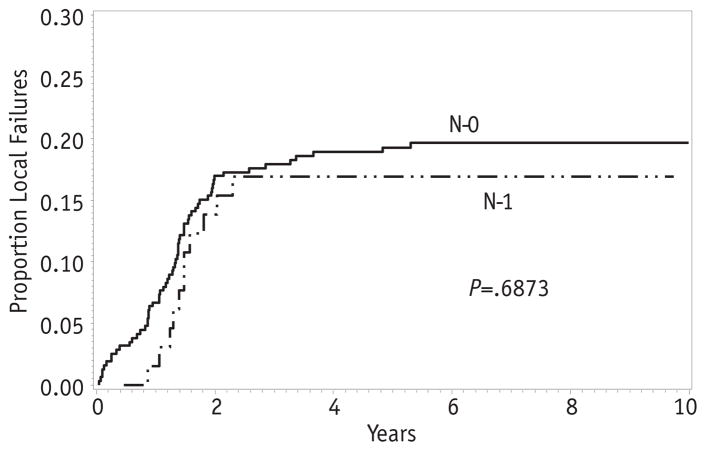
The overall 5-year EFS for patients with clinical group III disease was 70% (95% CI 65–74%), whereas the local failure was 19% (Fig. 2A, B). Therefore, relapse or progression at the primary site was the dominant type of failure for clinical group III patients. Among clinical group III patients, the rate of local failure did not differ by histologic subtype. The 5-year cumulative incidence was 20% for embryonal RMS and 17% for alveolar RMS (P = .97; Fig. 3). Likewise, there was no difference in local failure according to nodal status. The 5-year local failure rate was 19% for patients without lymph node involvement and 17% for those with positive nodes (P = .69; Fig. 4).
Fig. 2.
Event-free survival (A) and local failure (B) for all patients with clinical group III embryonal rhabdomyosarcoma.
Fig. 3.
Local failure by histologic subtype.
Fig. 4.
Local failure by lymph node status.
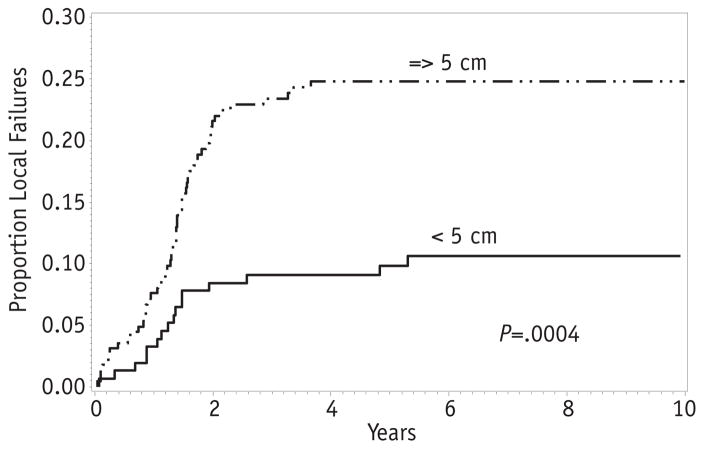
Tumor size was a strong predictor of local failure. The 5-year local failure was 10% for tumors <5 cm in maximum dimension and 25% for tumors ≥5 cm (P = .0004; Fig. 5). There was a trend for higher local failure among retroperitoneal tumors, with a 5-year local failure of 33%. Parameningeal tumors both with and without intracranial extension failed locally at a rate of 19%. The 5-year local failure for extremity tumors was 15%, whereas bladder/prostate and others had a local failure rate of 14% (P = .12; Fig. 5). The rates of local failure by primary site were similar regardless of histologic subtype.
Fig. 5.
Local failure by tumor size.
Because tumor size varied by primary site, with a greater proportion of retroperitoneal tumors being 5 cm or larger, a Cox regression model was performed for tumor size and primary site. After adjusting for tumor size, differences between primary sites diminished, suggesting that the increased risk of local failure for retroperitoneal tumors reflects the greater proportion of tumors ≥5 cm in this subgroup. To further investigate the relationship between primary site and tumor size, an estimate of the local failure was restricted to tumors >5 cm. Among large tumors, 5-year local failure for retroperitoneal and parameningeal tumors was the same (24%) and did not differ significantly from extremity or bladder/prostate (19%, P = .86).
Because of the difficulty in delivering RT to the retro-peritoneum, especially in children, the rate of protocol compliance was assessed to determine whether inadequate therapy might be a contributing factor to decreased local control. Among 47 patients with retroperitoneal primary tumors, 7 experienced failure before RT. Of the remaining 40, 35 had sufficient data for RT review, and only 3 cases were classified as having major deviations. On the basis of previous COG sarcoma protocols, this is a relatively low rate of RT deviation, and thus RT compliance does not seem to be a contributing factor (5).
Discussion
Historically, patients with clinical group I–II alveolar histology have EFS and overall survival outcomes inferior to their counterparts with embryonal histology (6). In COG D9803, with a strategy of intermediate-risk chemotherapy and postoperative RT, local control for this subgroup was excellent at 90%, and local failure accounted for less than one-third of events. It may be reasonable for future protocols to study reductions in postoperative RT doses to reduce the risk of late effects. Efforts to improve outcomes for patients in this category will focus on strategies to prevent distant metastases.
Conversely, local failure accounted for the majority of failures for patients with clinical group III disease. Compared with embryonal RMS, alveolar RMS is associated with poorer EFS (2). However, the rates of local failure for clinical group III patients were similar for both histologies. Reports focusing on local control from large single institutions have also made this observation (7, 8). Therefore, for patients with alveolar RMS, strategies for prevention of distant metastases are important for improving EFS.
In our analysis of clinical group III patients, those with and without involved regional lymph nodes had similar rates of local control. This is in contrast to IRS-III, which had shown lymph node status to be predictive of local failure. These data and other local control comparisons with IRS-III and IRS-IV are displayed in Table 1 (6, 9, 10). The reason this finding was not reproduced in the more modern study is uncertain but could potentially be related to improvements in imaging technology or definition of local failure. In IRS-III, the local failure rate among node-positive patients was unusually high at 32%, whereas that of node-negative patients was 16%, much closer to the 19% rate in the present study. Lymph node staging has improved significantly over successive studies, with more routine use of fluorodeoxyglucose positron emission tomography scans and/or sentinel node biopsies to clarify regional node status. On the basis of the data from COG D9803, provided that positive lymph nodes are appropriately identified and adequately treated with radiation, it does not seem that lymph node disease imparts a worse prognosis.
Table 1.
Comparison of local failure rates for intermediate-risk patients in IRS-III, IRS-IV, and D9803
| Patient subsets | IRS-III (%) | IRS-IV (%) | D9803 (%) |
|---|---|---|---|
| All patients | 19 | 13 | 19 |
| Histology | |||
| Embryonal | 19 | 20 | |
| Alveolar (+ UDS for IRS-III) | 17 | 17 | |
| Site | |||
| Parameningeal | 19 | 16 | 19 |
| Extremity | 17 | 7 | 15 |
| Bladder/prostate | 14 | 19 | 14 |
| Stage | |||
| N0 | 16 | 19 | |
| N1 | 32 | 17 | |
| Size (cm) | |||
| <5 | 16 | 10 | |
| ≥5 | 21 | 25 | |
Abbreviation: UDS = undifferentiated soft-tissue sarcoma.
Primary tumor site is one of the most important prognostic factors for survival in RMS. As with histology, the differences in EFS by primary site are largely related to the risk of distant failure rather than local failure. Although local control varied somewhat by site, the difference was not statistically significant. Retroperitoneal tumors seemed to be outliers, with a local failure rate of 33%, but this higher rate was due to the fact that almost all were ≥5 cm. Patients with retroperitoneal tumors (considered a “trunk” primary site) were eligible for DPE at week 12. A previous publication documented that of 74 patients with trunk primary tumors on D9803, 30 underwent DPE and 25 had resection of all gross disease. This made them eligible for an RT dose reduction to 36–41.4 Gy, depending on margin status. Local failure for these 30 patients with trunk primary tumors undergoing DPE was 20%. It was concluded that local control outcomes with DPE and reduced-dose RT were similar to historic controls with RT alone (4).
Large primary tumor size at diagnosis was the only significant predictor of local failure among patients with clinical group III tumors. Tumors ≥5 cm had a 25% local failure rate, compared with 10% for smaller tumors. Efforts to improve local control for larger tumors should be studied. One strategy could include DPE when feasible for larger tumors. As above, DPE combined with dose-reduced RT on COG D9803 achieved local control rates similar to historical controls for tumors of all sizes at selected sites (4). However, DPE may not be possible without major anatomic sacrifice, particularly for large tumors. Of the 61 patients who achieved complete tumor resection at DPE in D9803, 7 did have loss of a limb, organ, or function (4).
Alternatively, improving technologies for conformal radiation may allow a boost to residual tumor with acceptable toxicity. Image guidance, intensity modulated RT, and proton beams help to minimize normal tissue exposure. One argument against this approach is that dose escalation with hyperfractionation in IRS-IV failed to show a benefit in local control (10). This is a valid point, yet it is possible that the radiobiological assumptions used to calculate the hyperfractionated dosing, although still considered the gold standard, were not more biologically effective. Perhaps increasing dose with standard fractionation would be more effective.
Anatomic response to chemotherapy, which was given for 12 weeks before definitive radiation on this study, did not correlate with EFS (11). It is possible but very unlikely that a difference specifically in local control may correlate with anatomic response in any subset of patients, although this has been reported in single-institution series (12). A more promising direction of investigation may be the relationship between functional response assessed by positron emission tomography scan and local control. A strong correlation has been reported in a series of patients from Memorial Sloan-Kettering Cancer Center, but this finding requires validation in a larger, prospective protocol (13).
In conclusion, patients with nonmetastatic intermediate-risk RMS treated on COG D9803 had local control rates similar to previous studies. Patients with alveolar RMS and clinical group I/II disease have excellent local control but experience more distant failures. Conversely, local failure is the dominant form of relapse for clinical group III patients. No improvement in local control has occurred over recent COG studies despite technical advances in imaging and RT, hyperfractionated dose escalation, potentially radio-sensitizing chemotherapy, or DPE. Tumor size (≥5 cm) was the only significant risk factor for local failure. Future studies will attempt to refine risk assessment with functional imaging and will strive to enhance local therapy for these patients.
Summary.
Local failure was 10% for patients with clinical group I/II alveolar RMS, representing a small proportion of failures. Local failure was 19%, a more dominant form of failure, for patients with clinical group III RMS and did not vary by histology, nodal status, or primary site. Tumor size ≥5 cm was associated with a higher rate of local failure (25% vs 10%, P = .0004). Future protocols will explore methods for improving local control of larger tumors.
Acknowledgments
Supported by grants U10CA24507, U10CA072989, U10CA180886, U10CA180899, U10CA098543, and U10CA098413 from the National Cancer Institute/National Institutes of Health.
The authors thank Lawrence A. Herman for editorial assistance.
Footnotes
Conflict of interest: none.
References
- 1.Hawkins DS, Spunt SL, Skapek SX, et al. Children’s Oncology Group’s 2013 blueprint for research: Soft tissue sarcomas. Pediatr Blood Cancer. 2013;60:1001–1008. doi: 10.1002/pbc.24435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arndt CA, Stoner JA, Hawkins DS, et al. Vincristine, actinomycin, and cyclophosphamide compared with vincristine, actinomycin, and cyclophosphamide alternating with vincristine, topotecan, and cyclophosphamide for intermediate-risk rhabdomyosarcoma: Children’s Oncology Group study D9803. J Clin Oncol. 2009;27:5182–5188. doi: 10.1200/JCO.2009.22.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rudzinski ER, Teot LA, Anderson JR, et al. Dense pattern of embryonal rhabdomyosarcoma, a lesion easily confused with alveolar rhabdomyosarcoma: A report from the Soft Tissue Sarcoma Committee of the Children’s Oncology Group. Am J Clin Pathol. 2013;140:82–90. doi: 10.1309/AJCPA1WN7ARPCMKQ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodeberg DA, Wharam MD, Lyden ER, et al. Delayed primary excision with subsequent modification of radiotherapy dose for intermediate-risk rhabdomyosarcoma: A report from the Children’s Oncology Group Soft Tissue Sarcoma Committee. Int J Cancer. 2014;137:204–211. doi: 10.1002/ijc.29351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wharam MD, Meza J, Anderson J, et al. Failure pattern and factors predictive of local failure in rhabdomyosarcoma: A report of group III patients on the third Intergroup Rhabdomyosarcoma Study. J Clin Oncol. 2004;22:1902–1908. doi: 10.1200/JCO.2004.08.124. [DOI] [PubMed] [Google Scholar]
- 6.Crist WM, Anderson JR, Meza JL, et al. Intergroup rhabdomyosarcoma study-IV: Results for patients with nonmetastatic disease. J Clin Oncol. 2001;19:3091–3102. doi: 10.1200/JCO.2001.19.12.3091. [DOI] [PubMed] [Google Scholar]
- 7.Ladra MM, Edgington SK, Mahajan A, et al. A dosimetric comparison of proton and intensity modulated radiation therapy in pediatric rhabdomyosarcoma patients enrolled on a prospective phase II proton study. Radiother Oncol. 2014;113:77–83. doi: 10.1016/j.radonc.2014.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang JC, Wexler LH, Meyers PA, et al. Parameningeal rhabdomyosarcoma: Outcomes and opportunities. Int J Radiat Oncol Biol Phys. 2013;85:e61–e66. doi: 10.1016/j.ijrobp.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 9.Crist W, Gehan EA, Ragab AH, et al. The Third Intergroup Rhabdomyosarcoma Study. J Clin Oncol. 1995;13:610–630. doi: 10.1200/JCO.1995.13.3.610. [DOI] [PubMed] [Google Scholar]
- 10.Donaldson SS, Meza J, Breneman JC, et al. Results from the IRS-IV randomized trial of hyperfractionated radiotherapy in children with rhabdomyosarcoma–a report from the IRSG. Int J Radiat Oncol Biol Phys. 2001;51:718–728. doi: 10.1016/s0360-3016(01)01709-6. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg AR, Anderson JR, Lyden E, et al. Early response as assessed by anatomic imaging does not predict failure-free survival among patients with Group III rhabdomyosarcoma: A report from the Children’s Oncology Group. Eur J Cancer. 2014;50:816–823. doi: 10.1016/j.ejca.2013.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mandeville H, Ladra M, Padera T, et al. Local failure in parameningeal rhabdomyosarcoma: Does response to induction chemotherapy matter? Int J Radiat Oncol Biol Phys. 2013;87:S70. doi: 10.1016/j.ijrobp.2015.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casey DL, Wexler LH, Fox JJ, et al. Predicting outcome in patients with rhabdomyosarcoma: Role of [(18)f]fluorodeoxyglucose positron emission tomography. Int J Radiat Oncol Biol Phys. 2014;90:1136–1142. doi: 10.1016/j.ijrobp.2014.08.005. [DOI] [PubMed] [Google Scholar]