Abstract
We evaluated 44 novel cationic compounds for activity against metronidazole-sensitive and -resistant Trichomonas vaginalis isolates. Six compounds in three different structural classes demonstrated 50% inhibitory concentrations as low as 1 μM against both sensitive and resistant isolates, suggesting a mode of action independent of parasite biochemical pathways that confer resistance to 5-nitroimidazoles.
Trichomoniasis is a common sexually transmitted disease caused by the protozoan parasite Trichomonas vaginalis. An estimated 170 million persons are infected with T. vaginalis worldwide (24). Clinical manifestations range from an asymptomatic presentation to vaginitis, dyspareunia, and strawberry cervix in women and urethritis in men. Trichomoniasis has also been associated with premature birth, low infant birth weight, and increased susceptibility to human immunodeficiency virus infection (7, 20).
Metronidazole has been the principal drug used to treat trichomoniasis since it was introduced in 1960 (12). Although resistance to metronidazole was first reported in 1962 (17), the drug is still effective, successfully treating approximately 90 to 95% of infections (5). However, metronidazole treatment does not cure all patients, and recognition of resistance is increasing. Requests to the Centers for Disease Control and Prevention (CDC) for evaluation of metronidazole resistance in clinical isolates have increased in number from 15 in 1995 to over 100 in 2003 (W. E. Secor, K. A. Workowski, and D. J. Mosure, unpublished observations). In addition, side effects such as gastrointestinal discomfort and nausea are commonly reported (18) and, with hypersensitivity reactions (15), can be severe enough to preclude the use of metronidazole for treating some individuals.
It was recently reported that tinidazole is efficacious against T. vaginalis isolates at lower minimal lethal concentrations (MLCs) than metronidazole (8), a finding also supported by clinical observations (19). Like metronidazole, tinidazole is a 5-nitroimidazole, and for isolates with high levels of resistance to metronidazole, tinidazole MLCs are also high (8). In addition, although tinidazole use results in fewer common side effects than the use of metronidazole, it is possible that persons with hypersensitivity reactions to metronidazole may also have adverse reactions to tinidazole. Although tinidazole may prove to be useful in many cases of metronidazole treatment failure, the identification of nonnitroimidazole compounds that have efficacy against trichomonads is desirable.
Dicationic, aromatic diamidine compounds that bind the minor groove of DNA have activity against a wide spectrum of protozoan parasites (23). For example, pentamidine is used to treat African trypanosomiasis and antimony-resistant leishmaniasis. However, pentamidine must be administered parenterally; it causes potentially severe host side effects, and drug resistance among parasites is emerging. These factors have led to recent research on compounds that are structurally related to pentamidine and retain antiparasite activity but demonstrate decreased toxicity for mammalian cells. Chemically synthesized diamidine compounds have activity against Cryptosporidium parvum (2), Leishmania donovani (1, 22), Plasmodium falciparum (1), Trypanosoma brucei (14), and Trypanosoma cruzi (22) and the fungi Candida albicans, Cryptococcus neoformans, and Aspergillus fumigatus (10, 11). In addition, appropriate chemical design of prodrugs for these compounds can confer systemic bioavailability following oral administration (23). Such broad-spectrum activity for these related drugs stimulated us to evaluate them for activity against T. vaginalis.
Reference strains CDC085 and CDC520, which are metronidazole resistant and sensitive, respectively, were maintained at 37°C in Diamond's Trypticase-yeast-maltose medium (pH 6.0). Metronidazole, tinidazole, pentamidine, and Berenil were purchased from Sigma Chemical Co. (St. Louis, Mo.). Drugs DB75 (9), DB289 (3), DB181 and 249 (4), DB690 (13), DB673 (21), and DB818 (15a) were synthesized as previously reported; purity was determined by nuclear magnetic resonance (NMR) and thin-layer chromatography. The syntheses of DB507 and DB364 are outlined below.
4,4′-Bis{2-[(4-amidino) benzimidazolyl]}biphenyl tetrahydrochloride (DB507) was prepared by heating a mixture of 4,4′-diformyl-1,1′-biphenyl (0.21 g; 0.001 mol), 4-amidino-1,2-phenylenediamine hydrochloride hemihydrate (0.39 g; 0.002 mol), and 1,4-benzoquinone (0.216 g; 0.002 mol) in ethanol at reflux for 12 h. The solvent was reduced to one-third of the original volume, followed by dilution with ether and then filtration, yielding a dark solid. The solid was dissolved in a large volume of hot ethanol and filtered; the solution was treated with 10 ml of HCl gas-saturated ethanol and stirred. The solvent was reduced to one-third of the original volume and diluted with ether. A dark hydrochloride salt precipitated, which was filtered, washed with ether, and dried in vacuum at 75°C for 24 h to yield 0.43 g (66%). Melting point, >300°C decomposition; 1H NMR (dimethyl sulfoxide [DMSO]-d6): 8.35 (d, 4H, J = 7.6 Hz), 8.21 (s, 2H), 8.02 (d, 4H, J = 7.6 Hz), 7.85 (d, 4H, J = 8.4 Hz), 7.50 (d, 4H, J = 8.4 Hz). 13C NMR (DMSO-d6): 166.0, 153.2, 141.4, 137.5, 128.4, 127.8, 126.9, 123.4, 122.6, 116.2, 115.1. Fast atom bombardment mass spectra: m/e 483 (M+ + 1). Analysis calculated for C29H22N8 · 4HCl · 1.5H2O: C, 53.41; H, 4.46; N, 17.09. Found: C, 52.97; H, 4.61; N, 17.17.
2,5-Bis{2-[5-(N-isopropylamidino)benzimidazoyl]}benzo[b] furan tetrahydrochloride (DB364) was prepared using a protocol similar to that described for DB507. Condensation of benzo[b]furan-2,5-dicarboxaldehyde and 4-N-isopropylamidino-1,2-phenylenediamine gave a metallic green solid with a 69% yield. Melting point, 285 to 290°C. 1H NMR (DMSO-d6/80°C): 8.71 (s, 1H), 8.36 (d, 1H, J = 8.8 Hz), 8.08 (d, 2H, J = 9.2 Hz), 7.98 (d, 1H, J = 8.8 Hz), 7.96 (s, 1H), 7.85 (d, 1H, J = 8.8 Hz), 7.82 (d, 1H, J = 8.8 Hz), 7.64 (d, 1H, J = 8.8 Hz), 7.61 (d, 1H, J = 8.8 Hz), 4.02 (broad q, 2H, J = 6 Hz), 1.32 (broad d, 12H, J = 6 Hz). 13C NMR (DMSO-d6/D2O/80°C): 162.9, 162.6, 157.5, 152.9, 147.4, 145.3, 141.1, 138.7, 137.8, 134.9, 129.2, 126.9, 125.7, 125.0, 124.5, 123.8, 123.2, 121.4, 116.9, 116.1, 115.7, 115.5, 113.9, 109.4, 46.2, 46.1, 21.6 (signals overlap). Analysis calculated for C30H30N8 · 4HCl · 0.5H2O: C, 53.49; H, 5.23; N, 16.64. Found: C, 53.53; H, 5.29; N, 16.45.
Compounds were dissolved in DMSO (Sigma) and further diluted with Diamond's Trypticase-yeast-maltose medium to reach assay concentrations. Two types of assays were performed on the synthesized cationic compounds being evaluated. An initial screen was performed using the standard MLC assay (8, 16), with a maximum concentration of 20 μM for 44 test compounds. Each compound was tested at least twice under both aerobic and anaerobic conditions. Anaerobic conditions were generated with a GasPak jar and CO2-generating GasPak Plus anaerobic system envelopes (Becton Dickinson, Sparks, Md.) and monitored with GasPak disposable anaerobic indicator strips (Becton Dickinson). Compounds that showed no activity were not tested further.
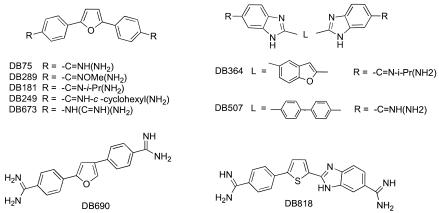
The structures of the six compounds that had some effect in the MLC assay as well as three related but ineffective compounds are shown in Fig. 1. To further evaluate their activities, a second type of assay was used to determine the concentration at which 50% of the parasite growth was inhibited (IC50). In these assays, 0.5 μCi of tritiated thymidine (Perkin-Elmer, Boston, Mass.) was added to each well of a standard assay at the initiation of culture. At 48 h of incubation under either aerobic or anaerobic conditions, cells were harvested onto glass fiber filters (Wallac, Turku, Finland) with a Tomtec (Hamden, Conn.) cell harvester. Incorporated thymidine was detected by using Betaplate (Wallac) scintillation fluid and plate reader. The resulting counts per minute over the compound's concentration range were utilized to calculate IC50s with Prism software (GraphPad Software, Inc., San Diego, Calif.).
FIG. 1.
Structures of dicationic compounds evaluated in this study.
Out of 44 dicationic compounds tested in the MLC assay, six compounds showed sufficient activity for further evaluation and IC50 calculation. Table 1 contains their in vitro IC50s against metronidazole-sensitive and -resistant T. vaginalis isolates as well as comparison data for the nitroimidazoles metronidazole and tinidazole. We also evaluated the classical antiprotozoan dicationic molecules pentamidine and Berenil. Interestingly, these two compounds are ineffective against T. vaginalis. In contrast, furamidine (DB75), the parent molecule in the 2,5-diphenylfuran family of diamidines, shows activity comparable to that of metronidazole against CDC520, the metronidazole-sensitive isolate. It is clearly more effective than either metronidazole or tinidazole against the resistant strain CDC085 under aerobic conditions. These data suggest a different mode of action for DB75 and the nitroimidazoles. The N-alkyl analogs of furamidine, DB181 and DB249, show similar in vitro effectiveness. Interestingly, DB673, a guanidino analog of DB75, and DB690, the 2,4-diphenylfuran isomer of DB75, are not effective against T. vaginalis in these assays. Thus, we find that the 2,5-diphenyl furan family of dications is quite effective against T. vaginalis in vitro but that the activity is quite sensitive to structure, as demonstrated by the lack of activity of DB690 and DB673. Not unexpectedly, DB289, the prodrug for DB75, was generally ineffective in vitro, as the mammalian biochemical pathways necessary to convert it to the active form were not present. Oral administration of DB289 provides systemic, efficacious concentrations of DB75, and this compound is currently in phase II trials for treatment of African trypanosomiasis (23). Our results suggest that it should also be evaluated for clinical efficacy against trichomonads, especially those that are resistant to metronidazole and tinidazole.
TABLE 1.
IC50s of compounds evaluated under aerobic and anaerobic conditions in this study
| Compound | Strain CDC085
|
Strain CDC520
|
||||||
|---|---|---|---|---|---|---|---|---|
| Aerobic
|
Anaerobic
|
Aerobic
|
Anaerobic
|
|||||
| na | IC50b | n | IC50 | n | IC50 | n | IC50 | |
| Metronidazole | 61 | 302.6 ± 22.2 | 62 | 12.3 ± 23.8 | 38 | 18.2 ± 4.25 | 31 | 1.89 ± 0.77 |
| Tinidazole | 5 | 45.1 ± 5.38 | 4 | 4.81 ± 1.17 | 2 | 1.48 ± 0.12 | 2 | 0.004 ± 0.0 |
| Pentamidine | 3 | No effect | 3 | No effect | 3 | No effect | 3 | No effect |
| Berenil | 3 | No effect | 3 | No effect | 3 | No effect | 3 | No effect |
| DB75 | 8 | 8.12 ± 2.45 | 7 | 18.6 ± 19.8 | 4 | 18.6 ± 6.43 | 4 | 57.9 ± 19.6 |
| DB289 | 3 | 39.1 ± 25.1 | 2 | No effect | 2 | No effect | 2 | No effect |
| DB181 | 7 | 6.44 ± 3.89 | 7 | 9.41 ± 7.92 | 7 | 6.60 ± 1.76 | 7 | 3.91 ± 1.06 |
| DB249 | 6 | 22.4 ± 13.7 | 6 | 10.2 ± 8.47 | 2 | 15.9 ± 0.74 | 3 | 13.9 ± 3.74 |
| DB364 | 6 | 7.27 ± 3.76 | 5 | 37.6 ± 43.9 | 2 | 44.7 ± 9.33 | 3 | 143.1 ± 127 |
| DB507 | 8 | 7.79 ± 4.20 | 6 | 25.3 ± 43.1 | 2 | No effect | 2 | No effect |
| DB690 | 7 | No effect | 6 | No effect | 3 | No effect | 3 | No effect |
| DB673 | 7 | No effect | 6 | No effect | 3 | No effect | 3 | No effect |
| DB818 | 7 | 0.27 ± 0.04 | 7 | 0.50 ± 0.42 | 6 | 0.98 ± 0.19 | 6 | 1.24 ± 0.39 |
n, number of times assay was performed. Drugs that did not show an effect in this assay were usually not tested more than two or three times.
IC50s are given as micromolar concentrations (means ± standard errors of the mean).
The bis-benzimidazoles DB364 and DB507 are also quite effective antitrichomonads. Interestingly, DB507 has activity against the metronidazole-resistant CDC085 strain but not against the metronidazole-sensitive CDC520 isolate. Thus, DB507 may be a useful tool to evaluate the biochemical basis of T. vaginalis resistance to metronidazole. The most effective dication studied is the mono-benzimidazole DB818. This compound demonstrated IC50s of 1 μM or less for both metronidazole-sensitive and -resistant isolates under either aerobic or anaerobic conditions. The mono-benzimidazole DB818 has been successfully used in vivo to treat a different protozoal infection in an experimental model without overt evidence of toxicity to the host (R. Brun and D. W. Boykin, unpublished observations). Clearly, like the 2,5-diphenylfurans, this compound merits further evaluation in vivo against T. vaginalis.
In conclusion, aromatic diamidines that bind to the minor groove of DNA at AT sites have potential as antitrichomonad agents. In addition, a recent paper (6) reports that another class of dicationic DNA minor-groove-binding compounds (bis-quaternary quinoliniums) is also effective against T. vaginalis. Together, these studies reinforce the utility of compounds that bind the minor groove of DNA as effective antitrichomonad agents and substantiate the need for further research of this interaction.
Acknowledgments
A.L.C. was supported by the Emerging Infectious Diseases Fellowship Program, administered by the Association of Public Health Laboratories. The Office of Minority and Women's Health, National Center for Infectious Diseases, CDC, funded the work. Compound synthesis activities were supported by NIH grant NIAID RO1AI46365. Additional support was provided by the VAMC Atlanta and the Atlanta Research and Education Foundation.
REFERENCES
- 1.Bell, C. A., J. E. Hall, D. E. Kyle, M. Grogl, K. A. Ohemeng, M. A. Allen, and R. R. Tidwell. 1990. Structure-activity relationships of analogs of pentamidine against Plasmodium falciparum and Leishmania mexicana amazonensis. Antimicrob. Agents Chemother. 34:1381-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blagburn, B. L., K. L. Drain, T. M. Land, R. G. Kinard, P. H. Moore, D. S. Lindsay, D. A. Patrick, D. W. Boykin, and R. R. Tidwell. 1998. Comparative efficacy evaluation of dicationic carbazole compounds, nitrazoxanide, and paromomycin against Cryptosporidium parvum infections in a neonatal mouse model. Antimicrob. Agents Chemother. 42:2877-2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boykin, D. W., A. Kumar, B. K. Bender, J. E. Hall, and R. R. Tidwell. 1996. Anti-pneumocystis activity of bis-amidoximes and bis-o-alkylamidoximes prodrugs. Bioorg. Med. Chem. Lett. 6:3017-3020. [Google Scholar]
- 4.Boykin, D. W., A. Kumar, G. Xiao, W. D. Wilson, B. K. Bender, D. R. McCurdy, J. E. Hall, and R. R. Tidwell. 1998. 2,5-Bis-[4(N-alkylamidino)phenyl]furans as anti-Pneumocystis carinii agents. J. Med. Chem. 41:124-129. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 1993. Sexually transmitted diseases treatment guidelines. Morb. Mortal. Wkly. Rep. 42(RR-14):70-72. [Google Scholar]
- 6.Chavalitshewinkoon-Petmitr, P., M. Ramdja, S. Kajorndechakiat, R. K. Ralph, W. A. Denny, and P. Wilairat. 2003. In vitro susceptibility of Trichomonas vaginalis to AT-specific minor groove binding drugs. J. Antimicrob. Chemother. 52:287-289. [DOI] [PubMed] [Google Scholar]
- 7.Cotch, M. F., J. G. Pastorek II, R. P. Nugent, S. L. Hillier, R. S. Gibbs, D. H. Martin, D. A. Eschenbach, R. Edelman, J. C. Carey, J. A. Regan, M. A. Krohn, M. A. Klebanoff, A. V. Rao, and G. G. Rhoads. 1997. Trichomonas vaginalis associated with low birth weight and preterm delivery. Sex. Transm. Dis. 24:353-360. [DOI] [PubMed] [Google Scholar]
- 8.Crowell, A. L., K. A. Sanders-Lewis, and W. E. Secor. 2003. In vitro metronidazole and tinidazole activities against metronidazole-resistant strains of Trichomonas vaginalis. Antimicrob. Agents Chemother. 47:1407-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das, B. P., and D. W. Boykin. 1977. Synthesis and antiprotozoal activity of 2,5-bis-(4-guanylphenyl)furans. J. Med. Chem. 20:531-536. [DOI] [PubMed] [Google Scholar]
- 10.Del Poeta, M., W. A. Schell, C. C. Dykstra, S. Jones, R. R. Tidwell, A. Czarny, M. Bajic, M. Bajic, A. Kumar, D. Boykin, and J. R. Perfect. 1998. Structure-in vitro activity relationships of pentamidine analogues and dication-substituted bis-benzimidazoles as new antifungal agents. Antimicrob. Agents Chemother. 42:2495-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Del Poeta, M., W. A. Schell, C. C. Dykstra, S. K. Jones, R. R. Tidwell, A. Kumar, D. W. Boykin, and J. R. Perfect. 1998. In vitro antifungal activities of a series of dication-substituted carbazoles, furans, and benzimidazoles. Antimicrob. Agents Chemother. 42:2503-2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durel, P., V. Roiron, A. Siboulet, and L. J. Borel. 1960. Systemic treatment of human trichomoniasis with a derivative of nitroimidazole 8823 R.P. Br. J. Vener. Dis. 36:21-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Francesconi, I., W. D. Wilson, F. A. Tanious, J. E. Hall, B. K. Bender, D. R. McCurdy, R. R. Tidwell, and D. W. Boykin. 1999. 2,4-Diphenyl furan diamidines as novel anti-Pneumocystis carinii pneumonia agents. J. Med. Chem. 42:2260-2265. [DOI] [PubMed] [Google Scholar]
- 14.Ismail, M., R. Brun, F. Tanious, W. D. Wilson, and D. W. Boykin. 2003. Synthesis and anti-protozoal activity of aza-analogs of furamidine. J. Med. Chem. 46:4761-4769. [DOI] [PubMed] [Google Scholar]
- 15.Kurohara, M. L., F. K. Kwong, T. B. Lebherz, and W. B. Klaustermeyer. 1991. Metronidazole hypersensitivity and oral desensitization. J. Allergy Clin. Immunol. 88:279-280. [DOI] [PubMed] [Google Scholar]
- 15a.Mallena, S., M. P. H. Lee, C. Reilly, S. Neidle, A. Kumar, D. W. Boykin, and W. D. Wilson. A thiophene based diamidine forms a “super” AT binding minor groove agent. J. Am. Chem. Soc., in press. [DOI] [PubMed]
- 16.Meingassner, J. G., and J. Thurner. 1979. Strain of Trichomonas vaginalis resistant to metronidazole and other 5-nitroimidazoles. Antimicrob. Agents Chemother. 15:254-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson, S. C. 1962. Trichomonal vaginitis resistant to metronidazole. Can. Med. Assoc. J. 86:665. [PMC free article] [PubMed] [Google Scholar]
- 18.Smilack, J. D., W. R. Wilson, and F. R. Cockerill III. 1991. Tetracyclines, chloramphenicol, erythromycin, clindamycin, and metronidazole. Mayo Clin. Proc. 66:1270-1280. [DOI] [PubMed] [Google Scholar]
- 19.Sobel, J. D., P. Nyirjesy, and W. Brown. 2001. Tinidazole therapy for metronidazole-resistant vaginal trichomoniasis. Clin. Infect. Dis. 33:1341-1346. [DOI] [PubMed] [Google Scholar]
- 20.Sorvillo, F., and P. Kerndt. 1998. Trichomonas vaginalis and amplification of HIV-1 transmission. Lancet 351:213-214. [DOI] [PubMed] [Google Scholar]
- 21.Stephens, C. E., F. A. Tanious, S. Kim, W. D. Wilson, W. A. Schell, J. R. Perfect, S. G. Franzblau, and D. W. Boykin. 2001. Diguanidino and “reversed” diamidino 2,5-diarylfurans as antimicrobial agents. J. Med. Chem. 44:1741-1748. [DOI] [PubMed] [Google Scholar]
- 22.Stephens, C. E., R. Brun, M. M. Salem, K. A. Werbovetz, F. A. Tanious, W. D. Wilson, and D. W. Boykin. 2003. The activity of diguanidino and “reversed” diamidino 2,5-diarylfurans versus Trypanosoma cruzi and Leishmania donovani. Bioorg. Med. Chem. Lett. 13:2065-2069. [DOI] [PubMed] [Google Scholar]
- 23.Tidwell, R. R., and D. W. Boykin. 2003. Dicationic DNA minor groove binders as antimicrobial agents, p. 416-460. In M. Demeunynck, C. Bailly, and W. D. Wilson (ed.), Small molecule DNA and RNA binders: from synthesis to nucleic acid complexes, vol. 2. Wiley-VCH, New York, N.Y. [Google Scholar]
- 24.World Health Organization. 1995. Global programme on AIDS, 1992-1993 progress report, p. 2-27. World Health Organization, Geneva, Switzerland.