Abstract
Background
The prevalence of pedal edema and its associations with abnormal cardiac structure/function, natriuretic peptides(NP), and incident heart failure (HF) is unknown, especially in community dwelling adults without a history cardiovascular disease(CVD).
Methods and Results
Out of 5004 MESA participants who had cardiac magnetic resonance imaging, 4196 had complete data and were included in this analysis (3501 for the Right Ventricle analysis). Logistic regression and Cox proportional hazard analyses were used to assess the associations among self-reported pedal edema(PE), 2 pillow orthopnea, paroxysmal nocturnal dyspnea(PND), left and right ventricular structure and function, NP levels and incident HF. PE was present in 28% of participants. PE was not associated with overt left or right ventricular systolic dysfunction (ejection fraction <50%). PE was associated with 2-pillow orthopnea (odds ratio [OR] 1.66 [95% confidence interval (CI) 1.30–2.12]), PND (OR 1.95 [95% CI 1.55–2.44]) and abnormal N-terminal pro-B-type natriuretic peptide (NTproBNP) levels (defined as >400pg/ml) (OR 1.80 [95% CI 1.21–2.68]) in adjusted models. After a mean of 10.2 years of follow up 184/4196 (4.4%) had an adjudicated incident HF hospitalization. PE was associated with incident HF hospitalization in models adjusted for age, gender and race (hazard ratio [HR] 1.44 [95% CI: 1.05–1.97]). This association persisted after adding additional covariates including comorbidities, baseline LVEF, and antecedent myocardial infarction (HR 1.43 [95% CI 1.02–1.99]). The association of PE with incident HF was attenuated by further adjustment for NT-proBNP.
Conclusions
PE is prevalent in community dwelling adults without clinically recognized CVD and associated with future hospitalized HF.
Keywords: pedal edema, heart failure, natriuretic peptides, orthopnea, paroxysmal nocturnal dyspnea, cardiac structure and function
Subject Codes: Epidemiology, Risk factors, Primary Prevention, Heart failure
Pedal edema (foot and ankle swelling) is one of the cardinal signs of congestive heart failure (HF) but can also be due to other systemic or local conditions, including chronic kidney disease, liver disease, thyroid disorders, venous insufficiency, and venous thrombosis1. Often times physicians are confronted with pedal edema as an isolated complaint in a relatively asymptomatic patient. The finding of pedal edema typically triggers investigations such as an echocardiogram and B-type natriuretic peptide (BNP) testing to rule out HF as the cause2. A normal BNP and normal biventricular systolic function are often considered helpful for excluding HF3. However, it is now clear that many patients have HF with a preserved ejection fraction (HFpEF) and that many patients with HFpEF have normal BNP levels4–6.
Currently there are no data on the prevalence of pedal edema in the general population without clinically recognized cardiovascular disease (CVD). It is also unclear whether isolated pedal edema is a benign process, a sign of early HF or a harbinger of future clinically overt (hospitalized) HF. Given the difficulty in recognizing (and under-appreciation) of the clinical syndrome of HFpEF, and the fact that epidemiological estimates of HF (including HFpEF) are based primarily on hospitalized HF, it is possible that pedal edema—when combined with other symptoms of HF, abnormalities in cardiac structure/function, and BNP levels—could provide insight into the true prevalence of HF (particularly early HF) in the community.
We therefore examined pedal edema in a multi-ethnic, community-based cohort free of overt CVD (the Multi-Ethnic Study of Atherosclerosis [MESA]), with the following hypotheses: (1) pedal edema is common in community dwelling adults without known CVD, and (2) despite multiple possible causes of pedal edema, it is frequently associated with indices of abnormal cardiac function, suggesting that early HF, particularly early HFpEF, is more common than previously appreciated. Here we report the association between self-reported pedal edema, symptoms of HF, biventricular structure and function, N-terminal proBNP (NTproBNP) levels, and incident hospitalized HF in MESA.
Methods
Study Population and Data Collection
A detail study design for MESA has been published elsewhere7. In brief, MESA is a prospective cohort study begun in July 2000 to investigate the prevalence, correlates, and progression of subclinical CVD in individuals without known CVD at baseline. The cohort includes 6814 women and men aged 45–84 years old recruited from 6 US communities (Baltimore, MD; Chicago, IL; Forsyth County, NC; Los Angeles County, CA; northern Manhattan, NY; and St. Paul, MN). MESA participants were 38% white (n = 2624), 28% black (n = 1895), 22% Hispanic (n = 1492) and 12% Chinese (n = 803). Individuals with a history of physician-diagnosed myocardial infarction, angina, heart failure, stroke or transient ischemic attack, or who had undergone an invasive procedure for CVD (coronary artery bypass graft, angioplasty, valve replacement, pacemaker placement or other vascular surgeries) were excluded. This study was approved by the Institutional Review Boards of each study site and written informed consent was obtained from all participants.
Demographics, medical history, anthropometric and laboratory data for this study were obtained at the first MESA examination (July 2000 to August 2002). Current smoking was defined as having smoked a cigarette in the last 30 days. Diabetes mellitus was defined as fasting glucose ≥126 mg 100 ml−1 or the use of hypoglycemic medications. Use of antihypertensive and other medications was based on the review of prescribed medication containers. Resting blood pressure was measured three times in seated position, and the average of the second and third readings was recorded. Hypertension was defined as a systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg or use of medication prescribed for hypertension. Body mass index was calculated as weight (kg)/height (m2). Total and high-density lipoprotein cholesterol were measured from blood samples obtained after a 12-h fast. Low-density lipoprotein cholesterol was estimated by the Friedewald equation8.
MESA Questionnaire Variables
During the baseline MESA examination, a questionnaire was administered to participants. Participants answered “yes”, “no” and “don’t know” to questions such as “do you experience swelling of your feet or ankles”, “how many pillows do you sleep on at night” and “do you sometimes wake up at night with trouble breathing”. Participants who responded “yes” having feet or ankle swelling were labelled as having pedal edema. Participants who sleep on more than 2 pillows were labelled as having orthopnea and those who wake up at night with trouble breathing were labelled as having paroxysmal nocturnal dyspnea (PND). Participants who responded “don’t know’ were excluded from the present analysis (n=15).
Cardiac Magnetic Resonance Imaging
Consenting participants underwent a cardiac MRI scan a median of 16 days after the baseline evaluation; 95% were completed within 11 weeks after the baseline examination. Participation in the MRI exam was voluntary unless participants have contra indications such as metal implants. All imaging was done with a four-element phased-array surface coil positioned anteriorly and posteriorly, electrocardiographic gating, and brachial artery blood pressure monitoring9. Imaging consisted of fast gradient echo cine images of the left ventricle with time resolution < 50 ms. Functional parameters and mass were determined by volumetric imaging. Imaging data were read using MASS software (version 4.2, Medis, Leiden, the Netherlands) at a single reading center by trained readers blinded to risk factor information. Papillary muscles were included in the LV volumes and excluded from LV mass. LV end-diastolic volume and LV end-systolic volume were calculated using Simpson’s rule (the summation of areas on each separate slice multiplied by the sum of slice thickness and image gap). LV mass was determined by the sum of the myocardial area (the difference between endocardial and epicardial contour) times slice thickness plus image gap in the end-diastolic phase multiplied by the specific gravity of myocardium (1.05 g/mL). LVEF was calculated as LV stroke volume/LV end-diastolic volume X 100. The interobserver variability in estimating LV parameters was: LVEF (5.1%, 95% CI 3.6, 6.7) and intraobserver variability in estimating LV parameters was: LVM (6.3 gm, 95% CI, 5.17, 7.38); LVEF (3.9%, 95% CI, 3.06, 4.72). Left ventricular systolic dysfunction (LVSD) was defined as LVEF <50%. 5004 out of 6814 MESA participants had cardiac MRI during the baseline examination.
The cardiac MRI protocol and interpretation of right ventricular (RV) parameters has previously been described10–12. The endocardial and epicardial borders of the RV were traced manually on the short-axis cine images at the end-systolic and end-diastolic phases. Full visualization of the correct placement of RV contours relied on evaluation of cine images to determine the demarcation between the right atrium and the RV. Contours were modified at basal slices of the heart by careful identification of the tricuspid valve so as to exclude the right atrium and to avoid overestimation of the volumes. The outflow tract was included in the RV volume. Papillary muscles and trabeculae were included in the RV volumes. RVEDV and RVESV were calculated with the Simpson rule by summation of areas on each slice multiplied by the sum of slice thickness and image gap. RV mass was determined at the end-diastole phase as the difference between end-diastolic epicardial and endocardial volumes multiplied by the specific gravity of myocardium (1.05 g/mL). RVSV was calculated by subtracting RVESV from RVEDV; RVEF was calculated by dividing RVSV by RVEDV. The intrareader intraclass correlation coefficient from random, blinded rereads of 229 scans for RVEDV and RVEF were 0.99 and 0.89 respectively. The interreader intraclass correlation coefficients from random, blinded rereads of 240 scans for RVEDV, and RVEF were 0.96, and 0.80, respectively. Right ventricular systolic dysfunction (RVSD) was defined as RVEF <50%13,14. 4204 out of the 5004 MESA participants who had baseline cardiac MRI had RV structure and function assessed.
NT ProBNP Assay
NT-ProBNP levels were measured from serum collected during the baseline MESA exam and stored at −70 degrees Celsius. The serum samples were thawed prior to testing (maximum of 3 freeze-thaw cycles). NT-ProBNP levels were measured using the Elecsys 2010 system (Roche Diagnostic, Indianapolis IN) at a core laboratory (Veteran’s Affairs San Diego Healthcare System, La Jolla, CA). Prior studies have shown that measurement of NT-ProBNP using this assay does not change after 5 freeze-thaw cycles 15,16. Intra-assay and interassay coefficient of variation at various concentrations of NT-ProBNP have been previously reported 16. The analytical measurement range for NT-ProBNP was 5–35,000 pg/dl. Abnormal NT-ProBNP levels was defined as >400pg/dl. 4196 out of the 5004 participants who had cardiac MRI had NTproBNP levels measured.
Ascertainment of Outcomes
Outcomes in MESA are adjudicated by a committee which includes a cardiologist, a cardiovascular physician-epidemiologist and a neurologist. Reviewers/adjudicators classified incident hospitalized HF as definite, probable, or absent. Definite or probable HF required heart failure symptoms, such as shortness of breath or edema; probable HF required HF diagnosed by a physician and patient receiving medical treatment for HF. Definite HF required one or more other criteria, such as pulmonary edema/congestion by chest X-ray; dilated ventricle or poor LV function by echocardiocardiography or ventriculography; or echocardiography evidence of left ventricular diastolic dysfunction. Individuals with adjudicated definite or probable HF were used in our analysis. All–cause mortality (death) was also adjudicated by committee.
Statistical Analysis
Demographic characteristics of participants who responded “yes” to ankle and feet swelling (pedal edema) and those who reported “No” (no pedal edema) are reported as mean±SD for continuous variables and as counts and percentages for categorical variables. T-tests were used for continuous variables and Chi squared tests were used for categorical variables for the comparison of baseline variables between those with vs. without pedal edema. Non-parametric tests were used for non-normally distributed continuous variables. Logistic regression analysis was used to assess the association between pedal edema and orthopnea, PND, LVSD, RVSD, and abnormal NT-ProBNP, on univariable and multivariable analyses. Covariates for the multivariable model were chosen based on clinical relevance and included the following potential confounders: age, gender, race/ethnicity, BMI, systolic BP, diabetes mellitus, cigarette smoking status and serum creatinine levels. A relatively small number of participants had LVSD and RVSD (Table 1), therefore as a sensitivity analysis, the association between pedal edema and RV ejection fraction and LV ejection fraction was also assessed using the general linear model.
Table 1.
Participant characteristics, stratified by presence or absence of pedal edema (N=4196)
| Variables | No Pedal edema (N=3010) (Mean± SD or %age) | Pedal edema (N=1186) (Mean± SD or %age) | P value |
|---|---|---|---|
|
| |||
| Age (yrs.) | 61.1± 10.2 | 63.5± 10.1 | <0.001 |
|
| |||
| Female (%) | 43.6 | 69.7 | < 0.001 |
|
| |||
| Race/Ethnicity (%) | <0.0001 | ||
| Caucasian | 40.5 | 36.7 | |
| Chinese | 17.4 | 6.8 | |
| African American | 19.9 | 30.4 | |
| Hispanic | 22.2 | 26.0 | |
|
| |||
| Body mass index(kg/m2) | 26.9± 4.5 | 29.7± 5.4 | <0.001 |
|
| |||
| Blood Pressure(mmHg) | |||
| Systolic | 123.8± 20.5 | 130.1± 30.1 | <0.001 |
| Diastolic | 71.9± 10.1 | 71.2± 10.8 | 0.86 |
|
| |||
| Blood Pressure Meds (%) | 26.3 | 43.5 | <0.001 |
| Any CCB | 9.8 | 17.4 | |
| Any Diuretics | 8.5 | 21.6 | |
| Any ACEI | 9.7 | 15.6 | |
| Any Beta Blocker | 7.6 | 12.4 | |
|
| |||
| Cigarette Smoking (%) | 0.41 | ||
| Never | 52.1 | 52.2 | |
| Former | 35.2 | 36.6 | |
| Current | 12.7 | 11.2 | |
|
| |||
| Cholesterol(mg/dl) | |||
| Total | 194.4± 34.9 | 194.2± 37.2 | 0.86 |
| LDL | 117.7± 31.0 | 115.1± 32.2 | 0.002 |
| HDL | 50.4 ±14.8 | 52.4± 15.0 | 0.01 |
| Triglycerides | 133.0± 88.2 | 134.5± 84.9 | 0.60 |
|
| |||
| Diabetes Mellitus | 10.7 | 14.9 | 0.002 |
|
| |||
| Serum Creatinine(mg/dl) | 0.96± 0.23 | 0.96± 0.52 | 0.88 |
|
| |||
| Sleep on > 2 pillows | 6.4 | 13.8 | <0.001 |
|
| |||
| Wake up at night with trouble breathing | 8.1 | 15.3 | <0.001 |
|
| |||
| Estimated GFR(ml/min/1.73m2) | 81.1± 16.4 | 79.8± 20.4 | 0.11 |
|
| |||
| Urinary Albumin(mg/dl) Median(Q1-Q3) | 0.60(0.3–1.2) | 0.7(0.3–1.5) | 0.002 |
|
| |||
| LVEF (%) | 68.7± 7.5 | 69.8 ±7.4 | 0.18 |
| ASLVD (<50%) | 66(2.1) | 15(1.3) | |
|
| |||
| LV mass Index (g/m2) | 78.7 ± 16.5 | 77.5 ± 16.6 | 0.35 |
|
| |||
| LV end diastolic Vol (ml/m2) | 68.9 ± 13.8 | 67.2 ± 13.2 | 0.08 |
|
| |||
| * RVEF (%) | 70.2± 6.5 | 71.2± 6.5 | 0.115 |
| RVSD (<50%) | 0.2 | 0.5 | |
|
| |||
| * RV mass Index(g/m2) | 11.5± 1.9 | 11.1± 1.9 | 0.13 |
|
| |||
| * RV end diastolic Vol(ml/m2) | 67.7± 12.7 | 64.8 ±12.4 | 0.003 |
|
| |||
| NT-ProBNP (pg/ml) Median (Q1-Q3) | |||
| Abnormal NT-ProBNP | 48.2(20.8–95.0) | 71.9(33.5–153.7) | <0.0001 |
| (> 400pg/ml) | 62.3 | 5.1 | |
Right ventricular (RV) measures available in 3501 out of 4196 participants. Q1-Q3: first-third quartiles
Cumulative hazard curves of participants with vs. without incident hospitalized HF were generated and evaluated using the log-rank test. Cox proportional hazard analyses were used to assess the association between pedal edema and incident HF adjusting for covariates (chosen based on clinical or pathophysiological relevance) in 3 models: Model 1 adjusted for age, gender and race/ethnicity; Model 2 adjusted for Model 1 covariates plus systolic BP, BMI, BP medication use, diabetes mellitus, cigarette smoking status, baseline LVEF, HDL, LDL cholesterol, serum creatinine levels, urinary albumin levels and antecedent MI which occurred during the follow up; and Model 3 adjusted for Model 2 covariates plus NT-proBNP levels. We also assessed the effects of mortality as a competing risk in our analyses using the sub distribution proportional hazard models proposed by Fine and Gray17. All statistical analyses were performed using SAS software (version 9.2, SAS Institute, Cary, NC).
Results
Out of the 5004 MESA participants who had cardiac MRI during the baseline exam, 4196 participants responded Yes/No to the questionnaire and were therefore included in this analysis. Supplemental Table 1 shows the demographics and CVD risk factor profile of the present study cohort compared with the total MESA cohort. Total MESA participants (N=6814) was older, and more likely to be female, smoker (former/current) and to have diabetes mellitus compared with the present study cohort (N=4196).
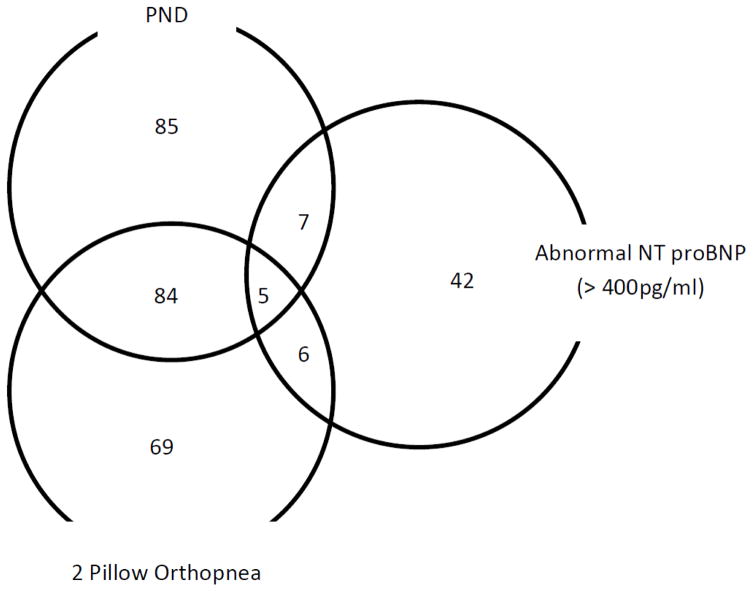
Only 3501 out of the 4196 had data on right ventricular structure and function. 1186/4196 (28.2%) admitted to having pedal edema. 129/4196(3.1%) had abnormal NT proBNP levels and 60/1186 (5.1%) of participants who admitted to having pedal edema also had abnormal NT proBNP. The Venn diagram shows the distribution PND +/−2 pillow orthopnea and an abnormal NT proBNP among MESA participants who reported having pedal edema (298/1186) (Figure 1). 18/256(7.0%) participants with PND+/− 2 pillow orthopnea had abnormal NT proBNP levels. Participants with pedal edema were older, more likely to be females and had worse cardiovascular risk factor distribution compared with those who denied having pedal edema (Table 1). Participants with pedal edema had similar LV/RV structure and functional parameters compared with those with no pedal edema except RV end diastolic volume index which was significantly higher in those with no pedal edema.
Figure 1.
Distribution of Paroxysmal nocturnal dyspnea (PND) +/− 2 pillow orthopnea and abnormal NT pro BNP (> 400pg/ml) among MESA participants with pedal edema (N=298).
In our logistic regression models, pedal edema was not associated with abnormal LV/RV systolic dysfunction in both univariate and multivariable models. However, participants with pedal edema were more likely to sleep on at least 2 pillows and wake up at night with trouble breathing (PND) in our multivariable logistic regression models [hazard ratio (95%CI): 1.66(1.30–2.12) and 1.95(1.55–2.44) respectively]. Pedal edema was also significantly associated with abnormal NT ProBNP levels in univariate and multivariable models [hazard ratio (95%CI): 2.27(1.60–3.20) and 1.80(1.21–2.68) respectively] (Table 2). Pedal edema was also not significantly associated with LV ejection fraction (continuous variable) and RV ejection fraction (continuous variable) in both the univariate (data not shown) and multivariable general linear models [β ± SE: −0.092± 0.26, p=0.72 and −0.162 ± 0.25, p=0.51 respectively].
Table 2.
Logistic regression models showing the association of self-reported pedal edema and left/right ventricular systolic dysfunction (EF <50%) and abnormal NT Pro BNP (>400pg/ml) in MESA
| Univariate Odds Ratio(95% CI) |
P value | Multivariable** Odds Ratio(95%CI) |
P value | |
|---|---|---|---|---|
| LVSD (LVEF < 50%) | 0.61(0.35–1.08) | 0.87 | 0.83(0.45–1.54) | 0.57 |
| RVSD (RVEF <50%)* | 0.70(0.42–1.19) | 0.19 | 0.67(0.38–1.19) | 0.82 |
| Abnormal NT ProBNP( >400 pg/ml) | 2.27(1.60–3.20) | <0.001 | 1.80(1.21–2.68) | 0.004 |
| Sleep on > 2 pillows | 2.36(1.90 – 2.94) | <0.001 | 1.66(1.30–2.12) | <0.001 |
| Wake up at night with trouble breathing | 2.06(1.67 – 2.53) | <0.001 | 1.95(1.55 – 2.44) | <0.001 |
LVSD: left ventricular systolic dysfunction. RVSD*: Only 3501 out of 4196 had RVEF available
adjusted for age, gender, race/ethnicity, BMI, systolic BP, diabetes mellitus, cigarette smoking status, serum creatinine levels
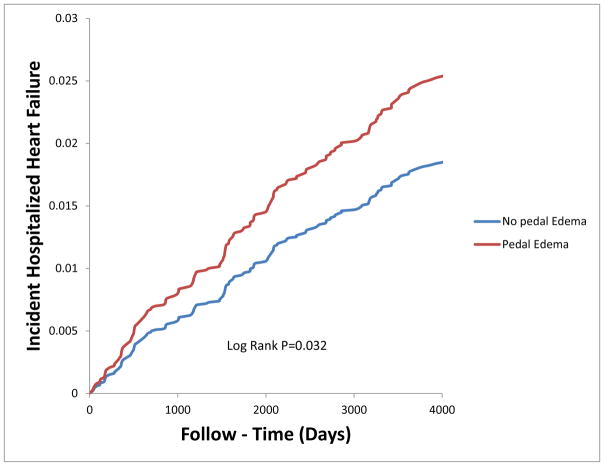
After 10 years of follow up, 184/4196(4.5%) of the participants had an adjudicated hospitalized heart failure [No Pedal edema= 4.0% vs. Pedal edema= 5.4%]. Pedal edema was a predictor of incident HF in univariate analysis (Table 3 and Figure 2). Pedal edema was an independent predictor of future adjudicated HF in our multivariable Cox models (Table 3). The association of pedal edema with incident heart failure was attenuated when NT ProBNP levels (continuous and categorical) was added to the multivariable Cox model. Our sub distribution proportional hazard models assessing mortality as a competing risk of HF yielded similar estimates and confidence intervals as in Table 3. Two pillow orthopnea and PND were not associated with incident CHF in univariate and multivariable models (data not shown) and therefore we not treated as confounders of the association between pedal edema and incident hospitalized HF. However, forcing two pillow orthopnea or PND into our multivariable Cox models did not change our point estimates or 95% confidence intervals.
Table 3.
Cox proportional hazard models showing the association between self-reported pedal edema and incident congestive heart failure
| # of Events | Unadjusted Hazard Ratio (95%CI) |
Model 1* Hazard Ratio (95%CI) |
Model 2** Hazard Ratio (95%CI) |
Model 3*** Hazard Ratio (95%CI) |
|
|---|---|---|---|---|---|
| Pedal Edema | 184/4196 | 1.39(1.03–1.88) P value = 0.032 |
1.44(1.05–1.97) P value= 0.022 |
1.43(1.02–1.99) P value =0.038 |
1.27(0.94–1.79) P value = 0.166 |
Model 1* adjusted for age, gender and race/ethnicity
Model 2** = model1 + systolic BP, BP meds, diabetes mellitus, BMI, cigarette smoking status, baseline LVEF, LDL and HDL cholesterol, serum creatinine and urinary albumin level and antecedent myocardial infarction which occurred during the follow up.
Model # 3*** = model 2 + NT proBNP levels
Figure 2.
Cumulative Hazard curves of participants with and without pedal edema and Incident Hospitalized Heart failure over the follow up period. MESA
Discussion
The goal of this study was to assess the prevalence of pedal edema in community-dwelling adults without clinically recognized CVD, the associations of pedal edema with other symptoms of HF and abnormalities in indices of cardiac structure/function, and the association of pedal edema and incident hospitalized HF. Our data showed that pedal edema is prevalent (28%) in community dwelling adults, is not associated with overt LV or RV systolic dysfunction, but is associated with abnormal levels of NTproBNP and incident hospitalized HF. Thus pedal edema in asymptomatic community dwelling adults may be a sign of early HF (particularly early HFpEF), and also may be a harbinger of future hospitalized HF. To our knowledge this is the first study that has provided (1) the prevalence of pedal edema in community dwelling adults with no history of CVD and (2) shown an association between pedal edema and incident hospitalized HF.
MESA is a population-based study that by design recruited adults with no prior clinical history (or clinical recognition) of CVD, including HF. However based on the present analysis, greater than one-fourth of the participants admitted to having pedal edema. Even though participants with pedal edema were not more likely to have overt LV or RV systolic dysfunction compared to those with no pedal edema, they were twice as likely to sleep on ≥2 pillows (orthopnea), wake up at night with trouble breathing (PND), and were more likely to have abnormal NTproBNP levels. Diastolic function was not assessed during the MESA baseline examination and therefore it is unknown if pedal edema is also associated with impaired LV relaxation, reduced LV compliance, or both. However the associations observed in the participants with pedal edema in MESA such as orthopnea and PND are suggestive of early signs and symptoms of HF. It is possible that targeting individuals in early stages of HF may reduce the prevalence of overt, hospitalized HF in the community. Based on our results, systematic programs for the early detection of community dwelling individuals at risk or with early signs and symptoms of HF are likely needed, and screening based on simple measures such as pedal edema, orthopnea, and PND with subsequent natriuretic peptide testing could be tested with the goal of reducing incident hospitalizations for HF.
HF is a leading cause of morbidity and mortality in the world today despite decades of research and multiple therapeutic options for chronic HF with reduced EF (HFrEF)18,19. Current data suggest that while the prevalence of HFrEF seems to have plateaued or on the decline, the prevalence of HFpEF is rising20,21. Unfortunately nearly all therapeutic HFpEF trials have produced null results 22,23. Historically, HFpEF clinical trials have included patients with relatively preserved LVEF and a clinical history of overt HF, defined as either a prior hospitalization of HF or elevated natriuretic peptides23. Such patients may already have very advance stages of HFpEF and therefore may be very difficult to either reverse or control with therapy. Prevention may be a better approach to reducing and controlling HFpEF and should target community-based adults with early stages of the HFpEF syndrome.
In response to increased myocardial stress due to volume/pressure overload states, the BNP gene is activated in cardiomyocytes resulting in the production of an intracellular precursor propeptide (proBNP100)3. Further processing of this propeptide result in the release of the biologically inert amino-terminal fragment (NT-ProBNP). Thus high levels of NT-ProBNP signals an increase in myocardial wall stress, an abnormality associated with HF syndromes24. However an invasive exercise hemodynamic study by Borlaug et al25 showed that patients with early HFpEF have low/normal natriuretic peptide levels and normal filling pressures at rest but increased filling pressures diagnostic of HF during exercise. High or abnormal NT proBNP levels at rest may therefore represent a more advanced stage of HFpEF than the very early stage studied by Borlaug et al25. However, the invasive nature of the approach used by Borlaug et al to screen/diagnose very early HFpEF makes it impractical for deployment on a wide scale as a screening tool compared with pedal edema. In the present study, NT proBNP levels were drawn at rest and participants with pedal edema were twice as likely to have high/abnormal NT-ProBNP compared with those without pedal edema. The addition of NT-proBNP to our full model also attenuated the association between pedal edema and incident HF. Thus in community dwelling adults without history of CVD, pedal edema may indicate high/abnormal NT-proBNP and may signal an increase in myocardial wall stress, a pathological process central to the development of clinical HF. Even though the vast majority of MESA participants with pedal edema had “normal” NTproBNP levels, the median values were 50% higher than in participants without pedal edema, and given the association with orthopnea and PND (with no overt LV systolic dysfunction), many of these participants likely had unrecognized early HFpEF. This finding underscores the possibility that the prevalence of (early) HF is likely higher than previously recognized in the general population, and that early HF, particularly HFpEF is quite common.
Our study found lower RV mass index (did not reach statistical significance) and RV end diastolic volume index in participants with pedal edema compared with those without pedal edema during the baseline MESA exam. Although this finding is unadjusted (Table 1), it is contrary to the accumulating data that right ventricular dysfunction is common in patients with HFpEF26–28. However, it should be noted that our study population consist of participants with no clinical diagnosis or established HFpEF7 and therefore those with pedal edema may represent early undiagnosed HFpEF while most of these studies27,28 used patients with diagnosed and more advanced stage of HFpEF26,27. In addition most of these advanced stage HFpEF patients had other significant comorbidities such as atrial fibrillation, significant tricuspid regurgitation, RV pacemaker insertions and pulmonary hypertension26–28, to name a few, all of which were absent in our cohort and are possible causes of right ventricular dysfunction. MESA also did not assess pulmonary hemodynamics so it is unclear whether the difference in RV parameters observed could be attributed to differences in pulmonary pressures in those with and without pedal edema. In the highly selected but clinically important retrospective and invasive study by Borlaug et al26 on early HFpEF, RV parameters and pedal edema were not reported in participants. Thus the RV dysfunction observed in prior HFpEF studies25–27 may either be a marker of clinically advanced stages of HFpEF or may be due to other comorbidities prevalent in clinically advanced HFpEF patients. Our study findings also suggest that pedal edema may predate RV dysfunction in early HFpEF.
Our study had several limitations, notably the lack of objective data on pedal edema. There may be recall bias in the self-reported pedal edema which may affect our results and conclusions. Data for validation of self-reported pedal edema is not available in MESA. We are also not aware of any data on the reliability of self-reported pedal edema. However, any discrepancy in self-reported vs. objective pedal edema would have likely attenuated the associations observed in our study. Our study is also an observational study, and although we adjusted for covariates, our results may be affected by residual confounding. The baseline MESA examination cardiac MRI did not evaluate for diastolic function or myocardial fibrosis, both of which could have added additional pathophysiologic insight into the association between pedal edema and HF, particularly HFpEF. However, it should be noted that HFpEF is now known to be associated with multiple cardiac and extracardiac pathophysiologic abnormalities beyond diastolic dysfunction and myocardial fibrosis. The current analysis also did not account for change in medications use, especially diuretics, ACE-I, ARBs, and beta blockers during the follow up period. About 10% of participants were taking calcium channel blockers (CCB), a group of drugs associated with pedal edema, during the baseline MESA exam. Sensitivity analyses eliminating participants on CCBs produced similar estimates and conclusions. We did not stratify our analysis by type of HF (HFrEF and HFpEF) due to the relatively small number of MESA participants with available data on LVEF at time of clinical HF diagnosis.
Conclusions
Pedal edema is present in nearly one-third of community dwelling adults without clinically recognized CVD. The presence of pedal edema is not associated with overt LV or RV systolic dysfunction but is associated with other symptoms of HF, abnormal NT-ProBNP levels, and incident hospitalized HF. These findings suggest that early HF, particularly early HFpEF, may be under-recognized in the general population, and may present an important opportunity for the prevention of progression of HF and HF-related morbidity/mortality.
Supplementary Material
Clinical Perspective.
Heart failure is a major cause of morbidity and mortality in the developed world. Prevention and early identification in asymptomatic community dwelling adults may help reduce the prevalence of heart failure. Pedal edema is one of the cardinal signs of heart failure. However, the prevalence, associations and prognosis of pedal edema in asymptomatic community dwelling adults without history of cardiovascular disease is unknown. We used data from participants of the ongoing Multi Ethnic Study of Atherosclerosis cohort to show that pedal edema is a) prevalent (~28%); b) is associated with other signs and symptoms of congestive heart failure such as orthopnea and paroxysmal nocturnal dyspnea; c) associated with abnormal NT-proBNP levels and d) associated with future hospitalized heart failure in community dwelling adults without history of cardiovascular disease. Pedal edema was not associated with reduced right and or left ventricular systolic function in this cohort. Thus despite the heterogenous causes of heart failure, a preventive approach targeting pedal edema as a symptom may help identify those with early heart failure or at risk for future hospitalized heart failure. These findings also suggest that early heart failure, particularly early heart failure with preserved ejection fraction, may be under-recognized in the general population, and may present an important opportunity for the prevention and progression of heart failure and heart failure-related morbidity/mortality.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org
Funding Sources
This research was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040 and UL1-TR-001079 from NCRR.
Footnotes
Disclosures
None.
References
- 1.Peacock WF, Emerman CL. Emergency Department Management of Patients with Acute Decompensated Heart Failure. Heart Failure Reviews. 2004;9:187–193. doi: 10.1007/s10741-005-6128-5. [DOI] [PubMed] [Google Scholar]
- 2.Kapoor JD, Perazella MA. Diagnostic and Therapeutic Approach to Acute Decompensated Heart Failure. American Journal of Medicine. 2007;120:121–127. doi: 10.1016/j.amjmed.2006.05.066. [DOI] [PubMed] [Google Scholar]
- 3.Kim HN, Januzzi JL. Natriuretic peptides testing in heart failure. Circulation. 2011;123:2015–2019. doi: 10.1161/CIRCULATIONAHA.110.979500. [DOI] [PubMed] [Google Scholar]
- 4.Parekh N, Maisel AS. Utility of B-natriuretic peptide in the evaluation of left ventricular diastolic function and diastolic heart failure. Curr Opin Cardiol. 2009;24:155–60. doi: 10.1097/HCO.0b013e328320d82a. [DOI] [PubMed] [Google Scholar]
- 5.Van Veldhuisen DJ, Linssen GCM, Jaarsma T, Van Gist WH, Hoes AW, Tijssen JGP, Paulus WJ, Voors AA, Hillege HL. B-type natriuretic peptide and prognosis in heart failure patients with preserved and reduced ejection fraction. J Am Coll Cardiol. 2013;62:1498–1506. doi: 10.1016/j.jacc.2012.12.044. [DOI] [PubMed] [Google Scholar]
- 6.Januzzi JL., Jr Natriuretic peptides, ejection fraction, and prognosis: parsing the phenotypes of heart failure. J Am Coll Cardiol. 2013;61:1507–9. doi: 10.1016/j.jacc.2013.01.039. [DOI] [PubMed] [Google Scholar]
- 7.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 8.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 9.Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch-Herold M, Pearson G, Sinha S, Arai A, Lima JA, Bluemke DA. Cardiovascular function in Multi-Ethnic Study of Atherosclerosis: normal values by age, sex, and ethnicity. Am J Roentgenol. 2006;186:S357–S365. doi: 10.2214/AJR.04.1868. [DOI] [PubMed] [Google Scholar]
- 10.Tandri H, Daya SK, Nasir K, Bomma C, Lima JA, Calkins H, Bluemke DA. Normal reference values for the adult right ventricle by magnetic resonance imaging. Am J Cardiol. 2006;98:1660–1664. doi: 10.1016/j.amjcard.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 11.Chahal H, Johnson C, Tandri H, Jain A, Hundley WG, Barr RG, Kawut SM, Lima JA, Bluemke DA. Relation of cardiovascular risk factors to right ventricular structure and function as determined by magnetic resonance imaging (results from the Multi-Ethnic Study of Atherosclerosis) Am J Cardiol. 2010;106:110–116. doi: 10.1016/j.amjcard.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawut SM, Lima JAC, Barr G, Chahal H, Jain A, Tandri H, Praestgaard A, Bagiella E, Kizer JR, Johnson WC, Kronmal RA, Bluemke DA. Sex and race differences in right ventricular structure and function. Circulation. 2011;123:2542–2551. doi: 10.1161/CIRCULATIONAHA.110.985515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pavlicek M, Wahl A, Rutz T, de Marchi SF, Hille R, Wustmann K, Steck H, Eigenmann C, Schwarzmann M, Seiler C. Right ventricular systolic function assessment: rank of echocardiographic methods vs. cardiac magnetic resonance imaging. Eur J Echocardiography. 2011;12:871–880. doi: 10.1093/ejechocard/jer138. [DOI] [PubMed] [Google Scholar]
- 14.Kovalora S, Necas J, Vespalec J. What is “normal” right ventricle? Eur J Echocardiography. 2006;7:293–297. doi: 10.1016/j.euje.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 15.Ordonez-Llanos J, Collinson PO, Christenson RH. Amino-terminal pro–B-type natriuretic peptide: analytic considerations. Am J Cardiol. 2008;101:9–15. doi: 10.1016/j.amjcard.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Roche Diagnostics. Elecsys proBNP package insert. Indianapolis, IN: 2003. [Google Scholar]
- 17.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 18.Yancy C, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:1810–52. doi: 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- 19.Hunt SA, Baker DW, Chin MH, Cinquegrani MP, Feldman AM, Francis GS, Ganiats TG, Goldstein S, Gregoratos G, Jessup ML, Noble RJ, Packer M, Silver MA, Stevenson LW, Gibbons RJ, Antman EM, Alpert JS, Faxon DP, Fuster V, Jacobs AK, Hiratzka LF, Russell RO, Smith SC, Jr American College of Cardiology/American Heart Association. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary. A report of the American College of Cardiology /American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2001;38:2011–2113. doi: 10.1016/s0735-1097(01)01683-7. [DOI] [PubMed] [Google Scholar]
- 20.Fox KA, Steg PG, Eagle KA, Goodman SG, Anderson FA, Jr, Granger CB, Flather MD, Budaj A, Quill A, Gore JM GRACE Investigators. Decline in rates of death and heart failure in acute coronary syndromes, 1999–2006. JAMA. 2007;297:1892–1900. doi: 10.1001/jama.297.17.1892. [DOI] [PubMed] [Google Scholar]
- 21.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 22.Shah SJ. Matchmaking for the optimization of clinical trials of heart failure with preserved ejection fraction. No laughing matter. J Am Coll Cardiol. 2013;62:1339–1342. doi: 10.1016/j.jacc.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelly JP, Mentz RJ, Mebazaa A, Voors AA, Butler J, Roessig L, Fiuzat M, Zannad F, Pitt B, O’Connor CM, Lam CS. Patient selection in heart failure with preserved ejection fraction clinical trials. J Am Coll Cardiol. 2015;65:1668–82. doi: 10.1016/j.jacc.2015.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32:670–679. doi: 10.1093/eurheartj/ehq426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;5:588–95. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iglesias-Garriz I, Olalla-Gómez C, Garrote C, López-Benito M, Martín J, Alonso D, Rodríguez MA. Contribution of right ventricular dysfunction to heart failure mortality: a meta-analysis. Rev Cardiovasc Med. 2012;13:e62–e69. doi: 10.3909/ricm0602. [DOI] [PubMed] [Google Scholar]
- 27.Melenovsky V, Huang SJ, Lin G, Redfield MM, Borlang BA. Right heart failure in heart failure with preserved ejection fraction. Eur Heart J. 2014;35:3452–3462. doi: 10.1093/eurheartj/ehu193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohammed SF, Hussian I, AbouEzzeddine OF, Takahama H, Kwon SH, Fortia P, Roger VL, Redfield MM. Right Ventricular function in heart failure with preserved ejection fraction. A community–based study. Circulation. 2014;130:2310–2320. doi: 10.1161/CIRCULATIONAHA.113.008461. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.