Abstract
The melaminophenyl arsenical melarsoprol is the main drug used against late-stage sleeping sickness caused by Trypanosoma brucei subspecies. Its active metabolite in the human body is melarsen oxide. Here, it is shown that this metabolite inhibits growth of the fission yeast Schizosaccharomyces pombe and that its toxicity can be abolished efficiently by thiamine (vitamin B1), thiamine analogues, and the pyrimidine moiety of the thiamine molecule. Uptake of melarsen oxide is mediated by a membrane protein (car1p), which is involved in the uptake of thiamine and its pyrimidine moiety. Melarsoprol is taken up by cells in a thiamine- and car1p-dependent manner but is not toxic to cells.
The melaminophenyl arsenical melarsoprol (Mel B) is the main drug used against late-stage sleeping sickness caused by forms of Trypanosoma brucei subspecies carried in the bloodstream (12). Unfortunately, Mel B causes severe side effects such as encephalopathy, and an alarming increase in Mel B-resistant strains has been reported (12, 16). Even though Mel B was introduced as an antitrypanosomiasis reagent many decades ago, its molecular mode of action is still poorly understood (9, 16). It inhibits glycolytic enzymes, phosphogluconate dehydrogenase, and (by forming a stable complex with trypanothione) trypanothione reductase. The active metabolite of Mel B in the human body is believed to be melarsen oxide (Mel Ox) (13). Mel Ox is taken up by the P2 (TbAT1) adenosine transporter in bloodstream forms of T. brucei brucei (5, 15). Additionally, other transporters, whose genes remain to be identified, may be involved in the uptake of the drug (16).
Here, I show that Mel Ox interferes with thiamine (vitamin B1) metabolism in the fission yeast Schizosaccharomyces pombe, which is an important model organism for eucaryotic cells (14). The thiamine molecule consists of a pyrimidine moiety, 4-amino-5-hydroxymethyl-2-methylpyrimidine (HMP), and a thiazole moiety, 5-(2-hydroxyethyl)-4-methylthiazole (MT), which are linked by a methylene bridge. Depending on the tissue and the organism, thiamine exists intracellularly in different amounts, as tri-, di-, and monophosphates and in its unphosphorylated form (10). The function of the thiamine diphosphate is best known: it acts as a cofactor of several enzymes which are mainly involved in carbohydrate metabolism, including pyruvate dehydrogenase, α-ketoglutarate dehydrogenase, pyruvate decarboxylase, transketolase, pyruvate oxidase, and acetohydroxy acid synthase, and is an important regulator of carbohydrate metabolism (11, 20). More recent data show that thiamine or its phosphorylated derivatives can bind to RNA without the need for protein cofactors and can regulate gene expression (24). A specific non-cofactor role for thiamine has been proposed for excitable cells for decades (1, 4). Thiamine triphosphate is involved in the modulation of ion channels and can act as a phosphate donor for the phosphorylation of proteins (2, 17).
S. pombe is prototrophic for thiamine; i.e., the organism can synthesize the vitamin itself and is not dependent on thiamine present in the growth medium. My colleagues and I showed earlier that growth conditions affect the synthesis and intracellular accumulation of thiamine and defined genes, which are responsible for the control of its metabolism (7, 8, 21, 22). Important for this study, we showed that the car1 gene encoding a potential 12-membrane-spanning protein is involved in the uptake of thiamine and HMP (18). Expression of the gene is repressed by thiamine and its pyrimidine moieties and is under the control of the same regulatory factors that regulate expression of genes involved in biosynthesis and dephosphorylation of thiamine. Mutants defective in the car1 gene are resistant to the diuretic amiloride, which has been shown to competitively inhibit thiamine uptake in neuroblastoma cells (3).
MATERIALS AND METHODS
Growth media and strains.
The heterothallic S. pombe wild-type strain 972 h− and the two car1 mutants (Δcar1::ura4 and car1-5) are from our collection and have been described previously (18). The Δcar1::ura4 ura4 strain D-18 has the car1 gene replaced by the ura4 gene, and the car1-5 mutant is a spontaneously arisen point mutant with serine at position 389 replaced by an asparagine residue (N. Naula and M. E. Schweingruber, unpublished data). Strains were cultivated in synthetic minimal medium (MM) described by Schweingruber and Edenharter (23). The medium contains no thiamine and was supplemented as indicated in the text. For growth analyses, two consecutive precultures were prepared by growing cells at 30°C in MM. For the main culture reagent, tubes containing 5 ml of MM and the appropriate supplements were inoculated with 100 μl of the second preculture on a rotary wheel at 30°C, and optical density at 600 nm (OD600; linear scale) of the culture was measured at different times. Each experiment was done twice, and the reported values represent mean values.
Chemicals.
Thiamine hydrochloride, oxithiamine, and pyrithiamine are from Sigma and were dissolved in H2O. Bacimethrin was a gift from T. Begley (Cornell University, Ithaca, N.Y.), and it was dissolved in methanol. HMP and MT were kindly provided by G. Moine (Hoffmann-La Roche & Co. AG, Basel, Switzerland). Mel B (dissolved in propylene glycol) and Mel Ox (dissolved in dimethyl sulfoxide) were generously supplied by R. Brun (Swiss Tropical Institute, Basel, Switzerland). Mel B dissolves poorly in MM; if Mel B is added to MM at concentrations higher than 100 μM, a faint milky precipitation is visible.
Determination of arsenic.
Cells were grown in 50 ml of MM containing Mel Ox, Mel B, and other supplements as indicated at 30°C on a rotary shaker, harvested at an OD600 of around 1, washed twice with H2O, and suspended in 1 ml of H2O. Cells were dissolved with 8.5% hydrogen peroxide and 37% nitric acid at 210°C in an MLS-ETHOS microwave oven for 20 min, and the arsenic content of the solution was determined with a Perkin-Elmer ELAN 6100 ICP mass spectrometer, according to the manufacturer's instructions. Rhodium added to the samples was used as an internal standard. Each probe was measured nine times. The standard deviation of the measurements was <2%.
RESULTS
Growth inhibition by Mel Ox and its relief by thiamine, thiamine analogues, and HMP.
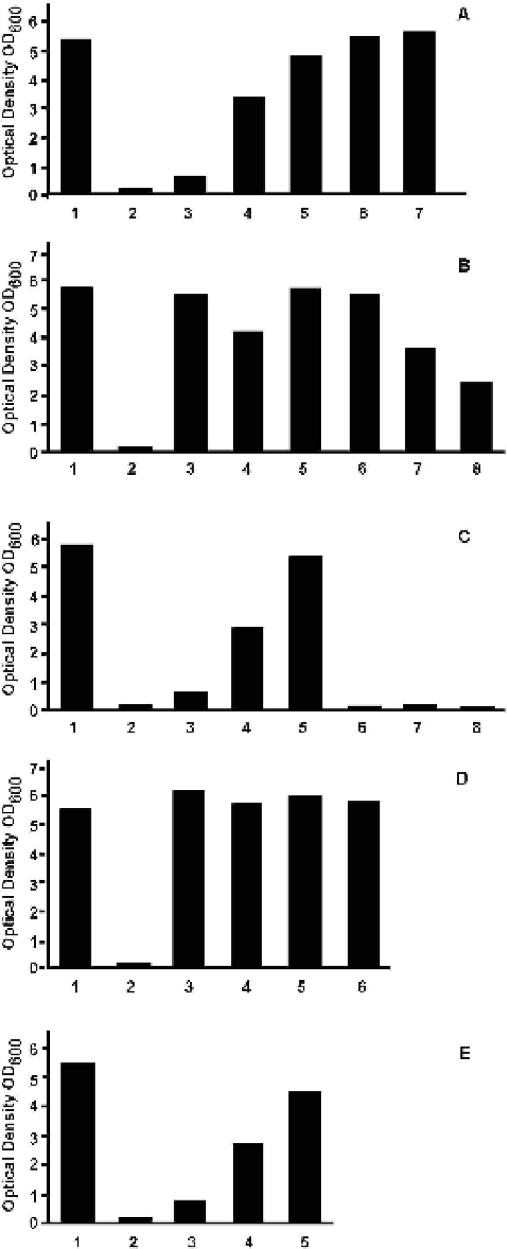
In MM containing no thiamine, Mel Ox (Fig. 1) inhibits growth of S. pombe in a dose-dependent manner. Inhibition starts at a concentration of around 0.1 μM and is complete at concentrations of 20 to 40 μM (results not shown). This effect is not observed in a medium containing yeast extract, indicating that yeast extract contains one or more compounds that can suppress the toxic effect of Mel Ox. I tested amino acids, pyrimidines, purines, and vitamins known to be present in yeast extract and found that the only compound able to efficiently abolish Mel Ox-induced growth inhibition was thiamine (Fig. 1). At a Mel Ox concentration of 35 μM, relief of growth inhibition starts at a thiamine concentration of approximately 10 nM and is complete at 1 μM (Fig. 2A).
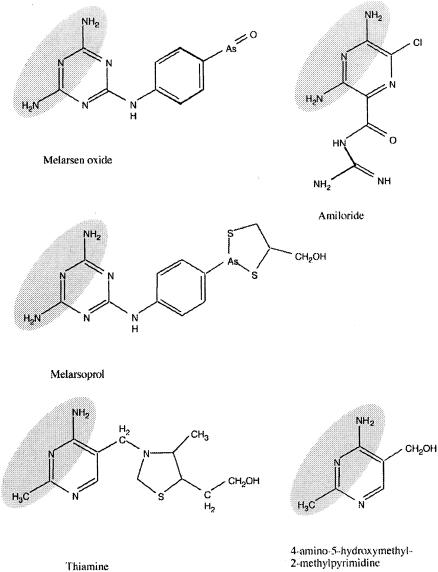
FIG. 1.
Structural formulas of compounds mentioned in the text. I propose that the shadowed structural elements of Mel Ox and amiloride compete with the shadowed elements in thiamine and HMP in reactions in which the pyrimidine moiety of thiamine is involved.
FIG. 2.
Growth of S. pombe wild-type and car1 mutant cells in the presence of Mel Ox, thiamine, thiamine antagonists, HMP, MT, and Mel B. Cells were cultivated in MM supplemented as indicated below, and growth (OD600) was measured at different times. OD600 after 40 h of growth (when cells in MM had reached stationary phase) is given. (A) Relief of Mel Ox-induced growth inhibition in the wild type by thiamine. Lanes: 1, MM; 2, MM containing 35 μM Mel Ox; 3 to 7, MM containing 35 μM Mel Ox and thiamine at 10 nM (lane 3), 50 nM (lane 4), 250 nM (lane 5), 1 μM (lane 6), or 5 μM (lane 7). (B) Effects of thiamine antagonists on Mel Ox-induced growth inhibition in the wild type. Lanes: 1, MM; 2, MM containing 35 μM Mel Ox; 4, 6, and 8, MM containing 35 μM Mel Ox and either 50 μM oxithiamine (lane 4), 100 μM bacimethrin (lane 6), or 0.2 μM pyrithiamine (lane 8) as an antagonist; 3, 5, and 7, MM and either 50 μM oxithiamine (lane 3), 100 μM bacimethrin (lane 5), or 0.2 μM pyrithiamine (lane 7) as an antagonist. (C) Effects of the pyrimidine and thiazole moieties on Mel Ox-induced growth inhibition in the wild type. Lane 1, MM; lane 2, MM containing 35 μM Mel Ox; lanes 3 to 8, MM containing 35 μM Mel Ox and HMP (lanes 3 to 5) and MT (lanes 6 to 8) at the following concentrations. For lane 3, 10 nM HMP; lane 4, 50 nM HMP; lane 5, 1 μM HMP; lane 6, 10 nM MT; lane 7, 50 nM MT; lane 8, 1 μM MT. (D) Growth of the wild-type and two car1 mutants in MM (lanes 1,3, and 5) and MM containing 100 μM Mel Ox (lanes 2, 4, and 6). Lanes 1 and 2, wild type; lanes 3 and 4, Δcar1::ura4 mutant; lanes 5 and 6, car1-5 mutant. (E) Release of Mel Ox-induced growth inhibition in the wild type by Mel B. Lane 1, MM; lane 2, MM containing 35 μM Mel Ox; lanes 3 to 5: MM containing 35 μM Mel Ox and either 17 μM (lane 3), 85 μM (lane 4), or 420 μM (lane 5) Mel B.
I also monitored cell proliferation in the presence of Mel Ox and various amounts of the structural thiamine analogues pyrithiamine, oxithiamine, and bacimethrin (Fig. 2B). These analogues are known to interact with different thiamine-dependent reactions (10, 19). Pyrithiamine is very toxic for S. pombe, and it completely inhibits cell proliferation at low micromolar concentrations. In contrast, the other two analogues inhibit cell proliferation only at concentrations above 50 μM. Interestingly, oxithiamine and bacimethrin both counteract the growth inhibition caused by Mel Ox, even when present in <2-fold excess over Mel Ox (Fig. 2B). Even the toxic analogue pyrithiamine exhibits a clear antagonistic effect on Mel Ox. I found that HMP (Fig. 1), but not MT, relieves Mel Ox-induced growth inhibition (Fig. 2C) as efficiently as thiamine, specifically indicating that the pyrimidine moiety of the thiamine molecule carries the structural information necessary for relief of growth inhibition.
Uptake of Mel Ox is mediated by car1.
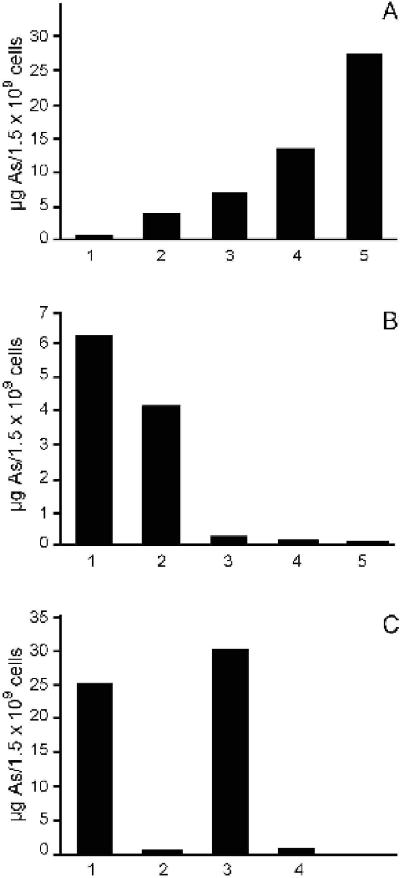
To demonstrate that Mel Ox interacts directly with cellular structures that recognize thiamine and its pyrimidine moiety and exerts its effect not only by some kind of indirect reaction, I set up experiments to specifically identify one of these structures in S. pombe. I found that the car1 gene is responsible for Mel Ox sensitivity. A car1 deletion strain (Δcar1::ura4) and a spontaneously arisen point mutant (car1-5) both grow normally at a Mel Ox concentration of 100 μM (Fig. 2D). To test more directly the involvement of thiamine and the car1 gene in Mel Ox uptake, I cultivated wild-type and car1 mutant cells in the presence of various amounts of Mel Ox and thiamine and measured the intracellular content of arsenic. In wild-type cells, intracellular arsenic increases with increasing Mel Ox concentrations in the absence of thiamine (Fig. 3A). It decreases when cells are grown at a constant Mel Ox concentration (1 μM) in the presence of increasing thiamine concentrations (Fig. 3B). For car1 mutant cells grown in the presence of Mel Ox, the amount of intracellularly accumulated arsenic is negligible (Fig. 3C). In conjunction, these results leave little doubt that thiamine competes with, or inhibits, Mel Ox uptake and that the gene car1 is involved in the uptake process.
FIG. 3.
Intracellular content of arsenic (As) in wild-type and car1 mutant cells grown in the presence of Mel Ox, Mel B, and thiamine. The samples were prepared and the intracellular arsenic content was determined as indicated in Materials and Methods. (A) Wild-type cells were cultivated in MM containing Mel Ox at the following concentrations. For lane 1, 0 μM; lane 2, 0.1 μM; lane 3, 0.5 μM; lane 4, 1 μM; lane 5, 5 μM. (B) Cells were cultivated in MM containing 1 μM Mel Ox and the following concentrations of exogenously added thiamine: for lane 1, 0 μM; lane 2, 0.01 μM; lane 3, 0.1 μM; lane 4, 1 μM; lane 5, 10 μM. (C) Cells of the wild type (lanes 1 and 3) and the Δcar1::ura4 mutant (lanes 2 and 4) were cultivated in MM containing 2 μM Mel Ox (lanes 1 and 2) or 20 μM Mel B (lanes 3 and 4).
Mel B is not toxic for S. pombe.
Patients suffering from sleeping sickness are treated with Mel B (Fig. 1) rather than Mel Ox. I found that Mel B does not inhibit growth in S. pombe under my experimental conditions. I observed, however, that it can relieve the toxic activity of Mel Ox in a dose-dependent manner (Fig. 2E) and that it is taken up by wild-type but not by car1 mutant cells (Fig. 3C). These results indicate that of the two melaminophenyl arsenicals tested, only Mel Ox is toxic for S. pombe. The results can best be explained by assuming that the nontoxic Mel B competes with its toxic metabolite Mel Ox.
DISCUSSION
My results show that both Mel B and Mel Ox are taken up by S. pombe cells but that only Mel Ox is toxic for cells. These findings are in good agreement with reports claiming that Mel Ox, rather than Mel B, is the active metabolite against trypanosomiasis in humans (13).
The toxic effect of Mel Ox is relieved by low concentrations of thiamine and its pyrimidine moiety. This observation is reminiscent of observations made previously about amiloride (18). This drug (Fig. 1) inhibits growth of S. pombe, and growth inhibition is relieved by thiamine and by its pyrimidine moiety alone. The effective concentrations of amiloride, thiamine, and HMP necessary to inhibit growth and to release growth inhibition are close to those observed for Mel Ox. Amiloride and Mel Ox share two identical structural elements in their molecules (Fig. 1). I suggest that these, together with other elements, are critical for the relief of their toxicity by thiamine. In thiamine and HMP, one of the proposed critical NH2 groups in amiloride, Mel Ox is replaced by a methyl group. I therefore propose that Mel Ox acts as a structural analogue of the pyrimidine moiety of thiamine and that its toxicity is due to its interference with one or more cellular reactions in which the pyrimidine moiety of the vitamin is essential. According to this hypothesis, relief of Mel Ox toxicity by thiamine can be explained by the competition of thiamine with Mel Ox.
One target of Mel Ox has been identified, the protein car1p, which is involved in uptake of thiamine and HMP. I suggest that this protein normally recognizes the structural element of the pyrimidine moiety of thiamine with high affinity (Fig. 1) but that it can also interact with the corresponding modified structures of amiloride, Mel Ox, and Mel B at a lower affinity. Not only thiamine but also oxithiamine, pyrithiamine, and bacimethrin relieve the growth inhibitory effects of Mel Ox. I found, however, that the car1 mutants are not resistant to these thiamine analogues (M. E. Schweingruber, unpublished data). This indicates that other Mel Ox targets in the S. pombe cell may exist, in addition to the car1p protein.
Mel Ox toxicity is also alleviated by thiamine in Escherichia coli and in Saccharomyces cerevisiae, a yeast that is evolutionarily very far apart from S. pombe (Schweingruber, unpublished). This indicates that interference of Mel Ox with thiamine metabolism may occur not only in fission yeast but also in many other organisms, including T. brucei and humans. In this context, it is interesting that the P2 (TbAT1) adenosine transporter in bloodstream forms of T. brucei brucei, which is responsible for the uptake of melaminophenyl arsenicals (5, 15), has been proposed to require an amidine group [NH2-C(R1) = NR2] that may be integrated into a pyridine or pyrimidine ring for optimal binding (6). The pyrimidine moiety of thiamine contains this structure, and I therefore wonder if the P2 transporter in T. brucei brucei is also involved in the transport of thiamine and/or its pyrimidine moiety or whether as-yet-unidentified thiamine transporters of the parasite are also involved in Mel Ox uptake.
In at least some of the reactions in which the pyrimidine moiety of thiamine is involved, humans and bloodstream-carried forms of T. brucei certainly differ, and it could be conceivable that interference of the drug with cellular structures that recognize the pyrimidine moiety of thiamine is the reason for the differential effects of the melaminophenyl arsenicals in trypanosomes and their hosts.
Acknowledgments
I thank K. Rieder and L. Muralt for the determinations of arsenic, A. M. Schweingruber, P. Mäser, and T. Seebeck for helpful comments on the manuscript, and R. Brun, T. Begley, and G. Moine for the drugs mentioned in Materials and Methods.
This work was supported by the Swiss National Foundation.
REFERENCES
- 1.Bettendorff, L. 1996. A non-cofactor role of thiamine derivatives in excitable cells? Arch. Physiol. Biochem. 104:745-751. [DOI] [PubMed] [Google Scholar]
- 2.Bettendorff, L., B. Hennuy, P. Wins, and E. Schoffeniels. 1993. Thiamin and derivatives as modulators of rat brain chloride channels. Neuroscience 52:1009-1017. [DOI] [PubMed] [Google Scholar]
- 3.Bettendorff, L., and P. Wins. 1994. Mechanism of thiamine transport in neuroblastoma cells. J. Biol. Chem. 269:14379-14385. [PubMed] [Google Scholar]
- 4.Bettendorff, L., and P. Wins. 1999. Thiamine derivatives in excitable tissues: metabolism, deficiency and neurodegenerative diseases. Recent Res. Devel. Neurochem. 2:37-62. [Google Scholar]
- 5.Carter, N. S., and A. H. Fairlamb. 1993. Arsenical-resistant trypanosomes lack an unusual adenosine transporter. Nature 361:173-176. [DOI] [PubMed] [Google Scholar]
- 6.De Koning, H. P., and S. M. Jarvis. 1999. Adenosine transporters in bloodstream forms of Trypanosoma brucei brucei: substrate recognition motifs and affinity for trypanocidal drugs. Mol. Pharmacol. 56:1162-1170. [DOI] [PubMed] [Google Scholar]
- 7.Fankhauser, H., and M. E. Schweingruber. 1994. Thiamine-repressible genes in Schizosaccharomyces pombe are regulated by a Cys6 zinc-finger motif-containing protein. Gene 147:141-144. [DOI] [PubMed] [Google Scholar]
- 8.Fankhauser, H., A. Zurlinden, A. M. Schweingruber, E. Edenharter, and M. E. Schweingruber. 1995. Schizosaccharomyces pombe thiamine pyrophosphokinase is encoded by gene tnr3 and is a regulator of thiamine metabolism, phosphate metabolism, mating, and growth. J. Biol. Chem. 270:28457-28462. [DOI] [PubMed] [Google Scholar]
- 9.Friedheim, E. A. H. 1949. Mel B in the treatment of human trypanosomiasis. Am. J. Trop. Med. Hyg. 29:173-180. [DOI] [PubMed] [Google Scholar]
- 10.Friedrich, W. 1987. Handbuch der Vitamine, p. 240-258. Urban and Schwarzenberg, Baltimore, Md.
- 11.Hohmann, S., and P. A. Meacock. 1998. Thiamin metabolism and thiamin diphosphate-dependent enzymes in the yeast Saccharomyces cerevisiae: genetic regulation. Biochim. Biophys. Acta 1385:201-219. [DOI] [PubMed] [Google Scholar]
- 12.Hubert, D., and M. P. Barrett. 2001. Uptake and mode of action of drugs used against sleeping sickness. Biochem. Pharmacol. 61:1-5. [DOI] [PubMed] [Google Scholar]
- 13.Keiser, J., O. Ericsson, and C. Burri. 2000. Investigations of the metabolites of the trypanocidal drug melarsoprol. Clin. Pharmacol. Ther. 67:478-488. [DOI] [PubMed] [Google Scholar]
- 14.MacNeil, S. A., and P. Nurse. 1997. Cell cycle control in fission yeast, p. 697-763. In J. P. Pringle, J. R. Broach, and E. W. Jones (ed.), The molecular and cellular biology of the yeast Saccharomyces, vol. 3. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. [Google Scholar]
- 15.Mäser, P., C. Sütterlin, A. Kralli, and R. A. Kaminsky. 1999. A nucleoside transporter from Trypanosoma brucei involved in drug resistance. Science 285:242-244. [DOI] [PubMed] [Google Scholar]
- 16.Mäser, P., A. Lüscher, and R. A. Kaminsky. 2003. Drug transport and drug resistance in African trypanosomes. Drug Resist. Updates 6:281-290. [DOI] [PubMed] [Google Scholar]
- 17.Nghiem, H. O., L. Bettendorff, and J. P. Changeux. 2000. Specific phosphorylation of Torpedo 43K rapsyn by endogenous kinase(s) with thiamine triphosphate as the phosphate donor. FASEB J. 14:543-554. [DOI] [PubMed] [Google Scholar]
- 18.Niederberger, C., H. Fankhauser, E. Edenharter, and M. E. Schweingruber. 1996. Amiloride toxicity in the fission yeast Schizosaccharomyces pombe is released by thiamine and mutations in the thiamine-repressible gene car1. Gene 24:119-122. [DOI] [PubMed] [Google Scholar]
- 19.Reddick, J. J., S. Saha, J. Lee, J. S. Melnick, J. Perkins, and T. P. Begley. 2001. The mechanism of action of bacimethrin, a naturally occurring thiamin antimetabolite. Bioorg. Med. Chem. Lett. 11:2245-2248. [DOI] [PubMed] [Google Scholar]
- 20.Schellenberg, A. 1998. Sixty years of thiamin diphosphate biochemistry. Biochim. Biophys. Acta 1385:177-186. [DOI] [PubMed] [Google Scholar]
- 21.Schweingruber, A. M., J. Dlugonski, E. Edenharter, and M. E. Schweingruber. 1991. Thiamine in Schizosaccharomyces pombe: dephosphorylation, intracellular pool, biosynthesis and transport. Curr. Genet. 19:249-251. [DOI] [PubMed] [Google Scholar]
- 22.Schweingruber, A. M., H. Fankhauser, J. Dlugonski, C. Steinmann-Loss, and M. E. Schweingruber. 1992. Isolation and characterization of regulatory mutants from Schizosaccharomyces pombe involved in thiamine-regulated gene expression. Genetics 130:445-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schweingruber, M. E., and E. Edenharter. 1990. Thiamin regulates agglutination and zygote formation in Schizosaccharomyces pombe. Curr. Genet. 17:191-194. [DOI] [PubMed] [Google Scholar]
- 24.Winkler, W., A. Nahvi, and R. R. Breaker. 2002. Thiamine derivatives bind messenger RNAs directly to regulate bacterial gene expression. Nature 419:952-956. [DOI] [PubMed] [Google Scholar]