Abstract
The activity of WCK 771, an experimental quinolone developed to overcome quinolone resistance in staphylococci and other bacteria, was determined against quinolone-susceptible and -resistant Staphylococcus aureus and S. epidermidis. WCK 771 MICs for 50 and 90% of the strains tested (MIC50 and MIC90, respectively) were 0.008 and 0.015 μg/ml for S. aureus (n = 43) and 0.015 and 0.03 μg/ml for S. epidermidis (n = 44) for quinolone-susceptible isolates, compared to ciprofloxacin values of 0.12 and 0.25 μg/ml and 0.25 and 0.5 μg/ml, respectively. Values for levofloxacin were 0.12 and 0.25 μg/ml and 0.12 and 0.25 μg/ml, those for clinafloxacin were 0.015 and 0.03 μg/ml and 0.015 and 0.03 μg/ml, those for moxifloxacin were 0.03 and 0.06 μg/ml and 0.06 and 0.12 μg/ml, and those for gatifloxacin were 0.06 and 0.12 μg/ml and 0.12 and 0.25 μg/ml, respectively. The WCK 771 MIC50 and MIC90, respectively, were 0.5 and 1 μg/ml for both species of staphylococci (n = 73 for S. aureus, n = 70 for S. epidermidis) for isolates highly resistant to ciprofloxacin (MIC50 and MIC90, >32 and >32 μg/ml, respectively). Values for levofloxacin were 8 and 32 μg/ml and 8 and 32 μg/ml, those for clinafloxacin were 1 and 2 μg/ml and 0.5 and 2 μg/ml, those for moxifloxacin 4 and >4 μg/ml and 4 and >4 μg/ml, and those for gatifloxacin were 4 and >4 μg/ml and 2 and >4 μg/ml, respectively. WCK 771 and clinafloxacin demonstrated MICs of 1 μg/ml against three vancomycin-intermediate strains. WCK 771 showed concentration-independent killing for up to 24 h at 2, 4, and 8 times the MICs against quinolone-resistant staphylococci and was also bactericidal after 8 h for high-density inocula (108 CFU/ml) of quinolone-resistant strains at 5 μg/ml, whereas moxifloxacin at 7.5 μg/ml was bacteriostatic. WCK 771 was not a substrate of the NorA efflux pump as evident from the similar MICs against both an efflux-positive and an efflux-negative strain. Overall, WCK 771 was the most potent quinolone tested against the staphylococci tested, regardless of quinolone susceptibility.
As the prevalence of multidrug-resistant strains of Staphylococcus aureus and coagulase-negative staphylococci has increased worldwide, there has been an attendant need for effective new agents. Strains of Staphylococcus species have showed increasing resistance to β-lactam compounds. As early as the 1970s, 70 to 85% of S. aureus isolates were penicillin resistant (4). Previously a problem of nosocomial transmission—methicillin-resistant S. aureus (MRSA) prevalence in hospitals was estimated by the Centers for Disease Control and Prevention to be approximately 50% in 1998—it is increasingly a problem in the community (4). Methicillin resistance is often accompanied by resistance to other antimicrobial agents, including quinolones. A Mexican study of 211 strains of S. aureus (30 methicillin resistant) and 998 strains of coagulase-negative staphylococci (533 methicillin resistant) showed only 10% ciprofloxacin susceptibility among S. aureus isolates and 43% susceptibility to ciprofloxacin in the coagulase-negative staphylococci, regardless of methicillin susceptibility (2). A European study of blood isolates showed similar results (9). Ciprofloxacin resistance as high as 24% in staphylococci has been reported in parts of Europe and is nearly 10% in Canada (28). In recent bloodstream isolates in the United States, susceptibility of methicillin-susceptible staphylococci to ciprofloxacin was high (94.3% for S. aureus and 89.9% for coagulase-negative staphylococci); however, 88.6% of methicillin-resistant S. aureus isolates were resistant to ciprofloxacin, as were 54.9% of coagulase-negative staphylococci (6). Isolates with reduced glycopeptide susceptibility have emerged in staphylococci, although their prevalence is currently low (0.25% of 15,439 S. aureus isolates, 1.9% of 6,350 coagulase-negative staphylococci) (6, 10). Recently, two strains of vancomycin-resistant S. aureus (VRSA) have been detected in the United States (3, 5).
There is, consequently, a need for newer agents with superior activity against methicillin- and quinolone-resistant staphylococci, particularly in view of emerging glycopeptide resistance. There is also a need for oral compounds for outpatient treatment of infections caused by staphylococci, particularly methicillin- and quinolone-resistant isolates. Introduced in the 1980s, fluoroquinolones initially fulfilled this need and remain important in the treatment of a wide range of infections. However, resistance to many members of this class of agent, particularly older ones such as ciprofloxacin, is increasing in staphylococci (13, 28). Development and spread of quinolone resistance among clinical isolates have been greatest in S. aureus, particularly among methicillin-resistant strains, in which both selection by quinolone exposure and transmission of clonal strains in health-care settings have contributed to the high prevalence of quinolone resistance in this species.
WCK 771 is a new, broad-spectrum, experimental quinolone under development by the Wockhardt Research Center, Aurangabad, India. This agent is the arginine salt of the active S-(−)-isomer of the racemic fluoroquinolone nadifloxacin, which has been developed for topical use because of solubility problems (17, 19-21, 27). The arginine salt formulation of S-(−)-nadifloxacin has overcome the solubility issue and allows parenteral administration of this agent. This study examined the in vitro antistaphylococcal activity of WCK 771, which was compared to those of ciprofloxacin, levofloxacin, gatifloxacin, moxifloxacin, clinafloxacin, vancomycin, and linezolid against both quinolone-susceptible and -resistant staphylococci. Additionally, kill kinetics and the effect of WCK 771 concentration on killing of high inocula of two quinolone-resistant staphylococcal isolates was determined.
MATERIALS AND METHODS
Antimicrobials.
WCK 771 and clinafloxacin were synthesized at the Wockhardt Research Centre (Aurangabad, India). Other antimicrobial agents were either recovered from their commercial preparations or obtained from their manufacturers.
MIC determination.
Broth microdilution methodology was used to test the staphylococcal isolates in Mueller-Hinton broth by using frozen commercially prepared trays (Trek Diagnostics, Westlake, Ohio) (16). Standard quality control strains, including Staphylococcus aureus ATCC 25923, were included in each run of broth microdilution testing (16). The staphylococcal species tested included 116 S. aureus and 114 S. epidermidis isolates. Forty-three (37%) of the S. aureus isolates were quinolone susceptible, as were 44 (39%) of the S. epidermidis isolates tested. Isolates originated from the United States, Canada, Mexico, Germany, Italy, Spain, and Hong Kong. There were 53 S. aureus and 55 S. epidermidis isolates that were methicillin resistant, all of them quinolone resistant. Additionally, WCK 771 was also comparatively evaluated with several fluoroquinolones against reference vancomycin-intermediate S. aureus (VISA) strains by the agar dilution method (16). The VISA strains used were ATCC 700698, ATCC 700699, and ATCC 700789, obtained from the American Type Culture Collection.
The antimicrobial agents and the ranges tested against the staphylococci included WCK 771 (tested at 0.008 to 4 μg/ml), levofloxacin (0.03 to 32 μg/ml), ciprofloxacin (0.06 to 32 μg/ml), clinafloxacin (0.008 to 8 μg/ml), moxifloxacin (0.015 to 8 μg/ml), gatifloxacin (0.015 to 4 μg/ml), vancomycin (0.25 to 4 μg/ml), linezolid (0.5 to 8 μg/ml), and oxacillin (0.06 to 4 μg/ml).
Effect of staphylococcal NorA efflux pump on quinolone MICs.
In order to determine whether WCK 771 is a substrate for the NorA efflux pump in comparison to other fluoroquinolones such as norfloxacin, ciprofloxacin, levofloxacin, gatifloxacin, moxifloxacin, and clinafloxacin, the MICs of these fluoroquinolones for norA-hyperexpressing S. aureus 1199B (NorA+) and the parent S. aureus 1199 (NorA−) were determined by the agar dilution method, with and without addition of the efflux pump inhibitor reserpine at a concentration of 20 μg/ml, as follows (25). A stock solution of 10 mg of reserpine (Sigma) per ml in dimethyl sulfoxide (DMSO; Sigma) was prepared, and 50 μl of this stock solution was added to 25 ml of molten Mueller-Hinton agar to provide a reserpine concentration of 20 μg/ml. An equal volume of DMSO (50 μl) was incorporated in reserpine-free, fluoroquinolone-containing plates and drug-free control plates to control for the use of DMSO as the solvent for reserpine. A fourfold or greater decrease in the fluoroquinolone MIC in the presence of reserpine was interpreted as evidence of an efflux effect.
Effect of WCK 771 concentrations on bactericidal activity.
Time-kill kinetic studies of WCK 771 were performed with MRSA 32 and MRSA 5023. Initial inocula of 106 CFU of log-phase cultures per ml at 35°C in Mueller-Hinton broth were prepared. The concentrations of WCK 771 used were 0.5 to 8 μg/ml, representing a concentration below the MIC to 8 times the MICs for both MRSA strains. Viable counts were determined at 0, 2, 4, 6, and 24 h. WCK 771 was considered bactericidal at the lowest concentration that reduced the original inoculum by ≥3 log10 CFU/ml (99.9% killing) in each of the time periods and bacteriostatic if the inoculum was reduced by <3 log10 CFU/ml. The problem of drug carryover was addressed by dilution as described previously (22).
High cell density kill kinetics.
To increase the stringency of assessment of bactericidal activity determination, the time-kill kinetics of WCK 771 at a concentration of 5 μg/ml and those of moxifloxacin at a concentration of 7.5 μg/ml against two methicillin- and quinolone-resistant S. aureus strains, MRSA 97 and MRSA 32, were studied by using higher cell density cultures (∼108 CFU/ml). To obtain log-phase high-density cultures, overnight shake flask-grown cultures (∼109 CFU/ml) were diluted 1:100 in fresh, warm Mueller-Hinton broth and brought to log phase to a density of 108 CFU/ml. To assess viability changes, the colony counts were measured at 4, 6, and 8 h by plating 0.1-ml volumes of serial 10-fold dilutions of cultures on drug-free medium. Antimicrobials were considered bactericidal on the basis of the criteria listed above.
RESULTS
The MICs for the 230 staphylococcal isolates (116 of S. aureus, 114 of S. epidermidis) tested are presented in Table 1. WCK 771 was the most potent agent tested against both species of staphylococci, with overall MICs for 50 and 90% of the strains tested (MIC50 and MIC90, respectively) of 0.5 and 1 μg/ml for S. aureus and 0.5 and 0.5 μg/ml for S. epidermidis.
TABLE 1.
Broth microdilution MICs for 230 staphylococci overall and by quinolone susceptibility
| Bacterial species (no. of isolates tested) and antimicrobial agent | MIC (μg/ml) for all isolates
|
Quinolone susceptibilitya
|
|||||
|---|---|---|---|---|---|---|---|
| Susceptible
|
Resistant
|
||||||
| Range | MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | |
| S. aureus (116) | |||||||
| WCK 771 | ≤0.008->4 | 0.5 | 1 | ≤0.008 | 0.015 | 0.5 | 1 |
| Levofloxacin | 0.06->32 | 4 | 32 | 0.12 | 0.25 | 8 | 32 |
| Ciprofloxacin | 0.12->32 | 16 | >32 | 0.25 | 0.5 | >32 | >32 |
| Clinafloxacin | ≤0.008->4 | 0.25 | 2 | 0.015 | 0.03 | 1 | 2 |
| Moxifloxacin | ≤0.015->4 | 2 | >4 | 0.03 | 0.06 | 4 | >4 |
| Gatifloxacin | ≤0.015->4 | 2 | >4 | 0.06 | 0.12 | 4 | >4 |
| Vancomycin | 0.5-2 | 1 | 1 | —b | — | — | — |
| Linezolid | 1-4 | 4 | 4 | — | — | — | — |
| Oxacillinc | 0.12->4 | 0.5 | >4 | — | — | — | — |
| S. epidermidis (114) | |||||||
| WCK 771 | ≤0.008->4 | 0.5 | 0.5 | 0.015 | 0.03 | 0.5 | 1 |
| Levofloxacin | 0.06->32 | 4 | 16 | 0.12 | 0.25 | 8 | 32 |
| Ciprofloxacin | ≤0.06->32 | 8 | >32 | 0.12 | 0.25 | >32 | >32 |
| Clinafloxacin | ≤0.008-4 | 0.25 | 1 | 0.015 | 0.03 | 0.5 | 2 |
| Moxifloxacin | ≤0.015->4 | 1 | 4 | 0.06 | 0.12 | 4 | >4 |
| Gatifloxacin | 0.03->4 | 2 | 4 | 0.12 | 0.25 | 2 | >4 |
| Vancomycin | 0.25-4 | 2 | 2 | — | — | — | — |
| Linezolid | 1-2 | 1 | 2 | — | — | — | — |
| Oxacillind | ≤0.06->4 | 2 | >4 | — | — | — | — |
Of the 116 S. aureus isolates, 43 were quinolone susceptible (ciprofloxacin MICs, ≤2.0 μg/ml) and 73 were quinolone resistant (ciprofloxacin MICs, >2.0 μg/ml). of the 114 S. epidermis isolates, 44 and 70 were susceptible and resistant, respectively. All values are in micrograms per milliliter.
—, not applicable.
43 (37%) oxacillin susceptible.
44 (39%) oxacillin susceptible.
WCK 771 was highly potent against quinolone-susceptible staphylococci, with an MIC90 of 0.015 μg/ml, compared to MIC90s of 0.06 to 0.5 μg/ml for currently available quinolones. Although MICs were higher, WCK 771 and clinafloxacin had superior potency against quinolone-resistant isolates, with MIC90s of 1 μg/ml and 2 μg/ml, respectively. In contrast, the MIC90s of the other quinolones tested against quinolone-resistant staphylococci were all >4 μg/ml. However, the relative differences between the MICs of quinolone-susceptible and -resistant isolates were similar for all of the quinolones tested in this study.
WCK 771 and clinafloxacin had the lowest MICs of the fluoroquinolones tested against VISA strains MU-3 and MU-50 (Table 2). With an MIC of 0.5 μg/ml, WCK 771 was found to be fourfold more active than clinafloxacin against the second VRSA isolate from Hershey Medical Center (1). Significantly, WCK 771 retained its bactericidal activity against this VRSA strain bearing a change from serine to leucine at position 84 in GyrA and a change from glutamic acid to lysine at position 471 in GrlB (1). The potencies of all of the other fluoroquinolones tested against VISA and VRSA strains were four- to eightfold lower than that of WCK 771.
TABLE 2.
MICs of WCK 771 and other agents against VISA isolates
| Compound | MIC (μg/ml)
|
||
|---|---|---|---|
| MU-3 ATCC 700698 | MU-50 ATCC 700699 | MRSA ATCC 700789 | |
| WCK 771 | 1 | 1 | 1 |
| Clinafloxacin | 1 | 1 | 1 |
| Moxifloxacin | 4 | 4 | 4 |
| Trovafloxacin | 2 | 2 | 2 |
| Levofloxacin | 16 | 8 | 8 |
| Ciprofloxacin | 32 | 32 | 32 |
| Vancomycin | 8 | 16 | 4 |
| Teicoplanin | 16 | 16 | 8 |
The impact of the staphylococcal NorA efflux pump on the MICs of WCK 771 and the other quinolones is shown in Table 3. The MIC of WCK 771 against the NorA+ strain was only onefold higher than that of the NorA− strain and was not affected by the presence of reserpine, indicating the lack of significant efflux of WCK 771 from the NorA+ strain. Under similar test conditions, the MICs of ciprofloxacin and clinafloxacin were 16 times higher and those of other fluoroquinolones were 2 to 4 times higher.
TABLE 3.
Effects of the NorA efflux pump and the efflux pump inhibitor reserpine on quinolone MICs
| Agent | MIC (μg/ml)
|
||
|---|---|---|---|
| S. aureus 1199 NorA− | S. aureus 1199B NorA+ | S. aureus 1199B NorA+ + reserpine (20 μg/ml) | |
| WCK 771 | 0.015 | 0.03 | 0.03 |
| Norfloxacin | 4 | 16 | 4 |
| Ciprofloxacin | 0.5 | 8 | 1 |
| Levofloxacin | 0.25 | 1 | 0.5 |
| Gatifloxacin | 0.12 | 0.5 | 0.25 |
| Moxifloxacin | 0.06 | 0.25 | 0.25 |
| Clinafloxacin | 0.015 | 0.25 | 0.06 |
Response of MRSA to increasing concentrations of WCK 771.
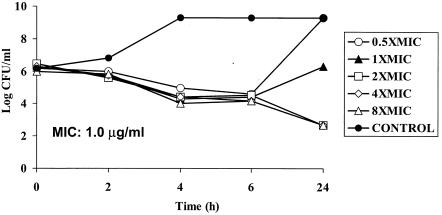
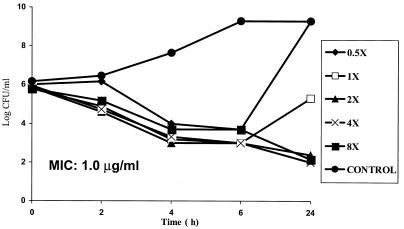
MRSA 32 and 5023 at densities of 106 CFU/ml were exposed to WCK 771 concentrations ranging from a concentration below the MIC (0.5 times the MIC) to 8 times the MIC (Fig. 1 and 2). Both of the strains treated with a concentration 0.5 times the MIC showed regrowth at 24 h, whereas there was initial killing with regrowth to <106 CFU/ml at the MIC. The MICs for isolates recovered after regrowth were the same as those for the initial isolates. Higher concentrations of WCK 771, ranging from 2.0 to 8.0 μg/ml, produced bactericidal effects that were concentration independent and resulted in 3-log10 killing by 24 h. WCK 771 produced transient killing of 1 to 1.5 log10 at 6 h at 0.5 times the MIC.
FIG. 1.
Time-kill study of WCK 771 against MRSA 32 showing concentration-independent bactericidal activity by 24 h.
FIG. 2.
Time-kill study of WCK 771 against MRSA 5023 showing concentration-independent bactericidal activity by 24 h.
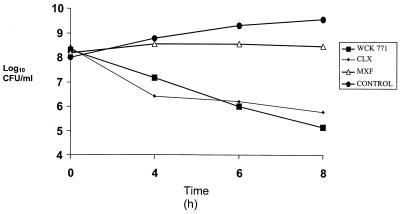
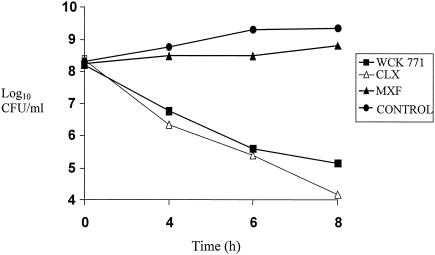
The results of high cell density time-kill experiments with the two quinolone-resistant MRSA strains are shown in Fig. 3 and 4. WCK 771 and clinafloxacin retained their bactericidal activity at 5 μg/ml, whereas moxifloxacin at 7.5 μg/ml showed no bactericidal effect during the 8-h exposure period. The bactericidal activities of WCK 771 and clinafloxacin against both MRSA strains at high cell density were similar.
FIG. 3.
Time-kill kinetics of WCK 771, clinafloxacin (CLX), and moxifloxacin (MXF) against a high-density inoculum of MRSA 97. Concentrations: WCK 771 and clinafloxacin, 5.0 μg/ml; moxifloxacin, 7.5 μg/ml. MICs: WCK 771, 1 μg/ml; CLX, 1 μg/ml; MXF, 4 μg/ml.
FIG. 4.
Time-kill kinetics of WCK 771, clinafloxacin (CLX), and moxifloxacin (MXF) against a high-density inoculum of MRSA 32. Concentrations: WCK 771 and clinafloxacin, 5.0 μg/ml; moxifloxacin, 7.5 μg/ml. MICs: WCK 771, 1 μg/ml; CLX, 1 μg/ml; MXF, 4 μg/ml.
DISCUSSION
WCK 771, an experimental quinolone under development for clinical use, is the arginine salt of the active isomer of nadifloxacin (19). Nadifloxacin has been reported to be very active against wild-type S. aureus and a grlA mutant, with only moderate decrease in activity against a gyrA mutant (27) and has been used topically to treat staphylococcal skin infections (15, 18). Initial studies, published in abstract form, have documented the excellent antistaphylococcal activity of WCK 771, even against quinolone-resistant strains (M. V. Patel, S. V. Gupte, S. K. Agarwal, D. J. Upadhyay, K. Sreenivas, Y. Chugh, N. Shetty, R. K. Beri, N. J. De Souza, and H. Khorakiwala, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-539, 2001; M. R. Jacobs, S. Bajaksouzian, A. Windau, M. V. Patel, N. De Souza, H. Khorakiwala, and P. C. Appelbaum, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-542, 2001). The present study reflects these preliminary findings, documenting antistaphylococcal MICs lower than any of the currently available quinolone agents. Additionally, WCK 771 was slightly more potent than clinafloxacin (MIC90s 1 dilution lower) against S. aureus and equally potent against S. epidermidis, including quinolone-susceptible and -resistant isolates. The MICs of other quinolones against the two staphylococcal species were consistent with those reported previously (14, 23, 24). At 0.5 to 1 μg/ml, WCK 771 and clinafloxacin had the lowest MICs of the fluoroquinolones tested against two VISA strains and one VRSA strain. While WCK 771 and clinafloxacin had the best potency of the quinolones studied, the relative differences between the MICs of all of the quinolones tested for quinolone-susceptible and -resistant isolates were similar, indicating similar effects of quinolone resistance mechanisms on the study agents.
The NorA efflux pump had no effect on the activity of WCK 771, with reserpine having no effect against the NorA-hyperexpressing strain S. aureus 1199B, in agreement with similar findings for the racemic precursor of WCK 771, nadifloxacin (21). However, MICs of norfloxacin, ciprofloxacin, and clinafloxacin against the NorA+ strain were significantly elevated, with fourfold or greater decreases in the MICs of these agents in the presence of reserpine (Table 2). As a large proportion of staphylococcal isolates express efflux-mediated fluoroquinolone resistance (25, 27), this is a significant advantage for WCK 771.
In kill kinetic studies, the interesting aspect of the bactericidal action of WCK 771 unraveled was its nondependence on its concentration. Thus, for both of the MRSA strains, concentrations ranging from two to eight times the MIC caused comparable extents of killing at various time points. However, in earlier comparative studies (D. J. Upadhyay, M. V.Patel, S. V.Gupte, S. K. Agarwal, M. A. Jafri, S. S. Bhagwat, N. J. De Souza, and H. F. Khorakiwala, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-534, 2001), concentration-dependent killing of MRSA was shown for clinafloxacin.
The bactericidal efficacy of WCK 771 against high-density MRSA cultures after exposure to a clinically relevant concentration of 5 μg/ml for 8 h would be therapeutically advantageous. This pharmacodynamic feature distinguishes WCK 771 from other, newer quinolones, such moxifloxacin, that lack this ability (12). This property has clinical relevance as the bacterial load in tissue can reach as high as 109 CFU/g in serious infections (7). A similar study comparing the bactericidal effects of ciprofloxacin against Escherichia coli at cell densities of 106 and 108/ml showed bactericidal activity against both inocula (11). Another rationale for testing a 100-fold-higher cell density is to expand subpopulations of fluoroquinolone-resistant clones present within MRSA cultures. It is conceivable that under such conditions, agents with superior affinity for molecular targets should demonstrate better bactericidal activity than agents that have relatively lower affinity for mutated targets. The response of two MRSA strains at high density clearly differentiated WCK 771 and clinafloxacin from moxifloxacin. This novel approach of evaluating antimicrobial agents against a denser pathogen population could lead to a new generation of antibacterials with superior pathogen eradication. While the recently introduced concept of the mutant prevention concentration (8) deals with a drug's ability to prevent the growth of resistant mutants from a high-density population, the approach of high cell density kill kinetics used in the present investigation deals with a drug's ability to effectively kill a larger pathogen population including potential mutants.
In summary, WCK 771 showed potency superior even to that of newer quinolones in clinical use against staphylococci. WCK 771 is a promising new agent that has high potency against staphylococci, including isolates resistant to other quinolones. If ongoing clinical studies yield a favorable safety profile, and if human pharmacokinetic studies support a susceptibility breakpoint of ≤2 μg/ml, WCK 771 will be active against all quinolone-susceptible staphylococci and >90% of quinolone-resistant staphylococci, features not achieved by currently available quinolones.
Acknowledgments
We thank the Wockhardt Research Center, Aurangabad, India, for supplying WCK 771 and G. W. Kaatz, Wayne State University, Detroit, Mich., for supplying S. aureus 1199 and 1199B.
REFERENCES
- 1.Bozdogan, B., D. Esel, C. Whitener, F. A. Browne, and P. C. Appelbaum. 2003. Antibacterial susceptibility of a vancomycin-resistant Staphylococcus aureus strain isolated at the Hershey Medical Center. J. Antimicrob. Chemother. 52:864-868. [DOI] [PubMed] [Google Scholar]
- 2.Calderon-Jaimes, E., L. E. Espinosa de los Monteros, and R. Avila-Beltran. 2002. Epidemiology of drug resistance: the case of Staphylococcus aureus and coagulase-negative staphylococci infections. Salud Publica Mex. 44:108-812. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2002. Public health dispatch: vancomycin resistant Staphylococcus aureus—Pennsylvania, 2002. Morb. Mortal. Wkly. Rep. 51:902. [PubMed] [Google Scholar]
- 4.Chambers, H. F. 2001. The changing epidemiology of Staphylococcus aureus? Emerg. Infect. Dis. 7:178-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang, S., D. M. Sievert, J. C. Hageman, M. L. Boulton, F. C. Tenover, F. P. Downes, S. Shah, J. T. Rudrik, G. R. Pupp, W. J. Brown, D. Cardo, and S. K. Fridkin. 2003. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N. Engl. J. Med. 348:1342-1347. [DOI] [PubMed] [Google Scholar]
- 6.Diekema, D. J., M. A. Pfaller, F. J. Schmitz, J. Smayevsky, J. Bell, R. N. Jones, and M. Beach. 2001. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997-1999. Clin. Infect. Dis. 32(Suppl. 2):S114-S132. [DOI] [PubMed] [Google Scholar]
- 7.Drlica, K. 2001. A strategy for fighting antibiotic resistance. ASM News 67:27-33. [Google Scholar]
- 8.Drlica, K., and M. Malik. 2003. Fluoroquinolones: action and resistance. Curr. Top. Med. Chem. 3:249-282. [DOI] [PubMed] [Google Scholar]
- 9.Fluit, A. C., M. E. Jones, F. J. Schmitz, J. Acar, R. Gupta, and J. Verhoef. 2000. Antimicrobial susceptibility and frequency of occurrence of clinical blood isolates in Europe from the SENTRY Antimicrobial Surveillance Program, 1997 and 1998. Clin. Infect. Dis. 30:454-460. [DOI] [PubMed] [Google Scholar]
- 10.Fridkin, S. K., J. Hageman, L. K. McDougal, J. Mohammed, W. R. Jarvis, T. M. Perl, and F. C. Tenover. 2003. Epidemiological and microbiological characterization of infections caused by Staphylococcus aureus with reduced susceptibility to vancomycin, United States, 1997-2001. Clin. Infect. Dis. 36:429-439. [DOI] [PubMed] [Google Scholar]
- 11.Gould, I. M., K. Milne, and C. Jason. 1990. Concentration-dependent bacterial killing, adaptive resistance and post-antibiotic effect of ciprofloxacin alone and in combination with gentamicin. Drugs Exp. Clin. Res. 16:621-628. [PubMed] [Google Scholar]
- 12.Herington, J. A., J. A. Fedirici, and J. M. Remy. 2000. Factors affecting the in vitro activity of moxifloxacin, p. 73-79. In D. Adam and R. Finch (ed.), Moxifloxacin in practice. Maxim Medical, Oxford, United Kingdom.
- 13.Hooper, D. C. 2002. Fluoroquinolone resistance among gram-positive cocci. Lancet Infect. Dis. 2:530-538. [DOI] [PubMed] [Google Scholar]
- 14.Jones, M. E., M. R. Visser, M. Klootwijk, P. Heisig, J. Verhoef, and F. J. Schmitz. 1999. Comparative activities of clinafloxacin, grepafloxacin, levofloxacin, moxifloxacin, ofloxacin, sparfloxacin, and trovafloxacin and nonquinolones linezolid, quinupristin-dalfopristin, gentamicin, and vancomycin against clinical isolates of ciprofloxacin-resistant and -susceptible Staphylococcus aureus strains. Antimicrob. Agents Chemother. 43:421-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimata, H. 1999. Effect of nadifloxacin on atopic dermatitis with methicillin-resistant Staphylococcus aureus in young children. Eur. J. Pediatr. 158:949. [DOI] [PubMed] [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard M7-A6. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 17.Nishijima, S., I. Kurokawa, and H. Nakaya. 2002. Susceptibility change to antibiotics of Staphylococcus aureus strains isolated from skin infections between July 1994 and November 2000. J. Infect. Chemother. 8:187-189. [DOI] [PubMed] [Google Scholar]
- 18.Nishijima, S., and M. Nakagawa. 1997. Sensitivity of antibacterials of Staphylococcus aureus isolated from impetigo patients. J. Int. Med. Res. 25:210-213. [DOI] [PubMed] [Google Scholar]
- 19.Nishijima, S., M. Nakagawa, N. Tsuboi, H. Akamatsu, T. Horio, M. Fujita, and S. Kawabata. 1996. Activity of nadifloxacin against methicillin-resistant Staphylococcus aureus isolated from skin infections: comparative study with seven other fluoroquinolones. J. Int. Med. Res. 24:12-16. [DOI] [PubMed] [Google Scholar]
- 20.Nishijima, S., S. Namura, H. Akamatsu, S. Kawai, Y. Asada, S. Kawabata, and M. Fujita. 1995. In vitro activity of nadifloxacin against both methicillin-susceptible and -resistant clinical isolates of Staphylococcus aureus from patients with skin infections. Drugs 49(Suppl. 2):230-232. [DOI] [PubMed] [Google Scholar]
- 21.Oizumi, N., S. Kawabata, M. Hirao, K. Watanabe, S. Okuno, T. Fujiwara, and M. Kikuchi. 2001. Relationship between mutations in the DNA gyrase and topoisomerase IV genes and nadifloxacin resistance in clinically isolated quinolone-resistant Staphylococcus aureus. J. Infect. Chemother. 7:191-194. [DOI] [PubMed] [Google Scholar]
- 22.Pankuch, G. A., M. R. Jacobs, and P. C. Appelbaum. 1994. Study of comparative antipneumococcal activities of penicillin G, RP 59500, erythromycin, sparfloxacin, ciprofloxacin, and vancomycin by using time-kill methodology. Antimicrob. Agents Chemother. 38:2065-2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pong, A., K. S. Thomson, E. S. Moland, S. A. Chartrand, and C. C. Sanders. 1999. Activity of moxifloxacin against pathogens with decreased susceptibility to ciprofloxacin. J. Antimicrob. Chemother. 44:621-627. [DOI] [PubMed] [Google Scholar]
- 24.Ryan, B. M., C. E. Mazzucco, L. E. Lawrence, H. Ho, G. Warr, J. F. Barrett, and M. Frosco. 2002. Comparison of the bactericidal activities and post-antibiotic effects of the Des-F6-quinolone BMS-284756, levofloxacin, and ciprofloxacin against methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis. 21:27-34. [DOI] [PubMed] [Google Scholar]
- 25.Schmitz, F. J., A. C. Fluit, M. Luckefahr, B. Engler, B. Hofmann, J. Verhoef, H. P. Heinz, U. Hadding, and M. E. Jones. 1998. The effect of reserpine, an inhibitor of multidrug efflux pumps, on the in-vitro activities of ciprofloxacin, sparfloxacin and moxifloxacin against clinical isolates of Staphylococcus aureus. J. Antimicrob. Chemother. 42:807-810. [DOI] [PubMed] [Google Scholar]
- 26.Smith, T. L., M. L. Pearson, K. R. Wilcox, C. Cruz, M. V. Lancaster, B. Robinson-Dunn, F. C. Tenover, M. J. Zervos, J. D. Band, E. White, and W. R. Jarvis. 1999. Emergence of vancomycin resistance in Staphylococcus aureus. N. Engl. J. Med. 340:493-501. [DOI] [PubMed] [Google Scholar]
- 27.Takei, M., H. Fukuda, R. Kishii, and M. Hosaka. 2001. Target preference of 15 quinolones against Staphylococcus aureus, based on antibacterial activities and target inhibition. Antimicrob. Agents Chemother. 45:3544-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomson, C. J. 1999. The global epidemiology of resistance to ciprofloxacin and the changing nature of antibiotic resistance: a 10 year perspective. J Antimicrob. Chemother. 43(Suppl. A):31-40. [DOI] [PubMed] [Google Scholar]