Abstract
A relatively fast and reproducible CE separation was developed for the glycoform analysis of α1-acid glycoprotein (AGP). Factors that were considered included the pH for this separation and various techniques for coating the capillary and/or to minimize electroosmotic flow and protein adsorption. Optimum resolution of the AGP glycoforms was obtained at pH 4.2 with a running buffer containing 0.1% Brij 35 and by using static and dynamic coatings of PEO on the capillary. These conditions made it possible to separate nine AGP glycoform bands in about 20 min. The limit of detection (based on absorbance measurements) ranged from 0.09 to 0.38 μM for these AGP glycoform bands, and the linear range extended up to a total AGP concentration of at least 240 μM. The migration times for the glycoform bands had typical within-day and day-to-day precisions of ± 0.16–0.23% or less, respectively, on a single treated capillary and the variation between capillaries was ± 0.56% or less. A charge ladder approach was employed to examine the mass or charge differences in the glycoforms that made up these bands, giving a good fit to a model in which the neighboring bands differed by one charge (e.g., from a sialic acid residue) and had an average mass difference of approximately 0.7–0.9 kDa. The approaches used to develop this separation method are not limited to AGP but could be extended to the analysis of other glycoproteins by CE.
Keywords: Alpha1-acid glycoprotein, Capillary electrophoresis, Glycoform analysis, Glycoproteins, Capillary coating, Charge ladder
1. Introduction
Alpha1-acid glycoprotein (AGP) is an acute phase glycoprotein and serum transport protein with a low isoelectric point (pI, 2.8–3.8) and a high carbohydrate content (40%) [1,2]. The normal concentration of this protein in human plasma is 0.5–1.0 g/L (or 12–24 μM); however, this concentration can increase by up to ten-fold in some diseases [1]. In theory, more than 105 glycoforms of AGP could exist as a result of the five glycosylation sites that are present on this protein; however, it has been stated that only 12–20 major glycoforms of AGP are typically detected in human serum [3]. The use of AGP glycoforms as biomarkers has been explored for the diagnosis of conditions such as ovarian cancer, leukemia and atherothrombosis [4,5]. Changes in the glycoforms of AGP have also been of interest in terms of how these variations may alter the binding of this protein to drugs [1–3,6].
Capillary electrophoresis (CE) is one technique that has been used to examine AGP and its glycoforms [4,5,7–12]. The potential advantages of this approach include its efficiency, fast analysis times and small sample requirements [13]. However, interactions with the capillary wall can present a challenge in the use of CE for AGP, glycoproteins and related biomolecules [14]. The presence of such interactions can lead to low separation efficiencies and alter the electroosmotic flow, which can also change the observed mobilities over time and result in poor precision or non-reproducible separations [14,15].
To overcome these issues, some previous studies have used capillaries that contained a cross-linked coating of dimethylpolysiloxane and a dynamic coating of (hydroxypropyl)methylcellulose or buffers containing the surfactant Tween 20 for the analysis of AGP glycoforms by CE [7,8,12]. The adsorption of AGP during CE has also been reduced by using capillaries that were covalently modified with succinyl-poly-L-lysine or by using a commercial capillary with a hydrophilic bonded phase (i.e., CElect-P1) [9]. Some limitations of these previous methods have been that they either involved tedious methods for capillary treatment or they required relatively expensive pre-treated capillaries [7–9,12]. Another approach has been to employ buffer additives such as putrescene or urea to disrupt interactions between AGP and the capillary wall and to reduce electroosmotic flow [4,5,10,11]. However, the migration times of AGP glycoforms cannot be easily used to make accurate peak assignments in such methods due to the variability in these values, the relatively large number of peaks that are present, and the similar migration times of the glycoform bands [4]. Thus, there is still a need for a simple and reproducible CE method for examining AGP glycoforms.
In this study, a relatively fast and reproducible CE method will be optimized and developed for analyzing the glycoform pattern of AGP. The effect of pH on the resolution of the glycoform pattern will be considered, as well as the use of capillaries that are modified with permanent coatings, static coatings or dynamic coatings based on various protein-repellent polymers. The use of other additives in the running buffer will also be examined to further improve the separation of AGP glycoforms. The final CE method will be evaluated with standard samples of human AGP to determine this technique’s resolution, speed, response, and precision. In addition, the results of this method will be analyzed with a charge ladder model to determine the general types of structural differences that are present between the AGP glycoforms that are present in each observed band.
2. Theory
Charge ladders have been used in the past with CE to characterize a series of related proteins [16,17]. In this current study, the electrophoretic mobility (μn) of a given AGP glycoform band (i.e., band number “n” in a series of bands) was described by using the following expression,
| (1) |
where z represents the charge of the species within the band, M is the average molar mass of these species, and α is an empirical parameter that is related to the shape and degree of solvation of the species (i.e., where α is generally believed to equal 2/3 for globular proteins) [16–19]. The term Cp in Eq. (1) is a constant that is related to the shape and solvation state of the glycoform species, as well as the ionic strength and viscosity of the running buffer [16,17]. The charge z in Eq. (1) can also be described by Eq. (2),
| (2) |
where z0 represents the charge of the AGP glycoform band with the lowest charge in a series of bands, and zseq is the charge difference between neighboring bands. Combining Eqs. (1–2) with a value for α of 2/3 gives the equivalent expressions in Eq. (3).
| (3) |
Over a small range of mass, such as represented by the product of the terms n and m, the electrophoretic mobility of an AGP glycoform band will be approximately proportional to (Note: see the Supplementary Materials, which demonstrate this concept using data from Ref. [18]). In addition, the average molar mass of the species in an AGP glycoform band can be represented by Eq. (4),
| (4) |
where M0 is the average mass of the AGP glycoform species in the band with the lowest charge, and m is the unit change in mass between the glycoform bands. When these expressions are placed into Eq. (3), the electrophoretic mobilities of the AGP glycoform bands can be described by the following approximate relationships.
| (5) |
For the expressions shown in Eq. (5), the product “nm” (i.e., which represents the change in mass between bands 0 and n) should often be quite small when compared to the overall and average mass M0 for the species with the lowest charge. This situation would be expected when the value of n is relatively low (e.g., nm was less than 10% of the value for M0 in this study when n was less than or equal to 6). This condition allows Eq. (5) to be simplified and rearranged into the form that is shown below.
| (6) |
According to Eq. (6), a plot of versus should result in a linear relationship for such a system (Note: in this study, the value of n was found by using the absolute difference in the migration order between a given AGP glycoform band and the glycoform band with the lowest charge). The ratio of the unit change in mass versus the overall mass, m/M0, can then be obtained by dividing the intercept by the slope for the resulting best-fit line. If M0 is also known, the value of m can be obtained by using the relationship m = (Intercept/Slope) M0.
3. Materials and methods
3.1. Reagents
The AGP (from pooled human plasma, ≥ 99% pure; catalog numbers, SLBJ6840V and SLBG6410V), poly(vinyl alcohol) (PVA, hydrolyzed; weight-average molar mass, 31–50 kDa; 98–99%), poly(ethylene oxide) (PEO; viscosity-average molar mass, 8,000 kDa), dextran (weight-average molar mass, 282 kDa), Brij 35 (number-average molar mass, 1.198 kDa) and thiourea were purchased from Sigma-Aldrich (St. Louis, MO, USA). All aqueous solutions and samples were prepared using water obtained from a Milli-Q Advantage A10 water purification system (EMD Millipore Corporation, Billerica, MA, USA).
3.2. Apparatus
The electrophoretic separations were carried out using a P/ACE MDQ instrument (Beckman Instruments, Fullerton, CA, USA). The capillary in this system was maintained at 25°C during the separation, which was performed in most experiments at an applied potential of −30 kV (i.e., in the reversed polarity mode). This system used 60.2 cm × 50 μm I.D. fused silica capillaries (Polymicro Technologies, Phoenix, AZ, USA) with an effective length to the detector of 50 cm. Absorbance detection was carried out at 200 nm, and the CE data were collected by using 32 Karat software 7.0 from Beckman. These data were analyzed by utilizing Peakfit 4.12 software (Systat Software, San Jose, CA, USA).
3.3. Capillary modification
Each capillary was activated by rinsing it for 30 min with 1.0 M sodium hydroxide and washing it with water for 10 min prior to use. The method that was used for the permanent capillary modification with PVA was the same as described previously [20]. The approach employed for placing a static coating of PVA, PEO or dextran on a capillary was also carried out according to a previous method [21] but used an automated rinsing protocol. In the static coating method, the capillary was cleaned for 5 min with 1.0 M sodium hydroxide, which was followed by a 3 min wash with water. The coating was then applied by using a 5 min rinse of 1.0 M hydrochloric acid and a 5 min rinse with a 0.2% (w/v) polymer solution that contained PEO, PVA or dextran along with 0.10 M hydrochloric acid. Application of the polymer solution was followed by a 5 min rinse with the running buffer that was to be used in the CE method. All rinses were performed at an applied pressure of 50 psi in the forward direction. A 0.05% (w/v) solution of the desired polymer (e.g., PVA, PEO, or dextran) was dissolved into the running buffer to create a dynamic coating during the CE separation.
The mobility due to electroosmotic flow (μosm) that resulted after each treatment method was characterized in triplicate by using thiourea as a neutral marker. The value of μosm was also measured on a bare fused silica capillary to determine the change in μosm that resulted from a given treatment technique. The value of μosm was calculated by using Eq. (7) [22],
| (7) |
where Ltotal is the overall length of the capillary, and Ldetector is the length of the capillary from the point of injection to the detector. The migration time of a neutral marker is represented in Eq. (7) by the term tmarker, and the applied voltage is represented by V.
4. Results and discussion
4.1. Effect of pH on the separation of AGP glycoforms
The effects of various factors were considered in the separation of AGP glycoforms by CE. In the ideal situation where longitudinal diffusion is the only source of band broadening, the resolution (Rs) between two neighboring peak in such a separation can be described by Eq. (8) [23],
| (8) |
where V is the applied voltage, D is the solute’s diffusion coefficient, μ1 and μ2 are the electrophoretic mobilities of the species in two neighboring peaks (i.e., peaks 1 and 2), μ̄ is the average electrophoretic mobility of these two peaks, and μosm is the mobility due to electroosmotic flow (Note: the value of 0.177 in this equation is a combination of constants that is equal to ).
Eq. (8) indicates that the resolution in a CE method will be increased by the presence of a large difference in the electrophoretic mobilities of the peaks (i.e., μ1 − μ2), a good balance between the size of the average mobility versus the mobility due to electroosmotic flow (i.e., a value for μ̄+μosm that approaches zero) and a high separation voltage [23,24]. However, a resolution that is lower than would be predicted by this equation is often seen for proteins in CE due to protein adsorption to the capillary, which can be a significant source of band broadening that is not considered in Eq. (8) [14,15].
The effect of changing the pH of the running buffer was one factor considered in the separation of AGP glycoforms by CE. This was evaluated by using a fused silica capillary that had a static coating of PEO to minimize electroosmotic flow, as will be discussed in more detail in the following section. An increase in the resolution of AGP glycoforms was seen as the pH was reduced from 5.0 to 4.2 (see Figure 1). AGP glycoforms can contain a variety of sialic acid residues, which results in a distribution of the isoelectric points (pI) for these glycoforms over a range of 2.8–3.8 [1]. As the pH decreases and approaches these pI values, some of the AGP glycoforms will take on a neutral or even a net positive charge, while others will retain some negative charge. This results in a large difference in the charge, and charge-to-size ratios, for the glycoforms. This effect would, in turn, be expected to lead to an increase in the difference in the electrophoretic mobilities of these glycoforms and an improvement in the resolution of these species, as is predicted by Eq. (8).
Figure 1.
Effect of pH on the resolution of AGP glycoforms. Conditions: sample, 4.0 g/L AGP in water; capillary conditions, 5 min rinse with 1.0 M sodium hydroxide, 3 min rinse with water, 5 min rinse with 1.0 M hydrochloric acid, 5 min rinse with 0.2% (w/v) PEO containing 0.1 M hydrochloric acid, 5 min rinse with buffer (pH 5.0, 4.6, 4.4, 4.2, 4.0, or 3.8, 20 mM sodium acetate); injection, 0.5 psi, 3 s; separation voltage, −30 kV.
A shift to longer migration times, and a less negative charge, was noted for all of the AGP glycoforms as the pH of the running buffer was decreased (see Figure 1). The contribution of electroosmotic flow to the overall observed mobilities was minimized in this case by treating the capillary with a static coating of PEO. Thus, it was concluded that the shift in the migration times and the reduction in electrophoretic mobilities was mainly was due to a change in the charges of the AGP glycoforms under these conditions. There was also an increase in the resolution between the bands for these glycoforms as the pH decreased from 5.0 to 4.2. However, a slightly lower resolution was again observed at pH 4.0 and 3.8. For instance, the resolution between the last two bands for the AGP glycoforms increased from 0.51 to 0.78 as the pH decreased from 5.0 to 4.2; this resolution then dropped from 0.78 to 0.68 in going from pH 4.2 to 4.0 and further decreased to 0.41 at pH 3.8 due to an increase in the broadening of these bands. This effect reflects, in part, an increase in the adsorption of the AGP glycoforms to the capillary as these glycoforms approach a neutral charge and become less soluble in the running buffer [15]. However, some of the apparent increase in band broadening at pH 4.0 and pH 3.8 was also due to the beginning of a partial separation for glycoform species with the same charge but slightly different masses (see Supplementary Materials and Section 4.4) [25]. Based on these results, a pH of 4.2 for the running buffer was chosen for use in all further studies in this current report. This value is consistent with the pH range of 4.2–4.5 that has been employed in previous CE methods for separating AGP glycoforms [4,7].
4.2. Capillary modification and characterization
Initial experiments were carried out with the separation of AGP glycoforms on a bare fused silica capillary. During these experiments it was found that there was a significant change in the migration times and observed peak areas for the glycoform bands over a series of consecutive injections, as is demonstrated in Figure 2. This trend led to a decrease in the migration times, which could not be reversed by rinsing the capillary between injections with 1.0 M sodium hydroxide. The suspected reason for these changes was that AGP was adsorbing to the surface of the capillary. Such an effect is known to occur for other proteins and to lead to non-reproducible separations, along with a loss of separation efficiency and decreased resolution [15].
Figure 2.
Separation of AGP glycoforms on an untreated silica capillary. Conditions: sample, 4.0 g/L AGP in water; capillary conditions, 5 min rinse with 1.0 M sodium hydroxide, 3 min rinse with water, 5 min rinse with pH 4.2, 20 mM sodium acetate buffer; injection, 0.5 psi, 3 s; separation voltage, +30 kV. A rinse with 1.0 M sodium hydroxide for 20 min was performed between the third and fourth injections.
Several approaches for capillary modification were examined to minimize these effects. Three types of polymers (i.e., PVA, PEO and dextrin, as shown in Figure 3) were considered for this purpose. PVA and PEO have been used previously as surface modification reagents to provide efficient separations for basic and/or acidic proteins in CE [20,21]; however, these agents have not been used in prior work with AGP. Dextran was also tested as a coating agent because glass surfaces that have been treated with this reagent have been shown previously to have reduced adsorption for other types of proteins [26,27].
Figure 3.
Structures of poly(vinyl alcohol), or PVA; poly (ethylene oxide), or PVA; and dextran. The structure shown for dextran is based on an α(1,6)-type linkage; less than 5% of the dextran had an α(1,3)-type linkage.
These agents were explored for use as permanent, static and dynamic coatings. A dynamic coating was created by simply adding a water-soluble polymer/coating agent into the running buffer [15]. A static coating was prepared by flushing the capillary with a solution that contained the coating agent and a coating-free running buffer before carrying out a separation by CE [15]. Permanent coatings are typically based on the covalent bonding of a polymer to the capillary’s surface [28], which avoids the need to regenerate the coating between runs (e.g., as is needed when a static coating is employed). In this study, a permanent coating of PVA was obtained through the crystallization of PVA onto a silica capillary’s internal surface at 140 °C, as has been described in prior work [20].
The ability of these agents and methods to coat the surface of a silica capillary was examined by measuring the reduction in electroosmotic flow that they each produced when the pH of the running buffer was 4.2. Table 1 shows the change in electroosmotic flow that was observed for each type of coating when compared to the results obtained with an untreated silica capillary. The use of PVA as a coating in the various tested formats resulted in a decrease in the electroosmotic flow of 48.0–66.8%. The best results were seen when PVA was utilized to give a permanent coating or permanent plus dynamic coatings. When PVA was used to give only a permanent coating, a 17.9% larger reduction in the electroosmotic flow was obtained than when only a static coating of the same agent was present. The addition of a dynamic coating of PVA gave a further reduction of 4.4% in the electroosmotic flow after the use of a static coating and an additional reduction of 1.0% after the use of a permanent coating. The best overall combination for PVA was the use of this agent to create permanent plus dynamic coatings.
Table 1.
Effect on electroosmotic flow when using various modification agents and methods to treat fused silica capillariesa
| Modification Agent & Method | Mobility due to electroosmotic flow, μosm (×10−3cm2min−1V−1) | Reduction in electroosmotic flow (%) | |
|---|---|---|---|
| PVA | Permanent coating | 7.05 (± 0.02) | 65.8 (± 1.4) |
| Permanent plus dynamic coating | 7.09 (± 0.23) | 66.8 (± 1.8) | |
| Static coating | 11.04 (± 0.27) | 48.0 (± 2.6) | |
| Static plus dynamic coating | 9.85 (± 0.06) | 52.3 (± 2.0) | |
| PEO | Static coating | 1.83 (± 0.01) | 91.1 (± 0.4) |
| Static plus dynamic coating | 0.222 (± 0.002) | 98.9 (± 0.1) | |
| Dextran | Static coating | 9.76 (± 0.01) | 52.8 (± 2.0) |
| Static plus dynamic coating | 9.89 (± 0.10) | 51.7 (± 2.1) | |
The mobility due to electroosmotic flow for a fused silica capillary was 20.66 (± 0.87) x 10−3 cm2min−1V−1. The electroosmotic flow was measured in pH 4.2, 20 mM acetate buffer with a sample containing 1.0 g/L thiourea in water. The temperature of the capillary was 25 °C. The running buffer contained 0.05% (w/v) PVA, PEO or dextran when these agents were used to create a dynamic coating. The samples were applied by using hydrodynamic injection at 0.4 psi for 3 s. All of the results represent the average of three trials, where the number in parentheses is a range of ± 1 S.D.
The use of dextran for creating a static coating gave a suppression of 52.8% in the electroosmotic flow. This level of suppression decreased slightly to 51.7% when dextran was also added to running buffer to give a dynamic coating. The overall reduction in electroosmotic flow that was obtained with dextran was comparable to that obtained with PVA when both were used in the same modification format. However, the ability to use PVA in making a permanent coating allowed this latter reagent to create a larger overall reduction in the electroosmotic flow.
An even greater decrease in the electroosmotic flow was seen when using PEO as a coating agent. For instance, the use of a static coating alone for PEO gave a 91.1% reduction in the extent of electroosmosis. The combined use of static and dynamic coatings based on PEO gave a 98.9% reduction in the electroosmotic flow. This type of decrease has been partly attributed in prior work to favorable non-covalent interactions (e.g., hydrogen bonds) between groups on PEO and silanol groups on the surface of a silica capillary [21]. Based on these results, a combination of static and dynamic coatings based on PEO was used in all later work in this study with AGP.
One disadvantage of using PEO as a coating agent was it required the use of an elevated temperature (80 °C), as utilized in prior work [29], and a relatively long period of stirring (i.e., around 2 hours) to fully dissolve this agent in the running buffers that were employed in this report. In contrast to this, PVA and dextran were more water soluble and easier to dissolve in the running buffers. However, a lower aqueous solubility may have also given PEO less bleeding from the capillary than these other coating agents, leading to a better surface coverage and a larger decrease in the electroosmotic flow.
The modified capillaries were next tested for use in separating AGP glycoforms. Figure 4 shows some typical electropherograms that were obtained. Partial resolution for eight glycoform bands, with Rs values ranging from 0.44–1.08, was accomplished within 20 min when a capillary was used that had been treated with both static and dynamic coatings of PEO. It took about 2.5 min longer to separate the same glycoform bands, and with a comparable resolution, when only a static coating of PEO was used. Similar separations took 45 to 90 min to complete when using the other capillary modification methods that were considered in this work. These differences in the separation time reflected the extent to which electroosmotic flow was reduced by each of the capillary modification methods.
Figure 4.
Separation of AGP glycoforms by capillaries that had been modified with various types of coatings: (a) static coating of PVA (range of peak resolution values, Rs, 0.53–0.74), (b) static and dynamic coatings of PVA (Rs, 0.41–0.84); (c) permanent coating of PVA (Rs, 0.48–0.85); (d) permanent and dynamic coatings of PVA (Rs, 0.68–0.76); (e) static coating of dextran (Rs, 0.43–0.98); (f) static and dynamic coatings of dextran (Rs, 0.42–0.87); (g) static coating of PEO (Rs, 0.71–0.95); and (h) static and dynamic coatings of PEO (Rs, 0.44–1.08). Conditions: sample, 4.0 g/L AGP in water; injection, 0.4 psi for 3 s; applied voltage, −30 kV.
4.3. Effect of a non-ionic surfactant on the separation of AGP glycoforms
The use of a non-ionic surfactant was considered as another option to improve the separation of AGP glycoforms. This was carried out by using capillaries that had also been treated with static and dynamic coatings of PEO. Brij 35 was chosen for this work because it is a non-ionic and mild surfactant (i.e., it is less likely to denature or unfold proteins when compared to ionic detergents) [30]. However, no prior studies have used this additive to improve the separation of AGP glycoforms.
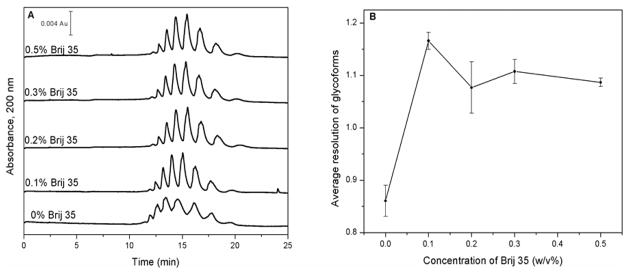
As is shown in Figure 5, sharper peaks for the AGP glycoforms were obtained when 0.1% Brij 35 was added to the running buffer versus when no surfactant was present. A total number of nine glycoform bands could now be partially separated under these conditions, as is shown in Figure 6; this was one more band than was observed without the addition of Brij 35 to the running buffer. The average efficiency increased by two-fold for the glycoform bands (i.e., going from a plate number of 2,500 to 5,100) when 0.1% Brij was present. The addition of this surfactant also resulted in an average 1.5-fold increase in the resolution of the AGP glycoform bands (new resolution range, 0.46–1.71). This resolution did not increase to any significant extent as the concentration of Brij 35 was further increased from 0.1% to 0.2–0.5%. The observed increases in the efficiency and resolution that were seen with the addition of Brij 35 were believed to be due to the ability of this surfactant to increase the solubility of AGP glycoforms in the running buffer, as has been proposed for FITC-labeled myoglobin [15]. Based on these results, all further work with the AGP glycoforms were carried out by using a running buffer that contained 0.1% Brij 35 and with the capillary still being treated with both static and dynamic coatings of PEO.
Figure 5.
(a) Electropherograms obtained for AGP glycoforms at various concentrations of Brij 35 in the running buffer, expressed as %(w/v), and (b) the average resolution of the AGP glycoform bands as a function of the concentration of Brij 35 that was placed into the running buffer. Conditions: running buffer, pH 4.2 acetate buffer containing 0.05% (w/v) PEO and various concentrations of Brij 35, sample, 4.0 g/L AGP in water; injection, 0.5 psi, 5 s; separation voltage, −30 kV.
Figure 6.
Validation of CE method for the separation of AGP standards: Sample, 4 g/L AGP dissolved with water; Background electrolyte, pH 4.2 acetate buffer with 0.05% (w/v) PEO and 0.1% (w/v) Brij 35; Separation voltage, −30 kV.
4.4. Response, precision and efficiency of CE method for AGP glycoform analysis
The final method that was developed for the separation and analysis of AGP glycoforms used both static and dynamic coatings of PEO in the capillary and a pH 4.2 running buffer that contained 0.1% Brij 35. This resulted in a separation of nine AGP glycoform bands within 20 min, with a resolution between neighboring bands that ranged from 0.46 to 1.71. The number of AGP glycoform bands that was observed under these conditions was one higher that has been noted in a previous report that used CE under other operating conditions [4].
The efficiency for this separation, as expressed in terms of the number of theoretical plates for the observed bands, ranged from 4,000 to 7,900. These relatively low values probably reflect the fact that each band consisted of multiple AGP glycoforms with the same charge but slightly different masses [3]. This was confirmed by experiments in which the pH of the running buffer was decreased from 4.2 to 3.8, making it possible for many of the glycoform bands to be separated into additional peaks (see Supplementary Materials). A pH below 4.2 was not used further in this study because of the increased adsorption of AGP, the loss in reproducibility, and the lower precision for the migration times that were seen under these conditions. However, these alternative separation conditions for AGP are being explored in on-going studies.
The response of this CE method was also evaluated, as based on absorbance detection. The limit of detection at a signal-to-noise ratio of three ranged from 0.09 to 0.38 μM for the nine observed AGP glycoform bands (see Supplementary Materials) when using hydrodynamic injection at 0.5 psi for 15 s. The linear range extended up to a total AGP concentration of approximately 240 μM under the same conditions. This range included those concentrations that would be expected in both normal human plasma and in patients with conditions that may increase the plasma concentration of AGP by up to ten-fold [1].
The precisions that were obtained for the migration times and peak areas were also evaluated for this technique. The within-day and day-to-day precisions for these parameters are summarized in Table 2. For glycoforms that made up 3% or more of the AGP sample (i.e., bands 3–8), the within-day precisions for the migration time ranged from ± 0.09–0.16% (n = 5) and the day-to-day precisions were ± 0.17–0.23% (n = 15) when measured on a single treated capillary. The variation noted for the migration time when using multiple capillaries was also tested, giving variations that ranged from ± 0.39–0.56% for three capillaries. These variations in the migration time were much smaller than the differences seen in the migration times for neighboring AGP glycoform bands, which differed by 1.7–8.6%. This difference in migration time between neighboring bands was 12- to almost 60-fold larger than the standard deviations that were observed in the migration time. As a result, it was possible with this method and by making only two to three replicate injections to distinguish between the neighboring glycoform bands at both the 95% and 99% confidence levels, based on the use of migration times.
Table 2.
Analytical characteristics of the CE method for examining AGP glycoformsa
| AGP peak number | Plate number, N (×103) | Migration time
|
Electrophoretic mobility (×10−3cm2min−1V−1) | Band Area
|
|||
|---|---|---|---|---|---|---|---|
| Average (min) (n = 15) | Within-day precision (%)(n = 5) | Day-to-day precision (%)(n = 15) | Within-day precision (%)(n = 5) | Day-to-day precision (%)(n = 15) | |||
| 1 | 3.8 (± 0.4) | 11.10 (± 0.03) | 0.40 | 0.31 | −9.28 (± 0.02) | 17.61 | 14.48 |
| 2 | 2.3 (± 0.2) | 11.91 (± 0.03) | 0.09 | 0.28 | −8.66 (± 0.02) | 4.00 | 6.56 |
| 3 | 7.9 (± 0.5) | 12.26 (± 0.03) | 0.09 | 0.23 | −8.42 (± 0.02) | 1.12 | 3.43 |
| 4 | 7.4 (± 0.4) | 12.83 (± 0.03) | 0.10 | 0.22 | −8.06 (± 0.02) | 1.16 | 2.92 |
| 5 | 5.9 (± 0.2) | 13.58 (± 0.03) | 0.10 | 0.22 | −7.63 (± 0.02) | 1.21 | 2.61 |
| 6 | 4.7 (± 0.1) | 14.51 (± 0.03) | 0.10 | 0.20 | −7.16 (± 0.01) | 1.35 | 2.42 |
| 7 | 4.2 (± 0.1) | 15.70 (± 0.03) | 0.11 | 0.18 | −6.63 (± 0.02) | 1.53 | 2.36 |
| 8 | 4.0 (± 0.1) | 17.17 (± 0.03) | 0.16 | 0.17 | −6.08 (± 0.02) | 2.47 | 2.79 |
| 9 | 3.3 (± 0.2) | 18.92 (± 0.03) | 0.25 | 0.18 | −5.54 (± 0.02) | 7.58 | 6.15 |
These results were measured for a series of injections for a standard containing 4.0 g/L AGP. The samples were injected by the hydrodynamic method at 0.5 psi for 15 s over three consecutive days and with five injections being made per day. The plate numbers given here were measured at the band’s half-height. The numbers in parentheses represent a range of ± 1 S.D; the within-day and day-to-day precision values are given as ± 1 R.S.D.
Table 2 also shows the precisions that were obtained for the areas of the AGP glycoform bands. When using pressure injection at 0.5 psi for 15 s, the within-day precision for the areas was ± 1.1–2.5% (n = 5) for glycoforms that made up 3% or more of the total AGP and when using a single treated capillary. The day-to-day precision for the same glycoforms on a single capillary was ± 2.4–3.4% (n = 15), and the capillary-to-capillary variation in the area was ± 3.6–5.7% (n = 9 for three capillaries).
4.5. Use of a charge ladder model to analyze AGP glycoform bands
In the next portion of this study a charge ladder model was used to examine the CE results to determine the types of structural differences that lead to the nine AGP glycoform bands. Figure 7 illustrates the analysis of these results by this approach, where the mobility of the AGP glycoform band that had the lowest charge (i.e., the lowest degree of sialylation) was designated as having the mobility value μ0. The entire data set was first tested with Eq. (6) to determine the average mass difference that was present between these bands (i.e., the value of m). This gave a fit with a correlation coefficient of 0.9995 and resulted in an initial estimate for m of 0.9 (± 0.2) kDa, as based on a one unit charge difference between each band (e.g., a difference of one sialic acid group). The use of a non-linear fit based on Eqs. (3) and (4) with the same data set gave a correlation coefficient of 0.9956 and a value for m of 1.3 (± 0.2) kDa. However, some of peaks that were used in this analysis (i.e., AGP glycoform peaks 1 and 2) had much lower areas and greater uncertainties in their mobilities than the rest of the observed bands.
Figure 7.
Observed relationship for a plot of versus for AGP glycoforms, where μ0 is the electrophoretic mobility of AGP glycoform with the least negative charge; μn is the electrophoretic mobility of an AGP glycoform that has an additional number (n) of charges (e.g., due to sialic acids) versus the glycoform with the least negative charge. The best-fit line obtained for this fit was y = −1810 (± 20) x − 29 (± 9), with a correlation coefficient of 0.9997 (n = 90).
AGP glycoform peaks 3 through 9 were next used to refine this analysis. This set of peaks had an overall change in mass that was less than 10% of the molar mass of AGP, which was now in line with the assumptions made in Eq. (5). A fit of this equation to the data gave a correlation coefficient of 0.9997 and a value for the ratio m/M0 of 0.016 (± 0.005). When an average molar mass of 42 kDa was used for AGP, the utilization of this value with the measured ratio of m/M0 gave a best estimate for m of 0.7 (± 0.2) kDa (Note: the use of a non-linear fit to this refined data set gave a correlation coefficient of 0.9972 and a value for m of 0.9 (± 0.2) kDa). One common type of sialylated glycan branch subunit that is found in AGP (NeuAc-Gal-GlcNAc; molar mass, 0.66 kDa) has a mass which fits well with these results. If a fucose residue were also included in this structure, the mass of this subunit would increase to 0.80 kDa, which also fell within estimated range of values for m.
4. Conclusion
In this report, a relatively fast and reproducible CE method was developed for the separation and analysis of AGP glycoforms. Factors that were considered in this work included the pH that was present for this separation, various techniques for coating the capillary to minimize electroosmotic flow and protein adsorption, and the possible use of a non-ionic surfactant in the running buffer. The final method used a pH 4.2 for separation of the AGP glycoforms, along with the use of static and dynamic coatings of PEO plus the addition of 0.1% Brij 35 to the running buffer. These conditions made it possible to minimize the electroosmotic flow and obtain nine AGP glycoform bands within 20 min. The approaches used to develop and optimize this method are not limited to AGP but could also probably be extended to the analysis of other glycoproteins by CE [31].
This method had good precision in terms of both the migration times and areas that were observed for the glycoform bands, with within-day variations in this parameter of less than ± 0.3% and ± 4%, respectively. The precision in the migration time was 10-fold better than noted in previous CE methods for AGP or other glycoproteins [4,7]. The response and linear range of this method, when using absorbance detection, spanned the typical concentrations that would be expected in serum from normal individuals or those with elevated AGP levels. A charge ladder model was also employed to examine the mass or charge differences in the glycoforms that made up each of these bands. A good fit was obtained for a model in which the bands differed by one charge (e.g., due to different numbers of sialic acids) and that had an average mass difference of approximately 0.7–0.9 kDa. Future work will explore the use of this method with AGP from various samples representing a number of diseases known or thought to alter AGP glycoform patterns [4,5].
Supplementary Material
Highlights.
CE was used to separate glycoforms of alpha1-acid glycoprotein (AGP).
Several operating parameters were considered in optimizing this method.
This method could separate nine glycoform bands within 20 min.
Good precision was obtained for both peak areas and migration times.
This method could be used at clinically-relevant concentrations with absorbance detection.
A charge ladder model was used to analyze the differences in the AGP glycoform bands.
Acknowledgments
This work was supported by the National Institutes of Health under grants R01 GM044931.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ceciliani F, Pocacqua V. The acute phase protein alpha1-acid glycoprotein: a model for altered glycosylation during diseases. Curr Protein Pept Sci. 2007;8:91–108. doi: 10.2174/138920307779941497. [DOI] [PubMed] [Google Scholar]
- 2.Fernandes CL, Ligabue-Braun R, Verli H. Structural glycobiology of human α1-acid glycoprotein and its implications for pharmacokinetics and inflammation. Glycobiol. 2015;25:1125–33. doi: 10.1093/glycob/cwv041. [DOI] [PubMed] [Google Scholar]
- 3.Fournier T, Medjoubi N, Porquet ND. Alpha-1-acid glycoprotein. Biochim Biophys Acta Protein Struct Mol Enzymol. 2000;1482:157–71. doi: 10.1016/s0167-4838(00)00153-9. [DOI] [PubMed] [Google Scholar]
- 4.Lacunza I, Sanz J, Diez-Masa JC, De Frutos M. CZE of human alpha-1-acid glycoprotein for qualitative and quantitative comparison of samples from different pathological conditions. Electrophoresis. 2006;27:4205–14. doi: 10.1002/elps.200600304. [DOI] [PubMed] [Google Scholar]
- 5.Puerta A, Díez-Masa JC, Martín-Álvarez PJ, Martín-Ventura JL, Barbas C, Tuñón J, Egido J, de Frutos M. Study of the capillary electrophoresis profile of intact α-1-acid glycoprotein isoforms as a biomarker of atherothrombosis. Analyst. 2011;136:816–22. doi: 10.1039/c0an00320d. [DOI] [PubMed] [Google Scholar]
- 6.Israili ZH, Dayton PG. Human alpha-1-glycoprotein and its interactions with drugs. Drug Metab Rev. 2001;33:161–235. doi: 10.1081/dmr-100104402. [DOI] [PubMed] [Google Scholar]
- 7.Kinoshita M, Murakami E, Oda Y, Funakubo T, Kawakami D, Kakehi K, Kawasaki N, Morimoto K, Hayakawa T. Comparative studies on the analysis of glycosylation heterogeneity of sialic acid-containing glycoproteins using capillary electrophoresis. J Chromatogr A. 2000;866:261–71. doi: 10.1016/s0021-9673(99)01080-8. [DOI] [PubMed] [Google Scholar]
- 8.Kakehi K, Kinoshita M, Kawakami D, Tanaka J, Sei K, Endo K, Oda Y, Iwaki M, Masuko T. Capillary electrophoresis of sialic acid-containing glycoprotein. Effect of the heterogeneity of carbohydrate chains on glycoform separation using an alpha1-acid glycoprotein as a model. Anal Chem. 2001;73:2640–7. doi: 10.1021/ac001382u. [DOI] [PubMed] [Google Scholar]
- 9.Pacakova V, Hubena S, Ticha M, Madera M, Stulik K. Effects of electrolyte modification and capillary coating on separation of glycoprotein isoforms by capillary electrophoresis. Electrophoresis. 2001;22:459–63. doi: 10.1002/1522-2683(200102)22:3<459::AID-ELPS459>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 10.Ongay S, Lacunza I, Díez-Masa JC, Sanz J, de Frutos M. Development of a fast and simple immunochromatographic method to purify alpha 1-acid glycoprotein from serum for analysis of its isoforms by capillary electrophoresis. Anal Chim Acta. 2010;663:206–12. doi: 10.1016/j.aca.2010.01.054. [DOI] [PubMed] [Google Scholar]
- 11.Morales-Cid G, Diez-Masa JC, De Frutos M. On-line immunoaffinity capillary electrophoresis based on magnetic beads for the determination of alpha-1 acid glycoprotein isoforms profile to facilitate its use as biomarker. Anal Chim Acta. 2013;773:89–96. doi: 10.1016/j.aca.2013.02.037. [DOI] [PubMed] [Google Scholar]
- 12.Sei K, Nakano M, Kinoshita M, Masuko T, Kakehi K. Collection of α1-acid glycoprotein molecular species by capillary electrophoresis and the analysis of their molecular masses and carbohydrate chains: basic studies on the analysis of glycoprotein glycoforms. J Chromatogr A. 2002;958:273–81. doi: 10.1016/s0021-9673(02)00353-9. [DOI] [PubMed] [Google Scholar]
- 13.Galievsky VA, Stasheuski AS, Krylov SN. Capillary electrophoresis for quantitative studies of biomolecular interactions. Anal Chem. 2015;87:157–71. doi: 10.1021/ac504219r. [DOI] [PubMed] [Google Scholar]
- 14.Kostal V, Katzenmeyer J, Arriaga EA. Capillary electrophoresis in bioanalysis. Anal Chem. 2008;80:4533–50. doi: 10.1021/ac8007384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lucy CA, MacDonald AM, Gulcev MD. Non-covalent capillary coatings for protein separations in capillary electrophoresis. J Chromatogr A. 2008;1184:81–105. doi: 10.1016/j.chroma.2007.10.114. [DOI] [PubMed] [Google Scholar]
- 16.Carbeck JD, Colton IJ, Gao J, Whitesides GM. Protein charge ladders, capillary electrophoresis, and the role of electrostatics in biomolecular recognition. Acc Chem Res. 1998;31:343–50. [Google Scholar]
- 17.Gao J, Gomez FA, Härter R, Whitesides GM. Determination of the effective charge of a protein in solution by capillary electrophoresis. Proc Natl Acad Sci USA. 1994;91:12027–30. doi: 10.1073/pnas.91.25.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rickard EC, Strohl MM, Nielsen RG. Correlation of electrophoretic mobilities from capillary electrophoresis with physicochemical properties of proteins and peptides. Anal Biochem. 1991;197:197–207. doi: 10.1016/0003-2697(91)90379-8. [DOI] [PubMed] [Google Scholar]
- 19.Adamson NJ, Reynolds EC. Rules relating electrophoretic mobility, charge and molecular size of peptides and proteins. J Chromatogr B. 1997;699:133–47. doi: 10.1016/s0378-4347(97)00202-8. [DOI] [PubMed] [Google Scholar]
- 20.Gilges M, Kleemiss MH, Schomburg G. Capillary zone electrophoresis separations of basic and acidic proteins using poly (vinyl alcohol) coatings in fused silica capillaries. Anal Chem. 1994;66:2038–46. [Google Scholar]
- 21.Iki N, Yeung ES. Non-bonded poly(ethylene oxide) polymer-coated column for protein separation by capillary electrophoresis. J Chromatogr A. 1996;731:273–82. [Google Scholar]
- 22.Landers JP. Handbook of Capillary and Microchip Electrophoresis and Associated Microtechniques. 3. Taylor and Francis; Boca Raton: 2008. [Google Scholar]
- 23.Jorgenson JW, Lukacs KD. Zone electrophoresis in open-tubular glass capillaries. Anal Chem. 1981;53:1298–302. [PubMed] [Google Scholar]
- 24.Jorgenson JW, Lukacs KD. Capillary zone electrophoresis. Science. 1983;222:266–72. doi: 10.1126/science.6623076. [DOI] [PubMed] [Google Scholar]
- 25.Wu Z, Wei B, Zhang X, Wirth MJ. Efficient separations of intact proteins using slip-flow with nano-liquid chromatography-mass spectrometry. Anal Chem. 2014;86:1592–8. doi: 10.1021/ac403233d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Massia SP, Stark J, Letbetter DS. Surface-immobilized dextran limits cell adhesion and spreading. Biomaterials. 2000;21:2253–61. doi: 10.1016/s0142-9612(00)00151-4. [DOI] [PubMed] [Google Scholar]
- 27.Österberg E, Bergström K, Holmberg K, Schuman TP, Riggs JA, Burns NL, Van Alstine JM, Harris JM. Protein-rejecting ability of surface-bound dextran in end-on and side-on configurations: comparison to PEG. J Biomed Mater Res. 1995;29:741–7. doi: 10.1002/jbm.820290610. [DOI] [PubMed] [Google Scholar]
- 28.Doherty EAS, Meagher RJ, Albarghouthi MN, Barron AE. Microchannel wall coatings for protein separations by capillary and chip electrophoresis. Electrophoresis. 2003;24:34–54. doi: 10.1002/elps.200390029. [DOI] [PubMed] [Google Scholar]
- 29.Preisler J, Yeung ES. Characterization of nonbonded poly(ethylene oxide) coating for capillary clectrophoresis via continuous monitoring of electroosmotic flow. Anal Chem. 1996;68:2885–9. doi: 10.1021/ac960260s. [DOI] [PubMed] [Google Scholar]
- 30.Klammt C, Schwarz D, Fendler K, Haase W, Dötsch V, Bernhard F. Evaluation of detergents for the soluble expression of α-helical and β-barrel-type integral membrane proteins by a preparative scale individual cell-free expression system. FEBS J. 2005;272:6024–38. doi: 10.1111/j.1742-4658.2005.05002.x. [DOI] [PubMed] [Google Scholar]
- 31.Mariño K, Bones J, Kattla JJ, Rudd PM. A Systematic Approach to Protein Glycosylation Analysis: A Path Through the Maze. Nature Chem Biol. 2010;6:713–23. doi: 10.1038/nchembio.437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.