Abstract
In this work we report the activity seen with combination therapy using sodium antimony gluconate in liposomes composed of egg phosphatidyl choline and stearylamine for elimination of Leishmania donovani parasites from the liver and spleen of BALB/c mice with established and chronic infections.
Despite extended treatment regimens, parenteral administration, and toxic side effects, the pentavalent antimonial sodium antimony gluconate (SAG) has remained the first-line treatment for visceral leishmaniasis (VL) or kala-azar for decades (19, 33). Frequent therapeutic failures with SAG necessitate the use of more toxic second-line drugs, amphotericin B and pentamidine (22, 23, 25). Recently several lipid-based formulations of amphotericin B with reduced levels of toxicity have been used (9, 11, 32). However, no commercial lipid formulations of SAG are available even though liposome-encapsulated SAG is 200 to 700 times more active than free SAG (2, 5, 28). In most of these studies the efficacy demonstrated by neutral and negatively charged liposomal formulations of SAG was determined purely on the basis of liver parasite burdens (2, 5, 21). These formulations either failed to contain parasites in the spleen (6) or required multiple doses for a significant clearance in an acute-infection model (7, 13). The spleen is a site of persistent infection with Leishmania donovani (1, 12, 32), and parasite removal from this organ is more difficult than parasite removal from liver (6, 8, 31, 32). Earlier, Dey et al. reported on a multiple-dose treatment of drug-free, positively charged liposomes comprising egg phosphatidylcholine (PC) and stearylamine (SA) for the elimination of parasites from the liver and spleen of BALB/c mice with established L. donovani infection (10). Herein we evaluate a single-dose treatment with PC-SA liposome encapsulating a suboptimal concentration of SAG against an established and a chronic infection of L. donovani at both the sites of infection.
To optimize phospholipid composition the nonderivative l-α-PC was replaced with dimyristoyl (DMPC), distearoyl (DSPC), and dipalmitoyl (DPPC) derivatives of PC in combination with SA in a 7:2 molar ratio as detailed earlier (10). For drug encapsulation the lipid film was dispersed in 20 mM phosphate-buffered saline (PBS; pH 7.0) containing 1 mg of SAG (Gluconate Health Limited, Calcutta, India)/ml and sonicated for 30 s in an ultrasonicator. To remove free drug, liposomes with entrapped SAG were washed in PBS with centrifugation (10,000 × g, 30 min, 4°C) (15). Approximately 300 to 400 μg of SAG was associated with 22 mg of lipid, estimated as described previously (2, 17).
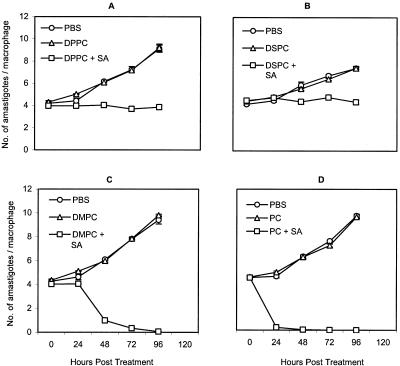
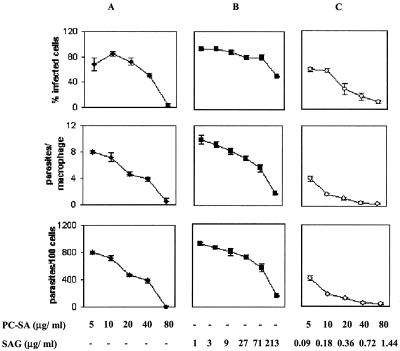
In vitro antileishmanial drug therapy was investigated as described earlier (10). Initially, four drug-free cationic liposomes, DSPC-SA, DMPC-SA, DPPC-SA, and PC-SA, each at 88 μg/ml, were tested against intracellular L. donovani amastigotes for optimum activity and minimum toxicity. Whereas DSPC-SA and DPPC-SA could not clear parasite infection by 96 h, DMPC-SA and PC-SA were almost equally effective at the time points studied (Fig. 1). However, while DMPC-SA induced 5% toxicity towards macrophages, as determined by lactate dehydrogenase release in supernatants (14) at 88 μg/ml, an identical dose of PC-SA exhibited no toxic effect. Hence, PC-SA was selected for combination therapy with SAG. Subsequently, the antileishmanial activity of SAG-loaded PC-SA liposomes was compared with the results seen with empty liposomes and free SAG. Liposomal SAG therapy was found significantly more potent than either of the monotherapies (Fig. 2). The 50% effective doses for suppression of parasite infection in macrophages calculated using sigmoidal regression analysis (Microsoft Excel) for empty PC-SA and free SAG were 38.26 and 69.57 μg/ml, respectively. In contrast, 0.5 μg of entrapped SAG in 22.39 μg of PC-SA/ml could induce an equal effect.
FIG. 1.
Effect of different phospholipids with SA-bearing liposomes on the survival of L. donovani amastigotes in mouse peritoneal macrophages. Cells were infected with L. donovani promastigotes and incubated with 88 μg/ml concentrations of DPPC-SA (A), DSPC-SA (B), DMPC-SA (C), and PC-SA (D) or an equivalent amount of respective neutral liposomes or PBS for 0 to 96 h at 37°C. Data represent the means ± standard errors from triplicate coverglasses of one experiment representative of two performed.
FIG. 2.
Activities of empty PC-SA liposomes, free SAG, and PC-SA-encapsulated SAG against L. donovani amastigotes in resident peritoneal macrophages from BALB/c mice. Cells were infected with L. donovani promastigotes and incubated with increasing concentrations of PC-SA (A), SAG (B), and PC-SA-entrapped SAG (C) for 72 h at 37°C. Levels of infection efficiency (percent infected cells [top panels]), intracellular growth (parasites per infected cell [middle panels]), and parasite survival (parasite per 100 cells [bottom panels]) of drug-treated cells are shown. Infected control macrophages contained 8.03 ± 0.117 amastigotes per macrophage. The bars show the standard errors for three replicates and are representative of two independent experiments.
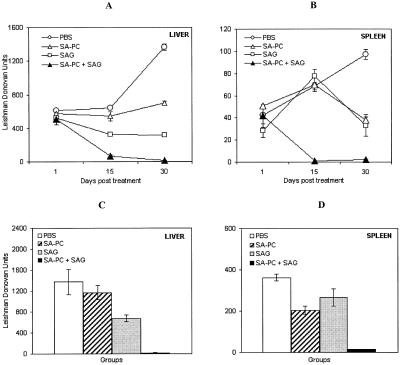
In vivo 8-week-infected BALB/c mice (10) were treated with a single dose of free SAG (16 mg/kg of body weight) or empty PC-SA (22 mg/mouse) or an equivalent dose of SAG entrapped in PC-SA (22 mg) (Fig. 3). Mice were sacrificed on days 1, 15, and 30 posttreatment, and levels of organ parasite burden were determined and expressed in Leishman Donovan units (1). Empty liposome or free SAG could suppress infection by 15 or 50%, respectively, only in the liver, with almost no reduction in the spleen 15 days posttreatment. On day 30, parasite suppression increased to 48% in liver and 60% in spleen (P < 0.05) with empty liposomes and to 76% in liver (P < 0.0001) and 65% in spleen (P < 0.05) with free SAG. Combination therapy was most effective, reducing the parasite burden by 89 and 98% in liver and 98 and 97% in spleen (P < 0.0001) at 15 and 30 days posttreatment, respectively, compared to the results seen with controls. The fall in parasitemia induced by combination therapy was significantly higher (P < 0.05) than that seen with either of the monotherapies in both the organs.
FIG. 3.
Combined therapy with PC-SA liposomes, free SAG, and PC-SA-encapsulated SAG for treatment of established and chronic murine VL. Eight-week-infected BALB/c mice were treated intravenously with a single dose of 22 mg of empty PC-SA liposomes or free SAG (16 mg/kg of body weight) or an equivalent dose of SAG entrapped in 22 mg of PC-SA liposomes. Control infected animals received only PBS. (A and B) Mice were sacrificed on days 1, 15, and 30 posttreatment. Levels of parasite burden in liver (A) and spleen (B) are expressed in Leishman Donovan units. (C and D) In the second set, efficacy of the combination therapy for treatment of heavy parasite infection was assessed (12 weeks). Liver (C) and spleen (D) parasite loads after 1 month of treatment are shown in Leishman Donovan units. Values represent the means ± standard errors of the means for four animals per group per time point.
Until recently splenectomy was the last recourse in cases of antimony resistance (25, 29). We thus examined the efficacy of combined treatment for mice after 3 months of infection, when splenic parasite load is well expressed (10). Interestingly, it was found that although the treatment efficiency declined with empty liposome and SAG to 16 and 50% (P < 0.05) in liver and 43 (P < 0.05) and 26% in spleen, respectively, at 30 days posttreatment, almost complete clearance of parasitemia from spleen (96%) and liver (98%), with a significant decrease in spleen weight (69%; P < 0.0001), was still achieved with the combination therapy in this model compared to the results seen with controls. Estimation of levels of serum alkaline phosphatase and glutamate pyruvate transaminase, specific enzymes related to liver dysfunction, and of levels of serum urea and creatinine, related to kidney dysfunction, 15 days postinjection of SAG entrapped in PC-SA liposome demonstrated levels within normal range, indicating no toxicity under the given conditions.
Herein we report a profound synergistic activity of SAG entrapped in PC-SA liposome in both in vitro and in vivo models of VL. To our knowledge this is the first report of a single dose of liposomal SAG treatment resulting in an almost complete elimination of parasites not only from the liver but also from the spleen of infected animals. An equivalent dose of free SAG or empty PC-SA liposome was only partially effective at clearing the intracellular amastigotes. Apart from a sphingomyelin-cholesterol-SA liposomal preparation of SAG, earlier formulations of SA-bearing liposomes demonstrated much less activity than negatively charged ones (2), resulting in follow-ups largely conducted with negatively charged (5, 21) and neutral (7, 28) vesicles. Although these formulations demonstrated spectacular success against liver parasites (2, 5, 6, 28), they failed to perform in spleens (6). There are considerable discrepancies in ideas as to how charges affect the interaction of liposomes with macrophages and their distribution in vivo. In contrast to previous observations (16, 20, 24), there are increasing reports of improved delivery of drug-entrapped positively charged liposomes to the liver as well as the spleen over the results seen with neutral and negatively charged vesicles (3, 18) and of better uptake by macrophages (27, 30) and efficient parasite clearance. The efficacies of most antileishmanials, including SAG, depend on the duration of infection, and the effect is more evident in the spleen than the liver (4, 26). A low-dose (16 mg/kg) activity of SAG observed even against a chronic infection in liver and spleen, hitherto not reported, can be attributed to a synergistic leishmanicidal activity of the PC-SA vesicles. Our preliminary investigations suggest reversible electrostatic interaction between PC-SA liposomes and parasite plasma membranes, damage of the cellular organization, and inhibition of parasite oxygen consumption. Studies are under way to optimize the combination therapy for complete cures.
Acknowledgments
This investigation received financial support from the Department of Science and Technology and Council of Scientific and Industrial Research, Government of India.
We thank Samir Bhattacharya, director of IICB, Calcutta, India, for supporting this work.
REFERENCES
- 1.Afrin, F., and N. Ali. 1998. Isotype profiles of Leishmania donovani-infected BALB/c mice: preferential stimulation of IgG2a/b by liposome-associated promastigote antigens. J. Parasitol. 84:743-748. [PubMed] [Google Scholar]
- 2.Alving, C. R., E. A. Steck, W. L. Chapman, Jr., V. B. Waits, L. D. Hendricks, G. M. Swartz, Jr., and W. L. Hanson. 1978. Therapy of leishmaniasis: superior efficacies of liposome-encapsulated drugs. Proc. Natl. Acad. Sci. USA 75:2959-2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aramaki, Y., K. Akiyama, T. Hara, and S. Tsuchiya. 1995. Recognition of charged liposomes by rat peritoneal and splenic macrophages: effects of fibronectin on the uptake of charged liposomes. Eur. J. Pharm. Sci. 3:63-70. [Google Scholar]
- 4.Baillie, A. J., T. F. Dolan, J. Alexander, and K. C. Carter. 1989. Visceral leishmaniasis in the BALB/c mouse: sodium stibogluconate treatment during acute and chronic stages of infection. Int. J. Pharm. 57:23-28. [Google Scholar]
- 5.Black, C. D., G. J. Watson, and R. J. Ward. 1977. The use of Pentostam liposomes in the chemotherapy of experimental leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 71:550-552. [DOI] [PubMed] [Google Scholar]
- 6.Carter, K. C., A. J. Baillie, J. Alexander, and T. F. Dolan. 1988. The therapeutic effect of sodium stibogluconate in BALB/c mice infected with Leishmania donovani is organ-dependent. J. Pharm. Pharmacol. 40:370-373. [DOI] [PubMed] [Google Scholar]
- 7.Carter, K. C., T. F. Dolan, J. Alexander, A. J. Baillie, and C. McColgan. 1989. Visceral leishmaniasis: drug carrier system characteristics and the ability to clear parasites from the liver, spleen and bone marrow in Leishmania donovani infected BALB/c mice. J. Pharm. Pharmacol. 41:87-91. [DOI] [PubMed] [Google Scholar]
- 8.Collins, M., K. C. Carter, and A. J. Baillie. 1992. Visceral leishmaniasis in the BALB/c mouse: antimony tissue disposition and parasite suppression after the administration of free stibogluconate. Ann. Trop. Med. Parasitol. 86:35-40. [DOI] [PubMed] [Google Scholar]
- 9.Davidson, R. N., L. di Martino, L. Gradoni, R. Giacchino, G. B. Gaeta, R. Pempinello, S. Scotti, A. Cascio, E. Castagnola, A. Maisto, M. Gramiccia, D. di Caprio, R. J. Wilkinson, and A. D. Bryceson. 1996. Short-course treatment of visceral leishmaniasis with liposomal amphotericin B (AmBisome). Clin. Infect. Dis. 22:938-943. [DOI] [PubMed] [Google Scholar]
- 10.Dey, T., K. Anam, F. Afrin, and N. Ali. 2000. Antileishmanial activities of stearylamine-bearing liposome. Antimicrob. Agents Chemother. 44:1739-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dietze, R., E. P. Milan, J. D. Berman, M. Grogl, A. Falqueto, T. F. Feitosa, K. G. Luz, F. A. Suassuna, L. A. Marinho, and G. Ksionski. 1993. Treatment of Brazilian kala-azar with a short course of amphocil (amphotericin B cholesterol dispersion). Clin. Infect. Dis. 17:981-986. [DOI] [PubMed] [Google Scholar]
- 12.Engwerda, C. R., M. L. Murphy, S. E. Cotterell, S. C. Smelt, and P. M. Kaye. 1998. Neutralization of IL-12 demonstrates the existence of discrete organ-specific phases in the control of Leishmania donovani. Eur. J. Immunol. 28:669-680. [DOI] [PubMed] [Google Scholar]
- 13.Everlien, H., and S. Hockertz. 1999. Combined liposomal immuno- and chemotherapy of visceral leishmaniasis. Arzneim.-Forsch. 49:954-961. [PubMed] [Google Scholar]
- 14.Filion, M. C., and N. C. Phillips. 1997. Toxicity and immunomodulatory activity of liposomal vectors formulated with cationic lipids toward immune effector cells. Biochim. Biophys. Acta 1329:345-356. [DOI] [PubMed] [Google Scholar]
- 15.Frezard, F., M. S. M. Michalick, C. F. Soares, and C. Demicheli. 2000. Novel methods for the encapsulation of meglumine antimoniate into liposomes. Braz. J. Med. Biol. Res. 33:841-846. [DOI] [PubMed] [Google Scholar]
- 16.Gregoriadis, G., and D. E. Neerunjun. 1974. Control of the rate of hepatic uptake and catabolism of liposome-entrapped proteins injected into rats. Possible therapeutic applications. Eur. J. Biochem. 47:179-185. [DOI] [PubMed] [Google Scholar]
- 17.Guru, P. Y., A. K. Agrawal, U. K. Singha, A. Singhal, and C. M. Gupta. 1989. Drug targeting in Leishmania donovani infections using tuftsin bearing liposome vehicles. FEBS Lett. 245:204-208. [DOI] [PubMed] [Google Scholar]
- 18.Heath, S., M. L. Chance, and R. R. New. 1984. Quantitative and ultrastructural studies on the uptake of drug loaded liposomes by mononuclear phagocytes infected with Leishmania donovani. Mol. Biochem. Parasitol. 12:49-60. [DOI] [PubMed] [Google Scholar]
- 19.Herwaldt, B. L., and J. D. Berman. 1992. Recommendations for treating leishmaniasis with sodium stibogluconate (Pentostam) and review of pertinent clinical studies. Am. J. Trop. Med. Hyg. 46:296-306. [DOI] [PubMed] [Google Scholar]
- 20.Hsu, M. J., and R. L. Juliano. 1982. Interactions of liposomes with the reticuloendothelial system. II: nonspecific and receptor-mediated uptake of liposomes by mouse peritoneal macrophages. Biochim. Biophys. Acta 720:411-419. [DOI] [PubMed] [Google Scholar]
- 21.Hunter, C. A., T. F. Dolan, G. H. Coombs, and A. J. Baillie. 1988. Vesicular systems (niosomes and liposomes) for delivery of sodium stibogluconate in experimental murine visceral leishmaniasis. J. Pharm. Pharmacol. 40:161-165. [DOI] [PubMed] [Google Scholar]
- 22.Jha, T. K. 1983. Evaluation of diamidine compound (pentamidine isethionate) in the treatment resistant cases of kala-azar occurring in North Bihar, India. Trans. R. Soc. Trop. Med. Hyg. 77:167-170. [DOI] [PubMed] [Google Scholar]
- 23.Jha, T. K., Y. N. Giri, T. K. Singh, and S. Jha. 1995. Use of amphotericin B in drug-resistant cases of visceral leishmaniasis in north Bihar, India. Am. J. Trop. Med. Hyg. 52:536-538. [DOI] [PubMed] [Google Scholar]
- 24.Juliano, R. L., and D. Stamp. 1975. The effect of particle size and charge on the clearance rates of liposomes and liposome encapsulated drugs. Biochem. Biophys. Res. Commun. 63:651-658. [DOI] [PubMed] [Google Scholar]
- 25.Khalil, E. A., A. M. el Hassan, E. E. Zijlstra, F. A. Hashim, M. E. Ibrahim, H. W. Ghalib, and M. S. Ali. 1998. Treatment of visceral leishmaniasis with sodium stibogluconate in Sudan: management of those who do not respond. Ann. Trop. Med. Parasitol. 92:151-158. [DOI] [PubMed] [Google Scholar]
- 26.Mullen, A. B., A. J. Baillie, and K. C. Carter. 1998. Visceral leishmaniasis in the BALB/c mouse: a comparison of the efficacy of a nonionic surfactant formulation of sodium stibogluconate with those of three proprietary formulations of amphotericin B. Antimicrob. Agents Chemother. 42:2722-2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakanishi, T., J. Kunisawa, A. Hayashi, Y. Tsutsumi, K. Kubo, S. Nakagawa, H. Fujiwara, T. Hamaoka, and T. Mayumi. 1997. Positively charged liposome functions as an efficient immunoadjuvant in inducing immune responses to soluble proteins. Biochem. Biophys. Res. Commun. 240:793-797. [DOI] [PubMed] [Google Scholar]
- 28.New, R. R. C., M. L. Chance, S. C. Thomas, and W. Peters. 1978. Antileishmanial activity of antimonials entrapped in liposomes. Nature 272:55-56. [DOI] [PubMed] [Google Scholar]
- 29.Prasad, L. S., and S. Sen. 1995. Migration of Leishmania donovani amastigotes in cerebrospinal fluid. Lancet 346:183-184. [DOI] [PubMed] [Google Scholar]
- 30.Schwendener, R. A., P. A. Lagocki, and Y. E. Rahman. 1984. The effects of charge and size on the interaction of unilamellar liposomes with macrophages. Biochim. Biophys. Acta 772:93-101. [DOI] [PubMed] [Google Scholar]
- 31.Seaman, J., C. Boer, R. Wilkinson, J. de Jong, E. de Wilde, E. Sondorp, and R. Davidson. 1995. Liposomal amphotericin B (AmBisome) in the treatment of complicated kala-azar under field conditions. Clin. Infect. Dis. 21:188-193. [DOI] [PubMed] [Google Scholar]
- 32.Sundar, S., N. K. Agrawal, P. R. Sinha, G. S. Horwith, and H. W. Murray. 1997. Short-course, low-dose amphotericin B lipid complex therapy for visceral leishmaniasis unresponsive to antimony. Ann. Intern. Med. 127:133-137. [DOI] [PubMed] [Google Scholar]
- 33.Thakur, C. P., G. P. Sinha, A. K. Pandey, N. Kumar, P. Kumar, S. M. Hassan, S. Narain, and R. K. Roy. 1998. Do the diminishing efficacy and increasing toxicity of sodium stibogluconate in the treatment of visceral leishmaniasis in Bihar, India, justify its continued use as first-line drug? An observational study of 80 cases. Ann. Trop. Med. Parasitol. 92:561-569. [DOI] [PubMed] [Google Scholar]