Abstract
Candida glabrata has recently emerged as a significant pathogen involved in both superficial and deep-seated infections. In the present study, a checkerboard broth microdilution method was performed to investigate the in vitro activities of voriconazole (VOR) in combination with terbinafine (TRB), amphotericin B (AMB), and flucytosine (5FC) against 20 clinical isolates of C. glabrata. Synergy, defined as a fractional inhibitory concentration (FIC) index of ≤0.50, was observed in 75% of VOR-TRB, 10% of VOR-AMB, and 5% of VOR-5FC interactions. None of these combinations yielded antagonistic interactions (FIC index > 4). When synergy was not achieved, there was still a decrease in the MIC of one or both drugs used in the combination. In particular, the MICs were reduced to ≤1.0 μg/ml as a result of the combination for all isolates for which the AMB MIC at the baseline was ≥2.0 μg/ml. By a disk diffusion assay, the halo diameters produced by antifungal agents in combination were greater that those produced by each drug alone. Finally, killing curves showed that VOR-AMB exhibited synergistic interactions, while VOR-5FC sustained fungicidal activities against C. glabrata. These studies demonstrate that the in vitro activity of VOR against this important yeast pathogen can be enhanced upon combination with other drugs that have different modes of action or that target a different step in the ergosterol pathway. Further studies are warranted to elucidate the potential beneficial effects of such combination regimens in vivo.
The patient populations at risk for serious fungal infections have increased dramatically in recent years. These populations include patients with AIDS, those receiving cancer chemotherapy or organ transplantation, and others receiving immunosuppressive medications (9). In addition, the spectrum of invasive fungal infections is changing, with the frequencies of infections due to non-Candida albicans Candida spp. on the rise (33).
C. glabrata has recently emerged as a significant pathogen, and increasing numbers of reports have shown its important role in either superficial or deep-seated infections (29, 30). The prominence of C. glabrata as a pathogen is of particular clinical concern because it is innately less susceptible to fluconazole and amphotericin B (AMB) than most other species of Candida (29).
Recently, voriconazole (VOR), a fluconazole derivative with improved antifungal activity and enhanced potency against fungal 14-alpha-demethylase, has been developed and approved for use for the treatment of acute invasive aspergillosis and other serious fungal infections (28). It possesses a wide spectrum of activity against yeasts and filamentous and dimorphic fungi. The in vitro activities of VOR against clinical isolates of C. glabrata are significantly higher than those reported for fluconazole (28).
A recent study analyzed the efficacy of VOR for the treatment of refractory and invasive fungal infections caused by less common agents (28). Although the overall treatment success rate for these patients was as high as 55%, it was significantly influenced by the species of Candida, with response rates of 100% for patients infected with C. parapsilosis but only 25% for patients infected with C. glabrata (28). This finding is quite disturbing.
Combination therapy might be a promising approach in such circumstances. The use of antifungal combinations may increase the rates of microbial killing, shorten the durations of therapy, avoid the emergence of drug resistance, and expand the spectrum of activity. It is important to evaluate carefully the effects of any combination therapy, since this approach carries a much higher cost and can increase the potential for drug interactions and toxicities.
Therefore, in this study, we investigated the in vitro activities of VOR, alone and in combination with three other antifungal agents, against clinical isolates of C. glabrata. The partner drugs were terbinafine (TRB), AMB, and flucytosine (5FC).
MATERIALS AND METHODS
Yeast isolates.
A total of 20 clinical yeast isolates of C. glabrata were used in this study. The isolates were recovered from blood, the gastrointestinal tract, the respiratory tract, the urinary tract, and other sterile body fluid specimens. Each strain represented a unique isolate from a patient. Yeast isolates were identified at the species level by conventional morphological and biochemical methods and stored at −70°C in 10% glycerol. Prior to initiation of the study, the yeast isolates were subcultured on antimicrobial agent-free medium to ensure viability and purity.
Antifungal agents.
Stock solutions of VOR (Pfizer, Rome, Italy), AMB (Sigma Chemical, Milan, Italy), and TRB (Sandoz Ltd., Basel, Switzerland) were prepared in dimethyl sulfoxide (Sigma). A stock solution of 5FC (Sigma) was prepared in sterile distilled water. Further dilutions of all drugs were prepared in the test medium.
Broth dilution assay.
Drug activity was assessed by a checkerboard method derived from the standardized procedure established by the National Committee for Clinical Laboratory Standards for broth microdilution antifungal susceptibility testing (22). Briefly, testing was performed in RPMI 1640 medium (Sigma) buffered to pH 7.0 with 0.165 M morpholinepropanesulfonic acid (MOPS; Gibco Laboratories, Milan, Italy) buffer. Volumes of 50 μl of each drug at a concentration of four times the targeted final concentration were dispensed in the wells of 96-well microtiter plates (Falcon 3072; Becton Dickinson). The final concentrations of the antifungal agents ranged from 0.008 to 8.0 μg/ml for VOR, 0.125 to 8.0 μg/ml for TRB and AMB, and 0.008 to 0.5 μg/ml for 5FC. Yeast inocula (100 μl), prepared spectrophotometrically and further diluted in order to obtain concentrations ranging from 1.0 × 103 to 5.0 × 103 CFU/ml (2× inoculum), were added to each well of the microdilution trays. The trays were incubated in air at 35°C and read at 48 h. Readings were performed spectrophotometrically with an automatic plate reader (model MR 700; Dynatech) set at 490 nm. MIC endpoints were considered the first concentration of the antifungal agent tested alone or in combination at which the turbidity in the well was 90% less than that in the control well. Both on-scale and off-scale results were included in the analysis. The high off-scale MICs were converted to the next highest concentration, while the low off-scale MICs were left unchanged. Drug interactions were classified as synergistic, indifferent (Loewe additivity), or antagonistic on the basis of the fractional inhibitory concentration (FIC) index (14, 24). The FIC index is the sum of the FICs of each of the drugs, which in turn is defined as the MIC of each drug when it is used in combination divided by the MIC of the drug when it is used alone. The interaction was defined as synergistic if the FIC index was less than or equal to 0.50, indifferent if the FIC index was greater than 0.50 and less than or equal to 4.0, and antagonistic if the FIC index was greater than 4.0 (14, 24).
Halo assay.
Halo assays were performed in Mueller-Hinton agar (Biogenetics srl, Padova, Italy) supplemented with 0.5 μg of methylene blue. Briefly, isolates were inoculated into liquid yeast peptone dextrose (2% glucose, 2% Bacto Peptone, 1% yeast extract; Difco Laboratories) and grown overnight at 35°C. The cells were then pelleted, washed three times with distilled water, and counted with a hemocytometer. Approximately 107 cells were inoculated into 80 ml of melted medium, and the mixture was poured onto 150-mm-diameter petri dishes and allowed to dry. The drugs and the solvent control were pipetted onto 6-mm-diameter BBL disks (Becton Dickinson & Co.). Disks were embedded with 10 μl of either drug alone or drugs in combination. VOR was used at a concentration of 1 μg, TRB and 5FC were used at concentrations at 10 μg, and AMB was used at a concentration at 0.5 μg. After the disks had dried, they were placed onto inoculated agar plates. The plates were incubated at 35°C, and inhibition zone diameters were measured at 24 and 48 h (25). Each disk diffusion assay was performed in duplicate, and mean diameters are reported.
Killing assay.
Three to five colonies of C. glabrata 4370 from a 48-h growth plate were suspended in 10 ml of sterile distilled water, and the turbidity was adjusted to a 0.5 McFarland standard (approximately 1 × 106 to 5 × 106 CFU/ml) by spectrophotometric methods. One milliliter of the adjusted fungal suspension was added to 9 ml of either RPMI 1640 medium buffered with MOPS buffer or a solution of growth medium plus an appropriate amount of each drug alone or in combination. VOR was used at 1.0 μg/ml (4 to 8 times the MIC), TRB was used at 8.0 μg/ml (the MIC), AMB was used at 1.0 μg/ml (0.5 times the MIC), and 5FC was used at 1.0 μg/ml (32 times the MIC). Test solutions were placed on a shaker and incubated at 35°C. At 0, 2, 6, and 24 h following the introduction of the test isolate into the system, 100-μl aliquots were removed from each test solution. After serial dilution 10-fold, a 50-μl aliquot from each dilution was streaked in triplicate onto Sabouraud dextrose agar plates for colony count determination. Following incubation at 35°C for 48 h, the number of CFU on each plate was determined. The limit of detection was 20 CFU/ml. Time-kill studies were also performed with nongrowing cells, in which RPMI 1640 was replaced by phosphate-buffered saline (pH 7.0) (5). Fungicidal activity was considered to have been achieved when the number of CFU per milliliter was <99.9% compared with the initial inoculum size. For time-kill studies, synergy was defined as a ≥100-fold increase in killing compared with that achieved with the most active single agent, while antagonism was defined as a ≥100-fold decrease in killing compared with that achieved with the most active single agent. If less than a 100-fold change from the effect of the most active single drug was observed, the interaction was considered indifferent (10). Experiments were performed in duplicate.
Statistical analysis.
Before statistical analysis the MIC data were transformed logarithmically to approximate a normal distribution. Continuous variables were compared by Student's t test or the Mann-Whitney test. A P value ≤0.05 was considered significant.
RESULTS
Three different approaches were used in this study to investigate the interactions between VOR and each of three other antifungal agents against clinical isolates of C. glabrata.
Broth dilution assay.
The susceptibility results obtained by the broth dilution assay for 20 clinical isolates of C. glabrata are reported in Table 1. VOR MICs ranged from 0.015 to 4.0 μg/ml, with the MIC at which 50% of isolates are inhibited (MIC50) and the MIC90 of 0.125 and 2.0 μg/ml, respectively. TRB MICs ranged from 1.0 to >8.0 μg/ml, with both the MIC50 and the MIC90 being greater than 8.0 μg/ml. AMB MICs ranged from 0.5 to 4.0 μg/ml, with both the MIC50 and the MIC90 being 2.0 μg/ml. 5FC MICs ranged from 0.015 to 0.25 μg/ml, with the MIC50 and the MIC90 being 0.03 and 0.06 μg/ml, respectively. Overall, VOR MICs were significantly lower than both the TRB and the AMB MICs (P < 0.0001) but were higher than the 5FC MICs (P < 0.0001).
TABLE 1.
In vitro activities of VOR, TRB, AMB, and 5FC, alone and in combination, by the broth dilution assay against 20 clinical isolates of C. glabrata
| Drug(s)a | MIC (μg/ml) reported as:
|
% Isolates showing the following interactions:
|
|||
|---|---|---|---|---|---|
| Geometric mean | Range | Synergism | Indifference | Antagonism | |
| VOR | 0.20 | 0.015-4.0 | |||
| TRB | 9.8 | 1.0->8.0 | |||
| VOR-TRB | 0.05a/1.2b | ≤0.008-2.0/≤0.125-4.0 | 75 | 25 | 0 |
| VOR | 0.18 | 0.015-4.0 | |||
| AMB | 1.5 | 0.5-4.0 | |||
| VOR-AMB | 0.08/0.39c | ≤0.008-2.0/≤0.125-1.0 | 10 | 90 | 0 |
| VOR | 0.20 | 0.015-4.0 | |||
| 5FC | 0.03 | 0.015-0.25 | |||
| VOR-5FC | 0.02a/0.02 | ≤0.008-2.0/≤0.008-0.06 | 5 | 95 | 0 |
P < 0.05 for the combination versus VOR alone.
P < 0.05 for the combination versus TRB alone.
P < 0.05 for the combination versus AMB alone.
When VOR was combined with TRB, there were significant reductions in the geometric mean VOR MIC (from 0.20 to 0.05 μg/ml; P = 0.005) and the geometric mean TRB MIC (from 9.8 to 1.2 μg/ml; P < 0.0001). Synergistic interactions were observed for 75% (15 of 20) of the isolates, while indifferent interactions were observed for the remaining 25% (5 of 20). When VOR was combined with AMB, there was a significant reduction in the geometric mean AMB MIC (from 1.5 to 0.39 μg/ml; P < 0.0001). Although the geometric mean VOR MIC dropped from 0.18 to 0.08 μg/ml when it was combined with the polyene, this reduction was not statistically significant (P = 0.149). Synergistic activity was noted against only two isolates (10%). The interactions were indifferent for 90% (18 of 20) of the isolates. Regardless of the type of interaction, the MICs dropped to ≤1.0 μg/ml when AMB was combined with VOR for all isolates (13 of 20) for which AMB MICs were ≥2.0 μg/ml at the baseline.
When VOR was combined with 5FC, there was a significant reduction in the geometric mean VOR MIC (from 0.20 to 0.02 μg/ml; P < 0.0001) but not the 5FC MIC (from 0.03 to 0.02 μg/ml; P = 0.333). These combinations were noted to have synergy against only a single isolate (5%). For 95% (19 of 20) of the isolates the interactions were indifferent. None of these combination studies yielded antagonistic interactions (Table 1).
Halo assay.
To further characterize the effects of all three combination therapies, we used a disk diffusion assay to test 20 clinical isolates of C. glabrata. The results are reported in Table 2. Both TRB and AMB alone inhibited growth only modestly, as shown by the smallest halo diameters produced. The halo diameters produced by combination therapies were generally larger that those produced by the drugs used alone. However, VOR combined with TRB or AMB showed an indifferent effect because the zone diameters never exceeded the largest diameter obtained with VOR alone. On the contrary, the mean sizes of the zones of inhibition of VOR and 5FC used in combination were superior to that of each of the drugs used alone (Table 2). Again, antagonism was never observed (i.e., the halo diameters of each drug combination were smaller than those produced by each drug alone).
TABLE 2.
In vitro activities of VOR, TRB, AMB, and 5FC, alone and in combination, against 20 clinical isolates of C. glabrata by the halo assay
| Drug | Halo diam (mm [mean ± SD]) with the following drugsa:
|
|||
|---|---|---|---|---|
| VOR | TRB | AMB | 5FC | |
| Alone | 18.6 ± 5.2 | 11.5 ± 3.5 | 10.0 ± 1.0 | 21.1 ± 4.3 |
| Combined | 19.8 ± 5.5 | 19.8 ± 7.4 | 28.1 ± 3.6b,c | |
VOR was used at 1 μg, TRB was used at 10 μg, AMB was used at 0.5 μg, and 5FC was used at 10 μg.
P < 0.05 for the combination versus VOR alone.
P < 0.05 for the combination versus FC alone.
Killing assay.
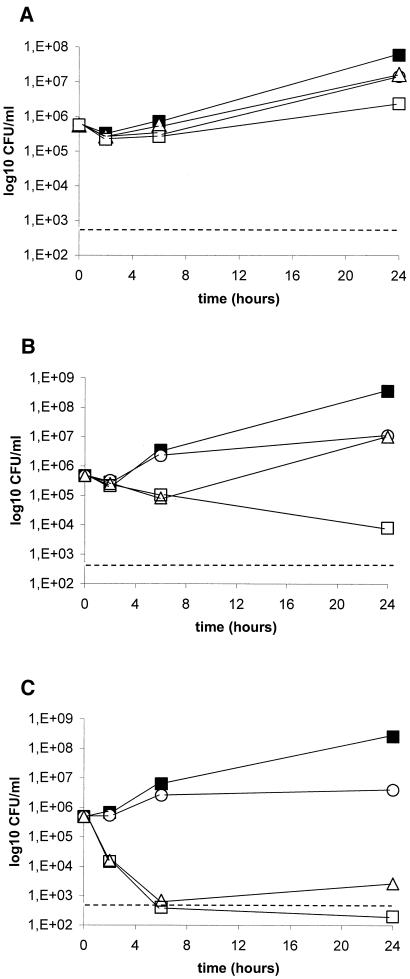
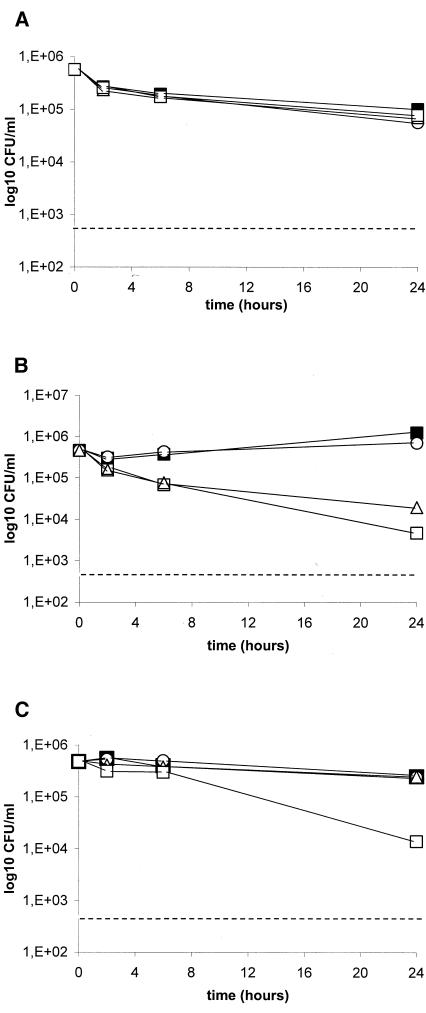
Killing studies were conducted with C. glabrata isolate 4370. This isolate was selected as a representative strain because VOR-TRB yielded a synergistic interaction, while indifference was observed with the other two combinations by the checkerboard dilution method (Table 3). Since the in vitro models that most closely mimic clinical infections are not known, we performed killing experiments by using both replicating and nonreplicating cells. The results of the experiments conducted with replicating cells are reported in Fig. 1 and Table 3. VOR combined with TRB exhibited an indifferent interaction (reduction of 0.9 log10 CFU/ml at 24 h; Fig. 1A). Cells incubated with AMB alone showed renewed growth after 6 h of incubation, and both agents used alone exerted similar activities at 24 h. On the contrary, combination therapy with VOR and AMB yielded sustained antifungal activity for up to 24 h (Fig. 1B). At that time this combination showed a synergistic effect (reduction of 3.1 log10 CFU/ml). Cells incubated with 5FC alone, which was almost fungicidal at 6 h, exhibited renewed growth throughout the end of the experiment. When 5FC was combined with VOR, the fungicidal activity documented was rapid and was maintained for up to 24 h. At this time, this combination exhibited an indifferent interaction (reduction of 1.1 log10 CFU/ml; Fig. 1C). The results of the experiments conducted with nonreplicating cells are reported in Fig. 2 and Table 3. Regardless of the time interval, VOR combined with TRB did not exhibit any antifungal effect compared with the findings for the growth control (Fig. 2A). Although VOR-AMB was the most effective antifungal regimen in this system, the combination yielded an indifferent interaction at 24 h (reduction of 0.6 log10 CFU/ml; Fig. 2B). Similar to the findings reported from experiments with replicating cells, VOR combined with 5FC showed an indifferent interaction at 24 h (reduction of 1.2 log10 CFU/ml; Fig. 2C). Antagonism was never observed.
TABLE 3.
Summary of drug interactions for C. glabrata 4370 by different methodologies
| Methodology | Results for the following combinations:
|
||
|---|---|---|---|
| VOR-TRB | VOR-AMB | VOR-5FC | |
| Checkerboard | Synergism | Indifference | Indifference |
| Time-kill studies with replicating cells | Indifference | Synergism | Indifference, fungicidal activity |
| Time-kill studies with nonreplicating cells | Indifference | Indifference | Indifference |
FIG. 1.
Time-kill studies conducted with replicating C. glabrata 4370 cells. VOR was combined with TRB (A), AMB (B), and 5FC (C). Black squares, controls; circles, VOR (1 μg/ml); triangles, TRB (8 μg/ml), AMB (1 μg/ml), and 5FC (1 μg/ml); white squares, combination therapies. Dashed lines represent a >99.9% growth reduction compared with the initial inoculum size. The limit of detection was 20 CFU/ml. Each datum point represents the means of two separate experiments with similar results.
FIG. 2.
Time-kill studies conducted with nonreplicating C. glabrata 4370 cells. VOR was combined with TRB (A), AMB (B), and 5FC (C). Black squares, controls; circles, VOR (1 μg/ml); triangles, TRB (8 μg/ml), AMB (1 μg/ml), and 5FC (1 μg/ml); white squares, combination therapies. Dashed lines represent a >99.9% growth reduction compared with the initial inoculum size. The limit of detection was 20 CFU/ml. Each datum point represents the mean of two separate experiments with similar results.
DISCUSSION
In this study we analyzed the in vitro interactions between the new triazole, VOR, and three established antifungals against clinical isolates of C. glabrata.
We decided to study this yeast species for three main reasons: first, C. glabrata is the most common cause of fungemia after C. albicans (29); second, infections due to C. glabrata are characterized by a high mortality rate (37); and third, C. glabrata infections are difficult to treat due to the reduced susceptibilities of the species to common antifungal agents (i.e., fluconazole) (29, 30, 39).
VOR, like other azoles, affects ergosterol biosynthesis via inhibition of 14-alpha-demethylase, an enzyme belonging to the cytochrome P-450 superfamily, and shows an extended spectrum of activity against different species of fungi (8, 39). Although two of the other three molecules selected as partner drugs inhibit ergosterol biosynthesis, they do so by acting at different steps of the same pathway. TRB affects ergosterol biosynthesis by inhibiting squalene epoxidase. This inhibition induces the intracellular accumulation of squalene, which disrupts fungal cell membranes. AMB binds to ergosterol and alters membrane permeability, which cause the leakage of cations and hydrogen ions. Finally, 5FC possesses a completely different mechanism of action, in that it inhibits protein synthesis (8, 39). Theoretically, a synergistic interaction is more likely to occur when two (or more) drugs possess a unique mode of action. However, recent experimental and clinical data have revealed that this rule is not always true in antifungal therapy (1-7, 11-21, 23, 26, 27, 31, 32, 34, 35, 36, 38).
Here we explored drug interactions by using different methods, including the classical checkerboard dilution method for determination of MICs, a disk diffusion assay, and killing curve assays.
Although TRB is generally used for the treatment of superficial fungal infections due to fungi other than yeasts, a body of literature emphasizes the potential utility of this drug in combination regimens against several yeast species and filamentous fungi (6, 7, 11, 13, 19-21, 27, 38). Recent in vitro reports have demonstrated that TRB in combination with several azoles exerts a synergistic effect against C. albicans (7, 38). Data from in vivo studies also support the use of these combinations for the treatment of infections due to Scedosporium prolificans (11, 13). Our checkerboard assay results confirmed previous findings presented by Perea et al. (27). Like those investigators, we found that synergism was the most frequent interaction encountered (75%) when the allylamine was combined with VOR. On the other hand, neither the halo assay nor the killing curve assay showed any interactions between these two drugs, although use of the combination gave a 0.9 log10 reduction in growth compared to that achieved with each agent alone at 24 h in killing experiments conducted with replicating cells.
Recently, AMB has been tested in combination with many other drugs to determine whether it possibly has enhanced activity when it is used in combinations (1, 4, 6, 16, 17, 21, 31, 32, 34, 35). In vitro and in vivo studies have shown wide variations in effects when the polyene is combined with fluconazole or itraconazole (4, 16, 17, 32, 34). A recent clinical trial comparing fluconazole alone or combined with AMB for the treatment of candidemia showed that the latter regimen tended to improve the treatment success rate and achieved a more rapid clearance of the organism from the bloodstream (31). Our investigations showed that synergism between VOR and AMB, measured by both the classical broth dilution method and the disk diffusion method, occurred rarely. However, it is interesting that the AMB MIC dropped to ≤1.0 μg/ml for all strains for which baseline AMB MICs were ≥2.0 μg/ml when AMB was combined with the triazole. Moreover, killing experiments conducted with replicating cells demonstrated that this combination exerted synergistic activity. This finding suggests the potential utility of such an approach.
Similar to the findings for VOR-AMB, the testing of VOR-5FC by the checkerboard dilution method showed that synergism occurred rarely. On the other hand, both the halo assay and the killing curve assay yielded very promising results. The halo assay showed a significant enhancement of growth inhibition with this combination regimen, while the killing experiments demonstrated that this combination regimen could be used to achieve fungicidal activity and to maintain that fungicidal activity against C. glabrata. Due to its unique mode of action, 5FC is quite suitable for study in combination with other antifungal drugs (3, 5, 8, 21, 23, 35). Classically, the pyrimidine derivative has been shown to increase the activity of AMB or fluconazole against infections due to Cryptococcus neoformans by both in vitro and in vivo experiments (3, 5, 23).
Our results also demonstrated that the type of interaction is method dependent. The reason for the discrepancies in the results among the different techniques is difficult to explain. We can hypothesize that the nature of a static system (i.e., the checkerboard dilution or halo assay) or a dynamic system (i.e., the killing curve assay) might influence the pharmacodynamics of drug interactions. The major drawback in the testing of AMB by the checkerboard dilution method is the problem with the medium. Tests performed with RPMI 1640 medium for AMB give MICs which cluster in a very narrow range, thereby reducing the possibility of detecting even a slight change in susceptibility (39). Therefore, a dynamic model, such as the killing curve assay, is necessary to effectively evaluate combination regimens containing the polyene derivative.
Also, because all isolates were already highly susceptible to 5FC, the checkerboard method was not able to detect any significant decrease in MICs due to the combination.
In conclusion, we demonstrated that VOR combined with TRB, AMB, or 5FC yielded several types of interactions in vitro, ranging from indifference to synergism. The results were greatly influenced by the method used, but it is encouraging that none of the methods showed antagonism. Although the killing experiments were performed with only one isolate, they gave promising results that showed that VOR and AMB can interact synergistically, while VOR and 5FC can yield sustained fungicidal activity against C. glabrata.
Therefore, these combination approaches merit further investigation in animal models of infections caused by this emerging and difficult-to-treat fungal pathogen.
Acknowledgments
This work was supported in part by a grant from the Istituto Superiore di Sanità, Rome, Italy (IV AIDS project, grant 50D.29).
REFERENCES
- 1.Arikan, S., M. Lozano-Chiu, V. Paetznick, and J. H. Rex. 2002. In vitro synergy of caspofungin and amphotericin B against Aspergillus and Fusarium spp. Antimicrob. Agents Chemother. 46:245-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachmann, S. P., G. Ramage, K. VandeWalle, T. F. Patterson, B. L. Wickes, and J. L. Lopez-Ribot. 2003. Antifungal combinations against Candida albicans biofilms in vitro. Antimicrob. Agents Chemother. 47:3657-3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barchiesi, F., A. M. Schimizzi, L. K. Najvar, R. Bocanegra, F. Caselli, S. Di Cesare, D. Giannini, L. F. Di Francesco, A. Giacometti, F. Carle, G. Scalise, and J. R. Graybill. 2001. Interactions of posaconazole and flucytosine against Cryptococcus neoformans. Antimicrob. Agents Chemother. 45:1355-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barchiesi, F., A. M. Schimizzi, F. Caselli, A. Novelli, S. Fallani, D. Giannini, D. Arzeni, S. Di Cesare, L. F. Di Francesco, M. Fortuna, A. Giacometti, F. Carle, T. Mazzei, and G. Scalise. 2000. Interactions between triazoles and amphotericin B against Cryptococcus neoformans. Antimicrob. Agents Chemother. 44:2435-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barchiesi, F., D. Gallo, F. Caselli, L. F. Di Francesco, D. Arzeni, A. Giacometti, and G. Scalise. 1999. In-vitro interactions of itraconazole with flucytosine against clinical isolates of Cryptococcus neoformans. J. Antimicrob. Chemother. 44:65-70. [DOI] [PubMed] [Google Scholar]
- 6.Barchiesi, F., L. F. Di Francesco, P. Compagnucci, D. Arzeni, A. Giacometti, and G. Scalise. 1998. In-vitro interaction of terbinafine with amphotericin B, fluconazole and itraconazole against clinical isolates of Candida albicans. J. Antimicrob. Chemother. 41:59-65. [DOI] [PubMed] [Google Scholar]
- 7.Barchiesi, F., L. F. Di Francesco, and G. Scalise. 1997. In vitro activities of terbinafine in combination with fluconazole and itraconazole against isolates of Candida albicans with reduced susceptibility to azoles. Antimicrob. Agents Chemother. 41:1812-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Como, J. A., and W. E. Dismukes. 1994. Oral azole drugs as systemic antifungal therapy. N. Engl. J. Med. 330:263-272. [DOI] [PubMed] [Google Scholar]
- 9.Edmond, M. B., S. E. Wallace, D. K. McClish, M. A. Pfaller, R. N. Jones, and R. P. Wenzel. 1999. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin. Infect. Dis. 29:239-244. [DOI] [PubMed] [Google Scholar]
- 10.Eliopoulos, G. M., and R. C. Moellering, Jr. 1996. Antimicrobial combinations, p. 330-396. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. The Williams & Wilkins Co., Baltimore, Md.
- 11.Gosbell, I. B., V. Toumasatos, J. Yong, R. S. Kuo, D. H. Ellis, and R. C. Perrie. 2003. Cure of orthopaedic infection with Scedosporium prolificans, using voriconazole plus terbinafine, without the need for radical surgery. Mycoses 46:233-236. [DOI] [PubMed] [Google Scholar]
- 12.Graybill, J. R., R. Bocanegra, L. K. Najvar, S. Hernandez, and R. A. Larsen. 2003. Addition of caspofungin to fluconazole does not improve outcome in murine candidiasis. Antimicrob. Agents Chemother. 47:2373-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howden, B. P., M. A. Slavin, A. P. Schwarer, and A. M. Mijch. 2003. Successful control of disseminated Scedosporium prolificans infection with a combination of voriconazole and terbinafine. Eur. J. Clin. Microbiol. Infect. Dis. 22:111-113. [DOI] [PubMed] [Google Scholar]
- 14.Johnson, M. D., C. MacDougall, L. Ostrosky-Zeichner, J. R. Perfect, and J. H. Rex. 2004. Combination antifungal therapy. Antimicrob. Agents Chemother. 48:693-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirkpatrick, W. R., S. Perea, B. J. Coco, and T. F. Patterson. 2002. Efficacy of caspofungin alone and in combination with voriconazole in a guinea pig model of invasive aspergillosis. Antimicrob. Agents Chemother. 46:2564-2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis, R. E., B. C. Lund, M. E. Klepser, E. J. Ernst, and M. A. Pfaller. 1998. Assessment of antifungal activities of fluconazole and amphotericin B administered alone and in combination against Candida albicans by using a dynamic in vitro mycotic infection model. Antimicrob. Agents Chemother. 42:1382-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louie, A., P. Banerjee, G. L. Drusano, M. Shayegani, and M. H. Miller. 1999. Interaction between fluconazole and amphotericin B in mice with systemic infection due to fluconazole-susceptible or -resistant strains of Candida albicans. Antimicrob. Agents Chemother. 43:2841-2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luque, J. C., K. V. Clemons, and D. A. Stevens. 2003. Efficacy of micafungin alone or in combination against systemic murine aspergillosis. Antimicrob. Agents Chemother. 47:1452-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meletiadis, J., J. W. Mouton, J. F. Meis, and P. E. Verweij. 2003. In vitro drug interaction modeling of combinations of azoles with terbinafine against clinical Scedosporium prolificans isolates. Antimicrob. Agents Chemother. 47:106-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meletiadis, J., J. W. Mouton, J. L. Rodriguez-Tudela, J. F. G. M. Meis, and P. E. Verweij. 2000. In vitro interaction of terbinafine with itraconazole against clinical isolates of Scedosporium prolificans. Antimicrob. Agents Chemother. 44:470-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosquera, J., A. Sharp, C. B. Moore, P. A. Warn, and D. W. Denning. 2002. In vitro interaction of terbinafine with itraconazole, fluconazole, amphotericin B and 5-flucytosine against Aspergillus spp. J. Antimicrob. Chemother. 50:189-194. [DOI] [PubMed] [Google Scholar]
- 22.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeast, 2nd ed. Approved standard M27-A2. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 23.Nguyen, M. H., L. K. Najvar, C. Y. Yu, and J. R. Graybill. 1997. Combination therapy with fluconazole and flucytosine in the murine model of cryptococcal meningitis. Antimicrob. Agents Chemother. 41:1120-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Odds, F. C. 2003. Synergistic, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 52:1. [DOI] [PubMed] [Google Scholar]
- 25.Onyewu, C., J. R. Blankenship, M. Del Poeta, and J. Heitman. 2003. Ergosterol biosynthesis inhibitors become fungicidal when combined with calcineurin inhibitors against Candida albicans, Candida glabrata, and Candida krusei. Antimicrob. Agents Chemother. 47:956-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perea, S., G. Gonzalez, A. W. Fothergill, W. R. Kirkpatrick, M. G. Rinaldi, and T. F. Patterson. 2002. In vitro interaction of caspofungin acetate with voriconazole against clinical isolates of Aspergillus spp. Antimicrob. Agents Chemother. 46:3039-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perea, S., G. Gonzalez, A. W. Fothergill, D. A. Sutton, and M. G. Rinaldi. 2002. In vitro activities of terbinafine in combination with fluconazole, itraconazole, voriconazole, and posaconazole against clinical isolates of Candida glabrata with decreased susceptibility to azoles. J. Clin. Microbiol. 40:1831-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perfect, J. R., K. A. Marr, T. J. Walsh, R. N. Greenberg, B. DuPont, J. de la Torre-Cisneros, G. Just-Nubling, H. T. Schlamm, I. Lutsar, A. Espinel-Ingroff, and E. Johnson. 2003. Voriconazole treatment for less-common, emerging, or refractory fungal infections. Clin. Infect. Dis. 36:1122-1131. [DOI] [PubMed] [Google Scholar]
- 29.Pfaller, M. A., S. A. Messer, L. Boyken, S. Tendolkar, R. J. Hollis, and D. J. Diekema. 2003. Variation in susceptibility of bloodstream isolates of Candida glabrata to fluconazole according to patient age and geographic location. J. Clin. Microbiol. 41:2176-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Redding, S. W., W. R. Kirkpatrick, S. Saville, B. J. Coco, W. White, A. Fothergill, M. Rinaldi, T. Eng, T. F. Patterson, and J. Lopez-Ribot. 2003. Multiple patterns of resistance to fluconazole in Candida glabrata isolates from a patient with oropharyngeal candidiasis receiving head and neck radiation. J. Clin. Microbiol. 41:619-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rex, J. H., P. G. Pappas, A. W. Karchmer, J. Sobel, J. E. Edwards, S. Hadley, C. Brass, J. A. Vazquez, S. W. Chapman, H. W. Horowitz, M. Zervos, D. McKinsey, J. Lee, T. Babinchak, R. W. Bradsher, J. D. Cleary, D. M. Cohen, L. Danziger, M. Goldman, J. Goodman, E. Hilton, N. E. Hyslop, D. H. Kett, J. Lutz, R. H. Rubin, W. M. Scheld, M. Schuster, B. Simmons, D. K. Stein, R. G. Washburn, L. Mautner, T. C. Chu, H. Panzer, R. B. Rosenstein, J. Booth, and National Institute of Allergy and Infectious Diseases Mycoses Study Group. 2003. A randomized and blinded multicenter trial of high-dose fluconazole plus placebo versus fluconazole plus amphotericin B as therapy for candidemia and its consequences in nonneutropenic subjects. Clin. Infect. Dis. 36:1221-1228. [DOI] [PubMed] [Google Scholar]
- 32.Sanati, H., C. F. Ramos, A. S. Bayer, and M. A. Ghannoum. 1997. Combination therapy with amphotericin B and fluconazole against invasive candidiasis in neutropenic-mouse and infective-endocarditis rabbit models. Antimicrob. Agents Chemother. 41:1345-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snydman, D. R. 2003. Shifting patterns in the epidemiology of nosocomial Candida infections. Chest 123:500S-503S. [DOI] [PubMed] [Google Scholar]
- 34.Sugar, A. M., C. A. Hitchcock, P. F. Troke, and M. Picard. 1995. Combination therapy of murine invasive candidiasis with fluconazole and amphotericin B. Antimicrob. Agents Chemother. 39:598-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Te Dorsthorst, D. T., P. E. Verweij, J. Meletiadis, M. Bergervoet, N. C. Punt, J. F. Meis, and J. W. Mouton. 2002. In vitro interaction of flucytosine combined with amphotericin B or fluconazole against thirty-five yeast isolates determined by both the fractional inhibitory concentration index and the response surface approach. Antimicrob. Agents Chemother. 46:2982-2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Te Dorsthorst, D. T. A., P. E. Verweij, J. F. G. M. Meis, N. C. Punt, and J. W. Mouton. 2002. Comparison of fractional inhibitory concentration index with response surface modeling for characterization of in vitro interaction of antifungals against itraconazole-susceptible and -resistant Aspergillus fumigatus isolates. Antimicrob. Agents Chemother. 46:702-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Viscoli, C., C. Girmenia, A. Marinus, L. Collette, P. Martino, B. Vandercam, C. Doyen, B. Lebeau, D. Spence, V. Krcmery, B. De Pauw, and F. Meunier. 1999. Candidemia in cancer patients: a prospective, multicenter surveillance study by the Invasive Fungal Infection Group (IFIG) of the European Organization for Research and Treatment of Cancer (EORTC). Clin. Infect. Dis. 28:1071-1079. [DOI] [PubMed] [Google Scholar]
- 38.Weig, M., and F. M. C. Müller. 2001. Synergism of voriconazole and terbinafine against Candida albicans isolates from human immunodeficiency virus-infected patients with oropharyngeal candidiasis. Antimicrob. Agents Chemother. 45:966-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White, T. C., K. A. Marr, and R. A. Bowden. 1998. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin. Microbiol. Rev. 11:382-402. [DOI] [PMC free article] [PubMed] [Google Scholar]