Abstract
The pharmacokinetic characteristics and tissue distribution of a novel water-soluble, nontoxic amphotericin B-arabinogalactan (AMB-AG) conjugate exhibited distinct differences from those of micellar and liposomal amphotericin B formulations. These differences included extended persistence of drug in the body and its accumulation in the lungs; thus, the AMB-AG conjugate was highly effective in treating systemic murine aspergillosis.
For many years, the classic amphotericin B-deoxycholate (AMB-DOC; Fungizone) conjugate has been considered to be the mainstay of antifungal therapy; however, AMB treatment is frequently associated with numerous toxicities that limit its use, including infusion-related adverse effects and, particularly, nephrotoxicity (3, 13, 15). Because these toxicities are partially ameliorated when a lipid carrier is used, three lipid formulations have recently been developed and licensed. One of these, liposomal AMB (L-AMB; AmBisome), is the most widely used; however, it is still associated with some toxicity and is not universally effective. Thus, alternative formulations are always desirable (1, 2, 7, 11).
One of the approaches for improving drug performance and reducing toxicity is conjugation to a polymeric carrier (5, 12). Our previous studies demonstrated that conjugation of AMB with oxidized arabinogalactan (AG) generated a highly water-soluble AMB-AG conjugate (8, 9) that was much safer and more therapeutically effective than AMB-DOC in different fungal and leishmanial mouse models (9, 10). The physicochemical characteristics of the AMB-AG conjugate differ from those of AMB in the micellar and lipid formulations (8, 9). We therefore studied the pharmacokinetics, distribution in tissue, and therapeutic efficacy of AMB-AG compared to those of L-AMB and AMB-DOC in mice.
Preparation of AMB formulations.
AMB was conjugated by a Schiff base reaction with oxidized AG via the formation of an imine bond between the AMB primary amine and the AG aldehyde group. The result was the production of an AMB-AG conjugate that was further reduced to generate the amine form, as previously described (8, 9). All of the AMB formulations were prepared for injection as previously described (9).
The three formulations of AMB (AMB-DOC, L-AMB, and AMB-AG) were administered intravenously (i.v.) by single injections of 0.2 ml for 5 consecutive days to groups of 10 healthy male albino BALB/c mice (weight, 20 to 25 g). On the seventh day, the mice were sacrificed, and the internal organs were removed and analyzed by high-pressure liquid chromatography (HPLC) for AMB content. Similarly, in order to assess the pharmacokinetics, the AMB concentrations in organs and plasma samples at different time points up to 24 h after drug administration were determined. The tissues were homogenized in methanol and incubated for 1 h at room temperature; the supernatant was assayed for AMB by HPLC (for experimental details, see Fig. 1 and 2).
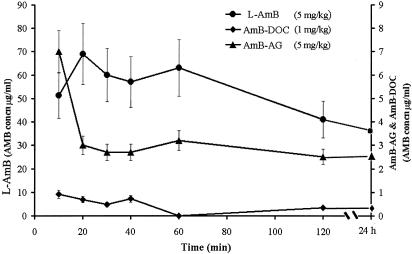
FIG. 1.
Profile of AMB concentrations in plasma (means ± standard deviations) following single i.v. administration of each of the AMB formulations to mice (10 per group). Blood was collected at the time points indicated, the plasma was separated immediately, and the AMB was extracted with methanol and analyzed by HPLC.
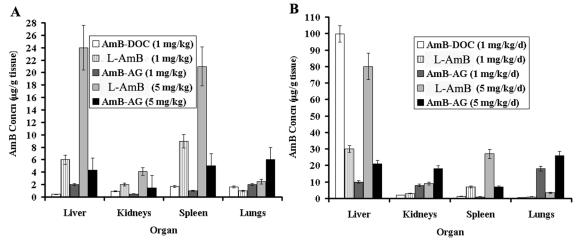
FIG. 2.
AMB concentrations (means ± standard deviations) in organs of mice (10 mice per group) after i.v. administration of various AMB formulations. The experiment was repeated three times. Organs were excised 2 h after a single drug administration (A) and on the seventh day after five daily consecutive injections of the AMB formulations (B).
HPLC analysis.
A sample of 40 μl was injected into a reverse-phase column (RP-8, 5 mm, 250 by 5 mm) with mobile phase (0.01 M ammonium acetate, pH 8, plus acetonitrile) at a rate of 1 ml min−1 and detection of AMB at 405 nm; the limit of detection was 0.1 μg/ml.
Systemic murine aspergillosis.
The murine aspergillosis model was modified slightly from that described by Denning et al. (4); the mice (male albino BALB/c mice weighing 20 to 25 g) were immunosuppressed 2 days prior to the inoculation with 5 × 106 conidia of Aspergillus fumigatus (ATCC 64026) and treated daily with one of the AMB formulations (see protocol in Fig. 2).
Pharmacokinetics and distribution in tissue.
The differences between the physicochemical properties of AMB and its conjugated form, AMB-AG, caused significant alterations in the time profile of the concentrations of the drug in both plasma and tissues following administration of either the AMB-AG conjugate or AMB in the micellar and liposomal formulations (Table 1; Fig. 1 and 2). Steady-state concentration of AMB in plasma (3 μg/ml) was attained 20 min after administration of AMB-AG and was maintained for up to 24 h (Fig. 1). The key pharmacokinetic parameters of AMB following administration in different formulations are summarized in Table 1. The modes of administration of AMB exerted a profound effect on both the distribution and elimination processes. The liposomal formulation significantly restricted the distribution of the drug in comparison to administration as a micellar dispersion, as evidenced by the volume of distribution at steady state (Vss) (68 versus 998 ml/kg of body weight, respectively). The reduced lipophilicity of the AMB-AG conjugate yielded an even larger Vss (1,438 ml/kg). The rate of elimination (indicated by the rate-of-elimination constant and the elimination half-life) was highest for AMB-DOC and lowest for the AMB-AG conjugate. These differences in distribution and elimination were associated with significant differences in drug concentration in plasma, as evidenced by the area under the concentration in blood-time curve. The values for mean residence time indicate that the liposomal formulation extended the duration of the drug in the body twofold compared to the micellar formulation and that the conjugate improved this parameter fivefold, a common increase for polymeric drugs (6). The concentrations of AMB in the urine were extremely low with all the formulations (data not shown).
TABLE 1.
Pharmacokinetic parametersa
| Drug (dose) | AUC (min · μg/ml) | CL (ml/min/kg) | kel (min−1 [103]) | t1/2 (min) | Vss (ml/kg) | MRT (min) |
|---|---|---|---|---|---|---|
| AMB-DOC (1 mg/kg) | 129 | 7.75 | 7.8 | 89 | 998 | 129 |
| L-AMB (5 mg/kg) | 3,083 | 0.33 | 4.8 | 144 | 68 | 208 |
| AMB-AG (5 mg/kg) | 386 | 2.59 | 1.7 | 410 | 1,438 | 556 |
Pharmacokinetic parameters were calculated by noncompartmental moment analysis of the mean plasma AMB concentration-time data following i.v. bolus administration of the AMB formulations to 10 healthy mice. The pharmacokinetics analysis was conducted on pooled data at each time point and normalized for a dose of 1 mg of AMB per kg of body weight. AUC, area under the concentration in blood-time curve; CL, clearance; kel, rate-of-elimination constant; t1/2, elimination half-life; MRT, mean residence time.
Investigation of the tissue distribution of AMB (Fig. 2) revealed that the highest concentrations of AMB after administration of the conjugate occurred in the lung, followed by the liver, spleen, and kidney, and this accumulation increased proportionally to the dose. Following administration of a single dose of one of the three AMB formulations, the drug accumulated mostly in the lungs after administration of the conjugate form (2 and 6 μg of drug/g of tissue for the low and high doses, respectively) (Fig. 2A), in the liver after administration of the liposomal formulation (6 and 24 μg of drug/g of tissue for the low and high doses, respectively) (Fig. 2A), and in the spleen after administration of the micellar formulation (1.7 μg of drug/g of tissue) (Fig. 2A).
The AMB levels in tissues increased significantly after the five-treatment regimen (Fig. 2B). The increase in concentration in the lungs was higher with the conjugate than that obtained with L-AMB and AMB-DOC; the concentration increased from 2 and 6 μg/g of tissue after single injections of low and high doses, respectively, to 18 and 26 μg/g after five injections (Fig. 2A and B). The accumulation of AMB from the conjugate was also observed in the kidney after five doses (8 and 18 μg/g of tissue for the low and high doses, respectively) (Fig. 2B); however, these concentrations were proven previously to be nontoxic (9). The AMB concentrations from the AMB-AG conjugate in the spleen and liver were significantly lower than those obtained with both low and high doses of L-AMB (Fig. 2A and B), and the concentrations of AMB in the spleen obtained with low doses of AMB-AG and AMB-DOC were similar. Interestingly, AMB from AMB-DOC accumulated to the largest extent in the liver. Only low concentrations of AMB were detected in the brains of mice treated with any of the formulations (<1.2 μg/g).
The AMB concentrations in the lung (2 to 26 μg/g) obtained with AMB-AG exceeded both the MICs and the minimal fungicidal concentrations against A. fumigatus (0.25 and 2 μg/ml, respectively) measured by us in vitro according to the NCCLS M38-A standard methods (14). Such information could potentially be of benefit for treatment of invasive fungal infections caused by airborne conidia, such as aspergillosis.
Therapeutic efficacy.
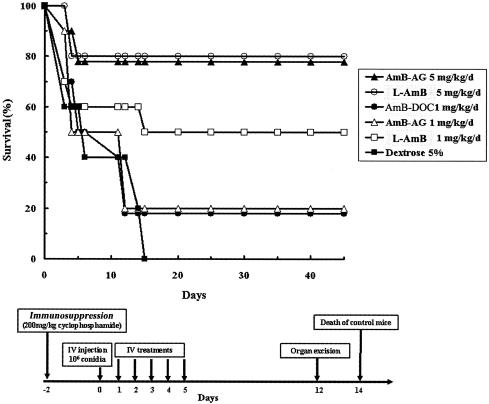
The parameter of therapeutic efficacy was based upon two criteria: survival of treated infected mice and eradication of the fungus from target organs. The high dose of the AMB-AG conjugate, similar to the high dose of L-AMB, was effective in prolonging the survival of infected mice (Fig. 3) and eradicating the fungus from the kidney and liver (Table 2) and may be more effective than high-dose L-AMB in eradicating Aspergillus from the lungs (90% versus 60% eradication compared to control) (Table 2). These results are in accordance with the high AMB concentrations found in the lungs of uninfected mice (Fig. 2A and B).
FIG. 3.
Therapeutic efficacy of different AMB formulations in murine systemic aspergillosis. Ten mice were included in each group; the experiment was repeated three times. The experimental protocol is outlined in the bottom part of the figure. The high doses of the AMB-AG conjugate, and L-AMB, were significantly more therapeutically effective (P < 0.005 by the Kolmogorov-Smirnov goodness-of-fit procedure) than low-dosage and control treatments. d, day.
TABLE 2.
Eradication of Aspergillus from mouse organs after treatment with AMB formulations
| Treatment (dosage)a | Mean no. of CFU/organ (range)
|
||
|---|---|---|---|
| Kidneys | Liver | Lungs | |
| Control (5% dextrose) | 120 (80-180) | 180 (100-240) | 100 (80-200) |
| AMB-DOC (1 mg/kg/day) | 120 (80-160) | 70 (60-100) | 110 (80-160) |
| L-AMB (1 mg/kg/day)b | 80 (0-120) | 50 (0-100) | 60 (0-80) |
| AMB-AG (1 mg/kg/day) | 90 (60-120) | 80 (60-100) | 80 (60-100) |
| L-AMB (5 mg/kg/day) | 0 | 10 (0-80) | 40 (0-100) |
| AMB-AG (5 mg/kg/day)c | 10 (0-80) | 50 (0-100) | 10 (0-60) |
There were 10 mice in each group; the experiment was repeated three times. The number of CFU was determined 12 days postinfection. The experimental protocol is outlined in Fig. 3.
Low-dose treatment with L-AMB was more effective than that with low-dose AMB-AG or AMB-DOC (P < 0.005 by the Kruskal-Wallis test followed by the Mann-Whitney rank-sum test between two groups).
Except for low-dose L-AMB in the liver, the treatment with high-dose AMB-AG was significantly more effective than the low dose of all the other formulations and the control treatment (P < 0.005) and as effective as the high-dose L-AMB in the kidney, liver, and lung (P < 0.01).
In conclusion, the characteristics that distinguish the AMB-AG conjugate from the other formulations—its pharmacokinetic properties, its accumulation in the lung, and its degree of efficacy in treatment of murine aspergillosis—indicate its potential to become an important medication for the treatment of invasive fungal infections.
Acknowledgments
This work was supported in part by a grant from the Chief Scientist of the Ministry of Education of Israel.
We thank Ahmad Nazer and Tirtsa Ehrenfreund for their guidance in this project and gratefully acknowledge JoAnne Johnson of the NIEHS for critically reviewing the manuscript. A.J.D. and A.H. are affiliated with the David R. Bloom Center for Pharmacy at the Hebrew University of Jerusalem.
REFERENCES
- 1.Adler-Moore, J., and R. T. Proffitt. 2002. AmBisome: liposomal formulation, structure, mechanism of action and pre-clinical experience. J. Antimicrob. Chemother. 49(Suppl. 1):21-30. [DOI] [PubMed] [Google Scholar]
- 2.Adler-Moore, J. P., and R. T. Proffitt. 1993. Development, characterization, efficacy and mode of action of AmBisome, a unilamellar liposomal formulation of AmB. J. Liposome Res. 3:429-450. [Google Scholar]
- 3.Butler, W. T. 1966. Pharmacology, toxicity, and therapeutic usefulness of amphotericin B. JAMA 195:371-375. [PubMed] [Google Scholar]
- 4.Denning, D. W., L. Hall, M. Jackson, and S. Hollis. 1995. Efficacy of D0870 compared with those of itraconazole and amphotericin B in two murine models of invasive aspergillosis. Antimicrob. Agents Chemother. 39:1809-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Domb, A. J., G. Linden, I. Polacheck, and S. Benita. 1996. Nystatin-dextran conjugates: synthesis and characterization. J. Polym. Sci. Part A Polym. Chem. 34:1229-1236. [Google Scholar]
- 6.Duncan, R., S. Gac-Breton, R. Keane, R. Musila, Y. N. Sat, R. Satchi, and F. Searle. 2001. Polymer-drug conjugates, PDEPT and PELT: basic principles for design and transfer from the laboratory to clinic. J. Control. Release 74:135-146. [DOI] [PubMed] [Google Scholar]
- 7.Dupont, B. 2002. Overview of the lipid formulations of amphotericin B. J. Antimicrob. Chemother. 49(Suppl. 1):31-36. [DOI] [PubMed] [Google Scholar]
- 8.Ehrenfreund-Kleinman, T., T. Azzam, R. Falk, I. Polacheck, J. Golenser, and A. J. Domb. 2002. Synthesis and characterization of novel water-soluble amphotericin B-arabinogalactan conjugates. Biomaterials 23:1327-1335. [DOI] [PubMed] [Google Scholar]
- 9.Falk, R., A. J. Domb, and I. Polacheck. 1999. A novel injectable water-soluble amphotericin B-arabinogalactan conjugate. Antimicrob. Agents Chemother. 43:1975-1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Golenser, J., S. Frankenburg, T. Ehrenfreund, and A. J. Domb. 1999. Efficacious treatment of experimental leishmaniasis with amphotericin B-arabinogalactan water-soluble derivatives. Antimicrob. Agents Chemother. 43:2209-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiemenz, J. W., and T. J. Walsh. 1996. Lipid formulations of amphotericin B: recent progress and future directions. Clin. Infect. Dis. 22(Suppl. 2):133-144. [DOI] [PubMed] [Google Scholar]
- 12.Maeda, M., C. Nishimura, D. Umeno, and M. Takagi. 1994. Psoralen-containing vinyl monomer for conjugation of double-helical DNA with vinyl polymers. Bioconjug. Chem. 5:527-531. [DOI] [PubMed] [Google Scholar]
- 13.Meyer, R. D. 1992. Current role of therapy with amphotericin B. Clin. Infect. Dis. 14(Suppl. 1):154-160. [DOI] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard M38-A, 2nd ed., vol. 22. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 15.Wingard, J. R., P. Kubilis, L. Lee, G. Yee, M. White, L. Walshe, R. Bowden, E. Anaissie, J. Hiemenz, and J. Lister. 1999. Clinical significance of nephrotoxicity in patients treated with amphotericin B for suspected or proven aspergillosis. Clin. Infect. Dis. 29:1402-1407. [DOI] [PubMed] [Google Scholar]