Abstract
A filter disk assay was used to investigate the penetration of antifungal agents through biofilms containing single and mixed-species biofilms containing Candida. Fluconazole permeated all single-species Candida biofilms more rapidly than flucytosine. The rates of diffusion of either drug through biofilms of three strains of Candida albicans were similar. However, the rates of drug diffusion through biofilms of C. glabrata or C. krusei were faster than those through biofilms of C. parapsilosis or C. tropicalis. In all cases, after 3 to 6 h the drug concentration at the distal edge of the biofilm was very high (many times the MIC). Nevertheless, drug penetration failed to produce complete killing of biofilm cells. These results indicate that poor antifungal penetration is not a major drug resistance mechanism for Candida biofilms. The abilities of flucytosine, fluconazole, amphotericin B, and voriconazole to penetrate mixed-species biofilms containing C. albicans and Staphylococcus epidermidis (a slime-producing wild-type strain, RP62A, and a slime-negative mutant, M7) were also investigated. All four antifungal agents diffused very slowly through these mixed-species biofilms. In most cases, diffusion was slower with biofilms containing S. epidermidis RP62A, but amphotericin B penetrated biofilms containing the M7 mutant more slowly. However, the drug concentrations reaching the distal edges of the biofilms always substantially exceeded the MIC. Thus, although the presence of bacteria and bacterial matrix material undoubtedly retarded the diffusion of the antifungal agents, poor penetration does not account for the drug resistance of Candida biofilm cells, even in these mixed-species biofilms.
Candida albicans is the major fungal pathogen of humans (10). During recent years this organism, together with related Candida species, has become one of the commonest agents of hospital-acquired infections (18). Many of these are implant-associated infections, in which adherent microbial populations, or biofilms, are found on the surfaces of devices, including catheters, prosthetic heart valves, endotracheal tubes, and joint replacements (15, 17). Such infections can be caused by a single microbial species or by a mixture of fungal or bacterial species (13, 28). Individual organisms in biofilms are embedded within a matrix of frequently slimy, extracellular polymers and typically display a phenotype that is very different from that of planktonic (free-floating) cells. In particular, biofilm cells are significantly less susceptible to antimicrobial agents (16, 17, 19, 34). As a result, drug therapy for an implant infection may be futile, and often, the only solution is mechanical removal of the implant (13).
Various model systems have been used to investigate the properties of Candida biofilms in vitro (17). These range from simple assays with catheter disks to more complex flow systems, such as the perfused biofilm fermentor (7). Biofilms of C. albicans usually consist of a mixture of yeasts, hyphae, and pseudohyphae and may have a basal yeast layer that anchors the biofilm to the surface (8). The cells are surrounded by a matrix of extracellular polymeric material, the synthesis of which markedly increases when developing biofilms are exposed to a liquid flow (24). Results from several studies have shown that Candida biofilms are resistant to clinically important antifungal agents, including amphotericin B, fluconazole, flucytosine, itraconazole, and ketoconazole (6, 11, 12, 23, 31, 37, 38). Newer azoles (voriconazole and ravuconazole) are also ineffective against biofilms (29), although some antibiofilm activity has been demonstrated with the echinocandin caspofungin in vitro (4, 29, 39). Mixed Candida-Staphylococcus biofilms are similarly resistant to fluconazole, and there is evidence that the bacteria can enhance Candida resistance (1).
The mechanisms of biofilm resistance to antimicrobial agents are not fully understood. One long-standing hypothesis for the resistance of bacterial biofilms is that the matrix material restricts drug penetration by forming a reaction-diffusion barrier (19) and that only the surface layers of a biofilm are exposed to a lethal dose of antibiotic. The extent to which the matrix acts as a barrier to drug diffusion would depend on the chemical nature of both the antimicrobial agent and the matrix material. Several research groups have investigated antibiotic penetration in Pseudomonas aeruginosa biofilms (26, 30, 42, 44). The overall conclusion from that work was that fluoroquinolones penetrate P. aeruginosa biofilms readily, whereas the penetration of aminoglycosides is retarded. Further studies suggested that aminoglycosides diffuse more slowly because they bind to matrix polymers such as alginate (20, 21, 36). Analogous investigations with Candida species have not been reported. However, the drug susceptibility profiles of C. albicans biofilms incubated statically (which have relatively little extracellular matrix material) were compared with those of biofilms incubated with gentle shaking (which produce much more matrix material). Biofilms grown with or without shaking did not exhibit significant differences in susceptibilities to amphotericin B, flucytosine, or fluconazole, suggesting that drug resistance is unrelated to the extent of matrix formation (9).
In the study described here, we have investigated the penetration of antifungal agents through Candida biofilms using a filter disk assay adapted from the technique reported by Anderl et al. (3) for bacterial biofilms. The abilities of flucytosine and fluconazole to permeate biofilms of C. albicans and related Candida species were evaluated in this way. In parallel, the viabilities of drug-treated biofilm cells were determined. The drug penetration of mixed-species biofilms containing C. albicans and Staphylococcus epidermidis (a slime-producing wild-type strain and a slime-negative mutant) was also assessed. S. epidermidis is the organism most frequently isolated from bacterial implant-associated infections and has also been found in polymicrobial infections with C. albicans (28). Voriconazole and amphotericin B, as well as flucytosine and fluconazole, were used in these mixed-species experiments.
MATERIALS AND METHODS
Organisms.
C. albicans GDH 2346 (NCYC 1467) and GDH 2023 were originally obtained from patients with denture stomatitis at Glasgow Dental Hospital; strain GRI 682 was from a vaginal smear at Glasgow Royal Infirmary. C. glabrata AAHB 12, C. tropicalis AAHB 73, and C. parapsilosis AAHB 4479 were isolated from patients with line infections at Crosshouse Hospital, Kilmarnock, Scotland. C. krusei was obtained from a clinical specimen and came from the Regional Mycology Reference Laboratory, Glasgow, Scotland. All strains were maintained on slopes of Sabouraud dextrose agar (Difco) and were subcultured monthly. Every 2 months, cultures were replaced by new ones freshly grown from freeze-dried stocks.
Two strains of S. epidermidis (RP62A and M7) were maintained on Colombia blood agar (Oxoid). Strain RP62A (ATCC 35984) is a known slime producer; strain M7 is a slime-negative mutant obtained after chemical mutagenesis of S. epidermidis RP62A with mitomycin C (41). The growth rate, initial adherence, cell wall composition, surface characteristics, and antimicrobial susceptibility profile of strain M7 are indistinguishable from those of wild-type strain RP62A (41).
Medium and culture conditions.
All Candida species were grown in yeast nitrogen base (YNB) medium (Difco) containing 50 mM glucose. Batches of medium (50 ml in 250-ml Erlenmeyer flasks) were inoculated from fresh slopes and incubated at 37°C for 24 h in an orbital shaker at 60 rpm. Cells were harvested and washed twice in 0.15 M phosphate-buffered saline (PBS; pH 7.2). Before use in biofilm experiments, all washed cell suspensions were adjusted to an optical density at 600 nm of 0.2.
Tryptic soy broth (Difco) was selected as the liquid medium best able to support the growth of both fungi and bacteria. C. albicans GDH 2346 and the two strains of S. epidermidis (strains RP62A and M7) grew at similar rates in this medium (1). Cultures were inoculated from fresh slopes and incubated with shaking at 37°C for 24 h. Cells were harvested, washed twice in PBS, and suspended to an optical density at 600 nm of 0.2 prior to use in biofilm experiments. For mixed-species biofilms, equal volumes of the standardized suspension of each organism were mixed immediately before use.
Biofilm formation.
Biofilms were grown on membrane filters resting on agar culture medium in petri dishes. For experiments with Candida species, polycarbonate membrane filters (diameter, 25 mm; pore size 0.2 μm; Whatman) were sterilized by exposure to UV radiation for 15 min on both sides prior to inoculation and were then placed on the surface of YNB agar containing 50 mM glucose. Tryptic soy agar was used for S. epidermidis and mixed-species biofilms. A standardized cell suspension (50 μl) was applied to the surface of each sterile membrane. All plates were incubated at 37°C for 24 h. The membrane-supported biofilms were then transferred to fresh agar for a further 24 h, giving a total incubation time of 48 h for biofilm formation.
Penetration of biofilms by antifungal agents.
Four clinically important antifungal agents were used in this study. Flucytosine and amphotericin B were obtained from Sigma. Fluconazole and voriconazole were kindly donated by Pfizer Limited. All drug solutions were prepared immediately before use. Flucytosine and fluconazole were dissolved in sterile distilled water and then added to molten culture medium at 50°C by use of a sterile filtration unit (Sartorius) to create antifungal agent-supplemented agar for the biofilm experiments. Voriconazole and amphotericin B were dissolved in dimethyl sulfoxide and filtered into the growth medium. The medium was buffered to pH 7 with 0.165 M morpholinepropanesulfonic acid buffer (Sigma). High drug concentrations were used in the antifungal agent-supplemented agar. They were selected on the basis of their ability to give large zones of growth inhibition in control assays for drug penetration outlined below. The concentrations used were as follows: flucytosine, 6 μg/ml (30 times the MIC for planktonic C. albicans GDH 2346); fluconazole, 24 μg/ml (60 times the MIC); voriconazole 10 μg/ml (220 times the MIC); and amphotericin B, 78 μg/ml (60 times the MIC).
Penetration of antifungal agents through biofilms was assessed by a modification of the filter disk technique described previously for bacterial biofilms (3). After biofilm formation on membrane filters, smaller polycarbonate membrane filters (diameter, 13 mm; pore size, 0.2 μm; Whatman) were sterilized by exposure to UV radiation for 15 min on both sides and were then carefully placed on top of the 48-h-old biofilms. Paper concentration disks (diameter, 6 mm; Becton Dickinson) were also sterilized by exposure to UV radiation for 15 min per side and then moistened with growth medium (normally 29 μl) prior to placement on top of the 13-mm-diameter membranes. Because of an occasional variation in disk thickness, a slightly higher or lower volume of medium was sometimes required to saturate the disks. Wetting of the disks helped prevent the capillary action of the antifungal medium through the biofilms. Biofilms sandwiched between the membranes and moistened disks were transferred to antifungal agent-containing agar medium. All plates were incubated for specified exposure times, namely, 60, 90, 120, 180, 240, or 360 min.
The amount of antifungal agent which had penetrated each biofilm and which had reached the concentration disk was determined by using the disk in a standard drug diffusion assay. Plates of YNB agar containing 200 mM glucose were seeded with 150 μl of a standardized suspension of planktonic C. albicans GDH 2346 (used here as an indicator organism and adjusted to an optical density at 520 nm of 1.0). After the appropriate exposure time, concentration disks were removed from the biofilm “sandwiches” and placed on the seeded plates, which were then incubated at 37°C for 24 h. The zones of growth inhibition were measured and used to determine the concentration of active antifungal agent in the disks by reference to a standard curve prepared by using drug solutions of different concentrations but fixed volumes. All drug penetration assays were carried out in duplicate on at least two separate occasions. In control assays, concentration disks were placed on the two-membrane system to which no cells had been added, i.e., the unit without the biofilm. The drug concentration that penetrated the biofilms (C) was divided by the drug concentration determined for the controls (C0) to provide a normalized penetration curve (3).
Viable counts of biofilm cells exposed to antifungal agents.
After biofilm formation on 25-mm-diameter membrane filters, biofilms were capped with sterile, 13-mm-diameter filters, transferred to antifungal agent-containing agar, and incubated at 37°C for 6 h (the maximum exposure period in drug penetration assays) or 24 h. After incubation, biofilm cells were gently scraped from the membranes with a sterile scalpel and resuspended in 10 ml of PBS. Serial dilutions (10−1 to 10−6) of each biofilm cell suspension were then prepared. Triplicate samples (0.1 ml) of the 10−4, 10−5, and 10−6 dilutions were spread on YNB agar containing 200 mM glucose, and the plates were incubated at 37°C for 24 h. In control assays, the membranes were transferred to growth medium containing no antifungal agent.
Scanning electron microscopy (SEM).
Biofilms of C. tropicalis and mixed-species biofilms of C. albicans GDH 2346 and S. epidermidis M7 formed on polycarbonate membranes were fixed with glutaraldehyde and then treated with osmium tetroxide and uranyl acetate as described previously (22). After dehydration in a series of ethanol solutions, samples were air dried in a desiccator for 48 h, coated with gold with a Polaron coater, and viewed under a Philips 500 scanning electron microscope.
RESULTS
In this study, we adapted a novel, filter disk assay devised by Anderl et al. (3) to investigate the penetration of antifungal agents through single- and mixed-species biofilms containing Candida. The technique involves the formation of a 48-h-old colony biofilm on a polycarbonate membrane filter and the capping of this biofilm with a second, smaller membrane filter and then a wetted paper disk of the type used in zone-of-inhibition bioassays. The assembly, which represents a primitive diffusion cell, is transferred to agar medium containing the antifungal agent. During subsequent incubation, the drug diffuses out of the agar and through the biofilm sandwich to the moistened paper disk. The drug concentration in the disk can finally be determined by measuring the zone of growth inhibition that it produces on medium seeded with an indicator strain of C. albicans in standard bioassays. The great advantage of this system is that because there is physical access to both sides of the biofilm, the penetration of solutes can be measured directly. Moreover, colony biofilms appear to lack the water channels that typically surround matrix-enclosed microcolonies in many other biofilms (46). The possibility that drugs simply move through water channels without reaching cells deep within the microcolonies is therefore largely eliminated by using this model system.
Flucytosine penetration through Candida biofilms.
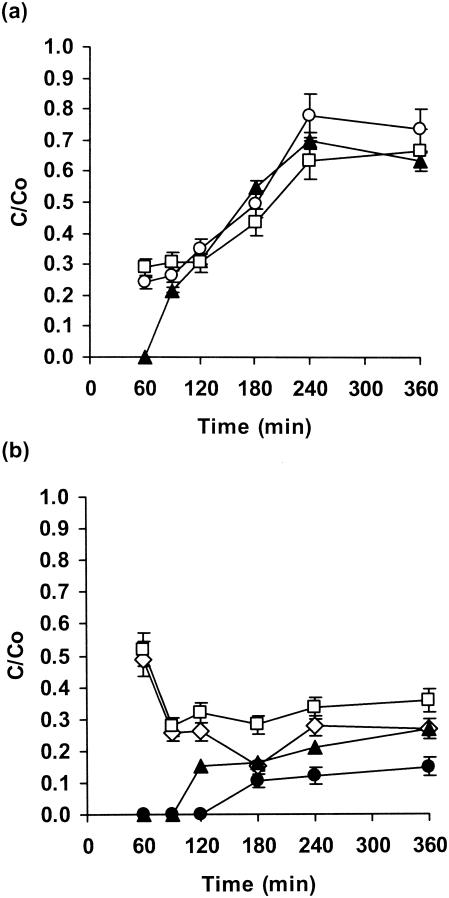
The levels of flucytosine penetration of biofilms of three C. albicans strains were similar, but they were initially lower with strain GDH 2346 (Fig. 1a). The drug concentration at the distal edge of the biofilm (i.e., distal with respect to the agar) was approximately 50% of that of the control value after 180 min. After 240 min it had reached 63 to 78% of that of the control value (Fig. 1a).
FIG. 1.
Penetration of flucytosine through biofilms of C. albicans GDH 2346 (closed triangles), C. albicans GDH 2023 (open squares), and C. albicans GRI 682 (open circles) (a) and through biofilms of C. krusei (open squares), C. glabrata (open diamonds), C. parapsilosis (closed triangles), and C. tropicalis (closed circles) (b). Error bars indicate the standard errors of the means. The mean C0 after 6 h was 22.7 μg of flucytosine/ml.
When biofilms of other Candida species were used, there was rapid penetration of the drug (approximately 50% of that for the control after 60 min) through both C. glabrata and C. krusei biofilms and then a decrease to a stable level of approximately 30% after 120 to 360 min. By contrast, there was slow diffusion through C. parapsilosis biofilms (25% by 360 min; Fig. 1b). However, the slowest penetration was observed with C. tropicalis biofilms (approximately 15% after 360 min; Fig. 1b).
Enzymatic degradation of flucytosine by C. glabrata and C. krusei did not occur. This was demonstrated by removing membrane-supported biofilms from antifungal agent-containing plates and spreading a sensitive strain (strain GDH 2346) of C. albicans onto the plates. These indicator organisms were unable to grow on any part of the plate, including the location that had been beneath the biofilm (results not shown).
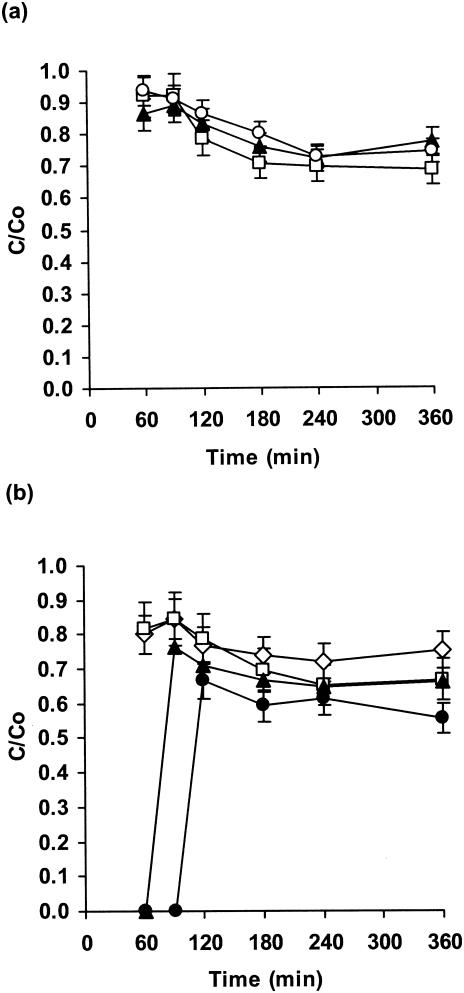
Fluconazole penetration through Candida biofilms.
Fluconazole penetration was similar and rapid for three C. albicans strains (approximately 90% of that for the control after 60 min; Fig. 2a). There was then a slight decrease and a leveling off at 70% of the control value after 360 min. Interestingly, diffusion was much more rapid with fluconazole than with flucytosine, but the final extents of drug penetration were similar after 360 min (Fig. 1a and 2a). However, this represents a higher drug concentration with fluconazole.
FIG. 2.
Penetration of fluconazole through biofilms of C. albicans strain GDH 2346 (closed triangles), C. albicans GDH 2023 (open squares), and C. albicans GRI 682 (open circles) (a) and through biofilms of C. krusei (open squares), C. glabrata (open diamonds), C. parapsilosis (closed triangles), and C. tropicalis (closed circles) (b). Error bars indicate the standard errors of the means. The mean C0 after 6 h was 26.6 μg of fluconazole/ml.
When non-C. albicans Candida species were used, there was rapid fluconazole penetration through biofilms of either C. glabrata or C. krusei, followed by a decrease to roughly 70% of the control value (Fig. 2b). The level of drug penetration through C. parapsilosis biofilms was zero after 60 min but then rose rapidly and leveled off at 65% of the control value. The slowest penetration was again with C. tropicalis biofilms (Fig. 2b). For all non-C. albicans species, the overall level of penetration of fluconazole (55 to 85%) was higher than that of flucytosine (15 to 50%; Fig. 1b and 2b).
Penetration of antifungal agents through single- and mixed-species biofilms of C. albicans and S. epidermidis.
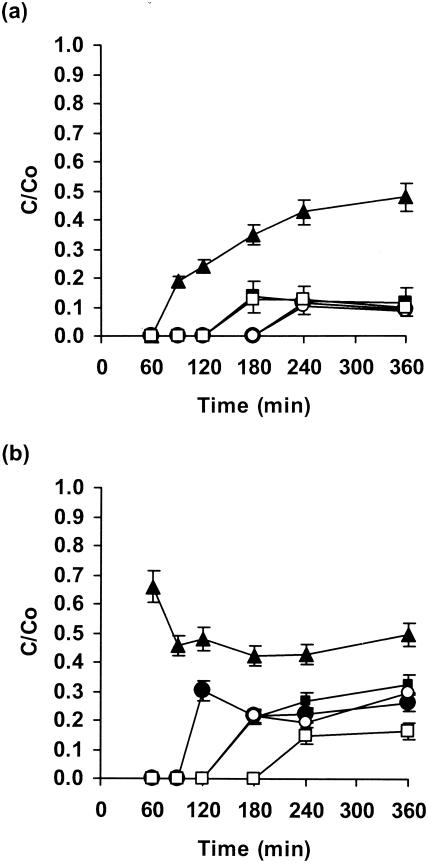
Flucytosine diffused through biofilms of C. albicans GDH 2346 fairly slowly, as noted above, with penetration of about 50% of the control value after 360 min (Fig. 3a). This value was slightly lower than that shown in Fig. 1a (65% after 360 min). In these experiments, however, the C. albicans biofilms, like the mixed-species biofilms, were grown on tryptic soy agar rather than YNB agar. By contrast, the drug penetrated S. epidermidis biofilms very poorly (Fig. 3a); there was approximately 12% penetration after 180 min for slime-negative mutant M7 and 10% penetration after 240 min for wild-type strain RP62A. Mixed fungal-bacterial biofilms showed similarly slow and poor drug penetration, although diffusion was more rapid with Candida-M7 biofilms than with Candida-RP62A biofilms (Fig. 3a).
FIG. 3.
Penetration of flucytosine (a) and amphotericin B (b) through single- and mixed-species biofilms of C. albicans and S. epidermidis. Biofilms contained C. albicans GDH 2346 (closed triangles), S. epidermidis RP62A (closed circles), S. epidermidis M7 (closed squares), C. albicans GDH 2346 and S. epidermidis RP62A (open circles), and C. albicans GDH 2346 and S. epidermidis M7 (open squares). Error bars indicate the standard errors of the means. Mean C0s after 6 h were 24.2 μg of flucytosine/ml and 120.6 μg of amphotericin B/ml.
Despite its low solubility in water, amphotericin B diffused rapidly through biofilms of C. albicans GDH 2346 (65% of the control value after 60 min; Fig. 3b). Penetration through S. epidermidis biofilms was slower and less extensive. With mixed fungal-bacterial biofilms, drug diffusion was also slow and poor, but in this instance diffusion was faster through biofilms containing wild-type strain RP62A than through those containing slime-negative mutant M7 (Fig. 3b). Surprisingly, the C0 of amphotericin B (Fig. 3 legend), like that of flucytosine (Fig. 1 legend), was higher than the drug concentration in the agar. The reason for this is not clear. However, it is conceivable that during the drug penetration assay some drugs bind to the disk cellulose (thus effectively reducing their concentration in solution) but then are released during the zone-of-inhibition assay.
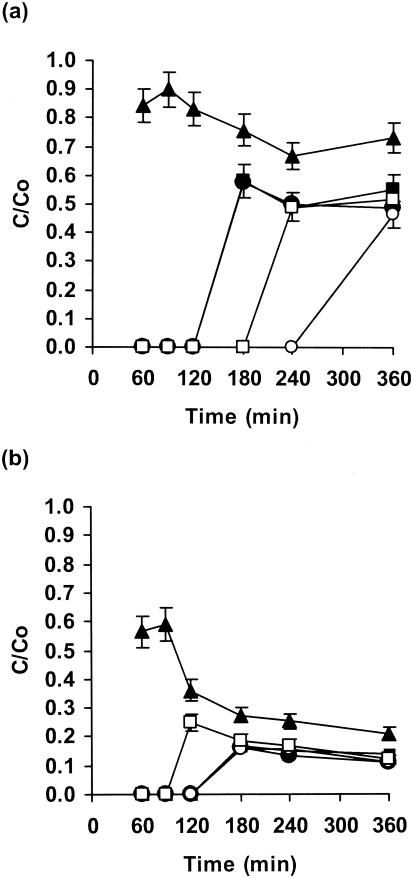
Both fluconazole and the newer azole voriconazole diffused rapidly through biofilms of C. albicans GDH 2346, although penetration by fluconazole was more extensive (Fig. 4a and b). There was slower and poorer penetration of S. epidermidis biofilms. These drugs also diffused through mixed fungal-bacterial biofilms slowly, but fluconazole penetrated mixed biofilms to a greater extent than any other antifungal agent tested (Fig. 4a). The diffusion of both azoles, like that of flucytosine, was more rapid with biofilms containing S. epidermidis M7 than with those containing wild-type strain RP62A (Fig. 4a and b).
FIG. 4.
Penetration of fluconazole (a) and voriconazole (b) through single- and mixed-species biofilms of C. albicans and S. epidermidis. Biofilms contained C. albicans GDH 2346 (closed triangles), S. epidermidis RP62A (closed circles), S. epidermidis M7 (closed squares), C. albicans GDH 2346 and S. epidermidis RP62A (open circles), and C. albicans GDH 2346 and S. epidermidis M7 (open squares). Error bars indicate the standard errors of the means. Mean C0s after 6 h were 27.0 μg of fluconazole/ml and 7.9 μg of voriconazole/ml.
Effects of antifungal agents on the viability of biofilm cells.
To assess the effects of the antifungal agents on biofilm cells, biofilms sandwiched between the two membranes, as in the drug penetration assay, were exposed to antifungal agent-containing agar at 37°C for 6 h (the time period during which drug penetration was determined) or 24 h. Antifungal agents (flucytosine or fluconazole) were present at concentrations identical to those used in the drug penetration assay. After incubation, the numbers of viable biofilm cells were determined by a standard procedure of serial dilution followed by plating. In no case did drug penetration result in the complete killing of biofilm cells (Table 1). C. glabrata AAHB 12 was wholly unaffected by fluconazole after 6 h, but many strains of this species are known to be resistant to fluconazole even when they are grown in planktonic culture (14). The results with fluconazole overall were not unexpected, despite the high concentration used, since this drug is generally considered to be fungistatic only. However, a recent study (35) has demonstrated that fluconazole can be fungicidal under certain conditions.
TABLE 1.
Viability of biofilm cells of Candida species after exposure to flucytosine or fluconazole for 6 or 24 ha
| Organism | Viability (%)
|
|||
|---|---|---|---|---|
| Flucytosine (6 μg/ml)
|
Fluconazole (24 μg/ml)
|
|||
| 6 h | 24 h | 6 h | 24 h | |
| C. albicans GDH 2346 | 34.0 ± 2.3 | 28.9 ± 0.5 | 64.1 ± 1.7 | 81.0 ± 1.5 |
| C. albicans GDH 2023 | 45.6 ± 1.5 | 29.9 ± 1.6 | 73.0 ± 2.0 | 62.1 ± 1.4 |
| C. albicans GRI 682 | 38.2 ± 2.2 | 29.4 ± 0.9 | 67.6 ± 1.6 | 78.7 ± 2.9 |
| C. glabrata AAHB 12 | 15.8 ± 1.2 | 24.2 ± 0.6 | 101.7 ± 1.1 | 81.1 ± 1.5 |
| C. krusei | 71.3 ± 1.3 | 66.9 ± 0.5 | 74.4 ± 0.6 | 47.8 ± 1.2 |
| C. parapsilosis AAHB 479 | 74.9 ± 0.7 | 62.1 ± 1.5 | 67.4 ± 3.0 | 51.1 ± 1.2 |
| C. tropicalis AAHB 73 | 65.0 ± 1.8 | 40.5 ± 1.4 | 49.6 ± 0.9 | 37.7 ± 2.1 |
Viability is expressed as a percentage of that of control cells incubated under identical conditions in the absence of antifungal agent. The results are means ± standard errors of the means of triplicate determinations.
SEM.
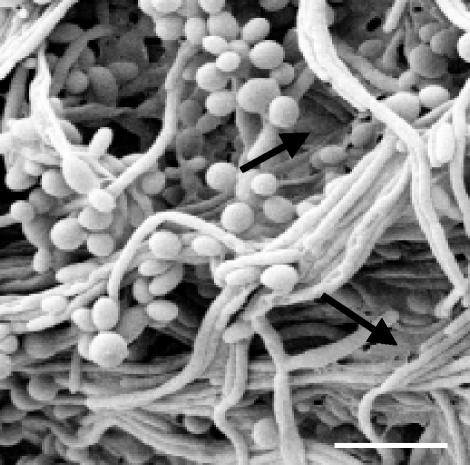
Biofilms of C. tropicalis had a very slimy appearance, suggestive of an extensive matrix, and were poorly penetrated by both flucytosine and fluconazole. C. tropicalis, whose ability to produce biofilms has received little attention, is able to grow in the form of yeast cells or filaments. Examination of the biofilms by SEM showed that they consisted of a dense cell network containing both morphological types. Many of the filaments appeared to lie parallel to each other in the form of bundles (Fig. 5). The procedure used for sample preparation allows clear visualization of biofilm cells but normally fails to preserve the biofilm matrix. However, fairly extensive matrix material could still be seen adhering to and linking some of the cells, a finding consistent with the slimy appearance of C. tropicalis biofilms.
FIG. 5.
Scanning electron micrograph of a 48-h-old colony (membrane-supported) biofilm of C. tropicalis. Arrows indicate extracellular matrix material. Bar, 10 μm.
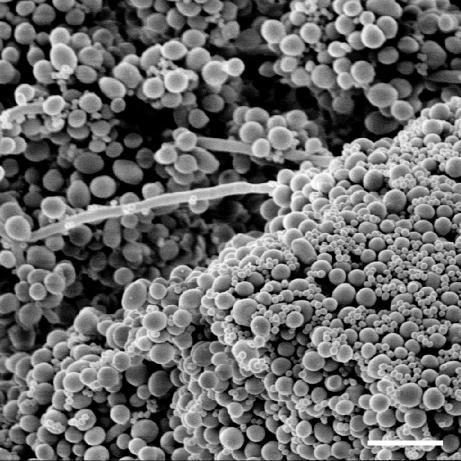
Previous work (1) demonstrated that both strains of S. epidermidis used here formed thick biofilms on catheter disks. However, wild-type strain RP62A, unlike mutant M7, produced abundant matrix material, or slime. The physical interactions between staphylococci and C. albicans were more easily seen with the slime-free mutant (1). A similar examination of mixed-species biofilms of C. albicans GDH 2346 and S. epidermidis M7 grown on polycarbonate membranes in this study also revealed multiple, adhesive interactions between bacterial and fungal cells. Staphylococci were clearly adherent to both yeasts and hyphae (Fig. 6).
FIG. 6.
Scanning electron micrograph of a 48-h-old colony (membrane-supported) biofilm of C. albicans GDH 2346 and S. epidermidis M7. Bar, 10 μm.
DISCUSSION
The mechanisms that protect microorganisms in biofilms from antibiotics and biocides are still being elucidated. Currently, four mechanisms are under study: (i) slow penetration of the antimicrobial agent into the biofilm, (ii) an altered chemical microenvironment within the biofilm leading to zones of slow or no growth, (iii) adaptive responses to environmental stress, and (iv) the existence of persister cells that are protected from all types of antimicrobial insult (43). Almost all of this work is being done with bacterial biofilms. At present, no single mechanism seems to account for the exceptional resistance of biofilm cells to a wide variety of antimicrobial agents. Instead, it is likely that two or more mechanisms operate together. All four mechanisms appear to depend on the multicellular nature of biofilms. For example, when an antimicrobial agent fails to penetrate a biofilm, it is because the drug is reactively neutralized as it diffuses into a cell cluster. This process may involve enzymatic degradation of the drug or drug binding to charged extracellular polymers, but it is effective only when microorganisms are aggregated and exert their collective neutralizing activity (43).
In this study, we have investigated the penetration of antifungal agents through single- and mixed-species biofilms formed by fungal pathogens in the genus Candida. Our results demonstrated that fluconazole permeated all single-species Candida biofilms more rapidly than flucytosine. The rates of diffusion of either drug through biofilms of three strains of C. albicans were similar. On the other hand, the rates of drug diffusion through biofilms of C. glabrata and C. krusei were faster than those through biofilms of C. parapsilosis and C. tropicalis. In all cases, the drug concentration reached at the distal edge of the biofilm was very high. However, drug penetration failed to produce complete killing of biofilm cells even when the incubation period was extended from 6 to 24 h. These results indicate that poor drug penetration is not a major resistance mechanism for Candida biofilms.
The biofilms showing the lowest levels of drug penetration, particularly with flucytosine, were those formed by C. parapsilosis and C. tropicalis. The strain of C. tropicalis used in these experiments, a clinical isolate, had a very slimy appearance on solid medium, and some of this slime, or matrix material, could be seen when biofilm preparations were viewed under a scanning electron microscope. As yet, nothing is known of the chemical composition of this material, but it could play a minor role in the drug resistance of C. tropicalis biofilms by slowing the diffusion of antifungal agents. The matrix of C. albicans biofilms has been isolated and shown to contain mainly carbohydrate and protein, with a relatively high proportion (16%) of glucose (9). Recent work with P. aeruginosa has led to the identification of periplasmic glucans in biofilm cells which appear to sequester antibiotics and slow their diffusion, perhaps preventing them from reaching their sites of action in the cytoplasm (33). It has also been postulated that the nature and amounts of extracellular glucans produced by oral streptococci from sucrose in dental plaque are major determinants retarding acid diffusion (25). It will be interesting to determine whether the matrix material of C. tropicalis biofilms is especially rich in glucan polysaccharides.
All four antifungal agents tested diffused very slowly through mixed-species biofilms containing C. albicans and either the wild-type or M7 mutant strain of S. epidermidis. In most cases, diffusion was slower with biofilms containing S. epidermidis RP62A, the wild-type, slime-producing strain. Curiously, however, amphotericin B penetrated biofilms containing the M7 mutant more slowly. In all of these experiments with mixed fungal-bacterial biofilms, the drug concentrations reaching the distal edges of the biofilms substantially exceeded the MIC for C. albicans. Thus, although the presence of bacteria and bacterial matrix material undoubtedly retarded the diffusion of the antifungal agents, poor penetration does not account for the drug resistance of Candida biofilm cells, even in these mixed-species biofilms.
The nature of the extracellular matrix of S. epidermidis biofilms is not fully established. It appears to contain a polymer of β-1,6-linked N-acetylglucosamine residues with some deacetylated amino groups, as well as succinate and phosphate substituents (the intercellular polysaccharide adhesin) (32). A 140-kDa accumulation-associated protein has also been identified (27). The M7 mutant, which fails to accumulate on glass surfaces (41) but which does form biofilms on polyvinyl chloride catheter disks (1), has been reported to lack this protein but nevertheless synthesizes intercellular polysaccharide adhesin (27). Interactions between these polymers and those produced by C. albicans in mixed-species biofilms might result in a more viscous matrix. Rheological interactions between matrix polysaccharides from Pseudomonas cepacia and P. aeruginosa have been shown to decrease the rates of diffusion and antimicrobial activities of antibiotics (2). On the other hand, a recent study of oral biofilms containing six microbial species, including C. albicans, suggested that retarded diffusion of fluorescent probes through the biofilm was due to tortuosity, i.e., the convoluted paths traversed by macromolecules during biofilm penetration (45).
Biofilm cells appear to grow slowly because of the limited availability of nutrients, especially at the base of the biofilm. Growth rate has therefore been considered as an important modulator of drug activity in biofilms (17, 19). A perfused fermentor was used to generate C. albicans biofilms at different growth rates, and the susceptibility of the biofilm cells to amphotericin B was compared with that of planktonic organisms grown at the same rates in a chemostat. The results indicated that biofilms were resistant to the drug at all growth rates tested, whereas planktonic cells were resistant only at low growth rates (5). An alternative mechanism of drug resistance might be upregulation of genes coding for multidrug efflux pumps in biofilm cells. C. albicans possesses two different types of efflux pump: ATP-binding cassette transporters and major facilitators, which are encoded by CDR and MDR genes, respectively. Recent work has shown that genes encoding both types of pump are indeed upregulated during biofilm formation and development. However, mutants carrying single or double deletion mutations in some of these genes were highly susceptible to fluconazole when they were growing planktonically but retained the resistant phenotype during biofilm growth (40). Overall, it seems probable that drug resistance in Candida biofilms, like that in bacterial biofilms, is a complex process involving more than one mechanism.
Acknowledgments
Mohammed Al-Fattani is the recipient of a research studentship from the Ministry of Health of Saudi Arabia.
We are indebted to Margaret Mullin for expert assistance with electron microscopy and to Pfizer Limited for a supply of fluconazole and voriconazole.
REFERENCES
- 1.Adam, B., G. S. Baillie, and L. J. Douglas. 2002. Mixed species biofilms of Candida albicans and Staphylococcus epidermidis. J. Med. Microbiol. 51:344-349. [DOI] [PubMed] [Google Scholar]
- 2.Allison, D. G., and M. J. Matthews. 1992. Effect of polysaccharide interactions on antibiotic susceptibility of Pseudomonas aeruginosa. J. Appl. Bacteriol. 73:484-488. [DOI] [PubMed] [Google Scholar]
- 3.Anderl, J. N., M. J. Franklin, and P. S. Stewart. 2000. Role of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob. Agents Chemother. 44:1818-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachmann, S. P., K. VandeWalle, G. Ramage, T. F. Patterson, B. L. Wickes, J. R. Graybill, and J. L. Lopez-Ribot. 2002. In vitro activity of caspofungin against Candida albicans biofilms. Antimicrob. Agents Chemother. 46:3591-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baillie, G. S., and L. J. Douglas. 1998. Effect of growth rate on resistance of Candida albicans biofilms to antifungal agents. Antimicrob. Agents Chemother. 42:1900-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baillie, G. S., and L. J. Douglas. 1998. Iron-limited biofilms of Candida albicans and their susceptibility to amphotericin B. Antimicrob. Agents Chemother. 42:2146-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baillie, G. S., and L. J. Douglas. 1999. Candida biofilms and their susceptibility to antifungal agents. Methods Enzymol. 310:644-656. [DOI] [PubMed] [Google Scholar]
- 8.Baillie, G. S., and L. J. Douglas. 1999. Role of dimorphism in the development of Candida albicans biofilms. J. Med. Microbiol. 48:671-679. [DOI] [PubMed] [Google Scholar]
- 9.Baillie, G. S., and L. J. Douglas. 2000. Matrix polymers of Candida biofilms and their possible role in biofilm resistance to antifungal agents. J. Antimicrob. Chemother. 46:397-403. [DOI] [PubMed] [Google Scholar]
- 10.Calderone, R. A. (ed.). 2002. Candida and candidiasis. ASM Press, Washington, D.C.
- 11.Chandra, J., P. K. Mukherjee, S. D. Leidich, F. F. Faddoul, L. L. Hoyer, L. J. Douglas, and M. A. Ghannoum. 2001. Antifungal resistance of candidal biofilms formed on denture acrylic in vitro. J. Dent. Res. 80:903-908. [DOI] [PubMed] [Google Scholar]
- 12.Chandra, J., D. M. Kuhn, P. K. Mukherjee, L. L. Hoyer, T. McCormick, and M. A. Ghannoum. 2001. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J. Bacteriol. 183:5385-5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 14.Cox, G. M., and J. R. Perfect. 1993. Fungal infections. Curr. Opin. Infect. Dis. 6:422-426. [Google Scholar]
- 15.Donlan, R. M. 2001. Biofilms and device-associated infections. Emerg. Infect. Dis. 7:277-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donlan, R. M., and J. W. Costerton. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Douglas, L. J. 2003. Candida biofilms and their role in infection. Trends Microbiol. 11:30-36. [DOI] [PubMed] [Google Scholar]
- 18.Fridkin, S. K., and W. R. Jarvis. 1996. Epidemiology of nosocomial fungal infections. Clin. Microbiol. Rev. 9:499-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilbert, P., T. Maira-Litran, A. J. McBain, A. H. Rickard, and F. W. Whyte. 2002. The physiology and collective recalcitrance of microbial biofilm communities. Adv. Microb. Physiol. 46:203-256. [PubMed] [Google Scholar]
- 20.Gordon, C. A., N. A. Hodges, and C. Marriott. 1988. Antibiotic interaction and diffusion through alginate and exopolysaccharide of cystic fibrosis-derived Pseudomonas aeruginosa. J. Antimicrob. Chemother. 22:667-674. [DOI] [PubMed] [Google Scholar]
- 21.Hatch, R. A., and N. L. Schiller. 1998. Alginate lyase promotes diffusion of aminoglycosides through the extracellular polysaccharide of mucoid Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 42:974-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hawser, S. P., and L. J. Douglas. 1994. Biofilm formation by Candida species on the surface of catheter materials in vitro. Infect. Immun. 62:915-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hawser, S. P., and L. J. Douglas. 1995. Resistance of Candida albicans biofilms to antifungal agents in vitro. Antimicrob. Agents Chemother. 39:2128-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hawser, S. P., G. S. Baillie, and L. J. Douglas. 1998. Production of extracellular matrix by Candida albicans biofilms. J. Med. Microbiol. 47:253-256. [DOI] [PubMed] [Google Scholar]
- 25.Hojo, S., M. Huguchi, and S. Araya. 1976. Glucan inhibition of diffusion in plaque. J. Dent. Res. 55:169. [DOI] [PubMed] [Google Scholar]
- 26.Hoyle, B. D., J. Alcantara, and J. W. Costerton. 1992. Pseudomonas aeruginosa biofilm as a diffusion barrier to piperacillin. Antimicrob. Agents Chemother. 36:2054-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hussain, M., M. Herrman, C. von Eiff, F. Perdreau-Remington, and G. Peters. 1997. A 140-kilodalton extracellular protein is essential for the accumulation of Staphylococcus epidermidis strains on surfaces. Infect. Immun. 65:519-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jenkinson, H. F., and L. J. Douglas. 2002. Interactions between Candida species and bacteria in mixed infections, p. 357-373. In K. A. Brogden and J. M. Guthmiller (ed.), Polymicrobial diseases. ASM Press, Washington, D.C. [PubMed]
- 29.Kuhn, D. M., T. George, J. Chandra, P. K. Mukherjee, and M. A. Ghannoum. 2002. Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob. Agents Chemother. 46:1773-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumon, H., K. Tomochika, T. Matunuaga, M. Ogawa, and H. Ohmori. 1994. A sandwich cup method for the penetration assay of antimicrobial agents through Pseudomonas exopolysaccharides. Microbiol. Immunol. 38:615-619. [DOI] [PubMed] [Google Scholar]
- 31.Lewis, R. E., D. P. Kontoyiannis, R. O. Darouiche, I. I. Raad, and R. A. Prince. 2002. Antifungal activity of amphotericin B, fluconazole, and voriconazole in an in vitro model of Candida catheter-related bloodstream infection. Antimicrob. Agents Chemother. 46:3499-3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mack, D., W. Fischer, A. Krokotsch, K. Leopold, R. Hartmann, H. Egge, and R. Laufs. 1996. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear β-1,6-linked glucoaminoglycan: purification and structural analysis. J. Bacteriol. 178:175-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mah, T.-F., B. Pitts, B. Pellock, G. C. Walker, P. S. Stewart, and G. A. O'Toole. 2003. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 426:306-310. [DOI] [PubMed] [Google Scholar]
- 34.Mah, T.-F. C., and G. A. O'Toole. 2001. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 9:34-39. [DOI] [PubMed] [Google Scholar]
- 35.Moosa, M.-Y. S., J. D. Sobel, H. Elhalis, W. Du, and R. A. Akins. 2004. Fungicidal activity of fluconazole against Candida albicans in a synthetic vagina-simulative medium. Antimicrob. Agents Chemother. 48:161-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nichols, W. W., S. M. Dorrington, M. P. E. Slack, and H. L. Walmsley. 1988. Inhibition of tobramycin diffusion by binding to alginate. Antimicrob. Agents Chemother. 32:518-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramage, G., K. VandeWalle, B. L. Wickes, and J. L. Lopez-Ribot. 2001. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob. Agents Chemother. 45:2475-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramage, G., K. VandeWalle, B.L. Wickes, and J. L. Lopez-Ribot. 2001. Biofilm formation by Candida dubliniensis. J. Clin. Microbiol. 39:3234-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramage, G., K. VandeWalle, S. P. Bachmann, B. L. Wickes, and J. L. Lopez-Ribot. 2002. In vitro pharmacodynamic properties of three antifungal agents against preformed Candida albicans biofilms determined by time-kill studies. Antimicrob. Agents Chemother. 46:3634-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramage, G., S. Bachmann, T. F. Patterson, B. L. Wickes, and J. L. Lopez-Ribot. 2002. Investigation of multidrug efflux pumps in relation to fluconazole resistance in Candida albicans biofilms. J. Antimicrob. Chemother. 49:973-980. [DOI] [PubMed] [Google Scholar]
- 41.Schumacher-Perdreau, F., C. Heilmann, G. Peters, F. Gotz, and G. Pulverer. 1994. Comparative analysis of a biofilm-forming Staphylococcus epidermidis strain and its adhesion-positive, accumulation-negative mutant M7. FEMS Microbiol. Lett. 117:71-78. [DOI] [PubMed] [Google Scholar]
- 42.Shigeta, M., G. Tanaka, H. Komatsuzawa, M. Sugai, H. Suginaka, and T. Usui. 1997. Permeation of antimicrobial agents through Pseudomonas aeruginosa biofilms: a simple method. Chemotherapy (Basel) 43:340-345. [DOI] [PubMed] [Google Scholar]
- 43.Stewart, P. S. 2003. Multicellular nature of biofilm protection from antimicrobial agents, p. 181-189. In A. McBain, D. Allison, M. Brading, A. Rickard, J. Verran, and J. Walker (ed.), Biofilm communities: order from chaos? BioLine, Cardiff, United Kingdom.
- 44.Suci, P., M. W. Mittelman, F. P. Yu, and G. G. Geesey. 1994. Investigation of ciprofloxacin penetration into Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 38:2125-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thurnheer, T., R. Gmur, S. Shapiro, and B. Guggenheim. 2003. Mass transport of macromolecules within an in vitro model of supragingival plaque. Appl. Environ. Microbiol. 69:1702-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walters, M. C., III, F. Roe, A. Bugnicourt, M. J. Franklin, and P. S. Stewart. 2003. Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob. Agents Chemother. 47:317-323. [DOI] [PMC free article] [PubMed] [Google Scholar]