Abstract
R126638 is a novel triazole with in vitro activity similar to that of itraconazole against dermatophytes, Candida spp., and Malassezia spp. In animal models of dermatophyte infections, R126638 showed superior antifungal activity. R126638 inhibits ergosterol synthesis in Candida albicans, Trichophyton mentagrophytes, Trichophyton rubrum, and Microsporum canis at nanomolar concentrations, with 50% inhibitory concentrations (IC50s) similar to those of itraconazole. The decreased synthesis of ergosterol and the concomitant accumulation of 14α-methylsterols provide indirect evidence that R126638 inhibits the activity of CYP51 that catalyzes the oxidative removal of the 14α-methyl group of lanosterol or eburicol. The IC50s for cholesterol synthesis from acetate in human hepatoma cells were 1.4 μM for itraconazole and 3.1 μM for R126638. Compared to itraconazole (IC50 = 3.5 μM), R126638 is a poor inhibitor of the 1α-hydroxylation of 25-hydroxyvitamin D3 (IC50 > 10 μM). Micromolar concentrations of R126638 and itraconazole inhibited the 24-hydroxylation of 25-hydroxyvitamin D3 and the conversion of 1,25-dihydroxyvitamin D3 into polar metabolites. At concentrations up to 10 μM, R126638 had almost no effect on cholesterol side chain cleavage (CYP11A1), 11β-hydroxylase (CYP11B1), 17-hydroxylase and 17,20-lyase (CYP17), aromatase (CYP19), or 4-hydroxylation of all-trans retinoic acid (CYP26). At 10 μM, R126638 did not show clear inhibition of CYP1A2, CYP2A6, CYP2D6, CYP2C8, CYP2C9, CYP2C10, CYP2C19, or CYP2E1. Compared to itraconazole, R126638 had a lower interaction potential with testosterone 6β hydroxylation and cyclosporine hydroxylation, both of which are catalyzed by CYP3A4, whereas both antifungals inhibited the CYP3A4-catalyzed hydroxylation of midazolam similarly. The results suggest that R126638 has promising properties and merits further in vivo investigations for the treatment of dermatophyte and yeast infections.
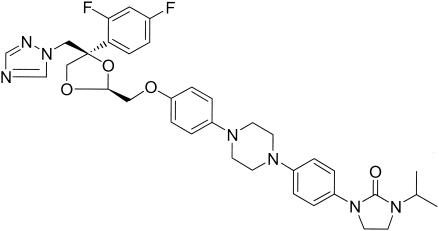
R126638 (Fig. 1) is a new triazole derivative with potent antifungal activities in vitro against Epidermophyton floccosum, Microsporum canis, Trichophyton spp., Malassezia spp., and Candida albicans comparable to those of itraconazole (14). In guinea pig models of cutaneous M. canis and Trichophyton mentagrophytes infections, R126638 consistently showed antifungal activity superior to that of itraconazole (14). Clinical studies are in progress to determine the value of R126638 for the oral treatment of dermatophytosis.
FIG. 1.
Chemical structure of R126638 (C35H39F2N7O4). Molecular weight, 659.74.
The antifungal activities of azole derivatives such as miconazole, clotrimazole, bifonazole, ketoconazole, itraconazole, fluconazole, and voriconazole arise from a complex multimechanistic process initiated by the inhibition of a cytochrome P450 (CYP) involved in the biosynthesis of ergosterol, namely, CYP51 (also called Erg11p, according to different gene-based nomenclatures) (6, 16, 20, 24). CYP51 (the product of the CYP51 gene) catalyzes the oxidative removal of the 14α-methyl group of lanosterol or eburicol.
Azole antifungal agents also affect other CYPs. At concentrations >100 nM, ketoconazole inhibits not only the mammalian CYP51 but also the 17-hydroxylase-17,20-lyase (CYP17), the cholesterol side chain cleavage enzyme (CYP11A1), and the 11β-hydroxylase (CYP11B1) (18). Itraconazole is almost devoid of effects on steroid metabolism (19). However, like ketoconazole (10, 29), itraconazole inhibits CYP3A4, a major drug-metabolizing P450 isoform in the human liver (10, 29). Lamb et al. (10) compared the inhibition by ketoconazole and itraconazole of human CYP3A4 and C. albicans CYP51 following heterologous expression in Saccharomyces cerevisiae. The 50% inhibitory concentrations (IC50s) of ketoconazole and itraconazole for CYP3A4 inhibition were 250 and 200 nM, respectively. The IC50s of ketoconazole and itraconazole for CYP51 inhibition were 8 and 7.6 nM, respectively. Fluconazole, miconazole (29), and voriconazole (6) are inhibitors of CYP2C9, which plays a major role in the metabolism of commonly prescribed drugs, such as phenytoin, S-warfarin, and a range of nonsteroidal anti-inflammatory drugs (11). Voriconazole also inhibits CYP2C19 (S-mephenytoin hydroxylase) and CYP3A4 (6, 7). The ideal azole antifungals are those that react strongly with fungal CYP51 and that have weak activities against mammalian CYPs.
Preclinical studies of new triazoles often consist of an in vitro test to help select congeners that will have the least drug-drug interactions in clinical trials.
In this study we describe the effects of R126638 and itraconazole on ergosterol synthesis in C. albicans, T. mentagrophytes, Trichophyton rubrum, and M. canis and on CYP-mediated reactions in mammalian cells, human liver and placental microsomes, rat testis microsomes, bovine adrenocortical and rat kidney mitochondria, and human skin epidermis.
MATERIALS AND METHODS
Antifungal drugs.
Analytical-grade powders of itraconazole and R126638, (2S-cis)-1-{4-[4-(4-{[4-(2,4-difluorophenyl)-4-(1H-1,2,4-triazol-1-ylmethyl)-1,3-dioxalan-2-yl]methoxy}phenyl)-piperazin-1-yl]phenyl}-3-(1-methyl-ethyl)-2-imidazolidinone, both from Janssen Pharmaceutica NV (Beerse, Belgium), were dissolved in dimethyl sulfoxide (DMSO). The stock solutions (10 mM) were further diluted in 100% DMSO and added to the incubation mixtures at a final solvent concentration of 0.1%.
Cells.
he C. albicans (B2630), T. rubrum (B68183), T. mentagrophytes (B32663), and M. canis (B68128) isolates were from the Janssen Pharmaceutica collection. The mammalian cells used were human hepatoma cells (HepG2, ATCC HB 8065), human tongue squamous carcinoma cells (SCC25, ATCC CRL 1628), and HD-11 cells (v-myc-transformed chicken myelomonocytic cell line; a gift from Thomas Graf, European Molecular Biology Laboratory, Heidelberg, Germany).
Inoculum preparation and culture conditions.
C. albicans (2.5 × 107 cells) was grown in 100 ml of CYG medium (casein hydrolysate [Merck, Darmstadt, Germany], yeast extract [Difco, Detroit, Mich.], and glucose, each at a concentration of 5 g liter−1) in 500-ml Erlenmeyer flasks in a reciprocating shaker, set at 100 strokes per min, at 37°C (9). T. rubrum, T. mentagrophytes, and M. canis were grown at 30°C on potato dextrose agar (39 g liter−1; Difco).
Ergosterol synthesis experiments.
To study the effects of R126638 and itraconazole on ergosterol synthesis in C. albicans, 100 ml of CYG medium was supplemented with 5 μCi of sodium [14C]acetate (specific activity, 58 μCi mmol−1; Radiochemical Center, Amersham, United Kingdom) and different concentrations of an azole antifungal and/or DMSO immediately before inoculation (12). Inoculation (2.5 × 105 cells ml−1) and growth conditions were as described above. After 24 h of growth, cell concentrations were determined with a Coulter counter, as described earlier (21). Cells were collected by centrifugation at 1,500 × g, and the cell pellet was washed twice with distilled water. The pellets were suspended in 8 ml of water and added to 5 g of acid-washed glass beads (diameter, 0.40 to 0.45 mm) in Packard Super polyethylene vials. Cells were shaken vigorously in a Retsch laboratory mixer mill 2000 set at maximum speed for 5 min. The homogenates were quantitatively separated from the glass beads, supplemented with 15% KOH dissolved in 90% ethanol, and saponified at 85°C. Nonsaponifiable lipids were extracted with 1 volume of n-heptane (spectrograde) (12).
M. canis, T. mentagrophytes, and T. rubrum conidia were collected after 2 to 3 weeks of growth by pipetting a solution of 0.01% Tween 40 onto the agar surface, followed by agitation. The culture suspensions were transferred to 500-ml Erlenmeyer flasks containing three layers of sterile glass beads (diameter, 0.40 to 0.45 mm). The flasks were shaken on a gyratory shaker at 250 rpm for 30 min, after which the suspensions were filtered through sterile glass wool and the filtrate was centrifuged aseptically for 20 min at 5,000 × g. The pellets were washed twice with physiological saline. To evaluate the effects of R126638 and itraconazole on ergosterol synthesis, T. mentagrophytes (2.5 × 105 conidia), T. rubrum (5 × 105 conidia), or M. canis (5 × 105 conidia) was inoculated in 75 ml of PYG medium, which contained 10 g of polypeptone, 10 g of yeast extract, and 40 g of glucose per liter, and were grown in 175-cm2 Falcon culture flasks at 30°C. Itraconazole or R126638 and/or DMSO was added to the medium immediately before inoculation. After 32 h (T. mentagrophytes), 40 h (T. rubrum), and 72 h (M. canis) of growth, 5 μCi of sodium [14C]acetate was added. T. mentagrophytes was grown for another 16 h, and T. rubrum and M. canis were grown for another 24 h. At the end of the incubation period, mycelium was collected by filtration on 8-μm-pore-size Millipore HAWP filters and washed with distilled water. The pellets were homogenized and saponified, and the nonsaponifiable lipids were extracted as described above for C. albicans. The total radioactivity in the extracts was determined by liquid scintillation counting (22).
The heptane extracts were dried under a stream of nitrogen and dissolved in minimal volumes of methanol-water (95:5). Sterols were separated by high-performance liquid chromatography (HPLC) on a Varian 9010 liquid chromatograph equipped with a Varian 9095 automatic injector, a Varian 9065 Polychrom detector, and a Berthold LB507A HPLC radioactivity monitor with Pico Aqua (Canberra Packard) as a scintillant and connected to a Compaq 386/33 computer (23).
Cholesterol synthesis.
The effects of R126638 and itraconazole on cholesterol synthesis from sodium [14C]acetate in human hepatoma cells (HepG2) were studied as described previously (22). Cholesterol and its precursors were separated by thin-layer chromatography on precoated silica gel plates (no. 5554-60F254; Merck) and developed in a solvent system consisting of heptane-diisopropylether-acetic acid-ethyl acetate (60:40:4:34.7; vol/vol/vol/vol). To visualize the radioactive fractions, the thin-layer chromatography plates were exposed to Kodak autoradiograph film for 3 days. The fractions were quantitated by digitizing the films with an RX5 video camera attached to a Macintosh IIfx computer. Integration of the density was done by means of Image (version 1.28) software.
Cholesterol side chain cleavage, 11β-hydroxylase, and androgen and estrogen biosynthesis.
Cholesterol side chain cleavage and 11β-hydroxylase were studied by using extracts from sonicated bovine adrenocortical mitochondria (25).
Androgen biosynthesis from [14C]pregnenolone in S 10,000 fractions (microsomes plus cytosol) of Wistar rat testes was studied as described previously (26).
The conversion of [3H]androstenedione to estrone by aromatase in human placental microsomes was carried out as described previously (22).
Retinoic acid metabolism.
Human skin was obtained from patients undergoing plastic surgery at local hospitals and was immediately transported to the laboratory in sterile transport medium (T medium) containing phosphate-buffered saline (PBS; Gibco) with calcium (CaCl2 · 2H2O; 0.132 g liter−1) and magnesium (MgCl2 · 6H2O; 0.10 g liter−1) but without sodium bicarbonate. T medium also contained 10 μg of gentamicin ml of PBS−1. Upon arrival, the skin was washed three times with T medium, and the adipose tissue was scraped off. Punch specimens of 0.8 cm in diameter were obtained with a Keyes' skin puncher and placed in cold normal human keratinocyte medium (NHK medium) (27). NHK medium contained 3 parts Dulbecco's modified Eagle's medium (DMEM; Gibco) and 1 part Ham's F-12 medium (Gibco) plus 10% fetal calf serum (FCS), 2 mM l-glutamine, 2 nM triiodothyronine (T3), 0.18 mM adenine, 0.1 nM cholera toxin, 5 μg of gentamicin, 0.4 μg of hydrocortisone, 5 μg of insulin, 5 μg of transferrin, and 10 ng of epidermal growth factor per ml of medium. Fifty skin punch specimens (diameter, 8 mm) were incubated overnight at 4°C in 15 ml of PBS (without calcium or magnesium) containing 2 ml of Dispase II (24 U ml−1; Boehringer, Mannheim, Germany). After incubation, the epidermis was separated from the dermis with sterile forceps and placed in a six-well plate (two epidermal sheets per well), dermal side down, on 2 ml of NHK medium containing 2 μl of drug and/or DMSO and 1 μCi of [11,12-3H(N)]all-trans retinoic acid (20 pmol; specific activity, 50.7 Ci mmol−1; New England Nuclear, Boston, Mass.). After 24 h of incubation at 37°C in a 5% CO2-humidified atmosphere, the medium was extracted for 30 min with 5 ml of ethyl acetate containing 0.005% (wt/vol) butylated hydroxytoluene (BHT). After evaporation of the organic solvent the samples were analyzed by HPLC as described by Van Wauwe et al. (28). All operations were carried out in a darkened room illuminated with yellow light.
25-Hydroxyvitamin D3-1α-hydroxylase.
v-myc-transformed chicken myelomonocytic (HD-11) cells (1) were grown on DMEM supplemented with 4 mM glutamine, 10% FCS, and 1% antibiotica-antimycotica mixture (Gibco). Monolayers of HD-11 cells were preincubated for 16 h in serum-free medium, after which the medium was replaced by 2 ml of fresh medium, to which drugs and/or DMSO was added. The reaction was started by adding 100 pmol of 25-hydroxyvitamin D3, of which 0.3 pmol was [26,27-3H]25-hydroxyvitamin D3 (specific activity, 165 Ci.mmol−1; New England Nuclear). At the end of the 3-h incubation period, the medium was transferred into a brown test tube containing 3 ml of chloroform and 0.5 ml of 10% formic acid. The cells were trypsinized with 1 ml of 0.125% trypsin-0.01% EDTA, and after 10 to 15 min of incubation at room temperature, the trypsinized cells were combined with the medium-chloroform mixture. The six-well plates were washed twice with 1.5 ml of methanol. After 20 min of extraction, the chloroform layer was separated by centrifugation (10 min at 2,400 rpm [MSE-GF8 centrifuge]) and dried under a stream of nitrogen. 25-Hydroxyvitamin D3 and its metabolites were separated by HPLC (Zorbax 5Si column; 250 by 4.5 mm; 5 μm; 30°C; flow rate, 1 ml min−1) by a modification of the method described by Jones et al. (9).
25-Hydroxyvitamin D3-24-hydroxylase.
Male Wistar rats (weight, 200 to 220 g) were pretreated (intraperitoneally) with 1,25-dihydroxyvitamin D3 (1 μg kg of body weight−1 per day) for three consecutive days. The animals were left for the last 24 h without food but with free access to water (8). Kidney mitochondria were isolated as described by Vieth and Fraser (30). They were suspended in ice-cold incubation medium, composed of 50 mM Tris-acetate buffer (pH 7.42), 10 mM MgCl2 · 6H2O, 12 mM isocitrate, and 2.5 μg of diphenyl-p-phenylenediamine. Incubations were carried out in brown tubes containing the following in a final volume of 1 ml of medium to which drugs and/or DMSO was added: 2 mg of mitochondrial protein, 500 pmol of 25-hydroxyvitamin D3, and 0.05 μCi of [26,27-3H]25-hydroxyvitamin D3 (specific activity, 165 Ci mmol−1; New England Nuclear). After incubation at 37°C for 30 min in a shaking water bath, the reaction was stopped by addition of 3.75 ml of methanol-chloroform (1:2; vol/vol) containing 0.01% BHT. Extraction and separation of the metabolites were performed as described above.
Catabolism of 1,25-dihydroxyvitamin D3.
Squamous carcinoma (SCC25) cells were seeded at a density of 2 × 105 cells per 2 ml in six-well plates on a 1:1 mixture of Ham's F-12 medium and DMEM (Gibco) containing 0.36 μg of hydrocortisone ml−1 and 10% FCS. The cells were grown for 4 days at 37°C in a humidified 5% CO2 atmosphere. At the end of the incubation period, the medium was replaced by serum-free keratinocyte medium (Gibco), and the confluent cells were incubated for an additional 3 days.
Human hepatoma (HepG2) cells were seeded at a density of 105 cells ml−1 in six-well plates (2 ml of cell suspension per well) and grown for 1 week in MEM-Rega 3 medium (Gibco) containing 10% FCS.
Sixteen hours before and at the onset of the experiment, the media were replaced by 2 ml of serum-free keratinocyte medium (SCC25 cells) or serum-free MEM-Rega 3 medium (HepG2 cells). The reaction was initiated by adding 0.1 μCi of 1α,25-[26, 27-3H]dihydroxyvitamin D3 (specific activity, 170 Ci mmol−1) in 10 μl of ethanol and 2 μl of drug and/or DMSO. At the end of a 3-h incubation period, substrate and metabolites were extracted and separated as described above.
Effects of R126638 on oxidative drug metabolism by human liver microsomes.
Microsomes were prepared from four human livers as described previously (3). All inhibition experiments were performed in a pooled batch of microsomes, which was characterized for the different CYP activities (3). Incubations were performed in triplicate at concentrations of 0, 1, 3, 10, and 30 mM R126638 for the CYP3A4 substrates cyclosporine, testosterone, and midazolam and the CYP2C19 substrate S-mephenytoin. Itraconazole was included as a reference compound. Inhibition of the other CYP forms was investigated at an R126638 concentration of 10 μM. The incubation conditions are summarized in Table 1.
TABLE 1.
Incubation conditions of the CYP probe substrates in human liver microsomes
| CYP substrate | CYP form | Substrate concn (μM) | Protein contenta (mg/ml) | Incubation
|
Analytical methodb | |
|---|---|---|---|---|---|---|
| Time (min) | Vol (ml) | |||||
| Caffeine | CYP1A2 | 10 | 1 | 30 | 0.5 | Radio-HPLC |
| Coumarin | CYP2A6 | 50 | 0.05-0.1 | 10 | 1 | Fluorimetry |
| Cyclosporine | CYP3A4 | 5 | 1 | 30 | 1 | Radio-HPLC |
| Testosterone | CYP3A4 | 100 | 0.5 | 25 | 0.5 | LC-MS |
| Midazolam | CYP3A4 and -5 | 50 | 0.5 | 10 | 1 | HPLC-UV |
| Phenytoin | CYP2C8 and -9 | 150 | 0.5 | 120 | 1 | Radio-HPLC |
| Tolbutamide | CYP2C8 and -9 | 1,200 | 0.5 | 90 | 1 | HPLC-UV |
| S-Mephenytoin | CYP2C19 | 100 | 0.5 | 30 | 0.5 | Radio-HPLC |
| Debrisoquine | CYP2D6 | 49.3 | 2 | 30 | 0.15 | Radio-HPLC |
| Chlorzoxazone | CYP2E1 | 500 | 0.5 | 20 | 1 | HPLC-UV |
Protein content was measured by the Lowry method, as modified by Miller (13), using bovine serum albumin as a standard.
Radio-HPLC, HPLC with online radioactivity detection; LC, liquid chromatography; MS, mass spectrometry.
The percent inhibition of the metabolism of a probe substrate or the percent inhibition of metabolite formation after incubation with R126638 or itraconazole was determined as follows: 100 − [(C+ inhibitor/Ccontrol) × 100], where C+ inhibitor and Ccontrol represent the overall metabolism or the relative amounts of the metabolites in the presence and the absence of R126638, respectively.
IC50s were determined from a plot of the percent inhibition against the logarithm of the R126638 or itraconazole concentration. The value was obtained by regression analysis of the linear part of the curve.
RESULTS
Effect on fungal CYP-dependent reactions.
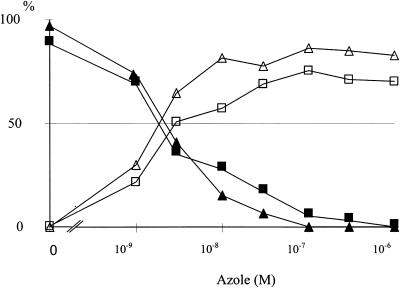
To prove that R126638 shares with other azole antifungal agents the property of CYP51-dependent ergosterol synthesis inhibition, its effects on ergosterol synthesis were studied in C. albicans, T. mentagrophytes, T. rubrum, and M. canis (Table 2). Itraconazole was used as the standard throughout the study. After 24 h of growth of C. albicans in CYG medium supplemented with [14C]acetate and R126638 or itraconazole, the IC50s of R126638 and itraconazole for ergosterol synthesis were 2.2 and 2.8 nM, respectively. Although the IC50s were almost identical, R126638 was a more potent inhibitor than itraconazole; 94% inhibition of [14C]acetate incorporation into ergosterol was achieved at 30 nM; at least 100 nM itraconazole was needed to achieve a similar level of inhibition (Fig. 2).
TABLE 2.
Effects of R126638 and itraconazole on ergosterol synthesis from [14C]acetate
| Organism | Incubation time (h)a | IC50 (nM)b for ergosterol synthesis
|
|
|---|---|---|---|
| R126638 | Itraconazole | ||
| C. albicans | 0 + 24 | 2.2 | 2.8 |
| M. canis | 72 + 24 | 280 | 310 |
| T. mentagrophytes | 32 + 16 | 22 | 82 |
| T. rubrum | 40 + 24 | 33 | 18.5 |
Azoles were added immediately before inoculation; [14C]acetate was added after 0, 72, 32, or 40 h of incubation.
Values are the means of at least three separate experiments.
FIG. 2.
Effects of R126638 (▴ and Δ) and itraconazole (▪ and □) on [14C]acetate incorporation into ergosterol by C. albicans. [14C]acetate, an azole antifungal, and/or DMSO was added immediately before inoculation. Cells were collected after 24 h of growth. The results are expressed as the percentage of radioactivity incorporated in desmethylsterols (ergosterol), 14-methylated sterols, and squalene. ▴ and ▪, ergosterol; Δ and □, 3,6-diol. Each symbol represents the mean of at least three experiments.
R126638 and itraconazole showed similar inhibitory activities against sterol synthesis in T. rubrum, with IC50s of 33 and 18.5 nM, respectively. R126638 (IC50 = 22 nM) was 3.7 times more active than itraconazole (IC50 = 82 nM) against ergosterol biosynthesis in T. mentagrophytes. Compared to itraconazole, R126638 had a similar activity against sterol synthesis in M. canis, with IC50s of 280 and 310 nM, respectively.
Inhibition of ergosterol synthesis in C. albicans coincided with the accumulation of 14α-methyl-ergosta-8,24(28)-dien-3β,6α-diol (3,6-diol) (Fig. 2); small amounts of two other 14-methylated sterols, i.e., obtusifoliol and eburicol (24-methylenedihydrolanosterol) also accumulated.
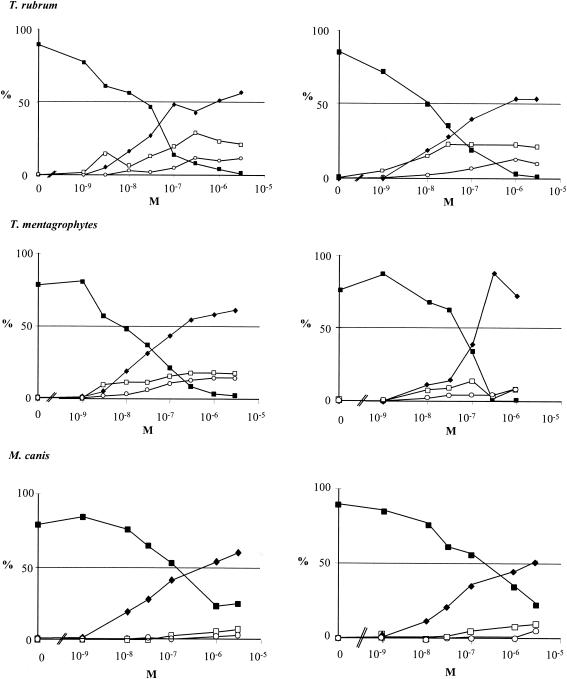
In the presence of either R126638 or itraconazole, T. rubrum, T. mentagrophytes, and M. canis accumulated much more eburicol than 3,6-diol and obtusifoliol (Fig. 3).
FIG. 3.
Effects of R126638 (left panels) or itraconazole (right panels) on [14C]acetate incorporation into ergosterol by T. rubrum, T. mentagrophytes, or M. canis. Azoles were added immediately after inoculation; [14C]acetate was added after 0, 72, 32, or 40 h of incubation. Thereafter, T. mentagrophytes was incubated for another 16 h and T. rubrum and M. canis were incubated for another 24 h. The results are expressed as the percentage of radioactivity incorporated into desmethylsterols (ergosterol), 14-methylated sterols, and squalene. ▪, ergosterol; ♦, eburicol; ○, obtusifoliol; □, 3,6-diol.
Effects on mammalian CYP-dependent reactions.
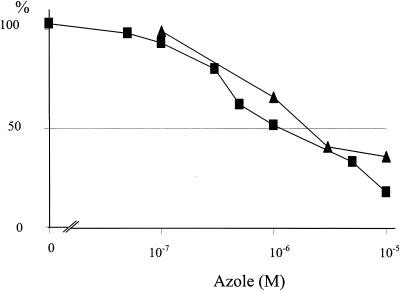
In contrast to the potent inhibitory action of R126638 on ergosterol biosynthesis in intact C. albicans cells, a R126638 concentration 440 times higher was needed to inhibit mammalian cholesterol synthesis (Fig. 4). Fifty percent inhibition of cholesterol synthesis from [14C]acetate was found when human hepatoma cells were incubated with 1.4 μM itraconazole or 3.1 μM R126638.
FIG. 4.
Effects of R126638 (▴) and itraconazole (▪) on cholesterol synthesis by human hepatoma (HepG2) cells. Cells were grown at 37°C for 24 h. [14C]acetate, R126638 or itraconazole, and/or DMSO was added and the cells were grown for another 24 h.
The adrenal mitochondrial cholesterol side chain cleavage enzyme (CYP11A1) and 11β-hydroxylase (CYP11B1), the 17-hydroxylase plus 17,20-lyase (CYP17) in the rat testis, the human placental aromatase (CYP19), and the 4-hydroxylation of all-trans retinoic acid (CYP26) in human skin epidermis were not affected at the concentrations of R126638 (10 μM) and itraconazole (5 μM) used.
As shown in Table 3, R126638 was also a poor inhibitor of the 1α-hydroxylation of 25-hydroxyvitamin D3 in chicken myelomonocytic cells; only 26% inhibition was achieved with 10 μM R126638. The IC50 of itraconazole for this CYP27B1-catalyzed reaction was 3.5 μM.
TABLE 3.
Effects of R126638 and itraconazole on mammalian CYP-dependent reactions
| Tissue or cell | Substrate | Product(s) formed | Cytochrome P450 | IC50 (μM)a
|
|
|---|---|---|---|---|---|
| R126638 | Itraconazole | ||||
| HD-11 cells | 25-Hydroxyvitamin D3 | 1,25-dihydroxyvitamin D3 | CYP27B1 | >10 (26)b | 3.5 |
| Rat kidney mitochondria | 25-Hydroxyvitamin D3 | 24,25-dihydroxyvitamin D3 | CYP24 | 3.9->10 | 2.8 |
| SCC25 cells | 1,25-dihydroxyvitamin D3 | 1,24,25-dihydroxyvitamin D3 + metabolites | CYP24 + other enzymesc | 0.17 | 0.42 |
| HepG2 cells | 1,25-dihydroxyvitamin D3 | 1,24,25-trihydroxyvitamin D3 + metabolites | CYP24 + other enzymes | 1.82 | 0.67 |
| Human liver microsomes | Testosterone | Testosterone 6β-hydroxylase | CYP3A4d | <1 (63) | 0.09 |
| Cyclosporine | Overall metabolism | CYP3A4 | >10 (19) | 1.21 | |
| Midazolam | 1′-Hydroxymidazolam | CYP3A4d | 2.28 | 1.98 | |
| 4-Hydroxymidazolam | CYP3A4d | 1.34 | 2.00 | ||
Values are means of at least three experiments.
Values in parentheses are the percent inhibition at the highest concentration tested.
Next to 1,24,25-trihydroxyvitamin D3 (calcitetrol), other degenerative products of vitamin D3 are 1α-hydroxy-23-carboxy-tetra nor-vitamin D3 (calcitroic acid) and 1,25-dihydroxyviamin D3-26,23 lactone (4).
CYP3A4 and CYP3A5 both contribute to midazolam and testosterone biotransformation in human liver microsomes, with CYP3A5 being metabolically much less active and less sensitive to ketoconazole (15).
Both R126638 and itraconazole inhibited the 24-hydroxylation of 25-hydroxyvitamin D3 at micromolar concentrations in rat kidney mitochondria. The catabolism of 1,25-dihydroxyvitamin D3 by human squamous carcinoma (SCC25) cells and human hepatoma (HepG2) cells was inhibited at lower concentrations. The IC50s of R126638 for the conversion of 1,25-dihydroxyvitamin D3 into more polar metabolites by SCC25 and HepG2 cells were 0.17 and 1.82 μM, respectively, and those of itraconazole were 0.42 and 0.67 μM, respectively.
Effects on oxidative drug metabolism by human liver microsomes.
The inhibitory effects of 10 μM R126638 on debrisoquine 4-hydroxylation (CYP2D6), coumarin 7-hydroxylation (CYP2A6), caffeine N3-demethylation (CYP1A2), tolbutamide hydroxylation (CYP2C8, -9, and -10), phenytoin hydroxylation (CYP2C8, -9, and -10), S-mephenytoin 4-hydroxylation (CYP2C19), and chlorzoxazone hydroxylation (CYP2E1) were studied in human liver microsomes. For R126638, no clear inhibition of the metabolism of the CYP probe substrates could be observed.
The effects of R126638 on the metabolism of the CYP3A4 substrates testosterone, cyclosporine, and midazolam were investigated in more detail. The inhibitory potential of R126638 was compared to the inhibitory capacity of itraconazole in the same set of experiments. On the basis of the testosterone 6β hydroxylation data and the overall metabolism data for cyclosporine, a lower intrinsic interaction potential with CYP3A4 substrates was observed for R126638 (Table 3) than for itraconazole. However, these triazole derivatives inhibited the formation of the 1′-hydroxy and 4-hydroxy metabolites from midazolam at similar concentrations (Table 3).
DISCUSSION
Imidazole and triazole derivatives exhibit their antifungal activities by inhibiting ergosterol biosynthesis. Ergosterol is an essential component of the fungal plasma membrane: it regulates membrane permeability and the activities of membrane-bound enzymes, is a major component of secretory vesicles, and has an important role in mitochondrial respiration (5, 20). The target enzyme of azole antifungal agents is the sterol 14α-demethylase, a CYP (CYP51) enzyme encoded by the CYP51 gene. Interaction with CYP51 results in a decreased availability of ergosterol and the accumulation of 14-methylsterols, such as 3,6-diol, obtusifoliol, and eburicol.
R126638 and itraconazole are almost equipotent inhibitors of ergosterol biosynthesis in C. albicans and the dermatophytes studied. The decreased synthesis of ergosterol and the concomitant accumulation of 14α-methylsterols provide indirect evidence that R126638 inhibits CYP51 in C. albicans, T. rubrum, T. mentagrophytes, and M. canis at nanomolar concentrations. The antifungal activity of R126638 in vivo was consistently superior to that of itraconazole (14). Since R126638 is an almost equipotent inhibitor of the 14α-demethylase as itraconazole, the higher degree of efficacy against guinea pig and mouse dermatophytoses (14) suggests that R126638 has a better pharmacokinetic profile in these particular animal models.
R126638 shares with other azole antifungal agents the capability to block mammalian cholesterol synthesis at CYP51. However, as with the other azoles (e.g., itraconazole) the concentration required to cause the same degree of inhibition is much higher than that required to inhibit C. albicans and dermatophytes. Although the effects of R126638 on the in vivo 14α-demethylation of lanosterol have not been studied, studies with itraconazole suggest that the interaction with mammalian cholesterol synthesis in vivo might be negligible. Eight days of treatment of female rats with itraconazole doses as high as 40 mg kg of body weight−1 did not affect liver cholesterol synthesis (17).
Micromolar concentrations of itraconazole are needed to inhibit the 25-hydroxyvitamin D3-1α-hydroxylase (CYP27B1) and 25-hydroxyvitamin D3-24-hydroxylase (CYP24); R126638 is even less active. Both triazoles are, at least in the models used, more active against the further metabolism of 1α,25-dihydroxyvitamin D3. In particular, the catabolism of 1α,25-dihydroxyvitamin D3 by human tongue squamous carcinoma cells is sensitive to inhibition. Again, however, R126638 affects 1,25-dihydroxyvitamin D3 at concentrations much higher than those needed to inhibit ergosterol biosynthesis in C. albicans.
Contrary to ketoconazole (28), both itraconazole and R126638 do not interfere with the in vitro metabolism of retinoic acid. R126638 at concentrations up to 10 μM did not affect CYP1A2, CYP2A6, CYP2D6, CYP2C8, CYP2C9, CYP2C10, CYP2C19, or CYP2E1 activity.
Back and Tjia (2) studied ketoconazole, itraconazole, and fluconazole for their effects on the metabolism of cyclosporine by human liver microsomes. Cyclosporine is metabolized by the major drug-metabolizing CYP isoform in human liver, CYP3A4. Ketoconazole caused marked inhibition of cyclosporine hydroxylase, with an IC50 of 0.24 μM; itraconazole was 10 times less potent, and the fluconazole IC50 was greater than 100 μM (2). In the present study the IC50 of itraconazole was similar; the inhibitory potency of R126638 was much lower. CYP3A4 also contributes to testosterone 6β hydroxylation. Again, R126638 showed a lower interaction potential with testosterone 6β-hydroxylase than itraconazole. Although the level of inhibition of these CYP3A4-catalyzed reactions by R126638 was much less than that by itraconazole, R126638 and itraconazole at similar concentrations inhibited the formation of the 1′-hydroxy and 4-hydroxy metabolites of midazolam, a well-known substrate of CYP3A4. The clinical relevance of these effects observed in vitro will be further studied.
Our results suggest that R126638 has promising properties and is deserving of further clinical investigations as a treatment for dermatophyte and yeast infections of the skin, since R126638 showed high degrees of efficacy in animal models and has a low affinity for most mammalian P450s studied.
REFERENCES
- 1.Adams, J. S., T. G. Beeker, T. Hongo, and T. L. Clemens. 1990. Constitutive expression of a vitamin D1-hydroxylase in a myelomonocytic cell line: a model for studying 1,25-dihydroxyvitamin D production in vitro. J. Bone Mineral. Res. 12:1265-1269. [DOI] [PubMed] [Google Scholar]
- 2.Back, D. J., and J. F. Tjia. 1991. Comparative effects of the antimycotic drugs ketoconazole, fluconazole, itraconazole and terbinafine on the metabolism of cyclosporin by human liver microsomes. Br. J. Clin. Pharmacol. 32:624-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bohets, H., K. Lavrijsen, J. Hendrickx, J. Van Houdt, V. Van Genechten, P. Verboven, W. Meuldermans, and J. Heykants. 2000. Identification of the cytochrome P450 enzymes involved in the metabolism of cisapride: in vitro studies of potential co-medication interactions. Br. J. Pharmacol. 129:1655-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouillon, R., W. H. Okamura, and A. W. Norman. 1995. Structure- function relationships in the vitamin D endocrine system. Endocrine Rev. 16:200-257. [DOI] [PubMed] [Google Scholar]
- 5.Daum, G., N. D. Lees, M. Bard, and R. Dickson. 1998. Biochemistry, cell biology and molecular biology of lipids of Saccharomyces cerevisiae. Yeast 14:1471-1510. [DOI] [PubMed] [Google Scholar]
- 6.Ghannoum, M. A., and D. M. Kuhn. 2002. Voriconazole—better changes for patients with invasive mycoses. Eur. J. Med. Res. 7:242-256. [PubMed] [Google Scholar]
- 7.Groll, A. H., H. Kolve, K. Ehlert, M. Paulussen, and J. Vormoor. 2004. Pharmacokinetic interaction between voriconazole and cyclosporin A following allogeneic bone marrow transplantation. J. Antimicrob. Chemother. 53:113-114. [DOI] [PubMed] [Google Scholar]
- 8.Hagenfeldt-Pernow, Y., Y. Ohyama, E. Sudjana-Sugiaman, K. Okuda, and I. Björkhem. 1994. Short-term starvation increases calcidiol-24-hydroxylase activity and mRNA level in rat kidney. Eur. J. Endocrinol. 130:608-611. [DOI] [PubMed] [Google Scholar]
- 9.Jones, G., D. Vriezen, D. Lohnes, V. Palda, and N. S. Edwards. 1987. Side-chain hydroxylation of vitamin D3 and its physiological implications. Steroids 49:29-53. [DOI] [PubMed] [Google Scholar]
- 10.Lamb, D. C., D. E. Kelly, B. C. Baldwin, and S. L. Kelly. 2000. Differential inhibition of human CYP3A4 and Candida albicans CYP51 with azole antifungal agents. Chem. Biol. Interact. 125:165-175. [DOI] [PubMed] [Google Scholar]
- 11.Lee, C. R., J. A. Goldstein, and J. A. Pieper. 2002. Cytochrome P450 2C9 polymorphism: a comprehensive review of the in vitro and human data. Pharmacogenetics 12:251-263. [DOI] [PubMed] [Google Scholar]
- 12.Marichal, P., J. Gorrens, L. Laurijssens, K. Vermuyten, C. Van Hove, L. Le Jeune, P. Verhasselt, D. Sanglard, M. Borgers, F. C. S. Ramaekers, F. Odds, and H. Vanden Bossche. 1999. Accumulation of 3-ketosteroids induced by itraconazole in azole-resistant clinical Candida albicans isolates. Antimicrob. Agents Chemother. 43:2663-2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller, G. L. 1959. Protein determination for large numbers of samples. Anal. Chem. 31:964-971. [Google Scholar]
- 14.Odds, F. C., J. Ausma, F. Van Gerven, L. Meerpoel, J. Heeres, H. Vanden Bossche, and M. Borgers. 2004. In vitro and in vivo activities of the novel azole antifungal agent R126638. Antimicrob. Agents Chemother. 48:388-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patki, K. C., L. L. von Moltke, and D. J. Greenblat. 2003. In vitro metabolism of midazolam, triazolam, nifedipine, and testosterone by human liver microsomes and recombinant cytochromes P450: role of CYP3A4 and CYP3A5. Drug Metab. Dispos. 31:938-944. [DOI] [PubMed] [Google Scholar]
- 16.Sanglard, D., and J. Bille. 2002. Current understanding of the modes of action of and resistance mechanisms to conventional and emerging antifungal agents for treatment of Candida infections, p. 349-383. In R. A. Calderone (ed.), Candida and candidiasis. ASM Press, Washington, D.C.
- 17.Vanden Bossche, H. 1987. Itraconazole: a selective inhibitor of the cytochrome P450 dependent ergosterol biosynthesis, p. 207-221. In R. A. Fromtling (ed.), Recent trends in the discovery, development and evaluation of antifungal agents. J. R. Prous Science Publishers, S.A., Barcelona, Spain.
- 18.Vanden Bossche, H. 1992. Inhibitors of P450-dependend steroid biosynthesis: from research to medical treatment. J. Steroid Biochem. Mol. Biol. 43:1003-1021. [DOI] [PubMed] [Google Scholar]
- 19.Vanden Bossche, H., D. Bellens, W. Cools, J. Gorrens, P. Marichal, H. Verhoeven, G. Willemsens, R. De Coster, D. Beerens, C. Haelterman, M.-C. Coene, W. Lauwers, and L. Le Jeune. 1986. Cytochrome P450: target for itraconazole. Drug Dev. Res. 8:287-298. [Google Scholar]
- 20.Vanden Bossche, H., M. Engelen, and F. Rochette. 2003. Antifungal agents of use in animal health—chemical, biochemical and pharmacological aspects. J. Vet. Pharmacol. Ther. 26:5-29. [DOI] [PubMed] [Google Scholar]
- 21.Vanden Bossche, H., P. Marichal, J. Gorrens, D. Bellens, H. Verhoeven, M.-C. Coene, W. Lauwers, and P. A. J. Janssen. 1987. Interaction of azole derivatives with cytochrome P450 systems in yeast, fungi, plants and mammalian cells. Pestic. Sci. 21:289-306. [Google Scholar]
- 22.Vanden Bossche, H., P. Marichal, G. Willemsens, D. Bellens, J. Gorrens, I. Roels, M.-C. Coene, L. Le Jeune, and P. A. J. Janssen. 1990. Saperconazole: a selective inhibitor of the cytochrome P-450-dependent ergosterol synthesis in Candida albicans, Aspergillus fumigatus, and Trichophyton mentagrophytes. Mycoses 33:335-352. [DOI] [PubMed] [Google Scholar]
- 23.Vanden Bossche, H., P. Marichal, L. Le Jeune, M.-C. Coene, J. Gorrens, and W. Cools. 1993. Effects of itraconazole on cytochrome P-450-dependent sterol 14α-demethylation and reduction in Cryptococcus neoformans. Antimicrob. Agents Chemother. 37:2101-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanden Bossche, H., G. Willemsens, W. Cools, W. Lauwers, and L. Le Jeune. 1978. Biochemical effects of miconazole on fungi. II. Inhibition of ergosterol biosynthesis in Candida albicans. Chem. Biol. Interact. 21:59-78. [DOI] [PubMed] [Google Scholar]
- 25.Vanden Bossche, H., G. Willemsens, W. Cools, and D. Bellens. 1984. Effects of etomidate on steroid biosynthesis in subcellular fractions of bovine adrenals. Biochem. Pharmacol. 33:3861-3868. [DOI] [PubMed] [Google Scholar]
- 26.Vanden Bossche, H., G. Willemsens, I. Roels, D. Bellens, H. Moereels, M.-C. Coene, L. Le Jeune, W. Lauwers, and P. A. J. Janssen. 1990. R76713 and enantiomers: selective nonsteroidal inhibitors of the cytochrome P450-dependent oestrogen synthesis. Biochem. Pharmacol. 40:1707-1718. [DOI] [PubMed] [Google Scholar]
- 27.Vanden Bossche, H., G. Willemsens, H. Schreuders, M.-C. Coene, C. Van Hove, and W. Cools. 1993. Use of human skin models in the study of retinoic acid metabolism and lipid synthesis, effects of liarozole, p. 61-76. In V. Rogiers, W. Sonck, E. Shephard, and A. Vercruysse (ed.), Human cells in in vitro pharmaco-toxicology. VUBPRESS, Brussels, Belgium.
- 28.Van Wauwe, J. P., M.-C. Coene, J. Goossens, G. Van Nyen, W. Cools, and P. A. J. Janssen. 1988. Ketoconazole inhibits the in vitro and in vivo metabolism of all-trans-retinoic acid. J. Pharmacol. Exp. Ther. 245:718-722. [PubMed] [Google Scholar]
- 29.Venkatakrishnan, K., L. L. von Moltke, and D. J. Greenblatt. 2000. Effects of antifungal agents on oxidative drug metabolism: clinical relevance. Clin. Pharmacokinet. 38:111-180. [DOI] [PubMed] [Google Scholar]
- 30.Vieth, R., and D. Fraser. 1979. Kinetic behaviour of 25-hydroxyvitamin D-1-hydroxylase and -24-hydroxylase in rat kidney mitochondria. J. Biol. Chem. 254:12455-12460. [PubMed] [Google Scholar]