Abstract
Background and Objectives:
Pneumoperitoneum during laparoscopy can produce changes in intraocular pressure (IOP) that may be influenced by several factors. In this study, we investigated changes in IOP during laparoscopy with different positioning.
Methods:
We recruited adult patients without eye disease scheduled to undergo laparoscopic operation requiring a reverse Trendelenburg tilt (rTr; group A; n = 20) or Trendelenburg tilt (Tr; Group B; n = 20). IOP was measured at 7 time points (T1–T7). All procedures were performed with standardized anaesthetic protocol. Mean arterial pressure (MAP), heart rate (HR), peak and plateau airway pressure, and end-tidal carbon dioxide (ETCO2) measurements were taken at each time point.
Results:
Both groups were similar in age, sex, mean body mass index (BMI), duration of surgery, and preoperative IOP. A decrease in IOP was observed in both groups after induction of anaesthesia (T2), whereas induction of pneumoperitoneum produced a mild increase in IOP (T3) in both groups. The Trendelenburg tilt produced IOP elevations in 80% of patients compared to 45% after the reverse Trendelenburg tilt (P = .012). A significant IOP increase of 5 mm Hg or more was recorded in 3 (15%) patients in the Trendelenburg tilt group and in none in the reverse Trendelenburg group. At T7, IOP had returned to preoperative levels in all but 3 (15%) in the Trendelenburg and 1 (5%) in the reverse Trendelenburg group. Reversible changes were observed in the MAP, HR, ETCO2, and airway pressures in both groups.
Conclusions:
IOP changes induced by laparoscopy are realigned after evacuation of pneumoperitoneum. A Trendelenburg tilt however produced significant changes that may require careful patient monitoring during laparoscopic procedures.
Keywords: Intraocular pressure, laparoscopy, position
INTRODUCTION
Visual impairment after nonophthalmic surgery is uncommon, and complete visual loss is rare. Studies from the United States have reported various incidences with different surgical procedures, with cardiac surgery (8.64/10,000) and spinal fusion (3.09/10,000) accounting for the commonest incidences, whereas appendectomy had a rate of postoperative visual loss (POVL) of only 0.12/10,000.1 Visual loss after surgery has been attributed to ischemic optic neuropathy (ION), cortical blindness (CB), or retinal vascular occlusion (RVO).2–4 Although the immediate cause of these remain unclear, reduction in ocular perfusion is central to all of the pathways.5,6 Ocular perfusion pressure, which is determined by the difference between main arterial pressure (MAP) and intraocular pressure (IOP) is known to be altered in surgical procedures where significant IOP fluctuations may occur.3,7
Laparoscopy and other forms of minimally invasive surgeries are increasingly popular in surgical practice. With many proven advantages over conventional open surgeries, laparoscopy is now increasingly used for most general abdominal and gynecological procedures.8–11 The pneumoperitoneum induced and maintained during laparoscopy, however, has been shown to produce significant increases in IOP that may potentially alter ocular perfusion, raising concerns about possibilities of POVL after laparoscopic surgery.12–14 There is an increasing number of reports of significant ocular complications and POVL after laparoscopic surgery.15–18 With this in mind, in many studies, researchers have investigated the relationship between IOP changes during laparoscopy and different anaesthetic agents employed, whereas few have investigated the role of steep Trendelenburg position on IOP changes.19–22 The changes in IOP with normal Trendelenburg and reverse Trendelenburg positions have not been fully investigated. We sought therefore to evaluate the changes in IOP with such changes in patient position during laparoscopic surgeries.
The severity of optic nerve damage from elevated IOP has been shown to be influenced by several factors, such as ethnicity, genetic susceptibility, age, sex, and other factors. To the best of our knowledge, IOP variations during laparoscopic surgical procedures with pneumoperitoneum have not been investigated in an indigenous African population. This study was therefore set to investigate IOP changes among indigenous African patients undergoing elective laparoscopic surgical procedures.
METHOD
We included adult patients aged 30–65 years of American Society of Anesthesiologists (ASA) physical status class 1 or 2 who were undergoing laparoscopic operation for upper (group A) or lower abdominal/pelvic (group B) conditions. The study excluded those with preoperative IOP more than 20 mm Hg, a known diagnosis of glaucoma, acute or chronic eye infection, and body mass index (BMI) greater that 35 kg/m2.
Each patient had preoperative evaluations during which we documented age, sex, BMI, and presence of comorbidities. Eye examinations and measurements were performed by experienced consultant ophthalmologists (OHO and OOA) to select those meeting the inclusion criteria. Baseline IOP in both eyes were recorded (T0) with a Perkin's Applanation Tonometer Mk2 (Haag Streit UK Ltd., Harlow, UK) after the instillation of topical Tetracain (Alcon, Fort Worth, Texas, USA) and preservative-free Minims fluorescein dye (iNova Pharmaceuticals, Chatsworth, NSW, Australia). All surgical procedures were performed under general anaesthesia with cuffed endotracheal intubation. Premedication was performed with atropine 0.6–1.0 m, and all patients had preoperative cefuroxime 1.5 g. Intravenous (IV) fentanyl 1–2 μg/kg, and propofol 1–2 mg/kg were used for induction of anaesthesia. IV suxamethonium 1–1.5 mg/kg was used, and endotracheal intubation secured. Anaesthesia was maintained with isoflurane 1%–2% in oxygen. Muscle relaxation was achieved with IV pancuronium 0.1 mg/kg stat, with top up as appropriate, with IV fentanyl for intraoperative analgesia. During surgery, we used a GE Dash 4000 Multiparameter monitor (GE Healthcare, Milwaukee, Wisconsin, USA) for monitoring of SaO2, end-tidal carbon dioxide (ETCO2), noninvasive blood pressure (NIBP), and electrocardiogram (ECG). At the completion of surgery, reversal was achieved with atropine 1.2 mg and neostigmine 2.5 mg. All patients received postoperative analgesia as appropriate.
During surgery, IOP, MAP, and (HR) were measured at the following time points: T1: before the induction of anaesthesia; T2: 5 min after the induction of anaesthesia, before pneumoperitoneum, in a supine, horizontal position, mechanically ventilated; T3: 5 min after the pneumoperitoneum was established; T4: pneumoperitoneum established, 5 min after a 15°–20° reverse Trendelenburg tilt (group A) or 15°–20° Trendelenburg tilt (group B); T5: pneumoperitoneum established, 5 min after return to the horizontal position; T6: 5 min after the pneumoperitoneum was evacuated; and T7: in the recovery room, half-seated, 20 min after tracheal extubation.
The peak and plateau airway pressures as well as the ETCO2 levels were recorded at times T2 through T6.
All laparoscopic procedures were performed by a single surgeon (AOA) adopting standard techniques. All patients were followed up in the outpatient clinic after discharge. Those with significantly elevated IOP during surgery were serially reviewed in the ophthalmology unit. The study protocol was approved by the Ethics and Research Committee of the hospital. Informed consent was obtained from all patients before they were recruited into the study.
Data generated were entered into a personal computer by using Stata, Version 13 (StataCorp., College Station, Texas, USA). Descriptive statistics were performed with frequencies, percentages, and frequency tables for categorical variables. The mean IOP changes in patients undergoing laparoscopy with different intraoperative position changes were calculated. Student's t test was performed at bivariate level to examine the mean difference of age, BMI, and bilateral preoperative IOP within the groups. For the categorical background variables (gender, duration of surgery), a χ2 test was performed to examine the associations.
RESULTS
Forty patients in 2 groups of 20 each were included in the study. Twenty had laparoscopic procedures requiring a reverse Trendelenburg tilt (group A) whereas 20 others had laparoscopic procedures requiring a Trendelenburg tilt (group B). Most patients (4 of every 5) were women. The age range was 19–69 years with a mean of 40.43 ± 12.93 years. Twenty-eight (70%) were ASA 1 and 12 (30%) were ASA 2. Mean BMI was 27.06 ± 5.74 kg/m2 (range, 18–35). Mean baseline IOPs of patients in both groups were 13.08 ± 3.17 in the right eye (RE) and 13.50 ± 2.94 in the left eye (LE). Mean interpalpebral fissures were 9.18 ± 2.873 cm RE and 9.19 ± 2.926 cm LE. There were no statistically significant differences between the 2 groups in age, sex, mean BMI, duration of surgery, and preoperative IOP (T0), as shown in Table 1.
Table 1.
Baseline Characteristics of Patients
| Group A (rTr) (n = 20) | Group B (Tr) (n = 20) | P | |
|---|---|---|---|
| Mean age (y) | 36.60 | 34.25 | 0.0604 |
| Gender | |||
| Male | 3 | 5 | |
| Female | 17 | 15 | 0.695 |
| Duration of surgery (min) | |||
| <60 | 8 | 7 | |
| 60–90 | 10 | 13 | 0.293 |
| >90 | 2 | 0 | |
| Mean preoperative IOP | |||
| RE | 13.70 | 12.45 | 0.217 |
| LE | 13.85 | 13.15 | 0.457 |
| Mean BMI (kg/m2) | 27.61 | 26.38 | 0.6629 |
Tr: Trendelenburg; rTr: reverse Trendelenburg.
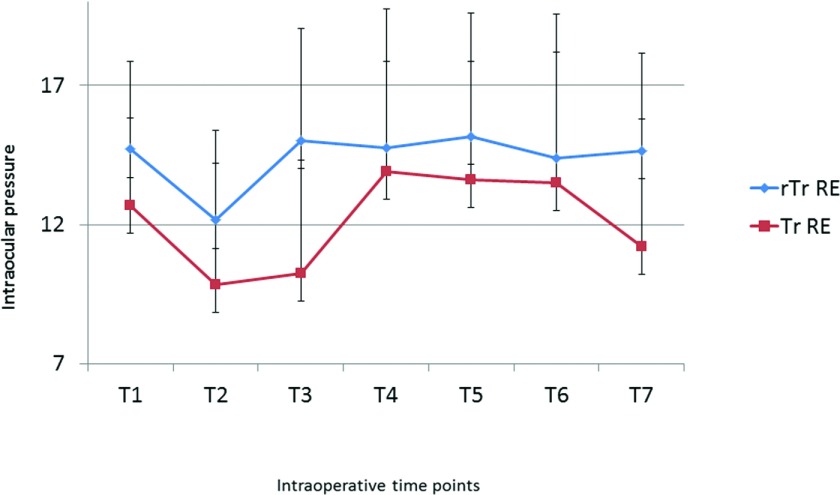
From baseline (T1), a decrease in IOP was observed in all patients in both groups after induction of anaesthesia (T2). The decrease ranged from 1 to 6 mm Hg (mean, 2.55) RE in the reverse Trendelenburg and 1–8 mm Hg (mean, 2.85) RE in the Trendelenburg group. On the contrary, pneumoperitoneum induction produced a mild increase in IOP (T3), with a mean difference of +2.85 mm Hg RE in the reverse Trendelenburg and +0.40 mm Hg in the Trendelenburg group (Figure 1, Table 2).
Figure 1.
Mean IOP changes during laparoscopic surgery. Tr: Trendelenburg; rTr: reverse Trendelenburg; RE: right eye.
Table 2.
Mean IOP Changes With Trendelenburg Positions
| T0 | T1 | T2 | T3 | T4 | T5 | T6 | T7 | |
|---|---|---|---|---|---|---|---|---|
| Trendelenburg | ||||||||
| RE | 12.45 | 12.70 | 9.85 | 10.25 | 13.90 | 13.60 | 13.50 | 11.20 |
| (SEM) | 0.731 | 0.704 | 0.723 | 0.906 | 1.121 | 0.996 | 1.151 | 0.783 |
| LE | 13.15 | 12.95 | 9.40 | 9.35 | 12.90 | 12.40 | 11.80 | 11.35 |
| (SEM) | .658 | .675 | .838 | .871 | 1.015 | .919 | 0.939 | 0.880 |
| Reverse Trendelenburg | ||||||||
| RE | 13.70 | 14.70 | 12.15 | 15.00 | 14.75 | 15.15 | 14.40 | 14.65 |
| (SEM) | 0.677 | 0.696 | 0.971 | 0.912 | 0.888 | 0.949 | 1.047 | 1.029 |
| LE | 13.85 | 16.30 | 12.75 | 14.55 | 13.50 | 13.95 | 13.65 | 14.05 |
| (SEM) | .642 | 0.653 | 1.066 | 0.884 | 0.686 | 0.796 | 0.847 | 0.803 |
Data are mean IOP with SEM. RE, right eye; LE, left eye.
When patients were tilted into the different positions, 36 (90%) had changes in IOP. Of these, 25 (62.5%) had IOP elevations, with 16 of 20 (80%) having elevations after Trendelenburg tilt and 9 of 20 (45%) having elevations in the reverse Trendelenburg group. On the contrary, only 2 of 20 (10%) had a reduction in IOP after the Trendelenburg tilt, whereas 9 of 20 (45%) recorded IOP reduction after the reverse Trendelenburg tilt. The difference was statistically significant (P = .012). A significant IOP increase of 5 mm Hg or more was recorded in 3 (15%) patients undergoing the Trendelenburg tilt and in none of the patients with the reverse Trendelenburg tilt. The difference was not statistically significant (P = .556).
After evacuation of pneumoperitoneum and extubation, IOP returned to preoperative levels in most patients in both groups. At time T7, only 4 (10%) of the patients had a significant increase (≥5 mm Hg)above the T1 level, including 3 (15%) patients in the Trendelenburg and 1 (5%) in the reverse Trendelenburg group. None of these required treatment on follow-up with resolution of the increase in all instances.
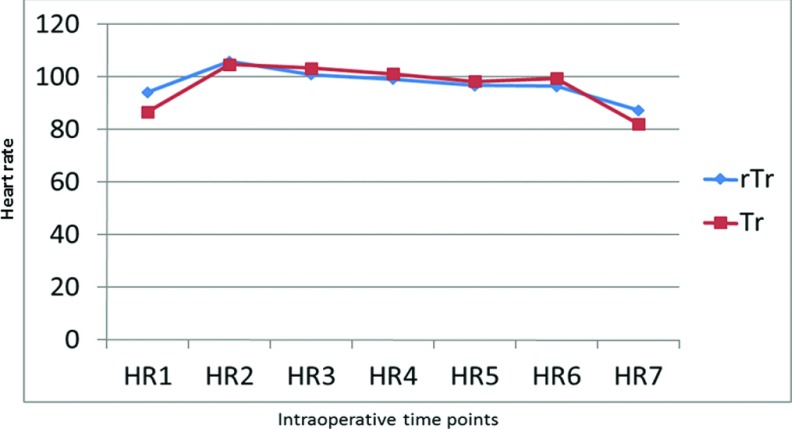
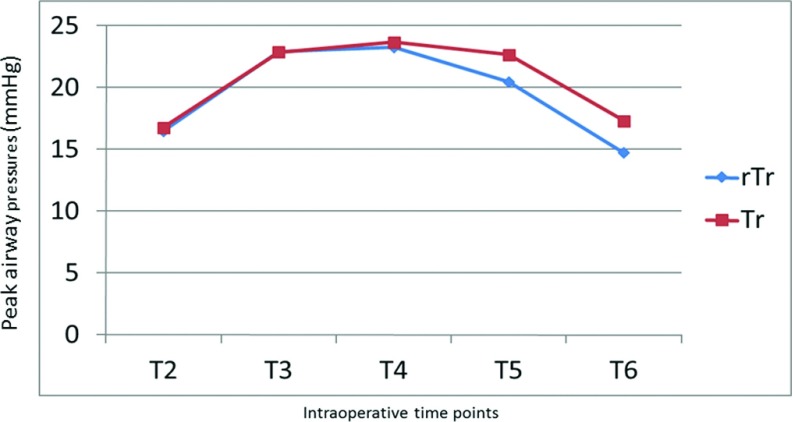
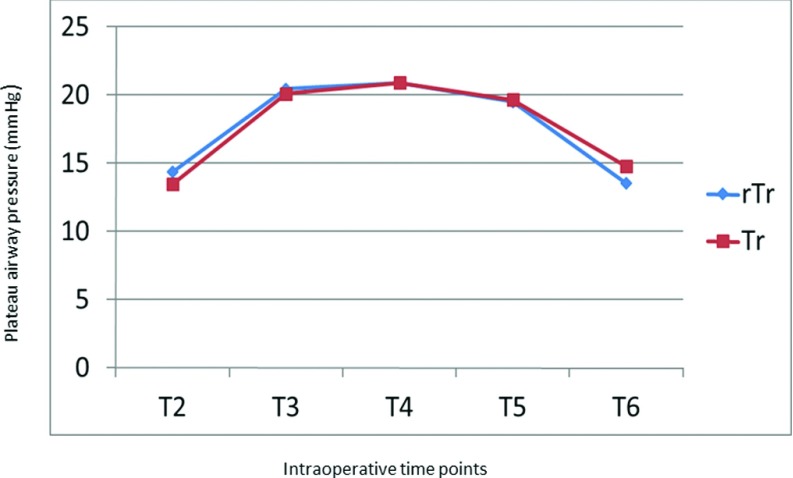
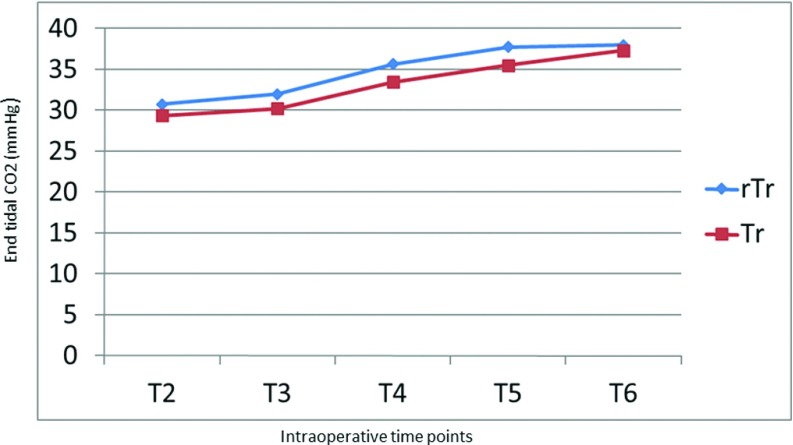
In all patients in both groups, MAP dropped by 14–22 mm Hg after induction of anaesthesia (T2) but rose by 5–10 mm Hg after abdominal insufflation to 14 mm Hg for pneumoperitoneum in both groups (T3). These values stabilized, however, and MAP reverted to normal levels after evacuation of pneumoperitoneum and patient extubation (Figure 2). A slight increase in HR accompanied induction of anaesthesia in all patients in both groups. HR, however, returned to preoperative values in all patients at time T7 (Figure 3). Both the peak and plateau airway pressures increased by a mean of 5 mm Hg after abdominal insufflation, but returned to a normal level after desufflation at time T6 (Figures 4 and 5). ETCO2 rose from the time of insufflation throughout the procedure until the time of abdominal desufflation. The values, however, remained within normal limits in all patients in both groups (Figure 6).
Figure 2.
MAP changes during laparoscopic surgery. Tr: Trendelenburg; rTr: reverse Trendelenburg.
Figure 3.
Changes in HR of patients during laparoscopic surgery. Tr: Trendelenburg; rTr: reverse Trendelenburg.
Figure 4.
Changes in peak airway pressure during laparoscopic surgery. Tr: Trendelenburg; rTr: reverse Trendelenburg.
Figure 5.
Changes in plateau airway pressure during laparoscopic surgery. Tr: Trendelenburg; rTr: reverse Trendelenburg.
Figure 6.
ETCO2 measurements during laparoscopic surgery. Tr: Trendelenburg; rTr: reverse Trendelenburg.
DISCUSSION
The potential of perioperative visual loss during laparoscopy and possible factors influencing it remain unclear. The present study was undertaken to evaluate changes in IOP that might be related to patient positioning during laparoscopic surgery. We recruited a similar number of patients (n = 20) in each of the 2 groups studied. Both groups had similar preoperative characteristics with no statistically significant difference in their preoperative IOP, age, sex, and mean BMI, which are some of the main factors influencing intraoperative changes in IOP. We equally standardized the anaesthetic processes, and all operations were performed by a single surgeon to eliminate most of the other factors that may influence the findings of this study.
We observed a significant decrease in IOP after establishment of anesthesia. This decrease was inversely related to the HR but directly related to the MAP of patients in both groups. Similar decreases unrelated to changes in HR and or MAP has been reported in other studies.20,23 This decrease was found to occur irrespective of the combination of anesthetic agents employed, but induction with propofol, which we used in our study, has been reported to produce the greatest decrease in IOP.20 Similarly, we observed elevation in IOP after abdominal insufflation with CO2 to create pneumoperitoneum in most patients in both groups. This finding is similar to those in other reports.12,13 The increase in IOP was directly related to MAP in our patients. The etiology of perioperative visual loss is thought to be directly influenced by alterations in ocular perfusion pressure from changes in MAP and to be indirectly related to IOP elevation.
The main finding of this study was that IOP increased with induction of pneumoperitoneum, but there was a trend toward a greater increase in IOP with standard Trendelenburg tilt compared to a reverse Trendelenburg tilt, which was associated with a slight reduction in IOP in several patients. These changes, however, were reversed in patients when intra-abdominal pressure was not more than 14 mm Hg and operative time was not beyond 90 minutes. Only 4 (10%) of patients in the study showed an elevation in IOP beyond the usual diurnal variation of 5 mm Hg, and none of the pressures reached glaucomatous range on follow-up. These findings suggests that perhaps a steep Trendelenburg positioning, as well as prolonged duration of surgery, are more important factors in attaining more dangerous elevations of IOP in patients undergoing laparoscopy than the standard pneumoperitoneum induction for routine cases. Many of the studies highlighting POVL and markedly elevated IOP changes (reaching glaucomatous range) after laparoscopic surgery had been performed with steep Trendelenburg position or in prolonged procedures.12–14,19,21,22,24,25 Mondzelewski et al22 observed a significantly elevated IOP in patients undergoing robot-assisted laparoscopic procedures in steep Trendelenburg position compared with IOP in those undergoing laparoscopy or open procedures in a horizontal position, further highlighting the role of positioning over and above that of pneumoperitoneum in significant IOP elevations. A recent study performed among patients undergoing laparoscopic colorectal surgery showed greater IOP elevation in both eyes of patients in Trendelenburg position during the surgical procedure, but found no substantial difference between the Trendelenburg and non-Trendelenburg group when IOP was measured 48 hours after surgery.14 The surgeons also did not use the steep positioning, suggesting that, as we found in the current study, the changes are reversed after pneumoperitoneum is evacuated if a steep positioning has not been used for a prolonged surgery. A larger population study to evaluate the long-term effects of IOP changes after such procedures would be useful for this purpose. Several groups are currently investigating the role of topical and or systemic agents in the prevention and management of significant intraoperative IOP elevations, with promising results, and some of these may become clinically relevant in the near future.25,26 It is pertinent, however, to adopt preventive measures, such as strict hemodynamic control, avoidance of prolonged steep Trendelenburg positioning, and reduction in total operative time, to prevent or reduce the chance of POVL.
In conclusion, this study has shown that IOP declines with induction of anesthesia, increases with induction of pneumoperitoneum and may rise further with placement in Trendelenburg positions during routine laparoscopic procedures. We found realignment of the IOP after evacuation of pneumoperitoneum with no long-term changes in IOP or permanent ocular damage. Further studies are desirable to determine the relationship between steep Trendelenburg positioning during prolonged procedures and prolonged IOP changes and to understand the pathogenesis of ocular damage after laparoscopy.
Contributor Information
Adewale O. Adisa, Departments of Surgery.
Oluwatoyin H. Onakpoya, Ophthalmology.
Anthony T. Adenekan, Anaesthesia and Intensive Care, Obafemi Awolowo University and Obafemi Awolowo University Teaching Hospitals Complex, Ile-Ife, Nigeria..
Oluwaseun. O. Awe, Ophthalmology.
References:
- 1. Shen Y, Drum M, Roth S. The prevalence of perioperative visual loss in the United States: a 10-year study from 1996 to 2005 of spinal, orthopedic, cardiac, and general surgery. Anesth Analg. 2009;109:1534–1545. [DOI] [PubMed] [Google Scholar]
- 2. Rubin DS, Parakati I, Lee LA, Moss HE, Joslin CE, Roth S. Perioperative visual loss in spine fusion surgery: ischemic optic neuropathy in the United States from 1998 to 2012 in the Nationwide Inpatient Sample. Anesthesiology. 2016;125:457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pinkney TD, King AJ, Walter C, Wilson TR, Maxwell-Armstrong C, Acheson AG. Raised intraocular pressure (IOP) and perioperative visual loss in laparoscopic colorectal surgery: a catastrophe waiting to happen? A systematic review of evidence from other surgical specialities. Tech Coloproctol. 2012;16:331–333. [DOI] [PubMed] [Google Scholar]
- 4. Lee LA, Roth S, Todd MM, et al. Risk factors associated with ischemic optic neuropathy after spinal fusion surgery. Anesthesiology. 2012;116:15–24. [DOI] [PubMed] [Google Scholar]
- 5. Ece I, Vatansev C, Kucukkartallar T, Tekin A, Kartal A, Okka M. The increase of intra-abdominal pressure can affect intraocular pressure. Biomed Res Int. 2015;2015:986895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Van Keer K, Breda JB, Pinto LA, Stalmans I, Vandewalle E. Estimating mean ocular perfusion pressure using mean arterial pressure and intraocular pressure. Invest Ophthalmol Vis Sci. 2016;57:2260. [DOI] [PubMed] [Google Scholar]
- 7. Kiel JW. Local determinants. In: Schmetterer L, Kiel J, eds. Ocular Blood Flow. Heidelberg: Springer-Verlag; 2012;211–241. [Google Scholar]
- 8. Keller DS, Champagne BJ, Reynolds HL, Stein SL, Delaney CP. Cost-effectiveness of laparoscopy in rectal cancer. Dis Colon Rectum. 2014;57:564–569. [DOI] [PubMed] [Google Scholar]
- 9. Fuks D, Cauchy F, Ftériche S, et al. Laparoscopy decreases pulmonary complications in patients undergoing major liver resection: a propensity score analysis. Ann Surg. 2016;263:353–361. [DOI] [PubMed] [Google Scholar]
- 10. Adisa AO, Lawal OO, Adesunkanmi ARK, Adejuyigbe O. Impact of introduction of laparoscopic surgery on management of unresolved intra-abdominal malignancies in a West African hospital. World J Surg. 2015;38:2519–2524. [DOI] [PubMed] [Google Scholar]
- 11. Burdall OC, Boddy AP, Fullick J, et al. A comparative study of survival after minimally invasive and open oesophagectomy. Surg Endosc. 2015;29:431–437. [DOI] [PubMed] [Google Scholar]
- 12. Lentschener C, Leveque JP, Mazoit JX, Benhamou D. The effect of pneumoperitoneum on intraocular pressure in rabbits with alpha-chymotrypsin-induced glaucoma. Anesth Analg. 1998;86:1283–1288. [DOI] [PubMed] [Google Scholar]
- 13. Lentschener C, Fredi-Reygrobellet D, Bouaziz H, Mazoit JX, Niessen F, Benhamou D. Effect of CO2 pneumoperitoneum on early cellular markers of retinal ischemia in rabbits with α-chymotrypsin-induced glaucoma. Surg Endosc. 2000;14:1057–1061. [DOI] [PubMed] [Google Scholar]
- 14. Grosso A, Scozzari G, Bert F, Mabilia MA, Siliquini R, Morino M. Intraocular pressure variation during colorectal laparoscopic surgery: standard pneumoperitoneum leads to reversible elevation in intraocular pressure. Surg Endosc. 2013;27:3370–3376. [DOI] [PubMed] [Google Scholar]
- 15. Koning JL, Nicolay LI, Jellison F, Heldt JP, Dunbar JA, Baldwin DD. Ocular complications after open and hand-assisted laparoscopic donor nephrectomy. Urology. 2011;77:92–96. [DOI] [PubMed] [Google Scholar]
- 16. Mizrahi H, Hugkulstone CE, Vyakarnam P, Parker MC. Bilateral ischaemic optic neuropathy following laparoscopic proctocolectomy: a case report. Ann R Coll Surg Engl. 2011;93:E53–E54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Molloy BL. Implications for postoperative visual loss: steep Trendelenburg position and effects on intraocular pressure. AANA J. 2011;79:115–121. [PubMed] [Google Scholar]
- 18. Nuzzi R, Tridico F. Ocular complications in laparoscopic surgery: review of existing literature and possible treatment and prevention strategies. Semin Ophthalmol. 2016;31:584–592. [DOI] [PubMed] [Google Scholar]
- 19. Yoo YC, Shin S, Choi EK, Kim CY, Choi YD, Bai SJ. Increase in intraocular pressure is less with propofol than with sevoflurane during laparoscopic surgery in the steep Trendelenburg position. Can J Anesthesiol. 2014;61:322–329. [DOI] [PubMed] [Google Scholar]
- 20. Agrawal M, Dureja V, Verma AP, Kang LS. A comparative study of four combinations of anesthetic drugs for assessing the intraocular pressure changes during gynaecological laparoscopic procedures. Anesth Essays Res. 2013;7:319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Raz O, Boesel TW, Arianayagam M, Lau H, Vass J, Huynh CCl. The effect of the modified Z Trendelenburg position on intraocular pressure during robotic assisted laparoscopic radical prostatectomy: a randomized, controlled study. J Urol. 2015;193:1213–1219. [DOI] [PubMed] [Google Scholar]
- 22. Mondzelewski TJ, Schmitz JW, Christman MS, et al. Intraocular pressure during robotic-assisted laparoscopic procedures utilizing steep Trendelenburg positioning. J Glaucoma. 2015;24:399–404. [DOI] [PubMed] [Google Scholar]
- 23. Awad H, Santilli S, Ohr M, et al. The effects of steep Trendelenburg positioning on intraocular pressure during robotic radical prostatectomy. Anesth Analg. 2009;109:473–478. [DOI] [PubMed] [Google Scholar]
- 24. Mowafi HA, Al-Ghamdi A, Rushood A. Intraocular pressure changes during laparoscopy in patients anesthetized with propofol total intravenous anesthesia versus isoflurane inhaled anesthesia. Anesth Anal. 2003;97:471–474. [DOI] [PubMed] [Google Scholar]
- 25. Joo J, Koh H, Lee K, Lee J. Effects of systemic administration of dexmedetomidine on intraocular pressure and ocular perfusion pressure during laparoscopic surgery in a steep Trendelenburg position: prospective, randomized, double-blinded study. J Korean Med Sci. 2016;31:989–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Molloy B, Cong X. Perioperative dorzolamide-timolol intervention for rising intraocular pressure during steep Trendelenburg positioned surgery. AANA J. 2014;82:203–211. [PubMed] [Google Scholar]