Abstract
Background
This study investigated the molecular mechanism of the effect of CD44 on the recurrence of EGC after ESD, including the potential regulator and signaling pathways of CD44.
Material/Methods
We searched the miRNA online database (www.mirdb.org) with the “seed sequence” located within the 3′-UTR of the target gene, and performed luciferase assay to test the miRNA/mRNA relationship. We also determined the expression of CD44 in the EGC and control samples. In addition, statistical analysis was used to explore the role of miR-328 as a biomarker to predict the recurrence after ECD.
Results
We validated CD44 to be the direct gene via luciferase reporter assay system. We also established the negative regulatory relationship between miR-328 and CD44 via studying the relative luciferase activity at different concentrations of miR-328 mimics. We also conducted real-time PCR and Western blot analysis to study the mRNA and protein expression level of CD44 among different groups (recurrence-positive and recurrence-negative) or cells treated with different concentrations of miR-328 mimics/inhibitors, indicating the negative regulatory relationship between miR-328 and CD44. We also investigated the relative viability of EGC cells when transfected with miR-328 mimics (50 nM and 100 nM) and miR-328 inhibitors (100 nM) to validate miR-328 to be negatively interfering with the viability of EGC cells. miR-328 was also recognized as a potential biomarker to predict recurrence after ESD in EGC patients via analysis of the recurrence-free rate among different groups of EGC patients.
Conclusions
The expression level of miR-328 can function as a predictive biomarker of recurrence after ECD in patients with EGC via targeting CD44.
MeSH Keywords: Antigens, CD44; MicroRNAs; Recurrence; Sphincterotomy, Endoscopic
Background
Gastric cancer is the second most common malignancy in the world, and early gastric cancer (EGC) has a better prognosis when treated properly [1]. Particularly, endoscopic treatment has been widely used to treat patients who have EGC, which improved prognosis of the patients to some extent. Consequently, numerous patients with EGC successfully avoided laparotomy and had a better quality of life.
Endoscopic submucosal dissection (ESD), which makes en bloc resection easier, has been regarded as a valuable procedure for treatment of EGC [2,3]. ESD has been accepted as standard treatment for EGC in some countries [4], and the procedure is gaining popularity on a global scale [5–8]. The frequency of residual lesions and recurrence rate of neoplasm after ESD have been decreased significantly when compared with that of conventional endoscopic mucosal resection (EMR) [2,3,8–10]; but the residual lesions and recurrence of neoplasm after non-curative resection by ESD are observed in a few cases [11–13].
CD44, an important adhesion molecule for the extracellular matrix, is involved in numerous physiological processes such as metastasis and invasion of tumor cells [14]. It has been confirmed that CD44 works as a cell surface marker and is related to cancer stem-like cells in a variety of solid malignancies [15,16]. Recently, it has been reported that xCT, a glutamate-cystine transporter, interacts with CD44 and is stabilized by CD44, leading to increased expression levels of glutathione (GSH) in cells, and a variant of CD44 presents improved ability to inhibit ROS production, leading to subsequent metastasis, recurrence, and therapeutic resistance of tumors [17–19]. MicroRNAs (MiRNAs) is a class of endogenous small noncoding RNAs that can bind to the 3′ untranslated region (3′UTR) of target mRNA sequences to modulate target gene expression at the posttranscriptional level [20]. Aberrant expressions of miRNAs are associated with development and origin of tumors, and miRNAs may function as either tumor suppressors or activators [21–23]. In the last few years, more and more studies have focused on the role of miRNAs as therapeutic, prognostic, diagnostic, or response-predictive biomarkers in malignancies [24,25].
It has been previously reported that a variant of CD44 was associated with the risk of recurrence after ESD [26], and CD44 has been shown to be a target gene of miR-328 in normal gastric mucosa [27] as well as cancer cells of other type [28]. This study focused on recurrence of EGC after treatment by ESD. These patients were randomly subclassified into 2 groups in accordance with their molecular findings after initial dissection, and the expression levels of CD44 and miR-328 were examined in those patients.
Material and Methods
Patients and ESD procedure
A total 230 EGC patients were recruited at the Affiliated Hospital of Qingdao University, Department of Gastroenterology, from September 2013 to February 2015. All of them were diagnosed with EGC, which was defined as malignancy in which invasion was restricted to the submucosal layer, irrespective of the absence or presence of lymph node metastasis. The expression of miR-328 was determined and those with miR-328 expression higher than the median were defined as “high miR-328 expression (N=127)” and the rest was defined as “low miR-328 expression (N=103)”. The patients were recruited when hospitalized in the department and the information was collected by interview or from the record. Written informed consent was obtained from each participant. The Institutional Review Board of Qingdao University approved the study.
Cell culture and treatment
The MKN28 cell line, purchased from ATCC (Manassas, VA), was kept in medium of RPMI 1640 with 10% fetal bovine serum in an environment with 5% CO2 at 37°C.
Quantitative real-time reverse transcription polymerase chain reaction A mirVana microRNA isolation kit purchased from Ambion (Austin, TX) was used to isolate total RNA in accordance with the instructions of the manufacturer. Using a NanoDrop ND-1000 spectrophotometer purchased from NanoDrop Technologies, all RNA samples were measured for absorbance ratio at 260 nm/280 nm in order to evaluate the concentration and purity (DE, USA). qRT-PCR was used to examine the expression levels of CD44 and miR-328. RNU6B was used to normalize the expression level of MiR-328. β-actin was used to normalize the expression level of CD44. A LightCycler 480 System II (Roche Diagnostics) was used to perform all the qRT-PCRs. The 2−ΔΔCt method was used to measure relative amounts. All qRT-PCRs were carried out in triplicate.
Transfection of miRNA
The mimic or inhibitory of miR-328 purchased from Applied Biosystems (Foster City, CA) was used to transfect the cells with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) in accordance with the protocol of manufacturer (Foster City, CA). A negative control purchased from Applied Biosystems (Foster City, CA) was used to verify the specificity of the transfection.
Luciferase assay
3′UTR of CD44 was amplified and inserted into the restriction sites of a pmirGLO (Promega). Site-directed mutagenesis primers were used to introduce vectors that contained mutant sequences of human CD44 3′-UTR targeted by miR-328. The wild-type or mutant and miR-328 or negative controls were used to transfect the MKN28 cells in 96-wells plates. The Dual-Glo™ Luciferase Assay System (Promega) was used to perform reporter assays 48 h after transfection to determine the Renilla and firefly luciferase activities. We conducted all the transfection experiments in triplicate.
Western blot analysis
Phosphate-buffered saline was used to wash the cells harvested or the tissue samples, followed by lysing them in radioimmunoprecipitation buffer containing phosphatase/protease inhibitor cocktail purchased from Thermo Scientific (Tokyo, Japan). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed with each protein sample, then we transferred the protein to a nitrocellulose membrane and incubated them with anti-CD44 and anti-β-actin primary antibodies (SCBT, Santa Cruz, CA). After incubating with secondary antibodies (SCBT, Santa Cruz, CA), the Enhanced Chemiluminescence Detection System, purchased from GE Healthcare, was used to detect signals (Little Chalfont, UK).
In vitro cytotoxicity assay
MKN28 cells which were transfected with mimic or control miR-328 were maintained in 96-well microplates at a density of 2×103 cells per well, cultured overnight, and then exposed to H2O2 for 3 days. The experiment was carried out in triplicate. A Cell Counting Kit-8, purchased from Dojindo Laboratories, was used to determine the number of viable cells.
Statistical analysis
The Kaplan-Meier test, the log-rank test, and the Fisher’s protected least significant difference test were used to perform the statistical comparisons among the 2 independent groups. The t test (for 2 groups) or one-way ANOVA were used to determine the difference between the groups. P<0.05 was considered statistically significant. SPSS 15.0 software was used to perform all statistical analyses (IBM Inc., Chicago, IL).
Results
CD44 was virtual target of miR-328
CD44 has been previously reported to be a promoting factor of invasion and metastasis, the expression level of which could affect the recurrence of EGC after ESD. In this study, we aimed to investigate the molecular mechanism, including the potential regulator and signaling pathways of CD44, that might be functionally involved in the control of the recurrence of EGC after ESD. As shown in Figure 1, we identified miR-328 as a potential regulator of CD44 by searching the miRNA online database (www.mirdb.org) with the “seed sequence” located within the 3′-UTR of the target gene.
Figure 1.

CD44 was identified as the candidate target gene of miR-328 in gastric cancer cells with the ‘seed sequence’ in the 3′UTR of CD44.
To validate the regulatory relationship between miR-328 and CD44, we also conducted luciferase activity reporter assay in gastric cancer cells by co-transfecting the cells with wild-type CD44 3′UTR constructs, and different concentration of miR-328 mimics (25 nM, 50 nM, and 100 nM). To verify the binding site of miR-328 in the mRNA, we also set up another reporter system with the wild-type replaced by the mutant. As shown in Figure 2 (P<0.01), compared with the scramble control, the relative luciferase activity of cells transfected with wild-type CD44 3′UTR constructs clearly decreased as the concentration of miR-328 mimics increased, exhibiting a negative regulation in a stepwise manner. On the contrary, cells carrying mutant CD44 3′UTR constructs exhibited comparable relative luciferase activity index when compared with the scramble controls, indicating CD44 as the direct target gene of miR-328 with the binding site located at the segment that has been mutated.
Figure 2.

Luciferase activity reporter assay was conducted to verify CD44 as the direct target gene of miR-328, as well as to validate the regulatory relationship between miR-328 and CD44. Compared with the scramble control, the relative luciferase activity of cells transfected with wild-type CD44 3′UTR constructs clearly decreased as the concentration of miR-328 mimics increased, exhibiting negative regulation in a stepwise manner. On the contrary, cells carrying mutant CD44 3′UTR constructs exhibited comparable relative luciferase activity index when compared with the scramble controls, indicating CD44 as the direct target gene of miR-328 with the binding site located at the segment that has been mutated.
Expression level of miR-328 and CD44 varied in different groups
To confirm reduced expression of miR-328 in EGC with early recurrence after ESD, we evaluated the expression of miR-328 in 230 recurrence-positive (N=96) and recurrence-negative (N=134) frozen EGC tissues using quantitative reverse transcriptase PCR (qRT-PCR). As shown in Figure 3 (P<0.01), the expression level of miR-328 in recurrence-positive tissues decreased substantially compared with recurrence-negative tissues.
Figure 3.

The recurrence-positive group (N=96) showed evidently lower miR-328 expression level when compared with the recurrence-positive group (N=134).
We further conducted real-time PCR and Western blot analysis to study the mRNA and protein expression level of CD44 among different groups. As shown in Figure 4, both the mRNA and protein expression level of CD44 of the recurrence-positive group were evidently higher when compared with the recurrence-negative group, indicating the negative regulatory relationship between miR-328 and CD44.
Figure 4.
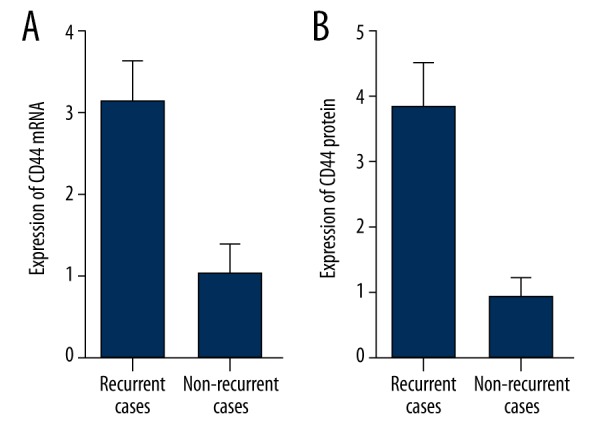
The mRNA (A) and protein (B) expression level of CD44 of the recurrence-positive group were evidently higher when compared with the recurrence-negative groups.
MiR-328 inhibits the expression of CD44
To further validate the hypothesis of the negative regulatory relationship between miR-328 and CD44, we investigated the mRNA/protein expression level of CD44 in GC cells treated with 50-nM miR-328 mimics, 100-nM miR-328 mimics, and 100-nM miR-328 inhibitors. As shown in Figure 5 (P<0.01), the CD44 protein (Figure 5A, 5C) and mRNA (Figure 5B, 5D) expression level of EGC cells treated with 50-nM miR-328 mimics were apparently lower than the scramble control, and those of the cells treated with 100-nM miR-328 mimics were even lower than the 50-nM treatment group, indicating a concentration-dependent effect of miR-328 on the expression of CD44. The miR-328 inhibitors treatment group showed evidently higher expression level of CD44 protein (upper panel) and mRNA (lower panel) when compared with the scramble controls and the miR-328 mimics treatment groups, validating the negative regulatory relationship between miR-328 and CD44.
Figure 5.

We investigated the mRNA/protein expression level of CD44 of EGC cells treated with 50-nM miR-328 mimics, 100-nM miR-328 mimics, and 100-nM miR-328 inhibitors to validate the negative regulatory relationship between miR-328 and CD44. The CD44 protein (A) and mRNA expression level (B) of EGC cells treated with 50-nM miR-328 mimics were apparently lower than the scramble control, while those of the sample group treated with 100-nM miR-328 mimics were even lower than the 50-nM treatment group. The miR-328 inhibitors treatment group showed evidently higher expression level of CD44 protein (C) and mRNA (D) when compared with the scramble controls and the miR-328 mimics treatment groups.
MiR-328 interfered with the viability of EGC cells
As shown in Figure 6, we also investigated the relative viability of GC cells when transfected with miR-328 mimics (50-nM and 100-nM) and miR-328 inhibitors (100-nM). Cells transfected with 100-nM miR-328 inhibitors showed evidently upregulated viability when compared with the scramble controls (P<0.01), while cells transfected with 50-nM/100-nM miR-328 mimics showed comparably lower viability, indicating miR-328 negatively interfered with the viability of EGC cells.
Figure 6.
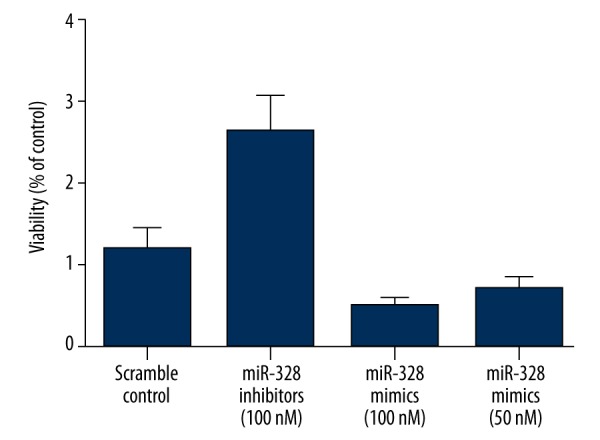
Cells transfected with 100-nM miR-328 inhibitors showed evidently upregulated viability when compared with the scramble controls, while cells transfected with 50-nM/100-nM miR-328 mimics showed comparably lower viability.
MiR-328 as a biomarker to predict recurrence after ESD in EGC patients
To study the association between miR-328 and risk of recurrence in EGC patients who received ESD treatment, we enrolled 230 EGC patients (including 127 subjects with high expression of miR-328 and 103 subjects with low expression of miR-328) to observe the time period between the ESD treatment and the recurrence using Cox proportional hazard model analysis. As shown in Figure 7, when the recurrence rate was analyzed via the log rank test, the recurrence-free rate was significantly longer in those with relatively high expression of miR-328 than in those with low miR-328 (P<0.01), indicating miR-328 might be a potential a biomarker to predict recurrence after ESD in EGC patients.
Figure 7.

The recurrence-free period was significantly longer in those with relative high expression of miR-328 than low miR-328, indicating miR-328 might be a potential biomarker to predict recurrence after ESD in EGC patients.
Discussion
In many cancers there is a low expression level of MiR-328. miR-328 has been reported to down-regulate levels of protein ABCG2 to reduce chemoresistance of GBM stem cells [29]. In addition, miR-328 presents decreased expression levels during progression of glioma (primary WHO grade II gliomas spontaneously turn into secondary WHO grade IV GBMs) [30]. These findings indicate that miR-328 plays a critical role in origination or/and progression of GBM. It was also shown that miR-328 targeted the 3′-UTR of CD44 directly to modulate CD44 expression of other cell types and confirmed that targeting sequences of miR-328 were located in 3′-UTR of CD44. A previous study reported that miR-328 targeted CD44 expression to regulate zonation morphogenesis [31]. In the present study we found that in gastric cancer cells, the relative luciferase activity of cells transfected with wild-type CD44 3′UTR constructs evidently decreased as the concentration of miR-328 mimics increased, exhibiting negative regulation in a stepwise manner. On the contrary, cells carrying mutant CD44 3′UTR constructs exhibited comparable relative luciferase activity index when compared with the scramble controls, indicating CD44 as the direct target gene of miR-328 with the binding site located at the segment that has been mutated. Furthermore, we showed that the expression level of miR-328 in recurrence-positive tissues decreased substantially compared with recurrence-negative tissues, while both the mRNA and protein expression level of CD44 of the recurrence-positive group were evidently higher when compared with the recurrence-negative group.
As an important receptor for hyaluronic acid and adhesion molecule, CD44 is involved in numerous physiological processes such as metastasis, invasion, and growth of cancer cells [32]. A previous study showed that a CD44 variant (CD44v), which could interact with xCT, induced the synthesis of the primary intracellular antioxidant glutathione, leading to reactive oxygen species (ROS) defense and promoting tumor growth [19,33]. Moreover, another study, which used flow-cytometric analysis, demonstrated that there was increased expression level of CD44v9 and CD44 in human gastric mucosa with H. pylori infection but not in gastric mucosa without H. pylori infection [34]. It has been found that there was a high expression level of CD44 in metaplastic cells of SPEM adjacent to tumors in C2mE/K19-Wnt1 mice and that tumor cells with high levels of CD44 had a much higher expression level of SPEM-related genes compared with CD44 tumor cells [35]. It was reported that CD44 acts as a critical CSC marker and plays an important part in ROS defense, leading to colonization of metastatic cancer cells and tumor development [19,17]. Consistent ROS stress might contribute to adaptive stress responses such as upregulation of survival factors, antioxidants, and redox-sensitive transcription factors. Adaptation of redox allows cancer cells surviving under increased ROS stress to develop resistance to some anticancer drugs [36,33]. CD44 expression is suppressed by several miRNAs by targeting the 3′-UTR of CD44, leading to suppression of metastasis and tumorigenesis. For example, miR-34a, a major negative regulative factor of prostate CSCs with CD44 positivity, repressed CD44 directly to inhibit metastasis, while CD44 was targeted by miR-199a in ovarian cancer-initiating cells with CD117/CD44 positivity, leading to suppression of multidrug resistance and tumorigenesis [37,38]. A potential target for therapy is provided by a signaling pathway related to CD44; however, it remains completely unknown how CD44 expression is regulated. In this study, we found that cells transfected with 100-nM miR-328 inhibitors showed evidently upregulated viability when compared with the scramble controls, while cells transfected with 50-nM/100-nM miR-328 mimics showed comparably lower viability, indicating miR-328 negatively interfered with the viability of EGC cells. We also studied the association between miR-328 and risk of recurrence in EGC patients who received ESD treatment. We enrolled 230 EGC patients to observe the time period between the ESD treatment and the recurrence using Cox proportional hazard model analysis. As shown in Figure 7, when the recurrence rate was analyzed via the log rank test, the recurrence-free rate was significantly longer in those with relatively high expression of miR-328 than low miR-328, indicating miR-328 might be a potential a biomarker to predict recurrence after ESD in EGC patients.
Conclusions
We validated CD44 as a target of miR-328 in gastrointestinal cancer, and found that the expression level of miR-328 is associated with the risk of recurrence of ECG after treatment with ESD. Therefore, miR-328 might be a potential predictive biomarker of recurrence after ESD.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Correa P. International cancer epidemiology meetings. Cancer Epidemiol Biomarkers Prev. 1992;1(3):245–57. [PubMed] [Google Scholar]
- 2.Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41(10):929–42. doi: 10.1007/s00535-006-1954-3. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka M, Ono H, Hasuike N, Takizawa K. Endoscopic submucosal dissection of early gastric cancer. Digestion. 2008;77(Suppl 1):23–28. doi: 10.1159/000111484. [DOI] [PubMed] [Google Scholar]
- 4.Nagano H, Ohyama S, Fukunaga T, et al. Indications for gastrectomy after incomplete EMR for early gastric cancer. Gastric Cancer. 2005;8(3):149–54. doi: 10.1007/s10120-005-0328-5. [DOI] [PubMed] [Google Scholar]
- 5.Toyokawa T, Inaba T, Omote S, et al. Risk factors for perforation and delayed bleeding associated with endoscopic submucosal dissection for early gastric neoplasms: analysis of 1123 lesions. J Gastroenterol Hepatol. 2012;27(5):907–12. doi: 10.1111/j.1440-1746.2011.07039.x. [DOI] [PubMed] [Google Scholar]
- 6.Jang JS, Choi SR, Graham DY, et al. Risk factors for immediate and delayed bleeding associated with endoscopic submucosal dissection of gastric neoplastic lesions. Scand J Gastroenterol. 2009;44(11):1370–76. doi: 10.3109/00365520903194609. [DOI] [PubMed] [Google Scholar]
- 7.Dinis-Ribeiro M, Pimentel-Nunes P, Afonso M, et al. A European case series of endoscopic submucosal dissection for gastric superficial lesions. Gastrointest Endosc. 2009;69(2):350–55. doi: 10.1016/j.gie.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 8.Takenaka R, Kawahara Y, Okada H, et al. Endoscopic submucosal dissection for cancers of the remnant stomach after distal gastrectomy. Gastrointest Endosc. 2008;67(2):359–63. doi: 10.1016/j.gie.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 9.Kakushima N, Ono H, Tanaka M, et al. Factors related to lateral margin positivity for cancer in gastric specimens of endoscopic submucosal dissection. Dig Endosc. 2011;23(3):227–32. doi: 10.1111/j.1443-1661.2010.01092.x. [DOI] [PubMed] [Google Scholar]
- 10.Park YM, Cho E, Kang HY, Kim JM. The effectiveness and safety of endoscopic submucosal dissection compared with endoscopic mucosal resection for early gastric cancer: A systematic review and metaanalysis. Surg Endosc. 2011;25(8):2666–77. doi: 10.1007/s00464-011-1627-z. [DOI] [PubMed] [Google Scholar]
- 11.Kato M, Nishida T, Tsutsui S, et al. Endoscopic submucosal dissection as a treatment for gastric noninvasive neoplasia: A multicenter study by Osaka University ESD Study Group. J Gastroenterol. 2011;46(3):325–31. doi: 10.1007/s00535-010-0350-1. [DOI] [PubMed] [Google Scholar]
- 12.Park JC, Lee SK, Seo JH, et al. Predictive factors for local recurrence after endoscopic resection for early gastric cancer: Long-term clinical outcome in a single-center experience. Surg Endosc. 2010;24(11):2842–49. doi: 10.1007/s00464-010-1060-8. [DOI] [PubMed] [Google Scholar]
- 13.Abe N, Gotoda T, Hirasawa T, et al. Multicenter study of the long-term outcomes of endoscopic submucosal dissection for early gastric cancer in patients 80 years of age or older. Gastric Cancer. 2012;15(1):70–75. doi: 10.1007/s10120-011-0067-8. [DOI] [PubMed] [Google Scholar]
- 14.Nagano O, Murakami D, Hartmann D, et al. Cell-matrix interaction via CD44 is independently regulated by different metalloproteinases activated in response to extracellular Ca(2+) influx and PKC activation. J Cell Biol. 2004;165(6):893–902. doi: 10.1083/jcb.200310024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalerba P, Dylla SJ, Park IK, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA. 2007;104(24):10158–63. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collins AT, Berry PA, Hyde C, et al. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65(23):10946–51. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 17.Yae T, Tsuchihashi K, Ishimoto T, et al. Alternative splicing of CD44 mRNA by ESRP1 enhances lung colonization of metastatic cancer cell. Nat Commun. 2012;3:883. doi: 10.1038/ncomms1892. [DOI] [PubMed] [Google Scholar]
- 18.Tsugawa H, Suzuki H, Saya H, et al. Reactive oxygen species-induced autophagic degradation of Helicobacter pylori CagA is specifically suppressed in cancer stem-like cells. Cell Host Microbe. 2012;12(6):764–77. doi: 10.1016/j.chom.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Ishimoto T, Nagano O, Yae T, et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(−) and thereby promotes tumor growth. Cancer Cell. 2011;19(3):387–400. doi: 10.1016/j.ccr.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 20.Ambros V. The functions of animal microRNAs. Nature. 2004;431(7006):350–55. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 21.Guessous F, Zhang Y, Kofman A, et al. microRNA-34a is tumor suppressive in brain tumors and glioma stem cells. Cell Cycle. 2010;9(6):1031–36. doi: 10.4161/cc.9.6.10987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449(7163):682–88. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 23.Sun L, Yan W, Wang Y, et al. MicroRNA-10b induces glioma cell invasion by modulating MMP-14 and uPAR expression via HOXD10. Brain Res. 2011;1389:9–18. doi: 10.1016/j.brainres.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 24.Corsini LR, Bronte G, Terrasi M, et al. The role of microRNAs in cancer: Diagnostic and prognostic biomarkers and targets of therapies. Expert Opin Ther Targets. 2012;16(Suppl 2):S103–9. doi: 10.1517/14728222.2011.650632. [DOI] [PubMed] [Google Scholar]
- 25.Schaefer A, Stephan C, Busch J, et al. Diagnostic, prognostic and therapeutic implications of microRNAs in urologic tumors. Nat Rev Urol. 2010;7(5):286–97. doi: 10.1038/nrurol.2010.45. [DOI] [PubMed] [Google Scholar]
- 26.Hirata K, Suzuki H, Imaeda H, et al. CD44 variant 9 expression in primary early gastric cancer as a predictive marker for recurrence. Br J Cancer. 2013;109(2):379–86. doi: 10.1038/bjc.2013.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishimoto T, Izumi D, Watanabe M, et al. Chronic inflammation with Helicobacter pylori infection is implicated in CD44 overexpression through miR-328 suppression in the gastric mucosa. J Gastroenterol. 2015;50(7):751–57. doi: 10.1007/s00535-014-1019-y. [DOI] [PubMed] [Google Scholar]
- 28.Ishimoto T, Sugihara H, Watanabe M, et al. Macrophage-derived reactive oxygen species suppress miR-328 targeting CD44 in cancer cells and promote redox adaptation. Carcinogenesis. 2014;35(5):1003–11. doi: 10.1093/carcin/bgt402. [DOI] [PubMed] [Google Scholar]
- 29.Li WQ, Li YM, Tao BB, et al. Downregulation of ABCG2 expression in glioblastoma cancer stem cells with miRNA-328 may decrease their chemoresistance. Med Sci Monit. 2010;16(10):HY27–30. [PubMed] [Google Scholar]
- 30.Malzkorn B, Wolter M, Liesenberg F, et al. Identification and functional characterization of microRNAs involved in the malignant progression of gliomas. Brain Pathol. 2010;20(3):539–50. doi: 10.1111/j.1750-3639.2009.00328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang CH, Lee DY, Deng Z, et al. MicroRNA miR-328 regulates zonation morphogenesis by targeting CD44 expression. PLoS One. 2008;3(6):e2420. doi: 10.1371/journal.pone.0002420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ponta H, Sherman L, Herrlich PA. CD44: From adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4(1):33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 33.Nagano O, Okazaki S, Saya H. Redox regulation in stem-like cancer cells by CD44 variant isoforms. Oncogene. 2013;32(44):5191–98. doi: 10.1038/onc.2012.638. [DOI] [PubMed] [Google Scholar]
- 34.Fan X, Long A, Goggins M, et al. Expression of CD44 and its variants on gastric epithelial cells of patients with Helicobacter pylori colonisation. Gut. 1996;38(4):507–12. doi: 10.1136/gut.38.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wada T, Ishimoto T, Seishima R, et al. Functional role of CD44v-xCT system in the development of spasmolytic polypeptide-expressing metaplasia. Cancer Sci. 2013;104(10):1323–29. doi: 10.1111/cas.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat Rev Drug Discov. 2009;8(7):579–91. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 37.Liu C, Kelnar K, Liu B, et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med. 2011;17(2):211–15. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng W, Liu T, Wan X, et al. MicroRNA-199a targets CD44 to suppress the tumorigenicity and multidrug resistance of ovarian cancer-initiating cells. FEBS J. 2012;279(11):2047–59. doi: 10.1111/j.1742-4658.2012.08589.x. [DOI] [PubMed] [Google Scholar]


