Abstract
In this study the effect of sweeteners with low glycemic index and non-cariogenic characteristics (isomaltulose, oligofructose and tagatose) in jelly prepared with citrus juice has been evaluated considering a citrus jelly formulated with sucrose as reference. The soluble solids, moisture content, pH, water activity, antioxidant capacity, optical and mechanical properties of jelly made using different sweeteners was determined during storage. Besides, mesophilic aerobics and moulds and yeasts was also counted to determine their stability over time. Sensory evaluation of the citrus jelly has also been done. The results showed the antioxidant activity decreased during storage in all formulations. Tagatose increased lightness whereas coordinates a*, b* and chrome of all the jellies prepared using new sweeteners were lower than jellies with sucrose. However, citrus jelly with only oligofructose or tagatose or with the mixture of isomaltulose and tagatose were most closely resembled to the control jelly with respect to mechanical properties. Jelly prepared with the combination of isomaltulose and tagatose in equal proportions obtained the best score in the sensorial analysis.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-016-2319-4) contains supplementary material, which is available to authorized users.
Keywords: Isomaltulose, Oligofructose, Tagatose, Antioxidants, Mechanical properties, Sensory evaluation
Introduction
Traditionally, jelly desserts are mainly produced with edible gelatine, water, sugar and flavors. Although jelly desserts have low content of gelatine this type of protein contains 18 different amino acids, including eight essential amino acids (GME 2015) being particularly rich in glycine, proline and hydroxyproline. Furthermore, gelatine is a natural colloide with properties of gelling and a stabilizing effect. Therefore, gelatine has a quite high nutritional value but with a low caloric power (17 kJ/kg or 4 kcal/g). Other important components of jelly desserts are sugars. It is widely known that their excessive consumption is related to tooth decay, diabetes and obesity (Edwards 2002; O’Donnell and Kearsley 2012), among other illnesses. Concretely, white sugar, which contains high percentage of sucrose, is one of the most usual sweetening agent in confectionary products but it requires calcium and potassium to be digested in detriment for vital organs (Shukla and Kandra 2015).
Despite the fact that this type of dessert is not considered with a high nutritional value, it is important to point out that this situation might change if natural vitamins and antioxidants provided from fruit juice were included in its formulation instead of the water.
Citrus fruits such as orange, lemon and mandarin orange have many beneficial properties due to their high content of fibre, vitamins, minerals, ascorbic acid and specially high content in antioxidant compounds, such as carotenoids, flavonoids and phenolic compounds (Álvarez et al. 2014). As far as we know, a jelly dessert prepared with a mixture of different citrus juices does not exist in the market and it could expand the possibilities of commercialization.
Currently awareness of health-related issues in society has increased the demand of new functional foods and consequently food industry must be constanly innovating to offer consumers new alternative products (Shukla and Kandra 2015). In the confectionary and beverage sectors this concern is mainly focused on the achievement of an adequate sweetness while improving health and appearance, and as a result the use of artificial sweeteners has increased. However artificial sweeteners, such as aspartame, acesulfame-k, saccharin and sodium cyclamate, or polyalcohols have negative connotations due to their possible risk to health and they must be subject to a rigorous assessment before their use in food products and beverages (de Queiroz Pane et al. 2015). In view of this the reformulation of jelly desserts with new non-cariogenic sweeteners available in the market could be a good chance to achieve this goal.
To cope with these issues nowadays there are natural sweeteners such as tagatose, isomaltulose and oligofructose (FDA 2005, 2010, 2011), which need to be studied in order to check their capacity to replace sucrose and other sugars in traditional foods as jelly desserts. In this sense, previous studies had been carried out to reformulate confectionary products with isomaltulose such as strawberry jam (Peinado et al. 2012, 2013), gummy confections (Periche et al. 2014) or marshmallows (Periche et al. 2015a). Tagatose and oligofructose have been also studied in orange marmalade (Rubio-Arraez et al. 2015) and the combination of isomaltulose, stevia and oligofructose in marshmallows (Periche et al. 2015b).
Oligofructose is an oligosaccharide derived from sucrose, which acts as dietary fibre regulating intestinal transit. It presents a prebiotic effect because it favours the selective growth of bifidus bacteria (Ledur et al. 2013). Besides, it reduces cholesterol and blood sugar levels (Chacón-Villalobos 2006) and improves calcium absorption (Van Den Heuvel et al. 1999). Nevertheless, it is highly soluble and possesses technological properties (sweet taste, stability…) analogous to sucrose (Pimentel et al. 2015). In 2011, oligofructose was recognized as safe (GRAS) (FDA 2011).
D-Tagatose (D-tag) is a ketohexose, a stereoisomer of d-fructose and it is found naturally in several foods, including cheese and yoghurt. Its texture is very similar to sucrose and almost as sweet as sucrose, with only 1.5 kcal/g and it does not provoke dental caries (Levin 2002; Oh 2007; Taylor et al. 2008; Calzada-León et al. 2013). Tagatose is very suitable for confectionary products, ice creams, soft drinks and breakfast cereals (Vastenavond et al. 2012). Tagatose is minimally absorbed by the upper gastrointestinal tract. The unabsorbed tagatose is fermented in the intestines, causing a change in the proportions of various short chain fatty acids (Taylor et al. 2008). Thus, it is considered a functional food and besides it performs functions as soluble fibre favouring lactic acid bacteria and Lactobacillus specie bacteria (Petersen-Skytte 2006). D-tagatose received GRAS status by the Food and Drug Administration in 2001 (Levin 2002; FDA 2010).
Isomaltulose is a reducing disaccharide which is naturally present in honey, and sugar cane juice, and its appearance, taste and viscosities of aqueous solutions are comparable to sucrose (Periche et al. 2014). Based on its chemical definition compared to sucrose or glucose, it is less insulinemic, less glycemic and is non-cariogenic (Lina et al. 2002). However, it has a third of the sweetening power of sucrose (Lina et al. 2002; De Oliva-Neto and Menão 2009; Peinado et al. 2012). In 2005, isomaltulose was recognized as safe (GRAS) (FDA 2005).
The aim of this paper was to evaluate the potential use as an alternative to sucrose in the development of jelly dessert along with the addition of fresh citrus juice on composition, mechanical, optical, and sensory properties.
Materials and methods
Materials of citrus jelly
Jelly was manufactured with citrus fruits juice (Citrus reticulata clementina, Citrus limon eureka, Citrus sinensis navelate), sugar/sweeteners and gelatine (Junca Gelatines S.L., Girona, Spain). Jelly was prepared using sucrose (Azucarera Iberia S.L., Madrid, Spain) as well as replacing with the different mixtures of oligofructose (Frutalose, Roosendaal, Netherlands), isomaltulose (Palatinose, Mannheim, Germany) and tagatose (Tagatesse, Heusden-Holder, Belgium). The jelly dessert was prepared using the same proportions of ingredients as in a commercial orange flavoured jelly powder (Royal, Kraft Foods, Madrid, Spain) which were: 85.2 % of sugars and 9.5 % of gelatine. It is important to point out that commercial jelly also contained vitamin C, acidity regulators (fumaric acid, sodium citrate), flavourings and colourants (E100: curcumine and E120: carminic acid) but these components were not included in the jelly of this study. Following the manufacturer’s instructions, the content of the powder was diluted with 500 g of water, leading to a final composition of 12.6 % of sugars and 1.6 % of gelatine. In the jelly prepared with citrus juice, the amount of sugars contained in the juice were taken into account when adding sweeteners in order to maintain the same proportion of sugars and gelatine as in the commercial formula. Furthermore, 50 % of the amount of water was replaced by citrus juice. The citrus juice was prepared with the following proportionos of each fruit: lemon juice 14 %, orange juice 43 % and mandarin orange juice 43 %.
Depending on the combination of sucrose/sweeteners used in jelly, the following notation was used: Control: 100 % sucrose; I50T50: 50 % isomaltulose and 50 % tagatose; T: 100 % tagatose; I: 100 % isomaltulose; I50O50: 50 % isomaltulose and 50 % oligofructose, and O jelly: 100 % oligofructose.
Jelly preparation
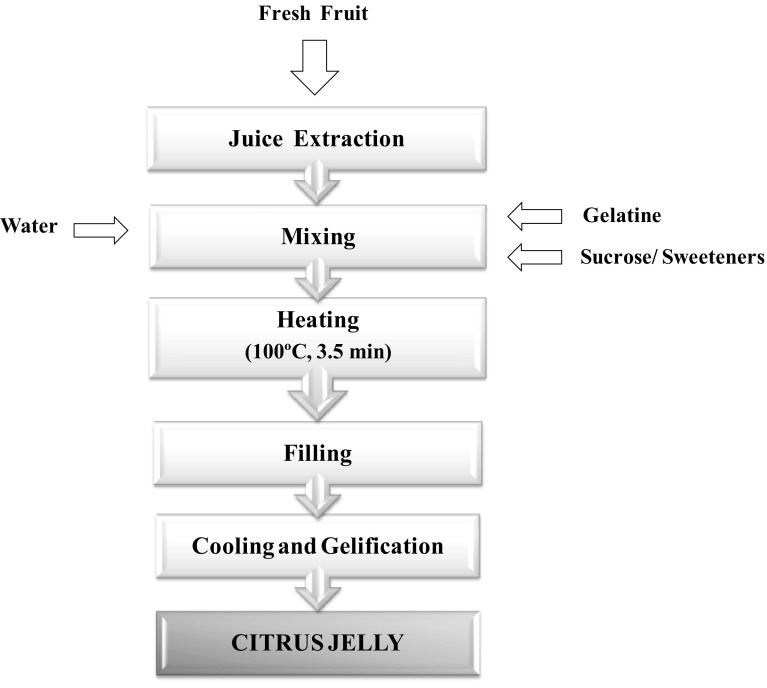
Figure 1 shows the flow chart of the stages required to prepare jelly for this study. The amounts of each component were weight in an analytical scale (Precisa Gravimetrics AG, model BJ 6100D, Dietikon, Switzerland). Juice was extracted using a liquidiser (Molinex, model vitapress, Mayenne, France). For the stages of mixing and blending, a thermal blender (Thermomix, model TM31, Vorwerk, Wuppertal, Germany) was used. Once the mixture was obtained, containers were filled with it and stored at refrigeration at 4 °C.
Fig. 1.
Flow chart of the manufacturing process of citrus jelly
Analytical determinations
Analysis of moisture content, Brix, pH, water activity, antioxidant capacity, optical and mechanical properties and microbiological analysis were performed for each formulation of citrus jelly at 1, 15, 30 and after 45 days of storage at 4 °C by triplicate. Next the methodologies followed for each case are described.
Moisture and soluble solids content, pH and water activity
Moisture content (x w: g water/g citrus jelly) was analysed gravimetrically following AOAC method (2000). Soluble solids content of samples were measured by a refractometer at 20 °C (Atago3T, Tokyo, Japan). pH was measured using a pH-meter (Mettler Toledo, model Seven Easy, Barcelona, Spain). Water activity (aw) was determined using a hygrometer (Decagon Devices, Inc., model 4TE, Pullman, Washington, USA), at 25 °C.
Determination of antioxidant capacity
The antioxidant activity of citrus jelly was analysed following the method described by Shahidi et al. (2006), based on the scavenging activity of the stable 2, 2-diphenyl-1-picrylhydrazyl (DPPH) free radical and measuring the absorbance change of samples at 515 nm in a spectrocolorimeter Thermo Fisher Scientific, Inc. (Helios Zeta UV-VIS, Waltham, Massachusetts, USA). The results were expressed as mg of Trolox equivalent per 100 g of citrus jelly.
Optical Properties
The optical properties of citrus jelly placed in 20 mm-wide cuvettes was measured using a spectrocolorimeter UV (Konica Minolta Inc., CM-3600d model, Tokyo, Japan). CIEL*a*b* coordinates were obtained using D65 illuminant and a 10º observer as reference system. Registered parameters were: L* (lightness), a* (red component), b* (yellow component), Chroma (C* = (a*2 + b*2)1/2) and hue (h* = arctg(b*/a*)).
Mechanical properties
The samples were evaluated with Texture Profile Analysis test (TPA) using a TA.XT plus Texture Analyser (Stable Micro Systems, Godalming, U.K.). Using a load cell of 50 kg and a 45 mm diameter cylindrical probe were used. The test conditions involved two consecutive cycles of 50 % compression with 15 s between cycles test speed of 1 mm/s for hardness, cohesiveness, adhesiveness and springiness.
Microbiological analysis
Mesophilic aerobic populations and yeast and molds colonies were determined following the procedure described by Rubio-Arraez et al. (2015). Microbial counts were expressed as CFU/g.
Sensorial analysis
An preliminary acceptance test using a 9-point hedonic scale (ISO 4121 2003; ISO 5492 2008) was used to evaluate the following attributes in the samples: color, flavor, texture, sweetness, global preference and intention of buying. The panel consisted of 30 trained panellists (aged from 20 to 50) who are regular consumers of this kind of dessert. Testing was conducted in a sensory evaluation laboratory built according to the international standards for test rooms. In this analysis the citrus jelly formulated using sweeteners containing only isomaltulose (I) and combination isomaltulose-oligofructose (I50O50), were not considered because the other samples of jelly were of a better quality.
Statistical analysis
Analyses of variance (multifactor ANOVA) were carried out by Statgraphics plus software (Statpoint Technologies, Inc., Centurion, Warrenton, Virginia, USA) to discern whether the effect of formulation or storage was significant on the citrus jelly studied with a significance level of 95 %. Interactions between factors were also considered.
Results and discussion
Compositional characterisation of citrus jelly
Table 1 shows the results of Total soluble solids content (Brix), moisture content (x w), and water activity (aw), pH, and antioxidant capacity of the jelly formulations with sucrose or new sweeteners (tagatose, oligofructose and isomaltulose). Initially, all jelly desserts reached a concentration of soluble solids around 22 ºBrix, but formulation that contained only oligofructose (O) had the highest values of ≈23 ºBrix unlike formulations contaning only isomaltulose (I) or tagatose (T) that showed the lowest values of ≈21 ºBrix. The storage decreased significantly ºBrix of formulation I50T50 but it increased in formulation T, being control and I50O50 the most stable formulations. Even though, values of soluble content were quite similar in all cases. In terms of moisture content, there were no significant differences due to formulation and only after 30 days of storage there was a significant increase but moisture content did not change even after 45 days in all cases. This fact could be due to the permeability to water vapour of the package and also because the relative humidity was not controlled to simulate the conditions of commercialization. Besides, water activity of all the formulations was 0.98, although formulation T showed the highest aw initially. The pH of all formulations of jelly did not differ, but it was initially lower in formulation T and control jelly, althougth all jellies presented similar values after 45 days of storage.
Table 1.
Values for moisture content (x w), Brix, Water activity (aw) and pH of citrus jelly formulated with sucrose (control) or with new sweeteners and their combinations (isomaltulose, oligofructose and tagatose) inicially, 15, 30 and 45 days of storage. Equal letters indicate homogeneous groups
| Formulation | Time (days) | Brix | xw (g water/ g citrus jelly) | aw | pH | Antioxidant capacity (mg Trolox/100 g Jelly) |
|---|---|---|---|---|---|---|
| Control | 1 | 21.41 ± 0.11b | 0.785 ± 0.001a | 0.9846 ± 0.0002c | 3.383 ± 0.006b | 53.2 ± 0.4e |
| 15 | 21.8 ± 0.2b | 0.785 ± 0.004a | 0.9819 ± 0.0001a | 3.543 ± 0.006d | 30.6 ± 1.4c | |
| 30 | 21.5 ± 0.5b | 0.91 ± 0.07b | 0.9839 ± 0.0002b | 3.523 ± 0.006d | 26.8 ± 1.1b | |
| 45 | 21.43 ± 0.12b | 0.817 ± 0.002a | 0.9848 ± 0.0002c | 3.51 ± 0.02d | 19.2 ± 0.2a | |
| T | 1 | 21.10 ± 0.11ab | 0.792 ± 0.004a | 0.9894 ± 0.0004d | 3.367 ± 0.006a | 62 ± 2g |
| 15 | 20.7 ± 0.3a | 0.64 ± 0.03a | 0.9829 ± 0.0002b | 3.533 ± 0.012d | 32 ± 2cd | |
| 30 | 22.2 ± 0.3bc | 0.88 ± 0.02b | 0.9841 ± 0.0001c | 3.637 ± 0.006f | 29 ± 2b | |
| 45 | 22.8 ± 0.3d | 0.804 ± 0.005a | 0.9851 ± 0.0001d | 3.64 ± 0.011f | 19.1 ± 0.2a | |
| I50T50 | 1 | 22.50 ± 0.06c | 0.781 ± 0.009a | 0.9855 ± 0.0002c | 3.677 ± 0.006g | 57.7 ± 5.9f |
| 15 | 21.46 ± 0.05b | 0.793 ± 0.003a | 0.9831 ± 0.0003b | 3.550 ± 0.011d | 27.4 ± 1.5b | |
| 30 | 21.3 ± 0.3b | 0.89 ± 0.05b | 0.9841 ± 0.0001c | 3.61 ± 0.03e | 25.4 ± 1.2b | |
| 45 | 20.7 ± 0.3a | 0.817 ± 0.003a | 0.9826 ± 0.0004b | 3.540 ± 0.011d | 19.6 ± 1.2a | |
| I | 1 | 20.11 ± 0.11b | 0.809 ± 0.004a | 0.9860 ± 0.0001c | 3.527 ± 0.006d | 54 ± 2e |
| 15 | 20.6 ± 0.2a | 0.804 ± 0.013a | 0.9856 ± 0.0004c | 3.587 ± 0.006d | 36.2 ± 1.2d | |
| 30 | 20.5 ± 0.2a | 0.88 ± 0.04b | 0.9826 ± 0.0002b | 3.487 ± 0.006c | 34.6 ± 1.0d | |
| 45 | 21.21 ± 0.11b | 0.818 ± 0.003a | 0.9804 ± 0.0003a | 3.593 ± 0.006d | 18.8 ± 0.3a | |
| O | 1 | 23.16 ± 0.05d | 0.775 ± 0.003a | 0.9886 ± 0.0001d | 3.640 ± 0.011f | 52.5 ± 1.3e |
| 15 | 23.03 ± 0.05d | 0.775 ± 0.004a | 0.9868 ± 0.0003c | 3.610 ± 0.011e | 31 ± 2c | |
| 30 | 22.63 ± 0.15c | 0.88 ± 0.04b | 0.9866 ± 0.0001c | 3.587 ± 0.006e | 27 ± 2b | |
| 45 | 22.56 ± 0.11c | 0.79 ± 0.02a | 0.9872 ± 0.0003c | 3.593 ± 0.006e | 17.9 ± 0.2a | |
| I50O50 | 1 | 21.36 ± 0.05b | 0.81 ± 0.05a | 0.9885 ± 0.0001d | 3.543 ± 0.006d | 54.73 ± 0.12e |
| 15 | 21.23 ± 0.05b | 0.86 ± 0.14ab | 0.9829 ± 0.0003b | 3.527 ± 0.006d | 31 ± 2cd | |
| 30 | 21.2 ± 0.3b | 0.897 ± 0.005b | 0.9831 ± 0.0002b | 3.50 ± 0.02d | 31.7 ± 0.4c | |
| 45 | 21.3 ± 0.2b | 0.81 ± 0.02a | 0.9826 ± 0.0004b | 3.52 ± 0.02d | 18.2 ± 0.3a |
Equal letters indicate homogeneous groups
Nomenclature correspond to the amount of sucrose/sweeteners in the % of sugars used in jelly. Control: 100 % sucrose; T: 100 % tagatose; I50T50: 50 % isomaltulose and 50 % tagatose; I: 100 % isomaltulose; O jelly: 100 % oligofructose, and I50O50: 50 % isomaltulose and 50 % oligofructose
Table 1 shows that all formulations of jelly prepared with citrus juice had the same antioxidant capacity except for I50T50 and T jellies which had the highest values due to presence of tagatose, which responsible for this behaviour. Other authors (Zeng et al. 2012) also reported an improvement in the radical scavenging activity and oxidation reduction potential of the hydrolysates of tune backbone with sugars like especially D-tag. However, in all cases there was a significant reduction of the antioxidant capacity over the storage period considered, approaching similar values after 45 days for all formulations as was also observed in previous studies of orange marmalade as a consequence of the oxidation of the components responsible of this capacity (Rababah et al. 2011).
Optical properties
The interaction charts of the colorimetric coordinates L*, a* and b*, chroma (C*) and hue (h*) of the citrus jellies as a function of sucrose/sweeteners and storage time are shown in Fig. 2. Initially control jelly desserts had more similarities in terms of lightness with jellies containing tagatose, but for coordinates a* and b* and for chroma formulation I50O50 was close to control jelly. It was also observed that the citrus jelly formulated with tagatose (T and I50T50) showed an increase of their luminosity after 45 days of storage in contrast with a decrease observed in formulations with isomaltulose and the combination of isomaltulose with oligofructose (I and I50O50) at the end of storage. This behaviour could be related with the low solubility of isomaltulose as reported earlier (Peinado et al. 2012). Coordinate a* in jellies containing only oligofructose or isomaltulose was the most stable but coordinate b* increased during storage in formulation I whereas it decreased in formulation O. At the end of storage a*, b* and C* of the new formulations of jellies were lower than in control jellies, except for a* of formulation I50T50 which was equal to the control jelly. In terms of h*, it was noteworthy that all formulations showed values around the results of the control jelly, being formulation I above control jelly in the whole period of storage and formulation O the most similar to control jelly. Thus, the effect of the different ingredients on the food system depends not only on their concentration or distribution but also on the interactions of the components (Peinado et al. 2013).
Fig. 2.
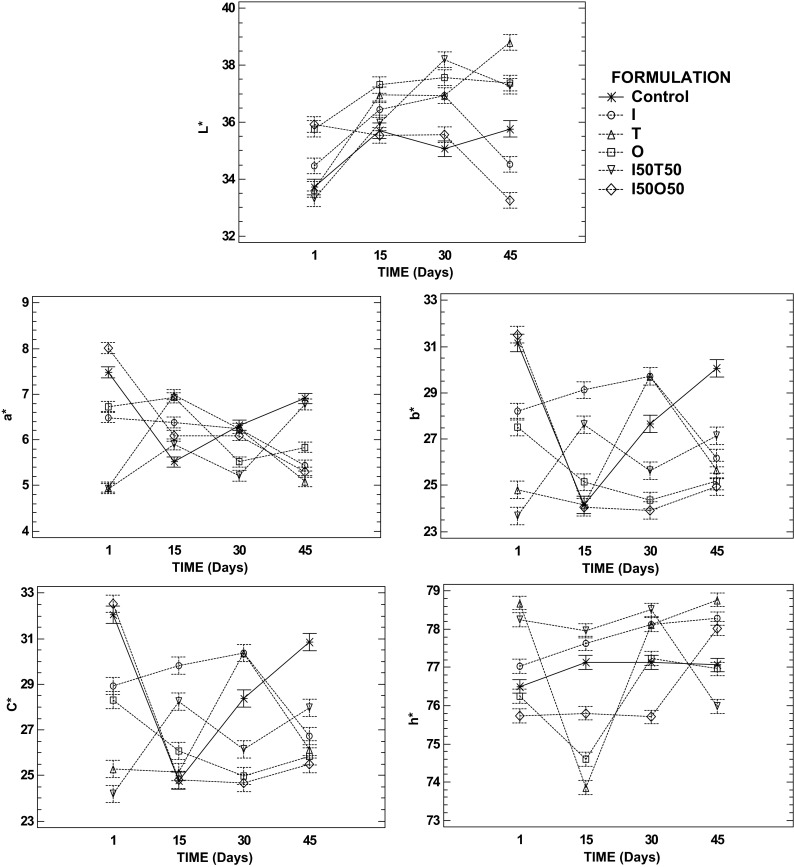
Interaction graphics (95% of significant level) of colour parameters: L*, a*, b* coordinates, chroma (C*) and hue (h*) of the citrus jelly as a function of the formulation and storage time
Mechanical properties
Figure 3 shows the results of the mechanical parameters for each formulation of jelly studied during storage. As can be seen, initially O jelly (formulated only with oligofructose), had the highest values of hardness without statistical differences respect to citrus jelly formulated with tagatose (T and I50T50), whereas samples prepared with isomaltulose showed the lowest hardness consistent with the results obtained by Peinado et al. (2012) in strawberry jams formulated with isomaltulose compared with those prepared with sucrose and also by Periche et al. (2014) in gummy confections in which sucrose and glucose syrup were replaced by isomaltulose and/or fructose. This behaviour gives evidence of the lowest capacity of isomaltulose to form gel structure. Additionally, formulations with only tagatose (T) and oligofructose (O) showed the highest hardness at the end of the storage (45 days). Even though, storage did not had a significant effect on most of the mechanical parameters of these jellies. However, the formulation composed of isomaltulose and oligofructose (I50O50) showed the highest values of adhesiveness. Furthermore, cohesiveness and springiness were also higher in that formulation and when there was only isomaltulose in the sweetener content of jelly (I). In contrast, gumminess was very low in formulation I50O50. Therefore, the most similar jellies to control samples were those prepared with the mixture of isomaltulose and tagatose (I50T50) followed by those prepared with only oligofructose (O) or tagatose (T).
Fig. 3.
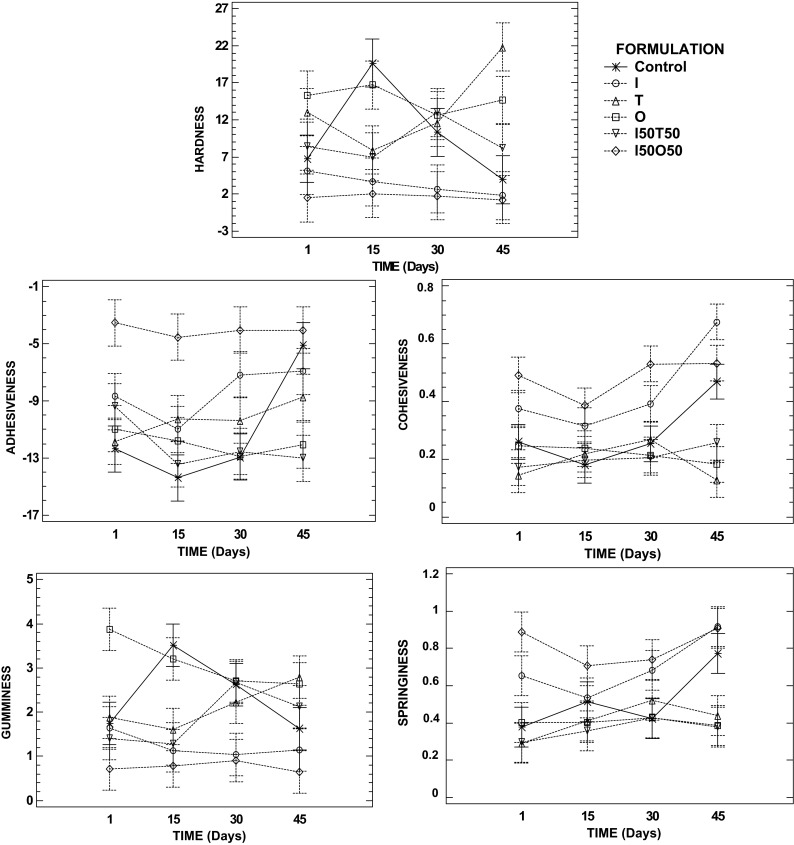
Interaction graphics (95 % of significant level) of hardness, adhesiveness, cohesiveness, gumminess and springiness of citrus jelly as a function of the formulation and storage time
Microbiological analysis
Microbial counts of mesophilic aerobics, yeasts and moulds were not found in any of the citrus jelly at 1, 15, 30 days of storage. However, at the end of storage (45 days) there were presence of mesophilic aerobics, yeasts and moulds, except for the formulation that only contained oligofructose. This protective effect of oligofructose could be due to its selective preference for the growth of bifidus bacteria (Ledur et al. 2013), which were not enhanced in the agars used for this analysis, specific for mesophilic aerobics, yeasts and moulds. According to Pascual and Calderón (2000), the microbial counts for jelly desserts must not exceed 5·102 CFU/g mesophilic aerobics and 5·101 CFU/g yeasts and moulds. Even though, the microbial count was below those limits (3·101 CFU/g mesophilic aerobics and 2·101 CFU/g yeasts and moulds) after 45 days in all cases. These results give evidence that the product was microbiologically stable for the studied period. The microbiological stability of the samples could be attributed to the acidity of citrus juice which gave place to a low pH (≈3.5) in citrus jellies.
Sensory analysis
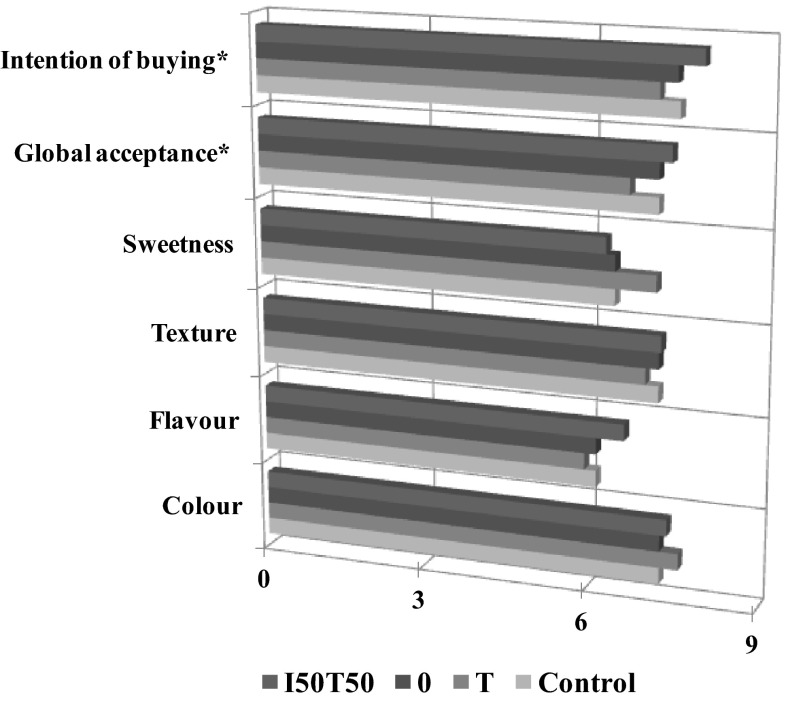
The results of sensory analysis of citrus jelly, depending on their formulation (control, T, I50T50, O), are presented in Fig. 4. T and I50T50 formulations showed the highest sweetness, due to their higher content of tagatose. This would coherent with the recommendations given by the manufacturer of the commercial tagatose (two tablespoons of sucrose provides the same sweetness as one tablespoon of tagatose), though as was mentioned in the introduction, tagatose should have similar sweetening power to sucrose (Oh 2007; Taylor et al. 2008; Calzada-León et al. 2013). It is noteworthy that the global preference and intention of buying of jelly formulated with equal proportion of tagatose and isomaltulose (I50T50) presented the better score. Therefore, the replacement of sucrose by a mixture of isomaltulose and tagatose in equal proportion would be feasible from a sensory point of view.
Fig. 4.
Sensory assessment of citrus jelly as a function of the formulation. Parameters marked with * mean that the studied formulations showed significant differences with 95 % of significant level
Conclusion
The reformulation of citrus jelly with non-cariogenic and low glycemic index sweeteners used in this research is viable. Besides, tagatose favoured the antioxidant capacity of citrus jelly initially, but not differences among all formulations were found after storage. In general, at the end of storage coordinates a*, b* and chrome of the new formulations of jellies were lower than in jellies with sucrose. From the mechanical point of view the recommended formulation would be oligofructose (O) or tagatose (T) or the mixture of isomaltulose and tagatose (I50T50). However the use of isomaltulose (I) or its combination with oligofructose (I50O50) reduced the capacity of gel formation. Citrus jellies with only oligofructose did not show presence of any microbial activity during storage. According to sensorial analysis, I50T50 was the best scored jelly.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors would like to thank the Serigó-Andrés family for donating the raw materials, and also the GVA projects GV/2013/029, GV/2014/012 as well as the Universitat Politècnica de València (Spain) for the financial support given to this research study (UPV PAID-06-12 SP20120889).
References
- Álvarez J, Pastoriza S, Alonso-Olalla R, Delgado-Andrade C, Rufián-Henares JA. Nutritional and physicochemical characteristic of commercial Spanish citrus juices. Food Chem. 2014;164:396–405. doi: 10.1016/j.foodchem.2014.05.047. [DOI] [PubMed] [Google Scholar]
- AOAC . Official methods of analysis of AOAC international. 17. MD: Gaithersburg; 2000. [Google Scholar]
- Calzada-León R, Ruiz-Reyes ML, Altamirano-Bustamante N, Padrón-Martínez MM. Features of the noncaloric sweeteners and their use in children. Acta Pediatr Méx. 2013;34(3):141–153. [Google Scholar]
- Chacón-Villalobos A. Current perspectives agribusiness oligofructosaccharides (FOS) Agron Mesoam. 2006;17(2):265–286. doi: 10.15517/am.v17i2.5165. [DOI] [Google Scholar]
- De Oliva-Neto P, Menão PTP. Isomaltulose production from sucrose by protaminobacter rubrum immobilized in calcium alginate. Bioresour Technol. 2009;100:252–4256. doi: 10.1016/j.biortech.2009.03.060. [DOI] [PubMed] [Google Scholar]
- de Queiroz Pane D, Dias CB, Meinhart AD, Ballus CA, Godoy HT. Evaluation of the sweetener content in diet/light/zero foods and drinks by HPLC-DAD. J Food Sci Tech . 2015;52(11):6900–6913. doi: 10.1007/s13197-015-1816-1. [DOI] [Google Scholar]
- Edwards WP. The science of goodies. Spain: Acribia S.A; 2002. [Google Scholar]
- Food and Drug Administration (FDA) (2005) GRAS Notification Isomaltulose (PALATINOSE). http://www.fda.gov/ucm/groups/fdagov-public/@fdagovfoodsgen/documents/document/ucm268989.pdf. Accessed 12 July 2015
- Food and Drug Administration (FDA) (2010) GRAS Notification Tagatose. GRN No.352.http://www.fda.gov/ucm/groups/fdagov-public/@fdagov-foods-gen/documents/document/ucm269560.pdf. Accessed 12 July 2015
- Food and Drug Administration (FDA) (2011) GRAS Notification Oligofructose. GRN No.392. http://www.fda.gov/ucm/groups/fdagov-public/@fdagov-foodsgen/documents/document/ucm277112.pdf. Accessed 12 July 2015
- GME (2015) Gelatine manufactured Europe gelatine properties. http://www.gelatine.org/en/about-gelatine/properties.html. Accessed 12 July 2015
- ISO . Sensory analysis. Guidelines for the use of quantitative response scales [ref. no.ISO 4121:2003] Geneva: International Organization for Standardization; 2003. [Google Scholar]
- ISO . Sensory analysis Vocabulary [ref. no.ISO 5492:2008] Geneva: International Organization for Standardization; 2008. [Google Scholar]
- Ledur MJ, Tessaro I, Zapata CP. Physicochemical characterization of Saccharides Powder obtained from Yacon Roots (Smallanthus sonchifolius) by membrane technology. Braz Arch Biol Technol. 2013;56(6):1024–1033. doi: 10.1590/S1516-89132013000600019. [DOI] [Google Scholar]
- Levin GV. Tagatose, the new GRAS sweetener and health product. J Med Food. 2002;5(1):23–36. doi: 10.1089/109662002753723197. [DOI] [PubMed] [Google Scholar]
- Lina BAR, Jonker G, Kozianowski G. Isomaltulose (Palatinose review of biological and toxicologycal studies) Food Chem Toxicol. 2002;40(10):1375–1381. doi: 10.1016/S0278-6915(02)00105-9. [DOI] [PubMed] [Google Scholar]
- O’Donnell K, Kearsley M. Sweeteners and sugar alternatives in food technology. 2. Chichester: Wiley; 2012. [Google Scholar]
- Oh DK. Tagatose: properties, applications, and biotechnological processes. Appl Microb Biotechnol. 2007;76(1):1–8. doi: 10.1007/s00253-007-0981-1. [DOI] [PubMed] [Google Scholar]
- Pacual MR, Calderón-Pascual V. Food Microbiology. Analytical methodology for foods and drinks. 2. Madrid: Diaz de Santos; 2000. [Google Scholar]
- Peinado I, Rosa E, Heredia A, Andrés A. Rheological characteristics of healthy sugar substituted spreadable strawberry product. J Food Eng. 2012;113(3):365–373. doi: 10.1016/j.jfoodeng.2012.06.008. [DOI] [Google Scholar]
- Peinado I, Rosa E, Heredia A, Escriche I, Andrés A. Optical, mechanical and sensorial properties of strawberry spreadable products formulated with isomaltulose. Food Bioprocess Tech. 2013;6(9):2353–2364. doi: 10.1007/s11947-012-0970-y. [DOI] [Google Scholar]
- Periche A, Heredia A, Escriche I, Andrés A, Castelló ML. Optical, mechanical and sensory properties of based-isomaltulose gummy confections. Food Biosci. 2014;7:37–44. doi: 10.1016/j.fbio.2014.05.006. [DOI] [Google Scholar]
- Periche A, Heredia A, Escriche I, Andrés A, Castelló ML. Potential use of isomaltulose to produce healthier marshmallows. LWT-Food Sci Technol. 2015;62(1):605–612. doi: 10.1016/j.lwt.2014.12.024. [DOI] [Google Scholar]
- Periche Á, Castelló ML, Heredia A, Escriche I (2015b) Stevia rebaudiana, Oligofructose and isomaltulose as sugar replacers in Marshmallows: stability and antioxidant properties. J Food Process Preserv. doi:10.1111/jfpp.12653
- Petersen-Skytte U. Tagatose. In: Mitchell H, editor. Sweeteners and sugar alternatives in food technology. Oxford: Blackwell Publishing; 2006. pp. 262–292. [Google Scholar]
- Pimentel TC, Madrona GS, Prudencio SH. Probiotic clarified apple juice with oligofructose or sucralose as sugar substitutes: sensory profile and acceptability. LWT–Food. Sci Technol. 2015;62(1):838–846. [Google Scholar]
- Rababah TM, Al-Mahasneh MA, Kilani I, Yang W, Alhamad MN, Ereifeja E, Al-U’datta M. Effect of jam processing and storage on total phenolics, antioxidant activity, and anthocyanins of different fruits. J Sci Food Agric. 2011;91:1096–1102. doi: 10.1002/jsfa.4289. [DOI] [PubMed] [Google Scholar]
- Rubio-Arraez S, Sahuquillo S, Capella JV, Ortolá MD, Castelló ML. Influence of healthy sweeteners (Tagatose and Oligofructose) on the physicochemical characteristics of orange marmalade. J Texture Stud. 2015;46(4):272–280. doi: 10.1111/jtxs.12127. [DOI] [Google Scholar]
- Shahidi F, Liyana-Pathirana CM, Wall DS. Antioxidant activity of white and black sesame seeds and their hull fractions. Food Chem. 2006;99(3):478–483. doi: 10.1016/j.foodchem.2005.08.009. [DOI] [Google Scholar]
- Shukla V, Kandra P. Development, physico-chemical and sensory evaluation of natural nutra candy. J Food Sci Tech Mys. 2015;52(11):7535–7539. doi: 10.1007/s13197-015-1810-7. [DOI] [Google Scholar]
- Taylor TP, Fasina O, Bell LN. Physical properties and consumer liking of cookies prepared by replacing sucrose with tagatose. J Food Sci. 2008;73(3):145–151. doi: 10.1111/j.1750-3841.2007.00653.x. [DOI] [PubMed] [Google Scholar]
- Van Den Heuvel EGHM, Muys T, Van Dokkum W, Schaafsma G. Oligofructose stimulates calcium absorption in adolescents. Am J Clinic Nutr. 1999;69:544–548. doi: 10.1093/ajcn/69.3.544. [DOI] [PubMed] [Google Scholar]
- Vastenavond CM, Bertelsen H, Hansen SJ, Laursen RS, Saunders J, Eriknauer K (2012) Tagatose (D-tagatose). In: Nabors L (ed) Alternative sweeteners. Boca Ratón, Florida, USA, p 197–222
- Zeng Y, Zhang X, Guan Y, Sun Y. Enzymatic hydrolysates from tuna backbone and the subsequent Maillard reaction with different ketohexoses. Int J Food Sci Technol. 2012;47:1293–1301. doi: 10.1111/j.1365-2621.2012.02973.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.