Abstract
Slurry ice possesses several advantages for seafood preservation, such as faster chilling rate, reduced physical damage, and complete coverage of the fish surface. In this paper, the efficiency of its employment, either applied alone or combined with ozone, was evaluated for bighead croaker and compared with traditional flake ice. Changes of a series of microbial, biochemical and sensory parameters including total viable count (TVC), sensory scores, total volatile basic nitrogen (TVB-N), peroxide value (PV), thiobarbituric acid (TBA), hardness and springiness were investigated up to 21 days. TVC, sensory attributes, hardness and springiness of fish stored in ozonised slurry ice, were significantly different from fish stored flake ice and slurry ice, indicating that a combination of slurry ice and ozone allowed better quality maintenance. Increase in TVB-N, PV and TBA values of the fish treated with ozonised slurry ice and slurry ice were found to be noticeably reduced compared to those of the flake ice ones. Based on the acceptable limits of TVC and other quantifiable attributes, a prolonged shelf life of 18 days was estimated for the bighead croaker stored in ozonised-slurry ice, whereas the counterpart batches treated with flake and slurry ice would exceed these limits after 9 and 15 days, respectively. Additionally, sodium dodecyl sulfate–polyacrylamide gel electrophoresis analysis and scanning electron microscope observation also revealed that ozonised-slurry ice could effectively retard the degradation of myofibrillar proteins and reduce the deterioration of microstructure.
Keywords: Slurry ice, Ozone, Quality, Bighead croaker, Shelf life
Introduction
Majority of (edible) fishery products are highly perishable and deteriorate rapidly post-mortem due to high water content, as well as high enzymatic and bacteriological activities, which inevitably result in loss of quality, decreased shelf life, and increased health risks to consumers (Gram and Dalgaard 2002). Rate of microbial spoilage depends on several factors, including the cultivation environment, type of fishery product, and postharvest handling, as well as storage conditions, with the last one being the critical factor (Okpala et al. 2014). Hence, a rapid and robust cooling strategy, which is required immediately after capture to maintain the fresh state of aquatic products, has always been of great necessity to slow down postharvest deterioration. Some preservation methods of interest that are widely reported have involved the use of conventional flake ice (Nunes et al. 1992), modified atmospheres (Bono et al. 2016), and chemical preservation agents (Zengin et al. 2011).
Slurry ice, also designated as flow ice, fluid ice, or liquid ice, is composed of two different phases, namely, liquid (water) and solid (ice). This novel chilling system enables the storage of marine species at subzero temperature ranging from −0.5 to −1.5 °C, whereas flake ice can only cool aquatic products to final temperatures of slightly above 0 °C. Hence, slurry ice is believed to be a very promising and valuable technique for preserving aquatic products supported by some advantages, such as: (1) faster chilling rate derived from higher heat-exchange capacity; (2) smaller physical damage on the fish surface by the microscopic spherical particles; (3) improved protection for aquatic products against the action of oxygen owing to complete coverage of the fish surface; and (4) more hygienic fish handling as a consequence of process automation (Rodríguez et al. 2006). Enhanced quality and extended shelf life could be obtained when marine species were stored in slurry ice (Losada et al. 2005). Slurry ice has also turned out to be a desirable pre-cooling medium, and hence it could be an alternative to traditional flake ice in transportation of marine species from ships to retail markets (Cakli et al. 2006). Furthermore, it was reported that slurry ice can also be combined with other additives to achieve additional effects. The combination of a preliminary treatment with antimelanosic agent prior to storage in slurry ice provided a better maintenance of quality, significantly reduced browning reactions, and extended shelf life of lobster as compared with the counterpart method, i.e., no pre-treatment with melanosis inhibitors (Aubourg et al. 2007). Likewise, slurry ice enriched with ozone was advisable for megrim, turbot, and sardine storage in previous studies (Aubourg et al. 2006; Campos et al. 2006, 2005).
Ozone, an approved disinfectant, has been widely used in a variety of fields because of its release of free oxygen atom along with molecular oxygen. Essentially, it exhibits potential oxidizing ability to destroy a wide range of food spoilage microorganisms (Greene et al. 2012; Okpala 2014a, b). Unstable ozone is degraded at a rapid rate into oxygen, which alleviates concerns about toxic residues and insures the safety of this sanitizing agent (Greene et al. 2012; Okpala 2014a, b). Hence, ozone has received global acceptance and attracted considerable attention on food safety uses. Ozone treatment has been applied to various kinds of fishery products, such as mussel, shrimp, sockeye salmon, Japanese flounder, jack mackerel, and rockfish (Naito 2012; Okpala 2014a, b). Reduced microbial load and extended shelf life was achieved for these species. However, undesirable events have a chance to probably occur due to the pro-oxidant effect of ozone on fish muscle/tissue constituents, such as phospholipids and polyunsaturated fatty acids (Feng et al. 2012; Greene et al. 2012; Okpala 2014a, b). Therefore, ozone concentration should be strictly controlled to avoid these negative effects.
Bighead croaker (Collichthys niveatus) is an abundant and economically important marine species distributed in the Western Pacific, Yellow Sea, and East China Sea (Shen et al. 2012). A total annual output in eastern coast of China, based on the data provided by Oceanic and Fishery Department of Zhejiang Province (2010), was about 0.32 million tons. In recent years, this species has attracted considerable attention due to its high nutritional content and delicious taste. However, it remains highly perishable at postharvest and therefore, it is critical and of great interest to explore effective preservative methods to reduce quality loss and extend shelf life.
Given the effectiveness of ozone in inactivating microorganisms, a chilling system combined with ozone and slurry ice was developed for bighead croaker in our laboratory. Specifically, the object of the current study was to evaluate the efficiency of this advanced refrigeration system using a number of microbial and biochemical parameters indicative of spoilage with two parallel batches of bighead croaker, which had been stored in flake ice and slurry ice alone. Additionally, variations of myofibrillar proteins were analyzed via sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE).
Materials and methods
Materials
Fresh bighead croaker (Collichthys niveatus), with an average length and weight of 14 cm and 50 g, respectively, was purchased from West wharf (Zhejiang, China). The fish was stored at −20 °C for further use after they were conveyed to the laboratory. All the chemicals employed in the present study were of analytical grade purity and commercially available.
Refrigeration facility
Slurry ice in this study was prepared by a RF-1000-SP prototype (Nantong Ruiyou Trade Co., Ltd., Jiangsu, China) from filtered seawater with salinity approximately 3.3%. The binary mixture, with a temperature of −1.5 °C, contained 40% ice and 60% water. Ozone, provided by RQ ozone generator (Ruiqing Ozone Equipment Co., Ltd, Shandong, China), was injected to slurry ice at a final concentration of 0.2 mg/L, whereas in the renewed mixtures, its concentration was controlled at 0.1 mg/L. The ozone concentration was inspected by redox potential in the liquid phase. In control batches, preparation of flake ice was conducted on an ice maker (DTY-ZBJ-100, Beijing, China) from tap water.
Sample treatment
To evaluate the preservation efficacy of ozonised slurry ice on bighead croaker, the fish were packed in a vacuum plastic bag and thawed for 12 h in a refrigerator at 4 °C before they were immersed in ozonised slurry ice with a fish/ice ratio of 1:1. Simultaneously, the fish was also placed in slurry ice, and samples surrounded with flake ice were taken as control group. Afterwards, all the three batches were stored at 2 °C in a refrigerator for 21 days. Ice was renewed when necessary during storage and ozone concentration was adjusted to 0.1 mg/L in the renewed mixtures to avoid detrimental effect. All analyses were performed in triplicate at an interval of 3 days.
Sensory analysis
Sensory analysis was performed according to odor, appearance, texture, and overall acceptability by 50 trained panelists from our Food Science and Technology Department. Importantly, consent was sought for prior to their participation. Each of them was asked to indicate their preferences independently on a discrete 9-point hedonic scale (1 = dislike extremely, 2 = dislike very much, 3 = dislike moderately, 4 = dislike slightly, 5 = neither like nor dislike, 6 = like slightly, 7 = like moderately, 8 = like very much and 9 = like extremely). The samples were placed in covered plastic cups and labeled with 3 random numbers. Sensory attributes were evaluated in a sensory laboratory with well equipped individual booths and average of 50 scores for each attribute was recorded. Odor was assessed by short sniffs immediately after opening the lids. Samples with scores higher than or equal to 5 were regarded as acceptable (Meilgaard et al. 2006).
Microbiological analyses
Samples (25 g) were aseptically introduced into 225 ml peptone water (0.1%) in a stomacher bag and were homogenized for 2 min. Subsequently, a serial of ten-fold dilutions from the microbial extracts were performed using the same diluent, 0.1 ml of which was pipetted onto the surface of the plate count agar in Petri dishes for total viable count (TVC). Agar plates were incubated at 30 °C for 48 h.
Chemical analyses
Determination of TVB-N
Fish muscle (5 g) was extracted with 45 ml of perchloric acid and mashed. After proper homogenization, the mixture was filtered and 5 ml of the filtrate was transferred into the digester tubes containing 5 ml of sodium hydroxide and 1 or 2 drops of phenolphthalein as the indicator. Distillation was performed and the distillate was collected in a conical flask containing 10 ml of boric acid (3%) and 2 or 3 drops of a mixed indicator produced from methyl red and methylene blue. Afterwards, the above solution was titrated with hydrochloric acid (0.1 mol/L). The results were expressed as mg TVB-N per 100 g samples and TVB-N value was determined according to the consumption of hydrochloric acid.
Determination of peroxide value (PV)
Approximately 2 g of samples were homogenized with 30 ml of glacial acetic acid and chloroform solution (ratio 3:2) in a conical flask, which was then shaken vigorously and added with 1 ml of saturated potassium iodide. After keeping the mixture in the dark for 5 min, 30 ml of distilled water and 1 ml of 1% starch were supplemented to generate an indicator solution. After shaking, the mixture was titrated with 0.01 mol/L sodium thiosulfate. Titration was terminated upon the disappearance of blue color. The peroxide values were expressed as milli equivalents (mEq) of active O2 per kg samples.
Determination of thiobarbituric acid (TBA)
Fish flesh (5 g) was homogenized with 50 ml of 7.5% trichloroacetic acid (TCA) containing 0.1% EDTA. The mixture was filtered after shaking for 25 min. Subsequently, 5 ml of the filtrate and equivalent amount of TBA (0.02 mol/L) were mixed and heated in a boiling water bath for 30 min. After cooling, the mixture was centrifuged at 12,000 rpm/min for 20 min and the supernatant was added with 5 ml of chloroform. After shaking, standing, and demixing of the mixture, the absorbance of the supernatant was measured at 532 nm and 600 nm, respectively. TBA was expressed as mg malonaldehyde (MDA) per kg samples and calculated as follows: TBA = [(A532 nm − A600 nm) × 0.05 × 72.6)]/(155 × m) × 1000, where m stood for the mass of the sample.
Texture profile analysis (TPA)
The texture features of fish samples were evaluated using a TMS-PRO texture analyzer (TMS-PRO, FTC, Virginia, USA) equipped with a 5 mm diameter cylindrical probe (P/5) under the conditions of 4 mm/s test speed and a trigger load of 0.6 N. Texture profile analysis was performed with the dorsal muscle above the lateral line of each fish (2 cm × 2 cm × 0.5 cm), which was compressed twice to 50% of its original height. The parameters, including hardness and springiness, were calculated as described by Bourne (2002).
SDS–PAGE analysis
The variations in protein profiles under different storage conditions were examined by using SDS–PAGE, which was carried out using 5% stacking gel and 12% separating gel. Chopped fish fillet (2 g) was homogenized with 18 mL of SDS (50 g/L, 85 °C). After homogenizing for 5 min, preserving at 85 °C for 1 h, and cooling down, the homogenate was centrifuged at 3500×g for 20 min. The supernatant was diluted 1:1 in loading buffer (0.01 mol/L Tris–HCl, pH 6.8, 10% SDS, 5% 2-mercaptoethanol, 10% glycerol and 0.1% bromophenol blue). The mixture was boiled for 5 min and then centrifuged at 12,000 r/min for 1 min. Aliquots of 10 μL of the supernatants were loaded into each well and electrophoresis was performed at 180 V during the run. A molecular weight (MW) marker, composing of a cocktail of proteins (10–200 kDa, TaKaRa Biotechnology Co., Ltd., Dalian, China) was also run. The protein bands on the gel were visualized by Coomassie Brilliant Blue R-250 staining and were de-stained in 10% acetic acid.
Microstructure observation by scanning electron microscope (SEM)
The microstructure of fish muscle was observed under SEM. Dimensions of 3 mm × 2 mm × 2 mm were scraped with a blade from samples stored in flake ice, slurry ice, and ozonised slurry ice, respectively, on the 8th day. Afterwards, fixation of these pieces was performed in 0.1 mol/L phosphate buffer (pH 7.4) containing 3% (v/v) glutaraldehyde and 2% (v/v) formaldehyde with a ratio of 4:1 at 4 °C for 24 h. The specimens were dehydrated stepwise after successive immersions in ethanol with graded concentrations of 30, 50, 70, 80, and 90% (v/v). The samples were maintained for 15 min in each ethanol concentration up to 90%, thereafter dehydrated 30 min in absolute ethanol three times. Subsequently, prior to examining by SEM, all samples were dehydrated on a lyophilizer and coated with a thin film of gold by an ion sputtering device.
Statistical analysis
Data were subjected to one-way analysis of variance (ANOVA) of SPSS software. Significant differences (p < 0.05) between mean values were determined using Duncan’s multiple range tests.
Results and discussion
Changes in the TVC
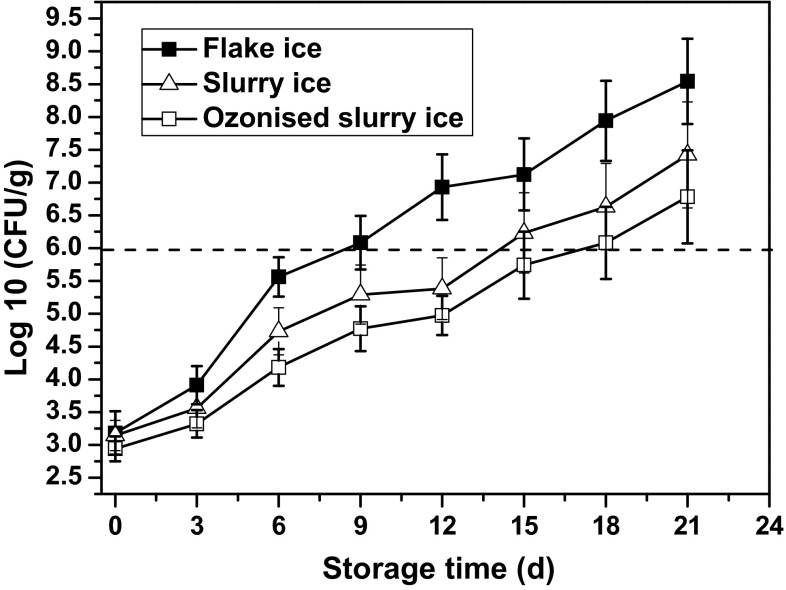
In the present study, changes in TVC of bighead croaker were monitored during storage in flake ice, slurry ice, and ozonised slurry ice (Fig. 1). The initial TVC was between 2.94 ± 0.19 to 3.18 ± 0.33 log CFU/g, reflecting relatively good state of the fresh fish. Steady increasing trends of bacterial load were observed across all the samples, although at different rates. Feasibly, microbial contents proliferated quickly when bighead croaker was subjected to flake ice storage. A similar microbial proliferation trend has been observed by Campos et al. (2005) in sardine muscle stored in flake ice. At day 6, TVC values were 0.83 and 1.38 log CFU/g lower for specimens stored in slurry and ozonised slurry ice, respectively, as compared with the counterpart ones in flake ice. Progress in storage revealed increase in differences among treatments, which could suggest good microbial control potential of slurry and ozonised slurry ice. Whilst TVC of flake ice stored samples reached 6.08 ± 0.41 log CFU/g by day 9, we respectively found 4.77 ± 0.34 and 5.29 ± 0.45 log CFU/g for samples stored in slurry and ozonised slurry ice (p < 0.05). In general, the acceptable microbial limit was set as 6 log CFU/g of TVC for cold-stored fishery products (Özogul et al. 2005), and in this sense, prolonged shelf life of 18 and 15 days was achieved for bighead croaker stored slurry ice and ozonised slurry ice, respectively, compared with 9 days for counterpart samples stored in flake ice.
Fig. 1.
Changes in TVC of bighead croaker stored in slurry ice, ozonised slurry ice and flake ice. Vertical bars represent standard error of the mean (n = 3)
It is believed that application of slurry ice technique began with Chapman (1990) with aim to preserve aquatic food products and it appeared as a novel chilling system and an effective method for maintaining product quality. The work of Chapman turned out to be validated by later authors who showed slurry ice was capable of inhibiting microbial growth and slow down the rate of spoilage of a wide range of fishery products. Obviously lower counts of aerobic mesophiles, anaerobes, coliforms, and proteolytic bacteria were detected in turbot muscle stored in the slurry ice batch than those in flake ice stored samples (Rodríguez et al. 2006). Campos et al. (2005) found that ozonised slurry ice resulted in notable reduction of microbial counts, including aerobic mesophiles, psychrotrophic bacteria, anaerobes, and coliforms in sardine muscle compared with samples stored in slurry ice alone, thereby demonstrating some bactericidal effect of ozone.
Changes in sensory attributes
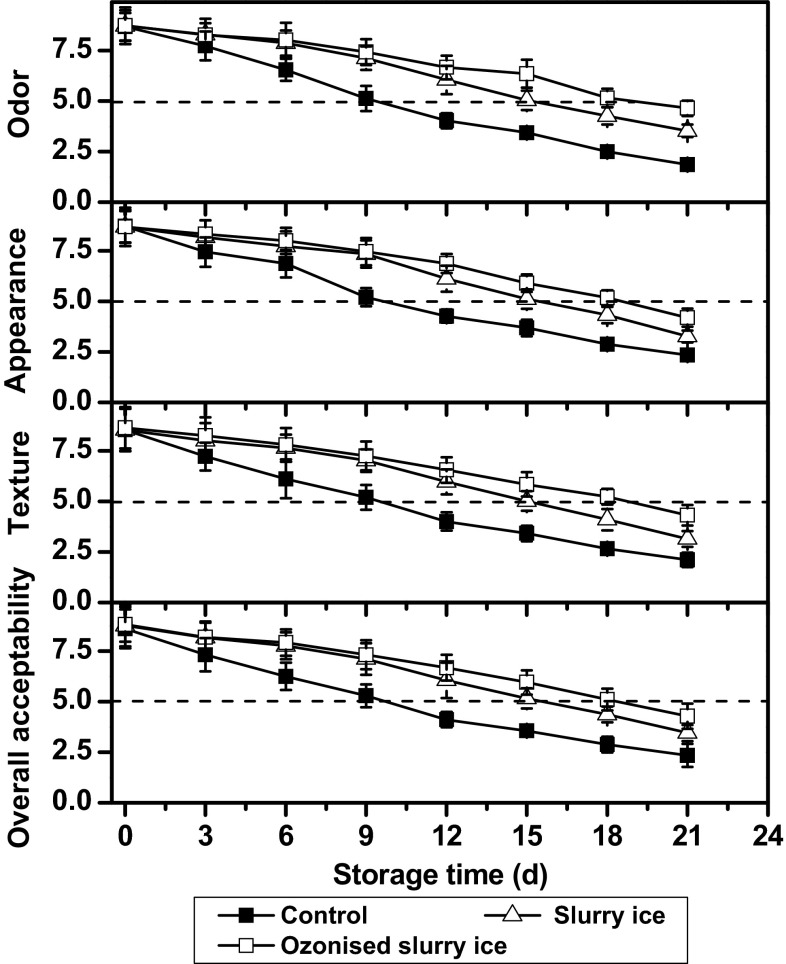
The results of the sensory evaluation of bighead croaker stored in flake, slurry, and ozonised slurry ice are shown in Fig. 2. At the start of storage, no significant differences (p > 0.05) were found such attributes as odor, appearance, texture, and overall acceptability, among the three aforementioned batch samples. All the scores appeared to be higher than 8 on a 9-point hedonic scale, which were between “like very much” and “like extremely”. All samples, as storage time progressed, displayed decreasing trends in sensory attributes, indicating gradual loss of freshness. However, samples stored in flake ice seemed to lose freshness more rapidly than those preserved in slurry ice and ozonised slurry ice, depicted by extremely low scores of the flake ice group, i.e., 1.87 ± 0.19, 2.34 ± 0.18, 2.11 ± 0.36, and 2.34 ± 0.37 for odor, appearance, texture, and overall acceptability, respectively. On the contrary, scores of the samples stored in slurry and ozonised slurry ice were significantly higher (p < 0.05), especially when ozone was present, thereby suggesting that both slurry ice and ozone allowed better maintenance of quality. Bighead croaker stored in ozonised slurry ice maintained “like moderately” to “like very much” quality up to day 9, and were acceptable up to day 18, whereas samples scored below 5 when slurry ice was used alone until day 15. Samples subjected to flake ice were no longer acceptable by day 9.
Fig. 2.
Changes in sensory properties of bighead croaker stored in slurry ice, ozonised slurry ice and flake ice. Vertical bars represent standard error of the mean (n = 3)
Feasibly, the use of slurry ice, either alone or in combination, would promise extension of shelf life. The presence of ozone might be playing a strong role, in this context. The shelf life of bighead croaker would likely be estimated at about 18, 15, and 9 days for samples processed by/stored in ozonised slurry, slurry, and flake ice, respectively. Predicting the shelf life by sensory attributes together with other means can be helpful; however, several factors, including chemical composition of fish, capturing method, state before capture, storage conditions and seasons, would influence sensory attributes (Erkan and Özden 2008).
Changes in TVB-N
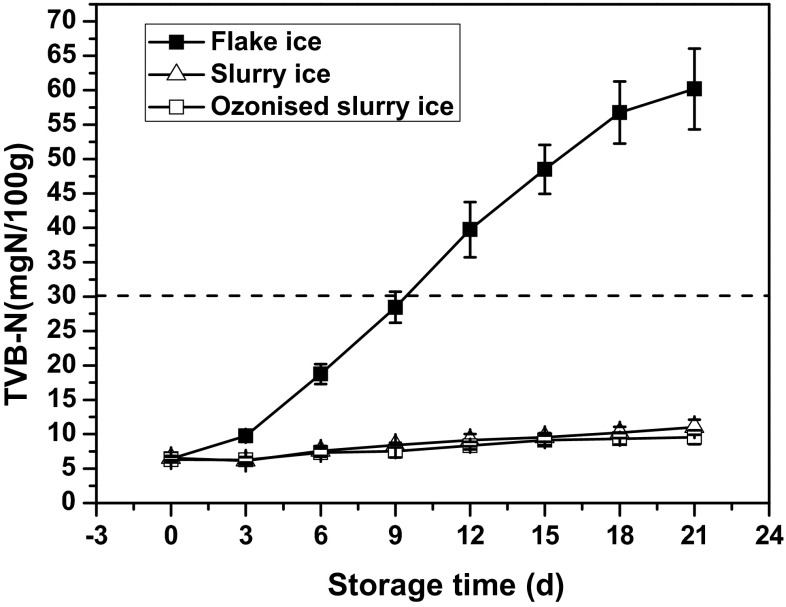
TVB-N is commonly regarded as a useful index of quality deterioration for seafood during storage (Erkan and Özden 2008; Okpala et al. 2014; Okpala 2014b). Changes in TVB-N of bighead croaker stored in flake, slurry, and ozonised slurry ice are presented in Fig. 3. There were no significant differences (p < 0.05) in initial TVB-N for all the three groups of samples. Subsequently, sharp increases in TVB-N values were observed for samples stored in flake ice from day 6. However, increase of TVB-N was substantially (p < 0.05) slower in samples stored in slurry and ozonised slurry ice than in the flake ice batch. TVB-N contents of bighead croaker stored in slurry and ozonised slurry ice were quite low throughout storage and they underwent slight increase, thereby indicating a considerably low formation of volatile bases in the fish muscle in these two batches. TVB-N of the flake ice batch was determined to be 28.46 ± 2.26 mg/100 g on day 9, whereas lower values of 8.42 ± 0.34, and 7.54 ± 0.93 mg/100 g, respectively, were achieved for slurry and ozonised slurry ice batches, respectively. At the end of the storage period, TVB-N value raised to 60.21 ± 5.87 mg/100 g, whereas counterpart batches stored in slurry and ozonised slurry ice displayed noticeably lower TVB-N contents of 11.02 ± 1.11 and 9.56 ± 1.02 mg/100 g, respectively. A level of 30 mg N/100 g TVB-N has been regarded as the acceptable limit for fishery products, above which they are considered spoiled and unfit for human consumption (Harpaz et al. 2003). TVB-N reached spoilage limit by day 9 for the flake ice group, while this parameter remained below the acceptable limit throughout the period for the other two groups, implying that slurry ice could effectively retard TVB-N formation. It should be stressed that all the samples subjected to slurry ice and slurry ice were acceptable according to TVB-N contents, even when the specimens were considered as rejected based on TVC and sensory analyses. Similar results were also obtained by Campos et al. (2005), indicating a limited effect of TVB-N production on the spoilage of sardine. It is reported that volatile ammonium containing compounds are mainly produced by Pseudomonas spp., which is one of the commonly known specific spoilage organisms (SSO) and it grows well on seafood. According to TVC and sensory analysis of the current study, the fish stored in slurry ice ought to be rejected by day 15, whereas those subjected to ozonised slurry ice ought to be unfit for human consumption after 18 days. However, based on the research of Okpala et al. (2014), which reported TVB-N scales of acceptability for raw seafood are: <12 mg N/100 g for fresh, 12–20 for edible but slightly decomposed, 20–25 for borderline, and >25 mg N/100 g for inedible and spoiled, TVB-N remained fresh level even at the end of storage period for slurry and ozonised slurry ice batches. The above findings strongly emphasized that TVB-N in our study might not be completely sufficient as an indicator of freshness and hence it is useful to monitor other freshness/spoilage parameters throughout storage. Similar results were also reported by other researchers, who observed that TVB-N increased only at the last stage of storage or after organoleptic rejection, making it not sufficient to present alone for chemical spoilage indices (Cakli et al. 2007; Parlapani et al. 2015).
Fig. 3.
Changes in TVB-N of bighead croaker stored in slurry ice, ozonised slurry ice and flake ice. Vertical bars represent standard error of the mean (n = 3)
Changes in PV and TBA
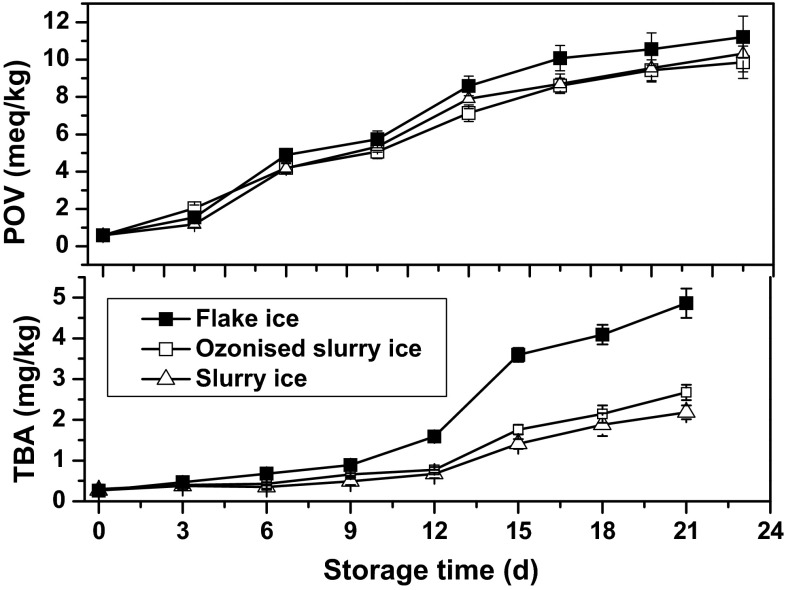
PV and TBA values have been widely employed for evaluating the degree of lipid oxidation, the former of which is an index of the first stage oxidation, while the latter represents the secondary oxidation from peroxides to aldehydes and ketones (Okpala et al. 2014; Okpala 2014a; Okpala et al. 2016). The development of rancidity of all samples was investigated by measuring PV and TBA values herein illustrated in Fig. 4. Increases in PV were detected with storage time, though occurring rather different rates as it reached 11.21 ± 1.12, 9.86 ± 0.87 and 10.32 ± 0.98 meq/kg for flake ice, slurry ice and ozonised slurry ice groups, respectively, on day 21 (Fig. 4a). PV, representing the hydroperoxides contents, were significantly higher (p < 0.05) for the flake ice group than those of the other two groups, indicating that slurry ice appeared effective in retarding the formation of PV, probably due to the inhibitory effect on endogenous protease or protease harboring microorganisms. Despite the positive effect of ozone, its oxidation capacity is considered as potential candidate able to accelerate lipid oxidation, which in turn might unintentionally aggravate quality deterioration of fish and fish products (Naito 2012; Okpala 2016; Okpala et al. 2016). Herein, the choice of ozone concentration in slurry ice demonstrated itself as a potential candidate to offer minimal effects on the production of primary lipid oxidation products. Moreover, the expected impact of ozone concentration in slurry ice also seems not clearly distinguishable comparing the PV values of both ozonised slurry-ice and slurry-ice group during the entire storage period of this study. This understanding was deduced based on the fact that the PV values of slurry ice group tended to resemble (p > 0.05) those of ozonised slurry iced samples. This may also be addressing the resultant minimal effects of ozone on lipid oxidation products in this study.
Fig. 4.
Changes in PV and TBA of bighead croaker stored in slurry ice, ozonised slurry ice and flake ice. Vertical bars represent standard error of the mean (n = 3)
Concerning TBA values also, which quantitatively detects malonaldehyde (MDA) content (Kamal-Eldin 2003; Okpala 2014a; Okpala et al. 2016), no significant differences (p < 0.05) were observed for initial TBA values of the three groups, i.e. 0.26 ± 0.02, 0.27 ± 0.02, and 0.30 ± 0.01 mg/kg for flake ice, slurry ice, and ozonised slurry ice groups, respectively. Subsequently, gradual increases in TBA occurred with the storage time for all three groups, indicating the formation of secondary lipid oxidation products (Kamal-Eldin 2003; Okpala 2014a, 2016). During the entire storage, significantly higher (p < 0.05) TBA contents were found in flake ice groups in comparison to slurry ice and ozonised slurry ice groups, suggesting that slurry ice was effective in slowing down the secondary oxidation rate. In addition, no obvious differences (p > 0.05) were observed between the TBA values of slurry iced samples and ozonised slurry iced ones, further demonstrating the current ozone concentration in slurry ice displayed somewhat slightly negative effect on lipid oxidation. In general, 8 mg malonaldehyde/kg flesh is considered the acceptable limit for most seafood (Okpala 2014a). Interestingly, TBA values of all three groups were below this limit, whereas at the end of the storage, obvious off-odor or taste has been detected by panelists in these samples. Hence, TBA values may not reflect actual rate of lipid oxidation since reactions might occur between malonaldehyde and other components of the fish, such as nucleic acid, nucleosides, proteins, amino acids and phospholipids (Bahmani et al. 2014).
Electrophoretic patterns of myofibrillar proteins
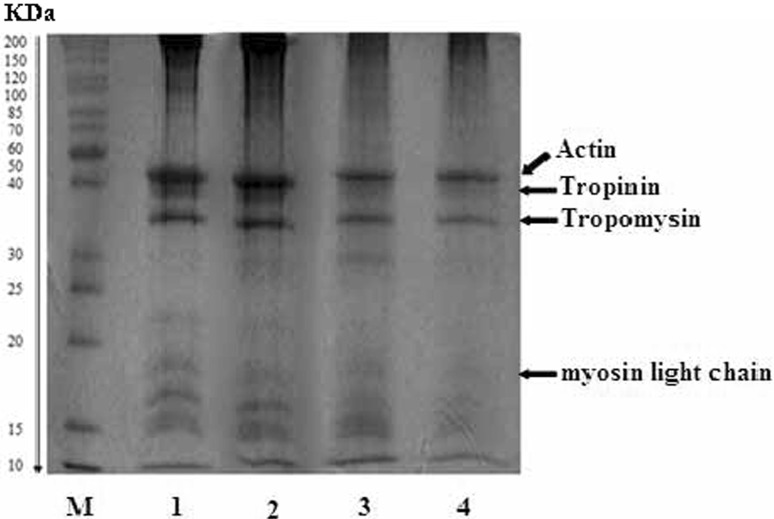
To identify changes in myofibrillar proteins profile of bighead croaker under different storage conditions, SDS–PAGE was performed as shown in Fig. 5. We could detect four major protein bands, including actin, troponin, tropomyosin, and myosin light chain, within the muscle of the fish, which presented a similar protein profile to previously reported results of muscle protein in other seafoods (Liu et al. 2014; Mignino and Paredi 2006). Samples stored in ozonised slurry ice assumed a protein profile closely matched with the fresh sample. The band intensity of actin, troponin, and tropomyosin did not exhibit significant differences for all the samples. The intensity of these three bands slightly decreased for samples stored in slurry ice and flake ice compared with those of fresh samples and the ones stored in ozonised slurry ice. However, significant decrease of the intensity of myosin light chain was observed when samples were stored in flake ice and slurry ice. A weak band of myosin light chain appeared in the fish subjected to slurry ice, while the band almost disappeared for the counterpart stored in flake ice. These findings suggested that samples stored in ozonised slurry ice exhibited rather decreasing proteolytic degradation, followed by those in slurry and flake ice. It is reported that protease activity, both endogenous and from some spoilage microorganisms, accounts for the protein degradation (Sánchez-Alonso et al. 2007). On this sense, changes in SDS–PAGE protein profiles of bighead croaker stored in slurry ice, ozonised slurry ice and flake ice were in well agreement with changes in TVC values.
Fig. 5.
Changes in SDS–PAGE protein profiles of fresh bighead croaker and samples stored in slurry ice, ozonised slurry ice and flake ice. M molecular weight of standard protein, 1, 2, 3, 4 fresh samples, samples stored in ozonised slurry ice, slurry ice and flake ice for 8 days
Microstructure analysis
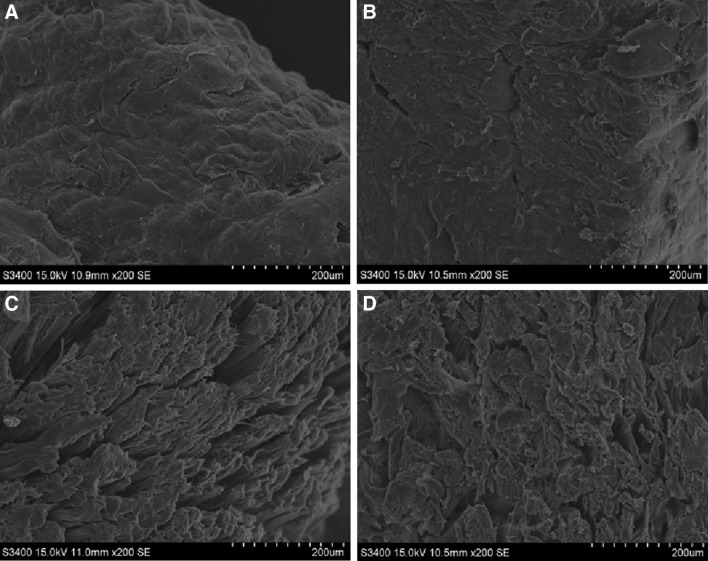
The microstructure of the muscle of bighead croaker stored in slurry ice, ozonised slurry ice and flake ice was observed under SEM (Fig. 6). The microstructure of muscle under different storage conditions displayed significant differences in myofibrils, as well as in intramuscular connective tissues. In case of fresh fish muscle, distinct and dense myofibrils structure was observed. Meanwhile, no obvious cracks were detected in intramuscular connective tissues for this batch. However, loose myofibrils structure and marked ruptures appeared when samples were stored in flake ice on day 8, which suggested the deterioration of microstructure. Those of slurry-iced samples seemed not too different, although the changes have indeed turned out to be much smaller, exhibiting reduction in the deterioration of microstructure. It could be observed that myofibril structure of ozonised slurry iced samples remained regular and dense. Moreover, the detectable connective tissues seemed to be adhered tightly to each other, which appeared to be similar to those of the fresh fish. These observations in microstructure changes of samples stored in different chilling media seemed to be in accordance with the above-mentioned analyses, including TVC, TVB-N, sensory properties, and SDS–PAGE patterns, further confirming the positive effect of slurry ice, especially the combined use of ozone, on cold-storage stability of microstructure.
Fig. 6.
Scanning micrographs of cross-section and surface of muscles of bighead croaker stored in slurry ice, ozonised slurry ice and flake ice. a Fresh muscle, b ozonised slurry ice muscle after 8-days storage at 4 °C, c slurry ice muscle after 8-days storage at 4 °C, d flake ice muscle after 8-days storage at 4 °C
TPA analysis
Texture attributes like hardness and springiness, which are related to the denaturation of the protein, are often used as freshness indicators for fish (Bourne 2002). As shown in Fig. 7a, the hardness of bighead croaker significantly decreased (p < 0.05) during 21 days storage for all the three groups, demonstrating that fish muscle might have softened quickly. Although initial hardness values of the three batches seemingly resembled (p > 0.05), the ozonised slurry iced samples displayed much reduced softening rate(p < 0.05), remaining approximately 37.72% of its original hardness on day 21, whereas muscles stored in slurry ice and flake ice lost about 72.38 and 85.94% of their initial harness values, respectively.
Fig. 7.
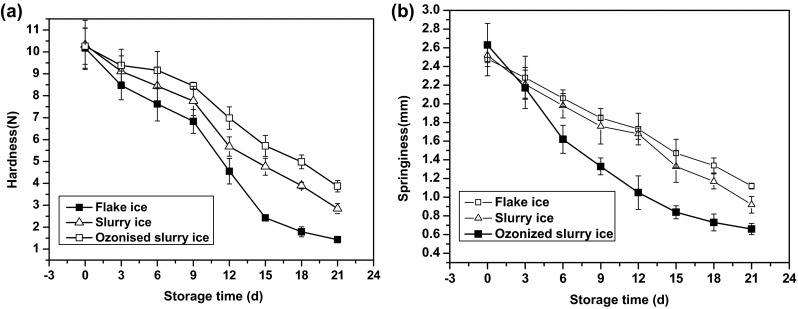
Changes in hardness and springiness of bighead croaker stored in slurry ice, ozonised slurry ice and flake ice. Vertical bars represent standard error of the mean (n = 3)
Regarding springiness, a decreasing trend, similar to that of hardness, was observed with storage time (p < 0.05) (Fig. 7b). At the end of the storage period, the ozonised slurry ice group obtained the highest springiness value (1.12 ± 0.03 mm), whereas the flake iced group reached the lowest springiness value (0.66 ± 0.06 mm). Authors believe that the samples stored in ozonised slurry ice appeared significantly more elastic (p < 0.05) relative to the other two groups, followed by those preserved in slurry ice. This tends to suggest an synergistic inhibitory effect of slurry ice and ozone to reduce texture changes.
The weakening of Z-discs of myofibrils, myosin actin junctions, coupled with degradation of connective tissue may soften and tenderize the fish muscle during chilled/cold storage (Okpala 2014a). Most likely, these causes may be owed to the action of endogenous enzymes and microbial activity. In fact, such autolytic enzymes as calpains, cathepsins as well as collagenases can facilitate the hydrolysis of myofibrillar protein (Cai et al. 2014). Moreover, the increasing microbial activity further contributes in the breakdown of the proteins. As earlier mentioned, microbial proliferation had occurred during chilled storage, and the variations in hardness and springiness of the fish muscle of all the three batches seemed to be connected with the microbial growth rate of corresponding batches. Feng et al. (2012) also observed that texture attributes of the black sea bream closely matched with the microbial activity. Double influences of the slurry ice immersion, i.e., stronger inhibition on endogenous enzymes and spoilage microorganisms, could probably account for the improved maintenance of muscle texture stability in slurry ice, compared with the flake ice ones. Furthermore, the addition of ozone in slurry ice could also be enhancing the double inhibitory effects, which might also be exerting an additional combined positive effect on the texture of fish muscle.
Conclusion
In this paper, the efficiency of chilling system combined with ozone and slurry ice was evaluated by a series of microbial and biochemical parameters during the storage of bighead croaker. Ozonised slurry ice treatment produced significant effects on TVC, sensory attributes, as well as hardness and springiness of the inspected fish samples during storage, which differed in many respects from the data obtained for flake ice and slurry ice alone. The TVB-N, PV and TBA of ozonised slurry ice and slurry ice groups obtained higher values compared to those of the flake ice. In addition, ozonised-slurry ice showed good promise towards reducing the degradation of myofibrillar proteins and deterioration of microstructure. Overall, we can infer that the combination of ozone and slurry ice allowed good maintenance of quality and promised further extension of product shelf life. The combined use of ozone and slurry ice proves useful candidate to assure for food safety competing effectively with other chilling methods utilized in the fisher industry/sector.
Acknowledgements
This work was supported by Program of Intl. S&T Cooperation, China (2012DFA30600), National Key Technologies R&D Program of China during the 12th Five-Year Plan Period (2012BAD29B06), Zhejiang Provincial Key Laboratory of Health Risk Factors for Seafood, Natural Science Fund for Young Scholars of Zhejiang Province, China (LQ15C200009) and ZheJiang Ocean University foundation (21135012614).
References
- Aubourg SP, Losada V, Gallardo JM, Miranda JM, Barros-Velazquez J. On-board quality preservation of megrim (Lepidorhombus whiffiagonis) by a novel ozonised-slurry ice system. Eur Food Res Technol. 2006;223:232–237. doi: 10.1007/s00217-005-0182-z. [DOI] [Google Scholar]
- Aubourg SP, Losada V, Prado M, Miranda JM, Barros-Velazquez J. Improvement of the commercial quality of chilled Norway lobster (Nephrops norvegicus) stored in slurry ice: effects of a preliminary treatment with an antimelanosic agent on enzymatic browning. Food Chem. 2007;103:741–748. doi: 10.1016/j.foodchem.2006.09.022. [DOI] [Google Scholar]
- Bahmani Z, Rezaei M, Hosseini SV, Hosseini SF, Alishahi A, Ahmad M, Regenstein JM. Effect of delayed icing on the microbiological, chemical, and sensory properties of caspian sea golden grey mullet (Liza aurata) J Aquat Food Prod Technol. 2014;23:542–551. doi: 10.1080/10498850.2012.731677. [DOI] [Google Scholar]
- Bono G, Okpala COR, Alberio GRA, Messina CM, Santulli A, Giacalone G, Spagna G. Toward shrimp consumption without chemicals: combined effects of freezing and modified atmosphere packaging (MAP) on some quality characteristics of Giant Red Shrimp (Aristaeomorpha foliacea) during storage. Food Chem. 2016;197:581–588. doi: 10.1016/j.foodchem.2015.10.146. [DOI] [PubMed] [Google Scholar]
- Bourne MC. Principles of objective texture measurement. In: Bourne MC, editor. Food texture and viscosity: concept and measurement. 2. San Diego: Academic Press; 2002. pp. 107–188. [Google Scholar]
- Cai LY, Wu XS, Dong ZJ, Li XP, Yi SM, Li JR. Physicochemical responses and quality changes of red sea bream (Pagrosomus major) to gum arabic coating enriched with ergothioneine treatment during refrigerated storage. Food Chem. 2014;160:82–89. doi: 10.1016/j.foodchem.2014.03.093. [DOI] [PubMed] [Google Scholar]
- Cakli S, Kilinc B, Dincer T, Tolasa S. Effects of using slurry ice during transportation on the microbiological, chemical, and sensory assessments of aquacultured sea bass (Dicentrarchus labrax) stored at 4 degrees C. Crit Rev Food Sci Nutr. 2006;46:453–458. doi: 10.1080/10408390500295425. [DOI] [PubMed] [Google Scholar]
- Cakli S, Kilinc B, Cadun A, Dincer T, Tolasa S. Quality differences of whole ungutted sea bream (Sparus aurata) and sea bass (Dicentrarchus labrax) while stored in ice. Food Control. 2007;18:391–397. doi: 10.1016/j.foodcont.2005.11.005. [DOI] [Google Scholar]
- Campos CA, Rodríguez O, Losada V, Aubourg SP, Barros-Velazquez J. Effects of storage in ozonised slurry ice on the sensory and microbial quality of sardine (Sardina pilchardus) Int J Food Microbiol. 2005;103:121–130. doi: 10.1016/j.ijfoodmicro.2004.11.039. [DOI] [PubMed] [Google Scholar]
- Campos CA, Losada V, Rodríguez O, Aubourg SP, Barros-Velazquez J. Evaluation of an ozone-slurry ice combined refrigeration system for the storage of farmed turbot (Psetta maxima) Food Chem. 2006;97:223–230. doi: 10.1016/j.foodchem.2005.03.040. [DOI] [Google Scholar]
- Chapman L. Making the grade. Ice slurries get top marks for quality products. Aust Fish. 1990;7:16–19. [Google Scholar]
- Erkan N, Özden Ö. Quality assessment of whole and gutted sardines (Sardina pilchardus) stored in ice. Int J Food Sci Technol. 2008;43:1549–1559. doi: 10.1111/j.1365-2621.2007.01579.x. [DOI] [Google Scholar]
- Feng LF, Jiang TJ, Wang YB, Li JR. Effects of tea polyphenol coating combined with ozone water washing on the storage quality of black sea bream (Sparus macrocephalus) Food Chem. 2012;135:2915–2921. doi: 10.1016/j.foodchem.2012.07.078. [DOI] [PubMed] [Google Scholar]
- Gram L, Dalgaard P. Fish spoilage bacteria—problems and solutions. Curr Opin Biotechnol. 2002;13:262–266. doi: 10.1016/S0958-1669(02)00309-9. [DOI] [PubMed] [Google Scholar]
- Greene A, Güzel-Seydim ZB, Seydim AC. Chemical and physical properties of ozone. In: O’Donnell C, Tiwari BK, Cullen PJ, Rice RG, editors. Ozone in food processing. Oxford: Wiley; 2012. pp. 19–32. [Google Scholar]
- Harpaz S, Glatman L, Drabkin V, Gelman A. Effects of herbal essential oils used to extend the shelf life of freshwater-reared Asian sea bass fish (Lates calcarifer) J Food Protect. 2003;66:410–417. doi: 10.4315/0362-028x-66.3.410. [DOI] [PubMed] [Google Scholar]
- Kamal-Eldin A. Lipid oxidation pathways. Uppsala: AOCS Press; 2003. [Google Scholar]
- Liu Q, Kong B, Han JC, Chen Q, He XY. Effects of superchilling and cryoprotectants on the quality of common carp (Cyprinus carpio) surimi: microbial growth, oxidation, and physiochemical properties. LWT Food Sci Technol. 2014;57:165–171. doi: 10.1016/j.lwt.2014.01.008. [DOI] [Google Scholar]
- Losada V, Pineiro C, Barros-Velazquez J, Aubourg SP. Inhibition of chemical changes related to freshness loss during storage of horse mackerel (Trachurus trachurus) in slurry ice. Food Chem. 2005;93:619–625. doi: 10.1016/j.foodchem.2004.09.041. [DOI] [Google Scholar]
- Meilgaard MC, Carr BT, Civille GV. Sensory evaluation techniques. 4. New York: CRC Press; 2006. [Google Scholar]
- Mignino LA, Paredi ME. Physico-chemical and functional properties of myofibrillar proteins from different species of mollusks. LWT Food Sci Technol. 2006;39:35–42. doi: 10.1016/j.lwt.2004.12.004. [DOI] [Google Scholar]
- Naito S. Ozone in seafood processing. In: O’Donnell C, Tiwari BK, Cullen PJ, Rice RG, editors. Ozone in food processing. Oxford: Wiley-Blackwell; 2012. pp. 137–162. [Google Scholar]
- Nunes ML, Batista I, Decampos RM. Physical, Chemical and sensory analysis of sardine (Sardina pilchardus) stored in ice. J Sci Food Agr. 1992;59:37–43. doi: 10.1002/jsfa.2740590106. [DOI] [Google Scholar]
- Okpala COR. Changes of physico-chemical properties of sequential minimal ozone treated ice-stored pacific white shrimp (Litopenaeus vannamei) Curr Nutr Food Sci. 2014;10:218–227. doi: 10.2174/1573401310666140306003557. [DOI] [Google Scholar]
- Okpala COR. Investigation of quality attributes of ice-stored pacific white shrimp (Litopenaeus vannamei) as affected by sequential minimal ozone treatment. LWT Food Sci Technol. 2014;57:538–547. doi: 10.1016/j.lwt.2014.02.007. [DOI] [Google Scholar]
- Okpala COR. Lipid autoxidation in ozone-processed crustacea under cold storage: a treatise. Lipid Technol. 2016;28:93–95. doi: 10.1002/lite.201600026. [DOI] [Google Scholar]
- Okpala COR, Choo WS, Dykes GA. Quality and shelf life assessment of Pacific white shrimp (Litopenaeus vannamei) freshly harvested and stored on ice. LWT-Food Sci Technol. 2014;55:110–116. doi: 10.1016/j.lwt.2013.07.020. [DOI] [Google Scholar]
- Okpala COR, Bono G, Cannizzaro L, Jereb P (2016) Changes in lipid oxidation and related flesh qualities of white shrimp (Litopenaeus vannamei) during iced storage: Effects on the use of increasing ozone exposures. Euro J Lipid Sci Technol. doi: 10.1002/ejlt.201500347 (in press)
- Özogul Y, Ozyurt G, Özogul F, Kuley E, Polat A. Freshness assessment of European eel (Anguilla anguilla) by sensory, chemical and microbiological methods. Food Chem. 2005;92:745–751. doi: 10.1016/j.foodchem.2004.08.035. [DOI] [Google Scholar]
- Parlapani FF, Haroutounian SA, Nychas GJE, Boziaris IS. Microbiological spoilage and volatiles production of gutted European sea bass stored under air and commercial modified atmosphere package at 2 °C. Food Microbiol. 2015;50:44–53. doi: 10.1016/j.fm.2015.03.006. [DOI] [PubMed] [Google Scholar]
- Rodríguez Ó, Barros-Velázquez J, Piñeiro C, Gallardo JM, Aubourg SP. Effects of storage in slurry ice on the microbial, chemical and sensory quality and on the shelf life of farmed turbot (Psetta maxima) Food Chem. 2006;95:270–278. doi: 10.1016/j.foodchem.2004.11.054. [DOI] [Google Scholar]
- Sánchez-Alonso I, Careche M, Borderías AJ. Method for producing a functional protein concentrate from giant squid (Dosidicus gigas) muscle. Food Chem. 2007;100:48–54. doi: 10.1016/j.foodchem.2005.09.008. [DOI] [Google Scholar]
- Shen Q, Guo R, Dai ZY, Zhang YP. Investigation of enzymatic hydrolysis conditions on the properties of protein hydrolysate from fish muscle (Collichthys niveatus) and evaluation of its functional properties. J Agr Food Chem. 2012;60:5192–5198. doi: 10.1021/jf205258f. [DOI] [PubMed] [Google Scholar]
- Zengin N, Yuzbasioglu D, Unal F, Yilmaz S, Aksoy H. The evaluation of the genotoxicity of two food preservatives: sodium benzoate and potassium benzoate. Food Chem Toxicol. 2011;49:763–769. doi: 10.1016/j.fct.2010.11.040. [DOI] [PubMed] [Google Scholar]