Abstract
Indinavir is currently used at a fixed dose of 800 mg either three times a day or twice a day in combination with 100 mg of ritonavir. Dosage individualization based on plasma concentration monitoring might, however, be indicated. This study aimed to assess the pharmacokinetic profile of indinavir in patients infected with human immunodeficiency virus to characterize interpatient and intrapatient variability and to build up a Bayesian approach for dosage adaptation. A population analysis was performed with the NONMEM computer program with 569 plasma samples from a cohort of 239 unselected patients receiving indinavir. A one-compartment model with first-order absorption was adapted, and the influences of clinical characteristics on oral clearance (CL) and distribution volume (V) were examined. Predicted average drug exposure and trough and peak concentrations were derived for each patient and correlated with efficacy and toxicity markers. The population estimates of CL were 32.4 liters/h for female and 42.0 liters/h for male patients; oral V was 65.7 liters; and the rate constant of absorption (Ka) was 1.0 h−1. CL decreased by 63% with ritonavir intake and was moderately correlated to body weight. Both interpatient variability, best assigned to oral CL (coefficient of variation [CV], 39%) and Ka (CV, 67%), and intrapatient variability were large (CV, 41%; standard deviation, 670 μg/liter). In conclusion, initial indinavir dosage should be decided according to ritonavir intake and sex, prior to plasma concentration measurements. The high interpatient pharmacokinetic variability represents an argument for therapeutic drug monitoring.
Indinavir is a protease inhibitor (PI) used in association with reverse transcriptase inhibitors in the treatment of human immunodeficiency virus type 1 (HIV-1) infection. The currently recommended adult dosage is either 800 mg t.i.d or 800 mg b.i.d in combination with low-dose ritonavir. Indinavir has unfavorable pharmacokinetic behavior, with high peak concentrations that create a risk of adverse reactions, such as nephrolithiasis (14), and minimum concentrations that are only slightly above the 95% inhibitory concentration of the virus under the t.i.d regimen, due to extensive cytochrome P450- mediated metabolism (http://www.eudra.org/humandocs/humans/epar/crixivan/crixivan.htm). Large interpatient and intrapatient variability in indinavir disposition, as reported in other population analyses (27, 29), and poor adherence to recommendations regarding food intake or drug interactions may further weaken the antiviral coverage in spite of a strict compliance with the every-8-h regimen. Insufficient concentrations in plasma are clearly associated with a rebound in viral load and an increased risk of emergence of viral resistances. Combining ritonavir as a kinetic booster in indinavir-containing regimens has been successful, allowing the daily doses of such regimens to be reduced while at the same time improving their antiviral efficacy (7, 10, 18, 28). New strategies based on target concentration dosage adaptation have gained increasing importance for the management of HIV-infected patients, in view of the relationship between plasma drug levels, therapeutic outcomes, and toxicity (2, 3, 8, 16, 17, 19). The evaluation of the benefits and indications of therapeutic drug monitoring still lacks prospective clinical trials, and the optimal concentration range for indinavir remains controversial (2, 25). The objectives of this study were to determine the population pharmacokinetic parameters of indinavir and its variability in patients receiving indinavir alone or in combination with ritonavir and to detect factors which might explain indinavir's pharmacokinetic variability. The population analysis was then used to build up a Bayesian strategy for dosage regimen individualization based on therapeutic drug monitoring.
MATERIALS AND METHODS
Study population.
Plasma population pharmacokinetics of indinavir were analyzed with sparse samples collected over a 40-month period. A total of 569 plasma indinavir levels from 239 patient samples, collected from 490 visits (median number of visits = 2; range, 1 to 8), were obtained. Most blood samples were drawn periodically at 1- to 3-month intervals on follow-up visits along with virological and hematological tests. Additional measurements were taken from seven patient samples to obtain a full concentration-time profile, with up to 10 blood samples drawn between 0.3 and 8 h after drug intake. Of the 569 samples, 94 (17%) were collected up to 2 h after dosing, 275 (48%) were obtained between 2 and 8 h after dosing, and the remaining 200 (35%) were taken >8 h after drug intake. All samples were obtained under steady-state conditions (i.e., the dosage was unchanged for at least 1 month). The patients received 800 mg of indinavir orally either three times a day (t.i.d.) (62 patients) or twice daily (b.i.d.) in combination with 100 mg of ritonavir and other antiretroviral agents (177 patients). For 21 patients, a reduction in dosage to 400 or 600 mg b.i.d had been already applied, due to the occurrence of side effects under the standard dosage regimen. In addition to dosing and sampling time information, the following data were recorded for each patient: sex, body weight, height, age, race, duration of antiretroviral treatment, duration of PI-containing regimens, duration of the present regimen, and concomitant medications which might influence drug therapy (Table 1). The study population included 169 male and 70 female patients, their median age was 40.1 years (range, 16.3 to 73.4 years), their median body weight was 66.8 kg (range, 41 to 116 kg), and their median height was 172 cm (range, 150 to 194 cm). Information on ethnic origin was available for 133 patients: 122 patients were Caucasian, 6 patients were black, 1 patient was Asian, and 4 patients were Hispanic.
TABLE 1.
Characteristics of 239 patients evaluated in the population pharmacokinetics analysis of indinavir
| Characteristic | Valueb | % of study population |
|---|---|---|
| Sex (male/female) | 169/70 | 71/29 |
| Median age (male/female) (yr) | 40 (16-73) | |
| Median body wt (kg) | 67 (41-116) | |
| Median ht (cm) | 172 (150-194) | |
| Median CD4 count (cells/mm3)a | 433 (12-1,491) | |
| ≥200 | 82 | 77 |
| <200 | 19 | 18 |
| Partially controlled over the study period | 5 | 5 |
| Median viral load (range) (copies/mm3)a | 400 (1-750,000) | |
| ≥400 | 44 | 42 |
| <400 | 50 | 47 |
| Partially controlled over the study period | 12 | 12 |
| Ethnicity | ||
| Caucasian | 122 | 92 |
| Black | 6 | 5 |
| Asian | 1 | 0.8 |
| Hispanic | 4 | 3 |
| PI (ritonavir) | 177 | 74 |
| Reverse transcriptase inhibitors | ||
| Efavirenz | 22 | 9 |
| Lamivudine | 176 | 74 |
| Zidovudine | 128 | 54 |
| Stavudine | 67 | 28 |
| Didanosine | 20 | 8 |
| Abacavir | 23 | 10 |
| Median length of therapy (mo) | ||
| All antiretroviral therapiesa | 58 (1-130) | |
| PI-containing regimens | 40 (1-86) | |
| Present regimen with indinavir | 13 (1-52) | |
| CYP P450 inducers (phenobarbital, carbamazepine, rifampin) | 3 | 1 |
| CYP P450 inhibitors (azithromycin, ciprofloxacin, clindamycin) | 10 | 4 |
CD4 cell and viral load counts were available for 106 patients, ethnicity data were available for 133 patients, and length of time on previous therapy was known for 82 patients.
Values in parentheses represent ranges.
A systematic evaluation of toxic side effects (urinary, dermatological, and gastrointestinal) was performed, together with the determination of CD4 lymphocyte count and viral load. This study was approved by the local Ethics Committee. An informed written consent was obtained from participants until indinavir determinations were included in the routine follow-up of patients.
Analytical method.
Blood samples (5 ml each) were collected into lithium heparin or potassium-EDTA Monovette syringes (Sarstedt, Nümbrecht, Germany). Plasma was isolated by centrifugation, inactivated for HIV infectivity in a water bath at 60°C for 60 min, and stored at −20°C until analysis. Plasma indinavir levels were determined by reverse-phase high-performance liquid chromatography according to a validated method, enabling the simultaneous quantification of HIV PIs (23). The limit of quantification of the assay was 250 μg/liter with a coefficient of variation (CV) of <20% over the whole dynamic range, shown to be linear up to 10,000 μg/liter. Despite less-accurate quantification, detectable concentrations below 250 μg/liter were included in the pharmacokinetic analysis, since they provided informative value for the description of indinavir kinetics; concentrations significantly higher than 10,000 μg/liter were reassessed after sample dilution.
Model-based pharmacokinetic analysis.
The analysis was performed with the NONMEM computer program (version V running with NM-TRAN version II) (NONMEM Users Guides, University of California, San Francisco). The analysis used mixed-effects regression (fixed and random) to estimate population means and variances of the pharmacokinetic parameters and to identify factors that influence them. A stepwise procedure was used to find the model that fitted the data best. First, one- and two-compartment models with first-order absorption from the gastrointestinal tract were fitted to the data from the seven patients whose data underwent intensive kinetic investigation. The analysis of the entire population was then conducted on the basis of these initial estimates. Since indinavir was only administered orally, the clearance (CL) and distribution volume (V) represent apparent values (CL/F and V/F, where F is oral bioavailability). Other pharmacokinetic parameters were derived from the final model, namely, elimination and absorption half-lives (t1/2 values) and time to maximal plasma drug concentration (Cmax), according to classical steady-state formulae for repeated oral dosing. Exponential errors following a log-normal distribution were assumed for the description of interpatient variability of the pharmacokinetic parameters, and a combined exponential and additive model was assigned to the intrapatient (residual) variability. Potential influencing covariates were incorporated sequentially into the structural model. At the end of the analysis, all patient characteristics that showed an influence on the parameters were evaluated again by comparison of the full model (with all factors included) with a model from which each of the factors was deleted sequentially.
Parameter estimation and model selection.
The data were fitted by use of the first-order conditional method. The change in the objective function (OF) resulting from the addition of a covariate approximates a χ2 distribution and was regarded as statistically significant (P < 0.05) if it exceeded 3.8 for one, 5.9 for two, and 7.8 for three additional parameters. Moreover, for parameters quantifying covariate influences on indinavir pharmacokinetics as well as for CL, V, and the rate constant of absorption (Ka), 95% confidence intervals (95% ICs) were estimated by means of a likelihood ratio profile (5). A simulation based on the final pharmacokinetic estimates was performed with NONMEM by using data from 1,000 individuals to calculate the 95% prediction interval. The concentrations encompassing the percentiles 2.5 and 97.5 at each time point were retrieved to construct the 95% prediction interval. The figures were generated with S-PLUS (Statistical Sciences, version 4.0, release 2) and Prism (Graphpad Software, Inc., version 3.00).
Dosage regimen individualization.
The results of the population pharmacokinetic analysis were used to build up a Bayesian approach for exploiting drug concentration measurements (26). When the average population value of the kinetic parameters (θpop) at a given level of influential covariates, their respective variances (ω2), the residual variability (σ2), and the observed plasma drug concentrations (Cobs) are considered, the maximum-likelihood a posteriori parameter estimates for an individual patient (θind) are those that minimize the function
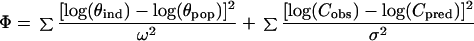 |
in which the predicted concentration of the drug in plasma (Cpred) is calculated from individualized parameters (θind). Because exponential (constant CV) error models were assumed during the population analysis, the θ and C values were entered as log values in the above equation. Individual Bayesian estimates of peak (Cmax), trough (Cmin), and average (Cav) plasma drug concentrations were used to explore their relationship with treatment outcome (viral load) and tolerability (adverse effects).
RESULTS
Population pharmacokinetic analysis.
The measured concentrations ranged between 8 and 17,870 μg/liter. Eighteen patients had on one single occasion a Cmin of 0 μg/liter while having levels above 1,000 μg/liter on other occasions under the same dosage regimen, suggesting poor compliance. These data were considered highly unlikely and were therefore removed from the model-building analysis.
A one-compartment model with first-order absorption from the gastrointestinal tract was found to describe the data appropriately. Despite a significant reduction in the OF (ΔOF = −28.3), a two-compartment model was not retained, since it did not remain significant after the assignment of ritonavir as a covariate on CL. Assignment of an interpatient variability on both CL (ΔOF = −72.2) and Ka (ΔOF = −21.6) improved the description of the data significantly, while no interpatient variability was an asset to V or F values (ΔOF < −1.2). The model-building steps for the covariate analysis are summarized in Table 2. Initial covariate analyses identified ritonavir (ΔOF = −280.4), body weight (ΔOF = −72.1), sex (ΔOF = −39.3), and height (ΔOF = −23.9) as significant covariates on CL, but none of these factors improved the fit when assigned on V. No significant influence from other demographic covariates (ethnicity and age) or comedication with CYP3A4 inducers (including efavirenz) or inhibitors could be detected (Table 2). Setting the HIV disease status (viral load, <400 copies/mm3; or CD4 count, >200 cells/mm3) as a covariate on CL did not improve the fit either (ΔOF = −2.6). A full model incorporating all the identified covariates was built up and further refined by setting them, one by one, to their null values. This step eliminated the influence of height on CL, but ritonavir, body weight, and sex remained statistically significant (ΔOF = −344.11).
TABLE 2.
Summary of the models used to examine the influence of patient covariates on indinavir oral CL and oral V
| Hypothesisa | Modela | Parameter
|
ΔOFb | |||
|---|---|---|---|---|---|---|
| θa | θb | θc | θd | |||
| Demographic characteristics | ||||||
| Does BW influence CL? | θa · (1 + θb · BW) | 20.1 | 0.4 | −72.1 | ||
| V? | 73.0 | <0.1 | −0.0 | |||
| Does HT influence CL? | θa · (1 + θb · HT) | 19.3 | 0.3 | −23.9 | ||
| V? | 73.0 | <0.1 | −0.0 | |||
| Does age influence CL? | θa · (1 − θb · age) | 16.4 | 0.4 | −0.7 | ||
| V? | 73.0 | <0.1 | −0.0 | |||
| Does sex influence CL? (male: sex = 1) | θa · (1 + θb · sex) | 12.2 | 0.6 | −39.3 | ||
| V? | 73.1 | <0.1 | −0.0 | |||
| Does ethnicity influence CL? | ||||||
| Black (q = 0) vs not Black (q = 1) | θa · (1 − q) + θb · q | 16.5 | 14.7 | −0.7 | ||
| Caucasian (q = 0) vs non-Caucasian (q = 1) | 13.6 | 16.5 | −1.4 | |||
| Asian (q = 0) vs non-Asian (q = 1) | 11.6 | 16.3 | −0.7 | |||
| Hispanic (q = 0) vs non-Hispanic (q = 1) | 21.6 | 16.2 | −1.0 | |||
| Concomitant HIV medications on CL | ||||||
| PIs (ritonavir) (PIs = 1 if present) | CL = θa · (1 + θb · ritonavir) | 39.1 | −0.7 | −280.4 | ||
| RTIs (RTI = 1 if present) | ||||||
| Efavirenz | CL = θa · (1 + θb · RTI) | 16.3 | <0.1 | −0.1 | ||
| 3TC, AZT, stavudine, didanosine, abacavir | 16.2 | <0.1 | −0.0 | |||
| Other concomitant medications on CL | ||||||
| CYP3A4 inducersc (inducer = 1 if present) | CL = θa · (1 + θb · inducer) | 16.9 | 0.2 | −0.5 | ||
| CYP3A4 inhibitors (inhibitor = 1 if present) | CL = θa · (1 − θb · inhibitor) | 16.0 | <0.1 | −0.0 | ||
| Virological and immunologic factors on CL | ||||||
| Viral load (≤400 copies/mm3 = 1) | CL = θa · (1 + θb · viral load) | 15.7 | 0.1 | −2.6 | ||
| CD4 count (≥200 cells/mm3 = 1) | CL = θa · (1 + θb · CD4 count) | 15.7 | 0.1 | −2.6 | ||
| Association of significant covariates on CL | ||||||
| Ritonavir + BW | CL = θa · (1 + θb · ritonavir) · (1 + θc · BW) | 39.5 | −0.63 | 0.3 | −313.6 | |
| Ritonavir + BW + sex | CL = θa · (1 + θb · ritonavir) · (1 + θc · BW) · (1 + θd · sex) | 32.4 | −0.63 | 0.16 | 0.30 | −344.1 |
BW, body weight; HT, height; RTIs, reverse transcriptase inhibitors; AZT, zidovudine; 3TC, lamivudine. BW, height, and age are expressed as the relative deviation of individual measurements from the population mean.
ΔOF, difference in the NONMEM OF compared to the basic structural model, including no covariates; estimates of CL = 16.3 liters; V = 73 liters; Ka = 0.95 h−1; and F = 1.
Inducers included efavirenz.
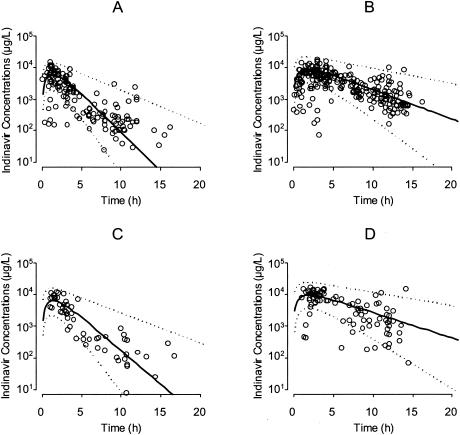
The population parameter values from the final model are given in Table 3. The oral CL of 32.4 liters/h in women and 42.0 liters/h in male patients decreases, respectively, to 12.1 and 15.7 liters/h in the presence of ritonavir and increases by a factor of 1.16 when body weight is doubled; oral V is 65.7 liters and t1/2 Ka is 41 min. The interindividual variability on CL was initially high (CV = 74.5%) and decreased after the introduction of the three significant covariates (CV = 39%). The interpatient variability on Ka remains important (CV = 67%). The residual intrapatient variability (CV = 41%; SD = 670 μg/liter) is of a magnitude similar to that of interpatient variability. The assignment of two distinct variabilities to the group of patients with or without ritonavir neither improved fit nor reduced the interpatient or intrapatient variability. The observed plasma indinavir concentrations are presented in Fig. 1, along with the average population prediction and 95% prediction intervals.
TABLE 3.
Population pharmacokinetic parameter estimates of indinavira
| Parameter | Population mean
|
Interindividual variabilityb
|
|||
|---|---|---|---|---|---|
| Estimate | 95% CI | % SEc | % Estimate | % SEc | |
| CLfemale (liters/h) | 32.4 | 27.8-37.2 | 13.5 | 39.0 | 44.8d |
| θmalee | 0.30 | 0.14-0.49 | 56.5 | ||
| θritonavirf | −0.63 | −0.6 to 0.64 | 6.5 | ||
| θBWg | 0.16 | 0.12-0.35 | 44.8 | ||
| V (liters) | 65.7 | 55.9-76.0 | 13.0 | ||
| Ka (h−1) | 1.0 | 0.80-1.41 | 21.8 | 67.0 | 65.5d |
| F | 1.0h | — | — | ||
| σ (CV%)i | 41 | 51.7d | |||
| σ (SD; μg/liter)j | 670 | 56.6 | |||
Final model for clearance: CL = CLfemale·(1 + θmale)·(1 + θritonavir)·[1 + θBW·(BW − 70)/70], with ritonavir, and sex (female or male) expressed as index variables.
Estimates of variability expressed as CV%.
SE, standard error of the estimates, expressed as CV%.
SE, standard error of the variance components, taken as 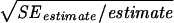 , expressed as a percentage.
, expressed as a percentage.
Relative increase in CL in male patients, compared to female patients.
Relative decrease in CL in presence of ritonavir.
Proportionality term relating CL to a relative increase or decrease in body weight (kg) from the average population value of 70 kg.
Set to 1 because intravenous drug administration not available, hence no 95% CI or % SE evaluable (—).
Residual variability in the plasma concentrations.
Additive component of the residual variability in the plasma concentration.
FIG. 1.
Plasma indinavir concentrations in samples from 239 HIV patients (circles). Samples from male patients receiving 800 mg of indinavir t.i.d. (A) or 800 mg of indinavir b.i.d with low-dose ritonavir (B) and from female patients receiving 800 mg of indinavir t.i.d. (C) or 800 mg of indinavir b.i.d. with low-dose ritonavir (D) are shown. Circles represent patient samples; solid line, average population prediction value; dashed lines, 95% prediction intervals.
Treatment efficacy and tolerability.
Viral load values ranged from 1 to 750,000 copies/mm3 (median, 400 copies/mm3). Viral suppression (according to the reference cutoff of ≤400 copies/mm3) was observed with 47% of patient samples. A very modest relationship between plasma indinavir concentration, estimated through Bayesian calculations, and viremia was observed both for Cav (r = 0.14; P = 0.018) and Cmax (r = 0.17; P = 0.057), but not for Cmin in this unselected patient population.
Urological complications (nephrolithiasis, pain on micturition, and nephritic colic) were observed in only seven patients (3%). In samples from these patients, Cmax estimates ranged from 5,163 to 11,731 μg/liter (mean = 8,629 μg/liter), but this trend did not reach statistical significance, probably due to the very limited number of affected patients and to indinavir dosage adjustments prior to enrollment in the study. No correlation between concentrations and other side effects was detected.
Dosage regimen adaptation.
A dosage adaptation is proposed based on the results of the population analysis, which assigned interpatient variabilities to oral CL and Ka. For female patients, the average population CL value (CLpop) without and with ritonavir was 32.4 and 12.1 liters/h, respectively; for male patients, CLpop without and with ritonavir was 42.0 and 15.7 liters/h. This value could be further multiplied by 1.16 times the relative deviation of the patient body weight from 70 kg. No covariate appeared to influence Ka, with a population value of 1.0 h−1. Thus, for a 70-kg male patient, the a priori predicted population, Cav and Cmin are 2,391 and 193 μg/liter under the standard regimen of 800 mg of indinavir t.i.d or 4,246 and 964 μg/liter with 800 mg of indinavir b.i.d with ritonavir. For a female patient, the values would be 3,086 and 466 μg/liter under the standard regimen of 800 mg of indinavir t.i.d and 5,510 and 1,839 μg/liter with 800 mg of indinavir b.i.d with ritonavir.
After having measured a single plasma concentration (Cobs) at time postdose, the a priori values of CLpop and Kapop can be altered according to the Bayesian strategy to meet a posteriori maximum-likelihood estimates of CL and Ka corresponding to the individual patient. The minimization of the function Φ has no analytical solution but can be solved numerically after integrating the population estimates of CLpop and Ka with their respective variances ωCL2 and ωKa2, the Cobs with its additive and multiplicative residual errors σadd and σprop, and the corresponding prediction Cpred given by the Bateman equation at a steady state.
Such individual estimates of CL and Ka enable the calculation of an a posteriori value of the patient's Cmin, which can be used to adapt indinavir dosing regimens to bring the concentrations into the effective target for optimizing viral suppression.
The weak relationship between drug exposure and therapeutic success or toxicity observed in the present study does not allow an estimation of the optimal therapeutic range of indinavir. According to the VIRADAPT study, indinavir Cmins should stay above the limit of 150 μg/liter (twofold 95% inhibitory concentration), as the limit of optimal plasma drug concentration (15) in treatment-naive patients. Other recent recommendations range from 80 to 250 μg/liter (2, 25). Because of the high residual variability in indinavir kinetics, the adjustment of Cmins to the consensual target of 150 μg/liter under the regimen of 800 mg of indinavir t.i.d without ritonavir would lead to subtherapeutic levels in about half of the patients. To ensure that a plasma drug concentration above the threshold of 150 μg/liter is maintained in 80% of the patients, an evaluation based on the intrapatient residual variability indicates that a Cmin of about 2,000 μg/liter should be targeted; such a dosage would also lead to Cmins above 4,000 μg/liter in 20% of the patients. This would a priori necessitate a ritonavir-boosted dosage of 1,700 mg b.i.d. in male patients or 900 mg b.i.d. in female patients, or even higher doses of a regimen based on indinavir only.
DISCUSSION
Heterogeneity in the response to antiretroviral therapy has been attributed to several factors, including genetic, immunologic, and virological ones, but pharmacological issues such as poor adherence to treatment, intolerance, and large interpatient differences in blood concentration following standard dosing regimens also represent important determinants of treatment failure. Despite unfavorable pharmacokinetic profiles and large interpatient and intrapatient variability, current practice still tends to administer a priori the same dose of indinavir to all patients without regard to differences in systemic blood and tissue disposition. Dosage individualization based on indinavir pharmacokinetic profiles and variability in the target population of patients receiving either indinavir alone or indinavir with low-dose ritonavir thus represents a suitable strategy for HIV treatment optimization.
The results of our population analysis are in agreement with previously reported population data (27). Indinavir has a short absorption t1/2 of 42 min with an important interpatient variability. Food has been shown to greatly influence the absorption and bioavailability of indinavir and may thus represent a relevant determinant of indinavir variability in this analysis, as the relationship between food and drug intake was neither controlled nor recorded (11; http://www.eudra.org/humandocs/humans/epar/crixivan/crixivan.htm). Without the specific assessment of the bioavailability of indinavir, the variability associated with this parameter was likely reported on both CL and residual error; this may also explain why the model was not ameliorated by associating interindividual variability with V. As expected, coadministration of low-dose ritonavir reduced oral CL by 63%, increasing indinavir elimination t1/2 from 1.4 to 3.8 h and explaining a significant part of the interpatient variability with oral CL (drop in CV from 75 to 48%). Interestingly, and in line with results observed clinically by Burger et al. (9) and in preclinical studies (22), it appeared that sex had a significant effect on oral clearance, resulting in a 30% increase in indinavir elimination in male patients versus female patients. Female patients may thus benefit from a better antiviral coverage than male patients under standard regimens but might also be more prone to side effect than male patients (12). A moderate further increase in oral CL with body weight was also observed, independently of sex, which is of limited clinical significance. Among the other demographic covariates tested, no influence of ethnicity on indinavir kinetics could be detected, but the presence of a majority of Caucasian patients in the present study may have limited the power to identify an association. These results are however in accordance with preliminary data (www.eudra.org/humandocs/humans/epar/crixivan/crixivan.htm).
Since indinavir is mainly metabolized by CYP3A4 enzymes, interactions with drugs acting on those isoforms were expected. Except for ritonavir, neither antiretroviral drugs (efavirenz or other reverse transcriptase inhibitors) nor known CYP3A4 inducers or inhibitors influenced indinavir kinetics significantly in this study. The small percentage of patients exposed to CYP3A4 inducers or inhibitors has most probably limited the power to detect such an association. Moreover, the presence of ritonavir, a potent CYP3A4 inhibitor coadministered in 74% of the study population, may have either compensated for decreases in drug exposure induced by efavirenz or other CYP3A4 inducers or masked the effect of less-potent inhibitors. In fact, studies conducted with both healthy volunteers and patients suggested that coadministration of efavirenz increased indinavir CL even with low-dose ritonavir or with nelfinavir and that low-dose ritonavir was not sufficient to fully compensate for efavirenz-induced decreases in drug exposure (1, 13, 24). Since the activity of cytochrome P450 varies greatly in the population, it is very difficult to estimate the magnitude and relevance of such a mutual interaction in unselected patients. Furthermore, other mechanisms involved in indinavir disposition, such as the inhibition of P-glycoprotein drug transport by ritonavir and not by efavirenz, may have independently contributed to alter indinavir elimination (20). Thus, in a regimen including low-dose ritonavir, dosage adjustment of indinavir is required, but evidence is lacking to recommend further systematic adaptation for comedications acting on CYP3A4.
Significant correlations between both Cmin and Cav and antiviral effectiveness have been demonstrated, mainly with PI-naive patients (3, 4, 8, 10, 16, 19, 25). Our study was conducted with a heterogeneous population that included both treatment-naive and -experienced patients: therefore, a potential selection bias, with patients achieving viral suppression being maintained on the initial regimen without ritonavir, could have confounded the relationship between drug exposure and treatment outcome. In particular, two patients receiving 1,000 and 1,200 mg of indinavir b.i.d without ritonavir presented a virological success, despite very low Cmins (<20 μg/liter). The therapeutic success in such patients might be explained by fairly adequate Cavs (around 2,000 μg/liter) leading to sustained intracellular concentrations despite low trough levels in blood, due to equilibration delays such as observed between plasma and cerebrospinal fluid (21). Our exploration of the concentration-outcome relationship questions whether average concentrations, better reflecting effective intracellular concentrations, could represent a more appropriate predictor of virological success or failure, as they are less affected by the oscillations related to the short t1/2 of the drug in plasma. This further emphasizes the potential interest of measuring intracellular concentrations.
Nephrolithiasis is the most commonly reported side effect of indinavir and has been related to maximal drug concentrations (7, 14). Twice-daily regimens of 800 mg of indinavir plus 100 to 200 mg of ritonavir are considered effective but poorly tolerated, and concerns about the increase in nephrotoxicity have been raised (6). A limited number of patients (n = 7) were affected in our population study, and only a marginal association between Cmax and this side effect was observed. However, among those seven patients, four presented plasma drug values of Cmax about two times higher than the study population average Cmax. Dosage reduction to 600 or 400 mg of indinavir had already been applied in 21 patients of this study population and might explain the lack of relationship between drug exposure and nephrotoxicity.
In conclusion, this study indicates that ritonavir and sex are major determinants of indinavir variability and should be considered for a priori dosage individualization. An association between drug concentrations and therapeutic success was reported in several studies and was observed even in this very heterogeneous population of treatment-naive and -experienced patients. This relationship, as well as the high interpatient variability, represents a strong argument in favor of the therapeutic drug monitoring of indinavir (2). However, a relatively high residual variability mainly reflecting an interoccasion variability, as recently reported (29), may limit the effectiveness of therapeutic drug monitoring. This could be circumvented by optimization of compliance, better adherence to the recommendations regarding food intake, and adoption of higher target levels, in particular for patients receiving indinavir for salvage therapy. The population parameters and the large residual variability suggest a target Cmin of 2,000 μg/liter to achieve effective levels in a majority of patients, and thus coadministration of ritonavir as a kinetic booster with indinavir must be recommended on principle; the treatment should be initiated at dosages as high as 1,700 mg b.i.d. in male patients or 900 mg b.i.d. in female patients, provided that therapeutic drug monitoring is performed for further dosage adaptation. The dosage of indinavir should be adapted carefully, especially with women, to avoid nephrotoxic levels. Whether dosage adaptation should preferably target adequate Cavs rather than Cmins remains an open question, and the range of adequate Cavs remains to be determined. This study proposes a Bayesian approach for the guidance of dosage adaptation based on population pharmacokinetic data as part of therapeutic drug monitoring of indinavir. Such an adaptative strategy still warrants prospective validation to establish its accuracy and clinical usefulness.
Acknowledgments
This study was made possible by a grant from the Novartis Foundation.
C. Csajka was supported by a grant from the Swiss National Science Foundation. C. Marzolini was supported by a grant from the Swiss National Science Foundation (3345-062092.99). K. Fattinger was supported by a grant from the Swiss National Science Foundation (3200-065173.01).
REFERENCES
- 1.Aarnoutse, R. E., K. J. T. Grintjes, D. S. C. Telgt, M. Stek, P. W. H. Hugen, P. Reiss, P. P. Koopmans, Y. A. Hekster, and D. M. Burger. 2002. The influence of efavirenz on the pharmacokinetics of a twice-daily combination of indinavir and low-dose ritonavir in healthy volunteers. Clin. Pharmacol. Ther. 71:57-67. [DOI] [PubMed] [Google Scholar]
- 2.Acosta, E. P., J. G. Gerber, and the Adult Pharmacology Committee of the AIDS Clinical Trials Group. 2002. Position paper on therapeutic drug monitoring of the antiretroviral agents. AIDS Res. Hum. Retrovir. 18:825-834. [DOI] [PubMed] [Google Scholar]
- 3.Acosta, E. P., K. Henry, L. Baken, L. M. Page, and C. V. Fletcher. 1999. Indinavir concentrations and antiviral effect. Pharmacotherapy 19:708-712. [DOI] [PubMed] [Google Scholar]
- 4.Anderson, P. L., R. C. Brundage, T. N. Kakuda, and C. V. Fletcher. 2002. CD4 response is correlated with peak plasma concentrations of indinavir in adults with undetectable human immunodeficiency virus ribonucleic acid. Clin. Pharmacol. Ther. 71:280-285. [DOI] [PubMed] [Google Scholar]
- 5.Bates, D. M., and D. G. Watts. 1988. Nonlinear regression analysis and its applications. J. Wiley and Sons, New York, N.Y.
- 6.Boubaker, K., P. Sudre, F. Bally, G. Vogel, J. Y Meuwly, M. P. Glauser, and A. Telenti. 1998. Changes in renal function associated with indinavir. AIDS 12:249-254. [DOI] [PubMed] [Google Scholar]
- 7.Burger, D., M. Boyd, C. Duncombe, M. Felderhof, A. Mahanontharit, K. Ruxrungtham, S. Ubolyam, M. Stek, D. Cooper, J. Lange, P. Phanupak, and P. Reiss. 2003. Pharmacokinetics and pharmacodynamics of indinavir with or without low-dose ritonavir in HIV-infected Thai patients. J. Antimicrob. Chemother. 51:1231-1238. [DOI] [PubMed] [Google Scholar]
- 8.Burger, D., P. Hugen, P. Reiss, I. Gyssens, M. Schneider, F. Kroon, G. Schreij, K. Brinkman, C. Richter, J. Prins, R. Aarnoutse, and J. Lange for the ATHENA Cohort Study Group. 2003. Therapeutic drug monitoring of nelfinavir and indinavir in treatment-naive HIV-1-infected individuals. AIDS 17:1157-1165. [DOI] [PubMed] [Google Scholar]
- 9.Burger, D. M., M. C. Siebers, P. W. H. Hugen, R. E. Aarnoutse, Y. A. Hekster, and P. P. Koopmans. 2002. Pharmacokinetic variability caused by gender: do women have higher indinavir exposure than men? J. Acquir. Immune Defic. Syndr. 29:101-102. [DOI] [PubMed] [Google Scholar]
- 10.Burger, D. M., P. W. H. Hugen, R. E. Aarnoutse, J. P. Dieleman, J. M. Prins, T. van der Pol, J. H. ten Veen, J. W. Mulder, P. L. Meenhorst, W. L. Blok, J. T. van der Meer, P. Reiss, and J. M. A. Lange. 2001. A retrospective, cohort-based survey of patients using twice-daily indinavir + ritonavir combinations: pharmacokinetics, safety and efficacy. J. Acquir. Immune Defic. Syndr. 26:218-224. [DOI] [PubMed] [Google Scholar]
- 11.Carver, P. L., D. Fleisher, S. Y. Zhou, D. Kaul, P. Kazanjian, and C. Li. 1999. Meal composition effects on the oral bioavailability of indinavir in HIV-infected patients. Pharm. Res. 16:718-724. [DOI] [PubMed] [Google Scholar]
- 12.D'Arminio Monforte, A., A. C. Lepri, G. Rezza, P. Pezzotti, A. Antinori, A. N. Phillips, G. Angarano, V. Colangeli, A. De Luca, G. Ippolito, L. Caggese, F. Soscia, G. Filice, F. Gritti, P. Narciso, U. Tirelli, M. Moroni, et al. 2000. Insights into the reasons for discontinuation of the first highly active antiretroviral therapy (HAART) regimen in a cohort of antiretroviral naive patients. AIDS 14:499-507. [DOI] [PubMed] [Google Scholar]
- 13.DiCenzo, R., A. Forrest, K. E. Squires, S. M. Hammer, M. A. Fischl, H. Wu, R. Cha, G. D. Morse, and the Adult AIDS Clinical Trials Group Protocol 368/886 Study Team. 2003. Indinavir, efavirenz, and abacavir pharmacokinetics in human immunodeficiency virus-infected subjects. Antimicrob. Agents Chemother. 47:1929-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dielman, J. P., I. C. Gyssens, M. E. Van der Ende, S. De Marie, and D. M. Burger. 1999. Urological complaints in relation to indinavir plasma concentrations in HIV-infected patients. AIDS 13:473-478. [DOI] [PubMed] [Google Scholar]
- 15.Durant, J., P. Cleenbergh, R. Garraffo, P. Halfon, S. Icard, P. Del Guidice, N. Montagne, J. M. Shapiro, and P. Dellamonica. 2000. Importance of protease inhibitor plasma level in patients treated with genotypic-guided therapy: pharmacological data from the VIRADAPT study. AIDS 14:1333-1339. [DOI] [PubMed] [Google Scholar]
- 16.Fletcher, C. V., P. L. Anderson, T. N. Kakuda, T. W. Schacker, K. Henry, C. R. Gross, and R. C. Brundage. 2002. Concentration-controlled compared with conventional antiretroviral therapy for HIV infection. AIDS 16:551-560. [DOI] [PubMed] [Google Scholar]
- 17.Fletcher, C. V. 1999. Pharmacologic considerations for therapeutic success with antiretroviral agents. Ann. Pharmacother. 33:989-995. [DOI] [PubMed] [Google Scholar]
- 18.Ghosn, J., C. Lamotte, H. Ait-Mohand, M. Wirden, R. Agher, L. Schneider, F. Bricaire, C. Duvivier, V. Calvez, G. Peytavin, and C. Katlama. 2003. Efficacy of a twice-daily antiretroviral regimen containing 100 mg ritonavir/400 mg indinavir in HIV-infected patients. AIDS 17:209-214. [DOI] [PubMed] [Google Scholar]
- 19.Kakuda, T. N., L. M. Page, P. L. Anderson, K. Henry, T. W. Schacker, F. S. Rhame, E. P. Acosta, R. C. Brundage, and C. V. Fletcher. 2001. Pharmacological basis for concentration-controlled therapy with zidovudine, lamivudine, and indinavir. Antimicrob. Agents Chemother. 45:236-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee, C., and M. M. Gottesman. 1998. HIV-1 protease inhibitors and the MDR1 multidrug transporter. J. Clin. Investig. 101:287-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Letendre, S. L., E. V. Capparelli, R. J. Ellis, J. A. McCutchan, and the HIV Neurobehavioral Research Center Group. 2000. Indinavir population pharmacokinetics in plasma and cerebrospinal fluid. Antimicrob. Agents Chemother. 44:2173-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin, J. H., M. Chiba, I. W. Chen, J. A. Nishime, and K. J. Vastag. 1996. Sex-dependent pharmacokinetics of indinavir. Drug Metab. Dispos. 24:1298-1306. [PubMed] [Google Scholar]
- 23.Marzolini, C., A. Telenti, T. Buclin, J. Biollaz, and L. A. Decosterd. 2000. Simultaneous determination of the HIV protease inhibitors indinavir, amprenavir, saquinavir, ritonavir, nelfinavir and the nonnucleoside reverse transcriptase inhibitor efavirenz by high-performance liquid chromatography after solid-phase extraction. J. Chromatogr. B Biomed. Sci. Appl. 740:43-58. [DOI] [PubMed] [Google Scholar]
- 24.Pfister, M., L. Labbé, S. M. Hammer, J. Mellors, K. K. Bennett, S. Rosenkranz, L. B. Sheiner, and the AIDS Clinical Trial Group Protocol 398 Investigators. 2003. Population pharmacokinetics and pharmacodynamics of efavirenz, nelfinavir, and indinavir: adult AIDS clinical trial group study 398. Antimicrob. Agents Chemother. 47:130-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rayner, C. R., K. J. Galbraith, J. L. Marriott, and G. J. Duncan. 2002. A critical evaluation of the therapeutic range of indinavir. Ann. Pharmacother. 36:1230-1237. [DOI] [PubMed] [Google Scholar]
- 26.Sheiner, L. B., S. Beal, B. Rosenberg, and V. V. Marathe. 1979. Forecasting individual pharmacokinetics. Clin. Pharmacol. Ther. 26:294-305. [DOI] [PubMed] [Google Scholar]
- 27.Shulman, N., A. Zolopa, D. Havlir, A. Hsu, C. Renz, S. Boller, P. Jiang, R. Rode, J. Gallant, E. Race, D. J. Kempf, and E. Sun. 2002. Virtual inhibitory quotient predicts response to ritonavir boosting of indinavir-based therapy in human immunodeficiency virus-infected patients with ongoing viremia. Antimicrob. Agents Chemother. 46:3907-3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Heeswijk, R. P. G., A. I. Veldkamp, R. M. W. Hoetelmans, J. W. Mulder, G. Schreij, A. Hsu, J. M. Lange, J. H. Beijnen, and P. L. Meenhorst. 1999. The steady-state plasma pharmacokinetics of indinavir alone or in combination with a low dose of ritonavir in twice daily dosing regimens in HIV-1-infected individuals. AIDS 13:95-99. [DOI] [PubMed] [Google Scholar]
- 29.Zhou, X. J., D. Havlir, D. Richman, E. P. Acosta, M. Hirsch, A. C. Collier, P. Tebas, J. P. Sommadossi, and the AIDS Clinical Trials Study 343 Investigators. 2000. Plasma population pharmacokinetics and penetration into cerebrospinal fluid of indinavir in combination with zidovudine and lamivudine in HIV-1-infected patients. AIDS 14:2869-2876. [DOI] [PubMed] [Google Scholar]