Abstract
In the study, osmotically dehydrated cherry tomatoes were partially dried to water activity between 0.746 and 0.868, vacuum-packed and stored at 4–30 °C for 60 days. Adaptive neuro-fuzzy inference system (ANFIS) was utilized to predict the physicochemical and microbiological parameters of these partially dried cherry tomatoes during storage. Satisfactory accuracies were obtained when ANFIS was used to predict the lycopene and total phenolic contents, color and microbial contamination. The coefficients of determination for all the ANFIS models were higher than 0.86 and showed better performance for prediction compared with models developed by response surface methodology. Through ANFIS modeling, the effects of storage conditions on the properties of partially dried cherry tomatoes were visualized. Generally, contents of lycopene and total phenolics decreased with the increase in water activity, temperature and storage time, while aerobic plate count and number of yeasts and molds increased at high water activities and temperatures. Overall, ANFIS approach can be used as an effective tool to study the quality decrease and microbial pollution of partially dried cherry tomatoes during storage, as well as identify the suitable preservation conditions.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-016-2339-0) contains supplementary material, which is available to authorized users.
Keywords: Cherry tomato, Partially dried, ANFIS, Lycopene, Phenolics, Microbiological analysis
Introduction
In the last decade, the consumption of snacking tomatoes, including cherry tomatoes, have been increasing continuously due to their rich nutritive contents, health-benefiting properties and convenience of use. There are many papers reporting that the ingestion of tomatoes and their derivatives is able to prevent cardiovascular diseases and several kinds of cancers, such as caners of prostate and digestive tract (Muratore et al. 2008; Nour et al. 2015). However, cherry tomato is a seasonal and highly perishable fruit due to rapid ripening and aging, pathogenic invasion and transpiration (Zapata et al. 2008). Thus, a large amount of researches have been conducted to preserve cherry tomatoes and extend their shelf-life.
Drying combined with osmotic dehydration pretreatment is an effective method for cherry tomato preservation. Meanwhile, the taste and texture of cherry tomatoes can be modified by drying and osmotic dehydration and their marketing values are enhanced simultaneously (Nabnean et al. 2016). There is a high demand for dried cherry tomatoes in the international market because of their flavor and taste. On the other hand, the development of cherry tomatoes with intermediate moisture has received much attention in recent years, which are characterized by increased stability due to low water activity, good nutritive values and satisfactory organoleptic features (Muratore et al. 2008). Partially dried cherry tomatoes can be used as a seasoning, snack and ingredient in daily cuisine as the alternative to fresh tomatoes. Today, the market shows increasing interest in cherry tomatoes with intermediate moisture.
Various technologies have been used for the dehydration of cherry tomatoes, such as sun drying (Nabnean et al. 2016), air drying (Bennamoun et al. 2015; Muratore et al. 2008), microwave drying (Heredia et al. 2007) and osmotic dehydration (Derossi et al. 2015). The drying kinetics of cherry tomatoes have been well investigated and simulated, while the effects of these technologies on the quality of cherry tomatoes have also been intensively studied. Meanwhile, a lot of studies have been performed to investigate the stability of bioactive compounds in tomatoes and tomato-related products, such as tomato juice, pulp and puree during storage. For example, a comprehensive review about lycopene stability has been made by Martínez-Hernández et al. (2016). Even so, very limited studies are concerned about the preservation of cherry tomatoes with intermediate moisture. According to our best knowledge, only Zanoni et al. (2000) studied the color variation and microbial stability of tomato halves with intermediate moistures during storage. However, the details about the effects of storage conditions on the main physicochemical and microbiological parameters of partially dried cherry tomatoes during storage, such as contents of lycopene and phenolics, color parameters, aerobic plate count, and number of yeasts and molds, are still unknown.
The variations of physicochemical and microbiological parameters of partially dried cherry tomatoes can be affected by a series of factors, such as water activity, temperature, oxygen, storage time, etc. (Martínez-Hernández et al. 2016). From a food engineering perspective, the development of mathematical models is effective to quantify the effects of various factors on the characteristics of partially dried cherry tomatoes and predict their quality. Among the existing modeling tools, adaptive neuro-fuzzy inference system (ANFIS) is a powerful tool to predict the properties of various food products and simulate the complex non-linear relationship between food properties and processing factors (Karaman and Kayacier 2011; Zheng et al. 2011; Rahman et al. 2012). More specifically, ANFIS is a multilayer feed-forward network that utilizes neural network learning algorithm and fuzzy inference systems to build the relationship between inputs and target output (Jang and Sun 1995). ANFIS combines the advantages of both artificial neural network (ANN) and fuzzy logic (FL) in such a way that the rules and membership functions of fuzzy systems are determined by ANN (Motahari-Nezhad and Mazidi 2016). Thus, ANFIS is a more versatile and effective modeling tool than ANN and FL alone. To date, there are no published papers about modeling the quality changes of cherry tomatoes during storage using ANFIS.
Therefore, the main objectives of this study were to (1) predict the physicochemical and microbiological parameters of partially dried cherry tomatoes during storage by means of ANFIS and (2) investigate the effects of different factors, including water activity, temperature and storage time on the determined properties of partially dried cherry tomatoes.
Materials and methods
Sample preparation
Mature cherry tomatoes (S. lycopersicum L.) without mechanical damage, cultivar “Sulong-1”, were collected from the farm of Mingyue Agricultural Co. Ltd. (Wuxi, China). Tomatoes were of same color and homogenous diameter (30.8 ± 1.5 mm). After collection, samples were washed with tap water, drained and stored at 4 °C in darkness until experiments.
To provide the products a sweet taste, cherry tomatoes were first treated by osmotic dehydration following the methods of Shi et al. (1997) and Azoubel and Murr (2004) with some modifications. Considering the high mass transfer resistance due to waxy skin of cherry tomatoes, all the samples were blanched in boiling water for 30 s and then pricked with needles to achieve a density of 40 holes/cm2. The osmotic solution with 50° Brix of sucrose concentration was prepared using sucrose. The Brix value of the solution was confirmed using an Abbe refractometer. The whole cherry tomatoes were then immersed in the sucrose solution at a ratio of 1:10 (fruit:solution, w:w). After osmotic dehydration at 50 °C for 5 h, tomatoes were washed with distilled water and blotted gently to remove the excess osmotic solution on the surface. The water loss and solid gain were then measured using the methods of Viana et al. (2014), which was found to be 27.11 ± 1.00 and 3.75 ± 0.09 %, respectively (% referred to 100 g fresh cherry tomatoes). At the same time, the total soluble solids value of cherry tomatoes after osmotic dehydration was measured by an Abbe refractometer, being 6.8° ± 0.2° Brix.
The samples were dried in a forced air-drying oven (DHG-9203A, Yiheng Tech Inc., Shanghai, China). At 70 °C and the air velocity was kept at 2.5 m/s. The selection of drying temperature was based on our preliminary results (Supplementary Table 1) and the literature about tomato drying. In our preliminary study, it was found that although magnitude of lycopene degradation increased with temperature rising during oven drying, the energy consumption reached minimum when drying temperature stayed at 70 °C.
During oven drying, cherry tomato samples were taken away from the oven periodically and mashed. Water activity was measured using a hygrometer (LabSwift-Novasina, Lachen, Switzerland). Drying process was terminated when water activity reached 0.746, 0.812 and 0.868, respectively. After drying, water content in tomatoes was measured using the AOAC (2000) method. Water activity of 0.746, 0.812 and 0.868 corresponded to the water content of 18, 22 and 25 % (wet basis), respectively.
Storage of partially dried cherry tomatoes
The aforementioned partially dried tomatoes with water activity of 0.746, 0.812 and 0.868 were vacuum-packed using airtight, water-proof and plastic bags. The samples were stored in dark at 4, 20 and 30 °C, respectively for 60 days. Physicochemical and microbiological parameters of cherry tomatoes were analyzed after storage for 0, 15, 30, 45 and 60 days. For each sampling, three groups of cherry tomato samples stored at the same conditions were selected for analysis. Thus, the total number of cherry tomato samples analyzed during storage was 105 (45 groups).
Physicochemical analysis
Color analysis
Color of partially dried cherry tomatoes was determined using a handheld tristimulus reflectance spectrocolorimeter (CR-400, Konica Minolta, Japan) with a 0.8-cm measuring aperture. Values were obtained for 2° observer and C illuminant. This instrument was calibrated with a white standard. Color was recorded using CIE L*a*b* (L*: brightness; a*: redness-greenness; b*: yellowness-blueness) system. Meanwhile, hue angle (h) and chroma (C) were calculated using the following equations:
| 1 |
| 2 |
For each measurement, ten samples stored at the same conditions were used. Three readings at three different positions in the equatorial zone of cherry tomatoes were taken. Then, the average values of each color parameter were calculated.
Determination of lycopene content
Lycopene content of cherry tomato was measured using the method of Demiray et al. (2013). Specifically, partially dried cherry tomatoes were first freeze-dried in a freeze drier (Freezezone 4.5, Labconoco, USA) under a pressure lower than 0.003 mBar at −45 °C for 48 h. Dried samples were then ground using a mortar manually. 1 g of powdered samples was added to 70 mL of ethanol-hexane solution (4:3, v:v) with 1 % butylated hydroxytoluene (w:v). After complete homogenization, the mixture was centrifuged at 11,000 rpm and 4 °C for 15 min. The supernatants were collected and filtered through 0.45-μm membrane filters.
In the next step, samples were analyzed by a HPLC system (Agilent 1200; GMI, Ramsey, MN, USA) connected with an Agilent ZORBAX SB-C18 column (250 × 4.6 mm, 5 μm). The column temperature was maintained at 25 °C and samples were detected at 470 nm using UV–Vis diode-array absorbance detection. The mobile phase was a mixture of acetonitrile, methanol, dichloromethane and hexane at a ratio of 40:20:20:20 (v/v/v/v) and the flow rate was 0.45 mL/min. Lycopene content was calculated from the calibration curve of pure lycopene (Sigma, St. 98 Louis, MO, USA) and the results were expressed as mg per 100 g of dry matter. All the samples were analyzed in triplicate.
Determination of total phenolic content
Total phenolic content of partially dried cherry tomatoes was determined using the well-established Folin-Ciocalteu method (Sricharoen et al. 2015) and expressed as mg gallic acid equivalents per 100 g of dry matter. All the samples were analyzed in triplicate.
Microbiological analysis
Microbiological analysis was carried out following the methods described by Aguayo et al. (2006). Briefly, 25 g of partially dried cherry tomatoes was mixed with 225 mL of sterile saline solution and aseptically homogenized using a Blender (JYL-A100, Joyoung Co., Ltd., China) for 1 min at 22,000 rpm. Next, the resulting homogenate was serially diluted with sterile saline solution. For the determination of aerobic plate count, 1 mL aliquot was spread in a Petri dish and then mixed well with 15 mL of plate count agar (yeast extract: 2.5 g, tryptone: 5.0 g, agar: 15.0 g, glucose: 1.0 g, distilled water: 1000 mL, pH: 7.0 ± 0.2, sterilization: 121 °C for 30 min). For the enumeration of molds and yeasts, 1 mL aliquot was spread in a Petri dish and mixed well with 15 mL of potato dextrose agar (peeled potato: 300 g, dextrose: 20 g, agar: 20 g, chloromycetin: 0.1 g, distilled water: 1000 mL, sterilization: 121 °C for 30 min). After agar solidification, the dishes containing plate count agar were incubated at 36 °C for 48 h, while the dishes containing potato dextrose agar were incubated at 28 °C for 5 days. Each microbial count was performed in duplicates and the results were expressed as log10 CFU/g (colony forming units per gram of sample).
ANFIS modeling
ANFIS combines both the learning ability of neural network and reasoning capacity of fuzzy logic (Rahman et al. 2012; Simha et al. 2016). ANFIS is based on a first-order Takagi–Sugeno inference system, in which the weights of neural networks correspond to the parameters of fuzzy system. The relationship between inputs and output are built using both the stipulated input–output data pairs and fuzzy if–then rules (Najafi et al. 2016). The parameters of first-order Takagi–Sugeno inference system can be identified through the back propagation method or a hybrid method composed of both back propagation method and least square method. There are five layers in ANFIS model, including fuzzification layer (Layer I), rule layer (Layer II), normalization layer (Layer III), defuzzification layer (Layer IV) and output layer (Layer V). The input signals are processed successively by the aforementioned layers and the overall output is derived from the last layer. Specifically, taking the ANFIS model with two inputs (x and y) and one output (f) for example, the fuzzy if–then rules can be expressed in the following forms:
| 3 |
| 4 |
where A 1, A 2, B 1, B 2 are the linguistic labels and p 1, p 2, q 1, q 2, r 1 and r 2 are the linear coefficients of the output function, f 1 and f 2 are first-order polynomials.
Layer I (fuzzification) transforms the input variables into a fuzzy set through membership functions. Every node in Layer I is an adaptive node and the corresponding node function is written as:
| 5 |
| 6 |
where and are the membership functions that could have various shapes.
Next, all the incoming signals in Layer II (rule layer) are multiplied by each other and the resulting outputs are expressed in the general form:
| 7 |
here, the output of each node i in this layer also represents the rule strength (Akbarpour et al. 2016).
In Layer III (normalization), all the firing strengths are normalized using the following equation:
| 8 |
where w i is the normalized strength.
Layer IV performs the defuzzification task to correlate the inputs and outputs:
| 9 |
where p i, q i and r i are the coefficients of f i.
Lastly, Layer V (output) summarizes all the incoming signals and the overall output is computed as:
| 10 |
During model construction, the whole dataset was divided into training (30 groups) and testing (15 groups) phases randomly. The water activity of cherry tomatoes, storage temperature and storage time were regarded as the inputs of ANFIS models, while physicochemical and microbiological parameters were taken as the outputs. The grid partition method was used for input data classification and rule establishment, since the gird partition is a suitable method to classify the data when a few input variables are considered (Jang and Sun 1995). Eight different membership functions were taken into account, including trapezoidal (trapmf), triangle (trimf), Gaussian (Gaussmf), generalized bell (gbellmf), Pi curve (pimf), two sided Gaussian (Gasuss2mf), difference between two sigmoidal functions (dsigmf) and product of two sigmoidal functions (psigmf). Meanwhile, the output membership function used was a linear function. Moreover, the hybrid learning algorithm was utilized to determine the optimum parameters of the Takagi–Sugeno inference system.
The predictive ability of constructed ANFIS models was evaluated using the coefficient of determination (R 2), root mean square error (RMSE), mean absolute percentage error (MAPE) and mean absolute error (MAE), which are defined using the following equations (Akbarpour et al. 2016):
| 11 |
| 12 |
| 13 |
| 14 |
where n is the total number of experimental data, Y i,e is the experimental data of the ith sample, Y i,p is the predicted data of the ith sample, Y m is the average value of all the experimental data. The predictive accuracy of a model is considered high if R 2 value is close to 1 and the values of RMSE, MPAE and MAE are low.
ANFIS modeling was performed in MatlabR2010a (The MathWorks, Inc., MA, USA) using the function anfis. Once ANFIS models with satisfactory predictive ability were obtained, 3D plots about the effects of storage conditions on the determined parameters of cherry tomatoes were generated in Matlab.
Statistical analysis
One-way analysis of variance (ANOVA) was conducted using SPSS 11.5 (SPSS, Inc., USA) to compare the means of physicochemical and microbiological data about partially dried cherry tomatoes. Significance was defined at p = 0.05 and the Least Significant Difference (Fisher’s LSD) test was used to separate the mean differences.
Results and discussion
General characteristics of partially dried cherry tomatoes during storage
The physicochemical and microbiological parameters of partially dried cherry tomatoes changed markedly after 60-day storage, especially at 30 °C. The characteristics of partially dried cherry tomatoes with different water activity before and after storage at 30 °C for 60 days are listed in Table 1. The initial content of lycopene and total phenolics in cherry tomatoes at water activity of 0.868 were the highest, followed by the samples at water activity of 0.812 and 0.747. Lycopene and total phenolics content in partially dried cherry tomatoes at water activity of 0.868 before storage were 212.6 ± 3.6 and 350.8 ± 2.7 mg/100 g dry matter, respectively, while the initial contents in partially dried tomatoes at water activity of 0.747 decreased to 197.5 ± 3.2 and 278.8 ± 2.2 mg/100 g dry matter, respectively. This phenomenon was reasonable since longer drying time was required to produce samples with lower water activity, whereas longer drying time was detrimental to the bioactive compounds of tomatoes (Demiray et al. 2013). Meanwhile, there were no significant difference (p ≥ 0.05) in L* values among samples before storage, while a* value at water activity of 0.812 was significantly (p < 0.05) lower than that of other samples. Furthermore, no bacteria, yeasts and molds were detected in all the processed cherry tomatoes prior to storage, probably due to initial sample selection, osmotic dehydration and subsequent drying treatment.
Table 1.
Characteristics of partially dried cherry tomatoes before and after storage at 30 °C for 60 days
| Water activity | Lycopene (mg/100 g dry matter) | Total phenolics (mg/100 g dry matter) | L* | a* | b* | C | h | Aerobic plate count (log10 CFU/g) | Yeasts and molds (log10 CFU/g) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Before storage | 0.746 | 197.5 ± 3.2c | 278.8 ± 2.2c | 30.18 ± 0.41a | 12.27 ± 0.27a | 6.83 ± 0.17b | 14.04 ± 0.25a | 1.61 ± 0.10a | <1.00 | <1.00 |
| 0.812 | 204.4 ± 1.4b | 314.6 ± 3.0b | 29.79 ± 0.44a | 10.95 ± 0.20b | 5.99 ± 0.38c | 12.91 ± 0.43b | 1.39 ± 0.20a | <1.00 | <1.00 | |
| 0.868 | 212.6 ± 3.6a | 350.8 ± 2.7a | 29.91 ± 0.75a | 12.12 ± 0.10a | 7.73 ± 0.06a | 13.91 ± 0.11a | 1.58 ± 0.06a | <1.00 | <1.00 | |
| After storage | 0.746 | 130.8 ± 1.9e | 196.6 ± 1.8f | 26.48 ± 0.13c | 5.29 ± 0.25d | 3.80 ± 0.42e | 8.64 ± 0.46c | 0.29 ± 0.08c | 2.95 ± 0.05a | 2.48 ± 0.09a |
| 0.812 | 131.9 ± 0.4e | 208.0 ± 1.8e | 25.50 ± 0.34d | 5.31 ± 0.30d | 3.65 ± 0.09e | 8.65 ± 0.15c | 0.29 ± 0.09c | 3.04 ± 0.06a | 2.60 ± 0.02b | |
| 0.868 | 134.9 ± 0.9d | 252.6 ± 6.2d | 27.15 ± 0.19b | 5.86 ± 0.33c | 5.13 ± 0.37d | 9.00 ± 0.38c | 0.43 ± 0.04b | 3.20 ± 0.03b | 3.08 ± 0.07c |
Data were mean value of three replicates ± SD
Values followed by different letters in each column indicated significant differences (p < 0.05)
After storage at 30 °C for 60 days, lycopene content in cherry tomatoes at water activity of 0.746, 0.812 and 0.868 decreased by 33.4, 35.5 and 36.5 %, respectively. Meanwhile, the loss of total phenolic content in cherry tomatoes at water activity of 0.746, 0.812 and 0.868 were 29.4, 33.9 and 28.0 %, respectively. Moreover, values of L*, a*, b*, C and h were significantly (p < 0.05) lower than that of samples before storage, indicating tissue browning and color degradation. On the other hand, more microorganisms were detected in samples at higher water activity. The total aerobic plate counts in cherry tomatoes at water activity of 0.746, 0.812 and 0.868 were 2.95 ± 0.05, 3.04 ± 0.06 and 3.20 ± 0.03 log10 units, respectively. The corresponding numbers of yeasts and molds were 2.48 ± 0.09 log10 units for samples at water activities of 0.746, 2.60 ± 0.02 log10 units for samples at water activities of 0.812, and 3.08 ± 0.07 log10 units for samples at water activities of 0.868. Similar results were reported by Arquiza and Hunter (2014), who reporting that low water activity inhibited fungal growth on solid foods.
ANFIS modeling
ANFIS was employed to model the changes in physicochemical and microbiological parameters of partially dried cherry tomatoes during storage quantitatively.
The performance of ANFIS models for prediction is strongly dependent on the number and type of membership function, as well as the number of training epoch. After training the ANFIS models with different numbers of membership function, it was found that satisfactory predictive results were obtained when the number of membership function was 2, whereas there was a substantial divergence between experimental and predicted results for the testing dataset once more than 2 membership functions were used. Therefore, in the constructed ANFIS models, 2 membership functions were assigned to each input variable.
Meanwhile, ANFIS models were trained individually using various types of input membership function. For the model of “aerobic plate count”, the predictive accuracy was the highest when “Gaussmf” was used. Regarding other ANFIS models, “trimf” resulted in the best performance of prediction. Both “Gaussmf” and “trimf” are the curves that specify how the points in the input space are mapped to a degree of membership between 0 and 1 (Zheng et al. 2011). “Gaussmf” (Eq. 15) and “trim (Eq. 16) membership functions are expressed in the following forms:
| 15 |
| 16 |
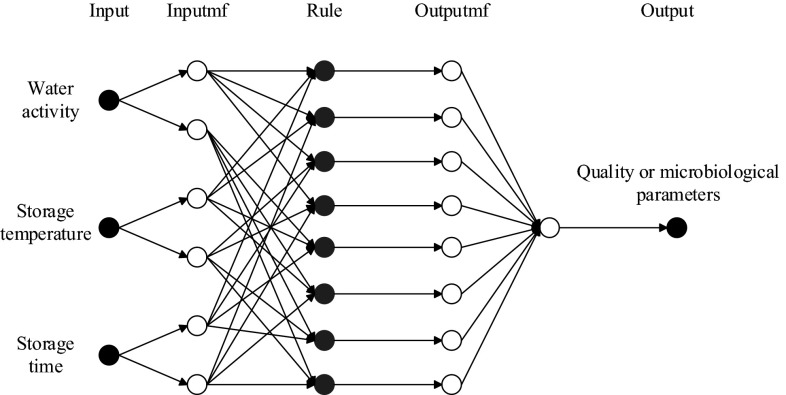
Furthermore, all the models were trained for up to 1000 epochs, so as to minimize the error measure. Details about ANFIS models are summarized in Table 2 and the general architecture of the Sugeno fuzzy models used to make ANFIS structure is illustrated in Fig. 1. In Fig. 1, the node in the output layer represented each physicochemical and microbiological parameter of partially dried cherry tomatoes.
Table 2.
Information about developed ANFIS models
| Characteristics | Models of “lycopene”, “total phenolics”, “L*”, “a*”, and “yeasts and molds” | Model of “aerobic plate count” |
|---|---|---|
| Fuzzy structure | Sugeno-type | Sugeno-type |
| Initial FIS for training | Genfis1 | genfis1 |
| Number of membership function | 2 2 2 | 2 2 2 |
| Input membership function | Triangle (trimf) | Gaussian (gaussmf) |
| Epoch | 1000 | 1000 |
| Output membership function | Linear | Linear |
| Training algorithm | Hybrid | Hybrid |
| Number of nodes | 34 | 34 |
| Number of linear parameters | 32 | 32 |
| Number of non-linear parameters | 18 | 12 |
| Total number of parameters | 50 | 44 |
| Number of training data pairs | 30 | 30 |
| Number of testing data pairs | 15 | 15 |
| Number of fuzzy rules | 8 | 8 |
Fig. 1.
Architecture of ANFIS models to predict physicochemical and microbiological parameters of partially dried cherry tomatoes during storage
It should be pointed out that the size of training data was lower than the number of modified parameters for all the ANFIS models in Table 2. To ensure good generalization of ANFIS model towards unseen data, the number of modified parameters needs to be lower than the number of training samples (Rahman et al. 2012). However, in the cases of small size of training dataset, it still can be acceptable if the performances of ANFIS models evaluated using both training and testing data are satisfactory (Akbarpour et al. 2016; Motahari-Nezhad and Mazidi 2016). The performance indicators relating to the training and testing of ANFIS models are listed in Table 3. As can be seen, all the ANFIS models had high values of R 2 (>0.86) using either training or testing data for evaluation. To obtain a satisfactory agreement between actual and predicted data, R 2 values of ANFIS models should be greater than 0.8 (Chong et al. 2015). Meanwhile, all the models had relatively low values of RMSE, MAPE and MAE, implying that the developed ANFIS models were reliable to simulate the changes of physicochemical and microbiological attributes of partially dried cherry tomatoes during storage. Furthermore, the comparisons between experimental and predicted data for all the parameters are plotted in Supplementary Fig. 1, showing an acceptable accordance between experimental data and ANFIS results.
Table 3.
Statistical analysis of the constructed ANFIS and RSM models
| Model | Parameter | Training phase | Testing phase | ||||||
|---|---|---|---|---|---|---|---|---|---|
| RMSE | R 2 | MAPE | MAE | RMSE | R 2 | MAPE | MAE | ||
| ANFIS | Lycopene | 1.319 | 0.997 | 0.623 | 1.147 | 2.495 | 0.993 | 1.146 | 2.017 |
| Total phenolics | 1.547 | 0.999 | 0.452 | 1.201 | 7.716 | 0.956 | 2.496 | 6.563 | |
| L* | 0.313 | 0.945 | 0.914 | 0.262 | 0.434 | 0.867 | 1.269 | 0.357 | |
| a* | 0.376 | 0.967 | 3.294 | 0.301 | 0.620 | 0.940 | 5.371 | 0.502 | |
| Aerobic plate count | 0.033 | 0.991 | 1.120 | 0.025 | 0.078 | 0.998 | 2.648 | 0.056 | |
| Yeasts and molds | 0.024 | 0.999 | 3.079 | 0.018 | 0.144 | 0.985 | 5.752 | 0.098 | |
| RSM | Lycopene | 2.519 | 0.987 | 1.122 | 1.976 | 2.779 | 0.986 | 1.198 | 2.082 |
| Total phenolics | 3.854 | 0.991 | 1.173 | 3.227 | 13.065 | 0.929 | 4.255 | 10.895 | |
| L* | 0.703 | 0.720 | 2.012 | 0.568 | 1.275 | 0.388 | 3.094 | 0.823 | |
| a* | 0.893 | 0.812 | 7.409 | 0.699 | 0.732 | 0.931 | 7.429 | 0.662 | |
| Aerobic plate count | 0.347 | 0.900 | 9.510 | 0.293 | 0.341 | 0.906 | 9.189 | 0.287 | |
| Yeasts and molds | 0.260 | 0.933 | 7.584 | 0.218 | 0.212 | 0.956 | 6.116 | 0.177 | |
On the other hand, the training dataset used for the construction of ANFIS model were fitted to the widely used response surface methodology (RSM) model, which is written as (Yadav et al. 2012):
| 17 |
where y denotes the physicochemical and microbiological parameters determined, x 1 is water activity of partially dried cherry tomato, x 2 is storage temperature, x 3 is storage time, a 1 to a 9 are the regression coefficients.
After the construction of RSM models (equations shown in Supplementary Table 2), the statistical indicators, namely RMSE, R 2, MAPE and MAE, were calculated using training and testing dataset same to that used by ANFIS models. The results are presented in Table 3. Compared with ANFIS models, RSM models were characterized by higher values of RMSE, MAPE and MAE, as well as low values of R 2. Therefore, ANFIS model was more accurate than RSM model to predict the changes of physicochemical and microbiological parameters of partially dried cherry tomatoes during storage. ANFIS model is able to correlate all the forms of non-linear relationships between variables, whereas RSM model restricts to second-order polynomial relationship (Desai et al. 2008).
Changes of physicochemical and microbiological parameters of partially dried cherry tomatoes as a function of water activity, storage temperature and storage time
After ANFIS modeling, the relationship between storage conditions and several parameters (lycopent content, a* and aerobic plate count) of partially dried cherry tomatoes is visualized in Fig. 2. The influences of storage conditions on total phenolic content, L* and number of yeasts and molds were similar to lycopent content, a* and aerobic plate count, respectively. The 3D surface plots about these three parameters are presented in Supplementary Fig. 2.
Fig. 2.
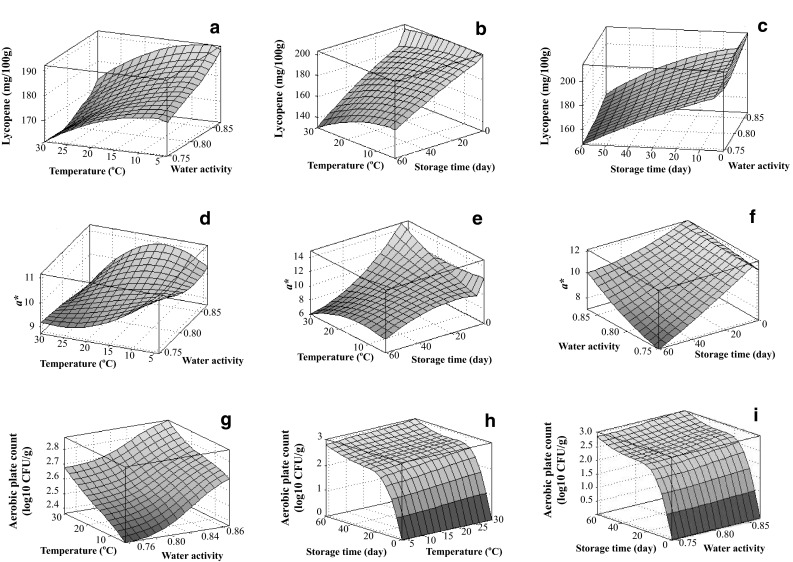
3D surface plots of lycopene content (a–c), a* (d–f) and aerobic plate count (g–i) of partially dried cherry tomatoes versus storage conditions
It can be seen from Fig. 2a–c and Supplementary Fig. 2a–c that the contents of lycopene and total phenolics decreased along with the increases of temperature and storage time. Meanwhile, the magnitudes of degradation of lycopene and phenolics were slightly higher at higher water activities. Thus, low water activity and low temperature can benefit the retention of bioactive compounds in partially dried cherry tomatoes during storage. Similar results were reported by Giovanelli and Paradiso (2002) and Martínez-Hernández et al. (2016) that the degradation of lycopene and phenolics in tomato-based products could be alleviated at low temperatures and low water activities. However, there are also several papers reporting contrary results. Lavelli and Giovanelli (2003) found that lycopene content was stable in several commercial tomato products during 90-day storage at 30–50 °C, while the total phenolic content increased gradually. Lavelli and Torresani (2011) revealed that lycopene degradation rate decreased as the water activity of dehydrated tomato by-produced increased during storage at 30 °C. Lycopene mainly exists in two different forms, all-trans and mono or poly-cis forms. Between them, cis-lycopene is more stable thermodynamically (Srivastava and Srivastava 2015). During storage, all trans-lycopene can be transferred to cis-lycopene due to reversible isomerization (Srivastava and Srivastava 2015). Moreover, individual phenolic compounds can react with each other through polymerization, copigmentation and other reactions (Balasundram et al. 2006). The discrepancies about lycopene and phenolics stability during storage in tomato-based products were probably because different chemical reactions about lycopene and phenolics occurred during storage. In summary, a group of abiotic factors can affect the stability of bioactive compounds in tomato-based products during storage, such as oxygen, light, metal ion, moisture content, packaging materials, et al. (Martínez-Hernández et al. 2016). Therefore, mathematical simulation can be a proper strategy to study the changes of bioactive contents in partially dried cherry tomatoes during storage, so as to control the product quality.
The effects of water activity and temperature on L* and a* of partially dried cherry tomatoes were relatively complicated (Fig. 2d–f and Supplementary Fig. 2d–f). At low water activities, there was a decreasing trend in L* and a* with the increase of temperature. On the contrary, at high water activities, L* and a* first increased to a peak value and then decreased gradually with the temperature rising. The change of L* during storage at different conditions was similar to that of a*, which was consistent of the changes of L* and a* in red grape juice during sonication (Tiwari et al. 2010). Lycopene is the major pigment responsible for the color properties of partially dried cherry tomatoes. Peasron’s correlation coefficient was calculated in SPSS 11.5 (SPSS, Inc., USA) to investigate the relationship between color parameters and lycopene content of partially dried cherry tomatoes. It was found that lycopene content was positively correlated with both L* and a* with high correlation coefficients (r = 0.78 and p < 0.01 for L* and lycopene; r = 0.87 and p < 0.01 for a* and lycopene). Therefore, to avoid color loss of partially dried cherry tomatoes during storage, the most important issue was to prevent the irreversible oxidation of lycopene (Srivastava and Srivastava 2015).
The contamination of tomato products with undesired microorganisms can occur during the storage period. In recent years, the frequency of foodborne illness outbreaks linked to tomato products has increased continuously (Guerreiro et al. 2016). To ensure the safety of partially dried cherry tomatoes, microbial growth, especially the growth of deterioration microorganisms and pathogens, should be strictly limited. Figure 2g–i and Supplementary Fig. 2g–i reveal the relationship of microbial growth in partially dried cherry tomatoes and storage conditions under investigation. It can be seen that high water activity and high temperature in the current experiment range facilitated the microbial growth in partially dried cherry tomatoes during storage. However, it should be pointed that no visual perception of microbial growth was detected during the entire storage period. In the study of Zanoni et al. (2000), molds were perceived by human eyes in vacuum-packed tomato halves at water activity of 0.90 after storage at 30 °C for 30 days. Thus, in order to inhibit the growth of microorganisms in cherry tomatoes during storage, their water activity is suggested to be lower than 0.90. In addition, the incorporation of antimicrobial agents into packaging materials can also inhibit the microbial growth in cherry tomatoes, thus extending their shelf-life (Fagundes et al. 2014). Future studies can pay some attentions to investigate quality change and microbial growth of partially dried cherry tomatoes packaged by various materials during storage.
Overall, the effects of storage conditions on the physicochemical and microbiological parameters of partially dried cherry tomato were more than linear relationship. ANFIS is a powerful tool to quantify the quality decrease and microbial pollution during the storage of partially dried cherry tomatoes.
Conclusion
ANFIS technique was successfully used to model the changes of physicochemical and microbiological parameters of partially dried cherry tomatoes during storage, as well as visualize the effects of water activity, temperature and storage time on these parameters. All the constructed models had satisfactory accuracy according to R 2, RMSE, MAPE and MAE values. Using the constructed ANFIS models, the values of mentioned physicochemical and microbiological attributes of partially dried cherry tomatoes during storage within the current experimental range can be predicted without chemical analysis. As a result, the quality of partially dried cherry tomatoes can be easily controlled. Future studies can be focused on minimizing quality loss and inhibiting microbial growth in partially dried cherry tomatoes, thus extending the shelf-life of this product.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and the Independent Innovation of Agricultural Sciences in Jiangsu Province, China coded CX(15)1026.
References
- Aguayo E, Escalona VH, Artés F. Effect of cyclic exposure to ozone gas on physicochemical, sensorial and microbial quality of whole and sliced tomatoes. Postharvest Biol Technol. 2006;39:169–177. doi: 10.1016/j.postharvbio.2005.11.005. [DOI] [Google Scholar]
- Akbarpour H, Mohajeri M, Akbarpour M. Pore diameter of nanoporous anodic alumina: experimental study and application of ANFIS and MLR. Chemometr Intell Lab. 2016;153:82–91. doi: 10.1016/j.chemolab.2016.02.012. [DOI] [Google Scholar]
- AOAC . Official methods of analysis of AOAC International. 17. Maryland: AOAC International; 2000. [Google Scholar]
- Arquiza JMRA, Hunter J. The use of real-time PCR to study Penicillium chrysogenum growth kinetics on solid food at different water activities. Int J Food Microbiol. 2014;187:50–56. doi: 10.1016/j.ijfoodmicro.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Azoubel PM, Murr FEX. Mass transfer kinetics of osmotic dehydration of cherry tomato. J Food Eng. 2004;61:291–295. doi: 10.1016/S0260-8774(03)00132-8. [DOI] [Google Scholar]
- Balasundram N, Sundram K, Samman S. Phenolic compounds in plants and agri-industrial by-products: antioxidant activity, occurrence, and potential uses. Food Chem. 2006;99:191–203. doi: 10.1016/j.foodchem.2005.07.042. [DOI] [Google Scholar]
- Bennamoun L, Khama R, Léonard A. Convective drying of a single cherry tomato: modeling and experimental study. Food Bioprod Process. 2015;94:114–123. doi: 10.1016/j.fbp.2015.02.006. [DOI] [Google Scholar]
- Chong SS, Aziz ARA, Harum SW, Arof H, Shamshirband S. Application of multiple linear regression, central composite design, and ANFIS models in dye concentration measurement and prediction using plastic optical fiber sensor. Measurement. 2015;74:78–86. doi: 10.1016/j.measurement.2015.06.019. [DOI] [Google Scholar]
- Demiray E, Tulek Y, Yilmza Y. Degradation kinetics of lycopene, β-carotene and ascorbic acid in tomatoes during hot air drying. LWT Food Sci Technol. 2013;50:172–176. doi: 10.1016/j.lwt.2012.06.001. [DOI] [Google Scholar]
- Derossi A, Severini C, Mastro AD, Pilli TD. Study and optimization of osmotic dehydration of cherry tomatoes in complex solution by response surface methodology and desirability approach. LWT Food Sci Technol. 2015;60:641–648. doi: 10.1016/j.lwt.2014.10.056. [DOI] [Google Scholar]
- Desai K, Survase SA, Saudagar PS, Lele SS, Singhal RS. Comparison of artificial neural network (ANN) and response surface methodology (RSM) in fermentation media optimization: case study of fermentative production of scleroglucan. Biochem Eng J. 2008;41:266–273. doi: 10.1016/j.bej.2008.05.009. [DOI] [Google Scholar]
- Fagundes C, Palou L, Monteiro AR, Pérez-Gago MB. Effect of antifungal hydroxypropyl methylcellulose-beeswax edible coatings on gray mold development and quality attributes of cold-stored cherry tomato fruit. Postharvest Biol Technol. 2014;92:1–8. doi: 10.1016/j.postharvbio.2014.01.006. [DOI] [Google Scholar]
- Giovanelli G, Paradiso A. Stability of dried and intermediate moisture tomato pulp during storage. J Agric Food Chem. 2002;50:7277–7281. doi: 10.1021/jf025595r. [DOI] [PubMed] [Google Scholar]
- Guerreiro D, Madureira J, Silva T, Melo R, et al. Post-harvest treatment of cherry tomatoes by gamma radiation: microbial and physicochemical parameters evaluation. Innov Food Sci Emerg. 2016;31:1–9. doi: 10.1016/j.ifset.2016.05.008. [DOI] [Google Scholar]
- Heredia A, Barrera C, Andrés A. Drying of cherry tomato by a combination of different dehydration techniques. Comparison of kinetics and other related properties. J Food Eng. 2007;80:111–118. doi: 10.1016/j.jfoodeng.2006.04.056. [DOI] [Google Scholar]
- Jang JSR, Sun CT. Neuro-fuzzy modeling and control. Proc IEEE. 1995;83:378–405. doi: 10.1109/5.364486. [DOI] [Google Scholar]
- Karaman S, Kayacier A. Effect of temperature on rheological characteristics of molasses: modeling of apparent viscosity using Adaptive Neuro–Fuzzy Inference System (ANFIS) LWT Food Sci Technol. 2011;44:1717–1725. doi: 10.1016/j.lwt.2011.03.004. [DOI] [Google Scholar]
- Lavelli V, Giovanelli G. Evaluation of heat and oxidative damage during storage of processed tomato products. II. Study of oxidative damage indices. J Sci Food Agric. 2003;83:966–971. doi: 10.1002/jsfa.1433. [DOI] [Google Scholar]
- Lavelli V, Torresani MC. Modelling the stability of lycopene-rich by-products of tomato processing. Food Chem. 2011;125:529–535. doi: 10.1016/j.foodchem.2010.09.044. [DOI] [Google Scholar]
- Martínez-Hernández GB, Boluda-Aguilar M, Taboada-Rodríguez A, Soto-Jover S, Marín-Iniesta F, López-Gómez A. Processing, packaging, and storage of tomato products: influence on the lycopene content. Food Eng Rev. 2016;8:52–75. doi: 10.1007/s12393-015-9113-3. [DOI] [Google Scholar]
- Motahari-Nezhad M, Mazidi MS (2016) An Adaptive Neuro-Fuzzy Inference System (ANFIS) model for prediction of thermal contact conductance between exhaust valve and its seat. Appl Therm Eng 105:613–621
- Muratore G, Rizzo V, Licciardello F, Maccarone E. Partial dehydration of cherry tomato at different temperature, and nutritional quality of the products. Food Chem. 2008;111:887–891. doi: 10.1016/j.foodchem.2008.05.001. [DOI] [Google Scholar]
- Nabnean S, Thepa S, Janjai S, Bala BK (2016) Drying kinetics and diffusivity of osmotically dehydrated cherry tomatoes. J Food Process Preserv (in press)
- Najafi G, Ghobadian B, Moosavian A, Yusaf T, Mamat R, Kettner M, Azmi WH. SVM and ANFIS for prediction of performance and exhaust emissions of a SI engine with gasoline–ethanol blended fuels. Appl Therm Eng. 2016;95:186–203. doi: 10.1016/j.applthermaleng.2015.11.009. [DOI] [Google Scholar]
- Nour V, Ionica ME, Trandafir I. Bread enriched in lycopene and other bioactive compounds by addition of dry tomato waste. J Food Sci Technol. 2015;52:8260–8267. doi: 10.1007/s13197-015-1934-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MS, Rashid MM, Hussain MA. Thermal conductivity prediction of foods by Neural Network and Fuzzy (ANFIS) modeling techniques. Food Bioprod Process. 2012;90:333–340. doi: 10.1016/j.fbp.2011.07.001. [DOI] [Google Scholar]
- Shi JX, Maguer ML, Wang SL, Liptay A. Application of osmotic treatment in tomato processing—effect of skin treatments on mass transfer in osmotic dehydration of tomatoes. Food Res Int. 1997;30:669–674. doi: 10.1016/S0963-9969(98)00031-3. [DOI] [Google Scholar]
- Simha HVV, Pushpadass HA, Franklin MEE, Kumar PA, Manimala K. Soft computing modelling of moisture sorption isotherms of milk-foxtail millet powder and determination of thermodynamic properties. J Food Sci Technol. 2016;53:2705–2714. doi: 10.1007/s13197-016-2242-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sricharoen P, Techawongstein S, Chanthai S. A high correlation indicating for an evaluation of antioxidant activity and total phenolics content of various chilli varieties. J Food Sci Technol. 2015;52:8077–8085. doi: 10.1007/s13197-015-1931-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Srivastava AK. Lycopene; chemistry, biosynthesis, metabolism and degradation under various abiotic parameters. J Food Sci Technol. 2015;52:41–53. doi: 10.1007/s13197-012-0918-2. [DOI] [Google Scholar]
- Tiwari BK, Patras A, Brunton N, Cullen PJ, O’Donnell CP. Effect of ultrasound processing on anthocyanins and color of red grape juice. Ultrason Sonochem. 2010;17:598–604. doi: 10.1016/j.ultsonch.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Viana AD, Corrêa JLG, Justus A. Optimisation of the pulsed vacuum osmotic dehydration of cladodes of fodder palm. Int J Food Sci Technol. 2014;49:726–732. doi: 10.1111/ijfs.12357. [DOI] [Google Scholar]
- Yadav BS, Yadav RB, Jatain M. Optimization of osmotic dehydration conditions of peach slices in sucrose solution using response surface methodology. J Food Sci Technol. 2012;49:547–555. doi: 10.1007/s13197-011-0298-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoni B, Pagliarini E, Foschino R. Study of the stability of dried tomato halves during shelf-life to minimise oxidative damage. J Sci Food Agric. 2000;80:2203–2208. doi: 10.1002/1097-0010(200012)80:15<2203::AID-JSFA775>3.0.CO;2-W. [DOI] [Google Scholar]
- Zapata PJ, Guillén F, Martínez-Romero D, Castillo S, Valero D, Serrano M. Use of alginate or zein as edible coatings to delay postharvest ripening process and to maintain tomato (Solanum lycopersicon Mill) quality. J Sci Food Agric. 2008;88:1287–1293. doi: 10.1002/jsfa.3220. [DOI] [Google Scholar]
- Zheng H, Jiang B, Lu H. An adaptive neural-fuzzy inference system (ANFIS) for detection of bruises on Chinese bayberry (Myrica rubra) based on fractal dimension and RGB intensity color. J Food Eng. 2011;104:663–667. doi: 10.1016/j.jfoodeng.2011.01.031. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.