Abstract
Thermal processing extends the shelf life of fruit and vegetables products by inactivating microorganisms and enzymes. The effect of a pasteurization (P) treatment, 90 ± 2 °C for 35 s, provided by continuous semi-industrial microwave (MW) under different conditions (high power/short time and low power/long time) or conventional pasteurization (CP) on orange-colored smoothies and their changes throughout 45 days of storage at 5 °C were investigated. Results indicated that vitamin C and antioxidant capacity (FRAP) in CP decreased dramatically in comparison with the unheated and MWP smoothies. On the contrary, all heating treatments increased the contents of total phenolic compounds and carotenoids. Based on the sensory quality and microbial counts, the shelf life of all those heated smoothies reached 45 days. No Listeria monocytogenes growth was found and all microbial counts were below the European legal limits (2007). MWP as compared to the CP method led to a greater reduction of mesophilic bacteria after 45 days at 5 °C (3.7 log cfu g−1 for CP and 1.6 log cfu g−1 for MWP). The highest power and the shortest time MWP treatments (3600 W for 93 s), resulted into better preservation of FRAP and vitamin C.
Keywords: Heat treatment, Beverage, Quality attributes, Microbial counts, Bioactive compounds
Introduction
Fruit and vegetables are a great dietary source of many natural antioxidants that provide protection against harmful free radicals. Antioxidants block the oxidation processes by neutralizing free radicals and therefore reducing the risk of certain types of cancer and other diseases (Azizah et al. 2009). The most important antioxidant compounds in fruits and vegetables include vitamins, pigments (such as lycopene), and phenolics (Podşedek et al. 2003). At present, consumers demand the best preservation of the sensory, nutritional and health-related characteristics of plant-derived food products. The nutritional quality of products depends not only on the nutrient content at harvest but also on the changes that occur during handling, processing, storage, and distribution (Favell 1998). Smoothies are a type of cold beverage made from fresh fruit and/or vegetables, and are entering the market healthy, nutritious products. The greatest benefit of consuming fruit and vegetables in the shape of a smoothie is the increased absorption of important nutrients.
Thermal processing is necessary for destroying harmful pathogenic microorganisms. Pasteurization is a relatively mild heat treatment, in which products are heated at a specific temperature (below 100 °C) for a stated duration (Park et al. 2014). Pasteurization also ensures the destruction of the natural enzymes that may cause negative modifications during product storage (Igual et al. 2010). Temperatures have to remain below certain levels in order to achieve the desired food product quality along with the maximum preservation of color, flavor and bioactive compounds (Knoerzer et al. 2009). Conventional thermal processing generally induces detrimental changes, lowering the quality attributes of products, especially nutritional value and sensory properties in terms of color and flavor (Igual et al. 2010; Math et al. 2014). In the food industry, microwave pasteurization (MWP) heat treatments have gained attention as an alternative to conventional pasteurization (CP) of liquid foods, such as milk and fruit juices because it is a fast heating method (Clare et al. 2005). Microwave (MW) heating efficiency is very dependent on the oven characteristics, the product itself, its geometry and physical properties, and should be optimized for each sample. Fratianni et al. (2010) evaluated the effects of MWP on quality parameters of orange juice such as cloud stability, color, carotenoids, and vitamin C content, and their results showed that the carotenoid content decreased by about 13 % after MWP at 70 °C for 1 min while the retention of vitamin C ranged from 96.1 to 97 %. Picouet et al. (2009) reported that MW treatment at 652 W for 35 s followed by 15 days of storage at 5 °C resulted in 50 % average loss of vitamin C in apple puree although the viscosity and titratable acidity were unaffected. The inactivation of microorganisms and enzymes through MWP, especially in continuous-flow systems, is preferred for pasteurization over conventional heating because it allows the smoothies to obtain a longer shelf life, while preserving nutrients and the “fresh” taste (Ahmed and Ramaswamy 2007). The inactivation of microorganisms and reduction of overall product quality are highly influenced by time–temperature treatments during pasteurization (Math et al. 2014). Also, continuous-flow MW systems deliver reduced thermal exposure to inactivate microorganisms while maintaining the high quality of the products. Since increasing MW power has an important effect on the reduction of heating time, a combination of high power and short time might be a solution for reducing the loss of quality. For all these reasons, the objective of the present work was to compare the effects of MWP and CP on the chemical, functional, microbial and sensorial parameters of a fresh orange-colored vegetable smoothie throughout 45 days of storage at 5 °C. To accomplish this task, a semi-industrial equipment of continuous-flow MW oven was used.
Methods
Sample preparation
After several preliminary compositional and sensory tests, an orange-colored smoothie was prepared with 126 g of tomato (Moneymaker cv.), 61 g of carrot (Nantesa cv.), 29 g of pumpkin (Crown Prince cv.), lemon juice (4 mL, to reach a pH of 4.45), mineral water (50 mL) and 0.3 g marine salt. All ingredients were blended for 3 min in a thermomix (Vorwerk elektrowerke, Model TM 31-1, France). Fresh control smoothies were chilled at 5 °C immediately after blending until subjected to subsequent processing.
Treatments
For MWP a semi-industrial prototype of continuous MW oven (Sairem Iberica S.L. SI-MAQ0101, Barcelona, Spain) was used for the current experiments. The continuous-flow system of the MW oven includes a feed belt that can move back and forth, an optimized heating chamber, new energy economizing filters, a computer interface and a fiber optic slip ring for online temperature measurements. To obtain MWP samples, three tempered and extra resistant MW glasses were used (Hostelvia, Vicrila, Leioa, Spain). These samples contained 200 mL of smoothie and they were heated at the same time. The glasses were placed in the feed system of the MW oven and treated under low power/long time (210 and 260 W for 646 and 608 s) and high power/short time (1600 and 3600 W for 206 and 93 s). Those power/time combinations of MWP were selected according to preliminary studies (Arjmandi et al. 2016). For CP, samples (600 mL) were heated in the thermomix. In both MWP and CP treatments, samples were heated from room temperature to a final temperature of 90 ± 2 °C, which was maintained for 35 s. For CP, the total heat process took 515 s. In all cases, the temperature was monitored with an automatic fiber optic thermometer (Neoptix, Quebec, Canada). After both kinds of pasteurization, the samples were packaged aseptically into plastic tubes and rapidly cooled (5 °C) with an ice-water bath. As control, fresh, unheated samples were stored. For each heating method, the full experiment was conducted independently three times, each experiment constituting a repetition. Microbial and sensorial analysis, total soluble solids, pH and titratable acidity, total polyphenol, antioxidant capacity, total vitamin C, and carotenoids were evaluated at day 0, and after 15, 30 and 45 days of storage at 5 °C.
Methods for quality determination
Microbial analysis
Mesophilic bacteria, molds, yeasts and Listeria monocytogenes counts were periodically evaluated. About 10 g of each sample were homogenized with 90 mL buffered peptone water (Scharlau Scharlab S.L., Spain, pH 7) for 60 s in a sterile stomacher bag (Steward Laboratory, London, UK) using a Masticator (Seward Medical, London, UK) at room temperature. Serial dilutions of the treated smoothies were performed in the above mentioned buffered peptone water. The agar medium and incubation conditions used were as described in Falagán et al. (2015), and the analysis performed in triplicate. Briefly, total aerobic bacterial counts were determined using the spread plate method; For mesophilic bacteria, 1 mL aliquots were aseptically pipetted, and for yeasts and molds, 0.1 mL were used. Plate Count Agar (Scharlau Scharlab S.L., Spain) was used for mesophilic bacteria and was incubated at 30 °C for 48 h. Yeasts and molds were counted using Rose Bengal Agar (Scharlau Scharlab S.L., Spain) after incubation at 25 °C for 5 and 7 days in the dark for yeasts and molds, respectively. For L. monocytogenes, 25 g of samples were put into 225 mL Fraster Listeria Broth Base (Cultimed, Panreac, Barcelona, Spain) and incubated at 37 °C for 24 h. L. monocytogenes was determined using the spread plate method with Listeria Oxford Selective Supplement (Scharlau Scharlab S.L., Barcelona, Spain) added to Oxford Agar Base (Scharlau Scharlab S.L. Barcelona, Spain). Each one of the three replicates was analyzed by triplicate. Microbial counts were reported as log10 colony forming units per gram of smoothies (log CFU g−1).
Physico-chemical analysis
Total soluble solids (TSS) of smoothies were determined using a digital refractometer (Atago, Tokyo, Japan) and expressed as °Brix. The pH of samples was measured with a pH-meter calibrated with phosphate buffers, pH 4 and 7 (Crison 2001 pH-meter, Crison Instruments S.A., Barcelona, Spain). Titratable acidity (TA) was performed by titrating 5 mL of homogenized sample with NaOH (0.1 N) to an end point of pH 8.1 (716 DMS Titrino, Metrohm, Herisau, Switzerland) (AOAC 1984).
Sensory analysis
Sensory analyses were performed according to international standards (ASTM STP 913 1986). Appearance, flavor and overall quality of smoothies were evaluated by a 12-persons (aged 24–67) panel at room temperature (20 °C) in a standard room (ISO 8589:2007) equipped with individual taste booths. The panelists were trained on the quality of fresh smoothies (unheated) on days 0 and 15, and for heat-treated (MWP and CP) samples stored at 5 °C for 0, 15, 30 and 45 days were used. They completed a rating sheet based on a nine-point scale where 1 = unacceptable, 3 = fair, low quality, 5 = moderate, 7 = high, 9 = very high quality.
Total polyphenol and antioxidant analysis
Homogenized samples (0.5 mL) were extracted with 2.5 mL methanol in an orbital shaker (SSL1, Stuart, UK) for 1 h at 200×g in darkness inside a polystyrene box with an ice bed. Afterwards, 2 mL of extracts were transferred into two 2 mL eppendorf tubes and centrifuged (Heraeus Fresco 21, Germany) at 15,000×g for 10 min at 4 °C. The supernatant was used to measure total phenolic compounds (TPC) and total antioxidant capacity (TAC) for each sample.
The TPC was assessed with the Folin–Ciocalteu colorimetric method. 19.2 µL of extracted sample were placed on a 96 well flat-bottom polystyrene plate (Greiner Bio-one, Frickenhausen, Germany) and 29 µL of Folin–Ciocalteu reagent 1 N were added. Samples were incubated for 3 min in darkness at room temperature. After incubation, 192 µL of a solution containing Na2CO3 (2 g 100 mL−1) and NaOH (0. 4 g 100 mL−1) was added and the reaction carried out for 1 h at room temperature in darkness. The absorbance was measured at 750 nm using a multiscan plate reader (Tecan Infinite M200, Männedorf, Switzerland). A standard calibration curve was prepared using different concentrations of chlorogenic acid in methanol. TPC was expressed as chlorogenic acid equivalents (ChAE) in mg 100 mL−1 smoothie. Each of the three replicates was analyzed by triplicate.
TAC was assessed chemically using the Ferric Reducing Antioxidant Power (FRAP) technique. The procedure used for the extraction was as described above. The FRAP technique depends upon the reduction of the ferric tripyridyl triazine (TPTZ) complex at low pH to the ferrous TPTZ by a reductant. The FRAP reagent was prepared by mixing 20 mL acetate buffer (300 mM, pH 3.6: 3.1 g sodium acetate (C2H3NaO2·3H2O) and 16 mL glacial acetic acid, with MilliQ water added for a final volume of 1000 mL), 2 mL TPTZ solution (0.1562 g of 2,4,6-tripyridyl-s-triazine in 0.166 mL hydrochloric acid) and 2 mL FeCl3·6H2O solution (0.5404 g of FeCl3·6H2O in 100 mL MilliQ water). This solution was incubated for 2 h at 37 °C. An aliquot of 6 µL of extracted sample or standard solution and 198 µL of reagent were placed on a 96-well flat-bottom polystyrene plate (Greiner Bio-one, Frickenhausen, Germany). The plate was kept for 30–40 min at room temperature in the dark for the reaction to take place. All of these stages were carried out on ice and in the dark. The absorbance of the extract was measured using the same device as for TPC at 593 nm. Results were expressed as mg ascorbic acid equivalent (AAE) per 100 mL smoothie. Each of the three replicates was analyzed by triplicate.
Total vitamin C
For the analysis of ascorbic acid (AA), 10 mL of smoothie were mixed with 10 mL of a solution containing 45 g L−1 of metaphosphoric acid and 7.2 g L−1 of DTT (dl-1,4-dithiothreitol). The mixture was centrifuged at 22,100×g for 15 min at 4 °C (Eppendrof, AG 22331, Germany), then the supernatant was filtered through four layers of cheesecloth and a 0.45 µm membrane (Chromafil® Xtra PA-45/25, Germany). The extracts were kept at −42 °C until required for analysis. The analysis of vitamin C was carried out by high performance liquid chromatography (HPLC). An aliquot of 10 µL was injected into a HPLC (Waters 2695, Detector UV–Vis 2687, Milford, USA) fitted with a reverse-phase C18 Spherisorb ODS2 (5 mm) stainless-steel column (4.6 × 150 mm). The mobile phase was a 0.1 % trifluoroacetic acid in ultrapure water. The flow rate was fixed at 1.4 mL min−1 at room temperature. Detection was performed with a UV–Vis at 260. Vitamin C was quantified through a calibration curve built with ascorbic acid pure standards and results were expressed as relative vitamin C concentration.
Carotenoids
Carotenoids were spectrophotometrically measured according to the method of Nagata and Yamashita (1992) with the slight modifications made by Navarro et al. (2010). 5 mL of smoothie were mixed with 20 mL acetone-hexane (4:6). After a few minutes, two phases separated and the upper phase was taken for lycopene and β-carotene measurements at 663, 645, 505 and 453 nm in a UV–visible spectrophotometer (Hewlet Packard, Model: 8453, Columbia, EEUU). Lycopene and β-carotene in acetone-hexane extracts were calculated according to the following equations:
Each one of the three replicates was analyzed in triplicate.
Statistical analysis
To find out the influence of pasteurization methods (conventional and different MW treatments) on the quality changes of smoothies, the data was analyzed using a classic randomized design with three replicates per treatment. For each day, the dependent variables were subjected to one-way analysis of variance (ANOVA, p ≤ 0.05 using Statgraphic Plus 5.1, Manugistic Inc, Rockville, MD, USA). Mean values were compared by LSD (multiple range least significant difference test) to identify significant differences among treatments.
Results and discussion
Microbial analysis
The unheated smoothies had a high mesophilic bacterial load (5.1 log CFU g−1) that was significantly reduced when any of the heat treatments were applied (<1 log CFU g−1) (Table 1). Yeast growth was also reduced from 2.5 log CFU g−1 in control samples to <2 log CFU g−1 in heated samples. The initial mold load was under the detection limit (<2 log CFU g−1), and it was maintained in all treated smoothies to the end of the storage time. The same stable behavior was found with yeast in heated samples. After 15 days of storage, yeast growth increased in unheated smoothies. When time of storage was extended, the mesophilic bacterial load increased in CP-treated smoothies compared to the slight increase obtained in MWP-treated ones (1.7, 3.6 and 3.7 log CFU g−1, at 15, 30 and 45 days, respectively). At day 45, the reduction differences between CP and MWP was about 1.7–2.2 log units, depending on the MWP treatments. Comparing among various MWP treatments, the combination of lowest power and longest heating time lead to a slightly higher mesophilic growth. No L. monocytogenes was detected in any of the heat treatments throughout the shelf life, in accordance to the European Commission (EC) Regulation (2007).
Table 1.
Microbial counts (log CFU g−1) in unheated (control), conventional (CP) and microwave (MWP; high power/short time and low power/long time doses) pasteurized smoothies throughout storage up to 45 days at 5 °C
| 0 days | 15 days | 30 days | 45 days | |
|---|---|---|---|---|
| Mesophilic | ||||
| Control | 5.1 ± 0.1 | 7.5 ± 0.3 | Not evaluated | Not evaluated |
| CP | <1 | 1.7 ± 0.3 | 3.6 ± 0.2 | 3.7 ± 0.1 |
| MWP: 210 W-646 s | <1 | 1.6 ± 0.2 | 1.4 ± 0.2 | 2.0 ± 0.1 |
| MWP: 260 W-608 s | <1 | 1.6 ± 0.3 | 1.4 ± 0.1 | 1.8 ± 0.2 |
| MWP:1600 W-206 s | <1 | 1.2 ± 0.1 | 1.4 ± 0.2 | 1.5 ± 0.2 |
| MWP: 3600 W-93 s | <1 | 1.1 ± 0.1 | 1.2 ± 0.2 | 1.6 ± 0.1 |
| Yeasts | ||||
| Control | 2.5 ± 0.1 | 6.1 ± 0.1 | Not evaluated | Not evaluated |
| CP | <2 | <2 | <2 | <2 |
| MWP (all doses) | <2 | <2 | <2 | <2 |
Values are means (n = 3) ± standard error. Mold load was <2 log CFU g−1 in all unheated and heated treatments
These results showed that MWP successfully eliminated vegetative bacteria in smoothies without compromising food quality. Inactivation of microorganisms and reduction of quality attributes are both strongly influenced by time–temperature treatments during pasteurization (Gentry and Roberts 2005). Using MW could significantly reduce heating time as compared to conventional-heating methods (Robinson et al. 2009), and in this case, MW was more efficient than CP for in reducing microbial counts in smoothies. These results are in agreement with those of Picouet et al. (2009), who reported that the MW processing of apple products (at 652 W for 35 s) reduced pathogenic microorganisms, while maintaining the nutritional and sensorial attributes that could be used to increase the competitiveness of the fruit sector. The same trend was found in microbial results from various beverages such as apple juice (Canumir et al. 2002), and orange juice (Tajchakavit and Ramaswamy 1995) by continuous-flow MW systems.
Physico-chemical analysis
TSS of unheated smoothie was 5.10 °Brix which increased to 5.13 in thermally treated samples (data not shown). No significant differences were found by type of thermal treatment or storage duration. The initial pH value (4.24) was not significantly affected by heat treatments and storage duration. These results are in agreement to those found by Quartey et al. (2012), who reported that a low pH (about 4.45) is an important qualitative parameter of tomato for consumer’s acceptance. TA was quite stable without significant differences among the differently treated samples and storage time, with 0.46 and 0.44 % citric acid for unheated and heated samples, respectively.
Sensory evaluation
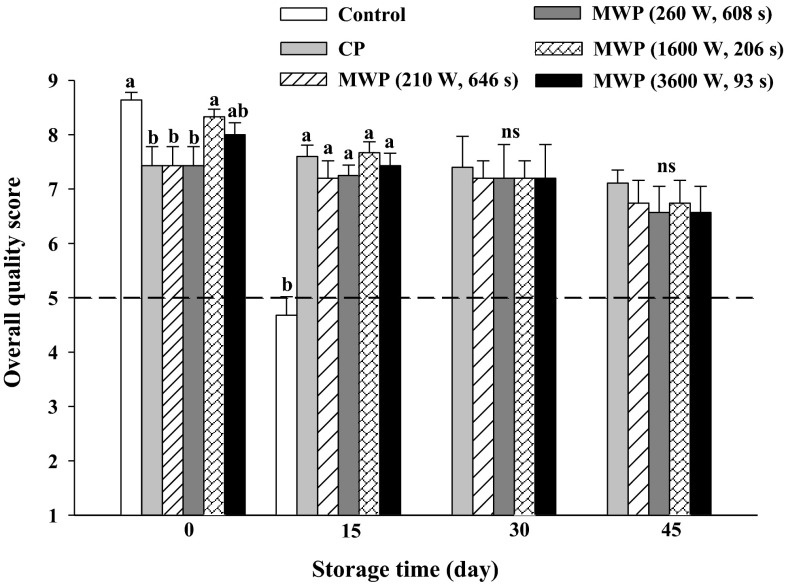
The shelf life of unheated smoothies was at most 15 days due to high mesophilic bacteria growth (Table 1) and low overall quality, resulting in significant differences (p < 0.05) among unheated and heat-treated smoothies for all sensory parameters (Fig. 1). At day 0, among the applied heating treatments, a similar appearance score was given to them (8.3–7.7). However, CP samples obtained the lowest flavor score (6.8 ± 0.4) on the processing day, while MWP, particularly using higher power/shorter time, such as 1600 W/206 s and 3600 W/93 s were scored as the best treatments (8.4 ± 0.2). The appearance, flavor and the overall quality decreased throughout commercial life in all of treated samples, probably due to enzymatic reactions and microbial spoilage. In all heated samples, overall quality scores were above the limit of acceptability for consumption after 45 days of cold storage. For this parameter, from 15 to 45 days of storage, no significant differences among CP and MWP were found. Polyphenoloxidase and perioxidase are related to discolouration (browning) with subsequent loss of sensorial properties such as texture and flavor in fruits and vegetables (Vámos-Vigyázó 1981; Macheix et al. 1990). Heat treatment led to enzyme inactivation, preserving the overall quality of products during the shelf life period. According to the aforementioned sensory results, the commercial life of pasteurized orange-colored smoothies, stored at 5 °C, was set to 45 days.
Fig. 1.
Overall quality (1–9) in unheated (control), conventional (CP) and microwave (MWP; high power/short time and low power/long time) pasteurized smoothies throughout storage up to 45 days at 5 °C. Means (n = 3) ± standard error. Different letters above the columns indicate significant differences among mean values (p < 0.05), and “ns” means there were no significant differences
Total phenolic content
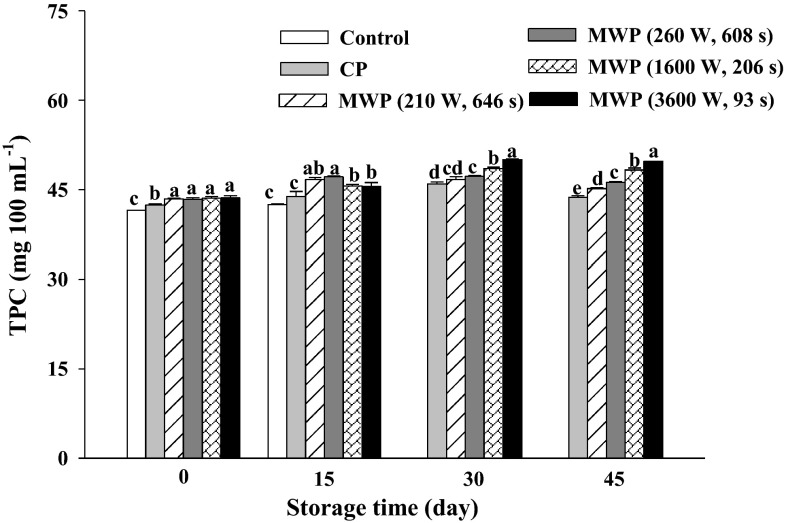
The initial TPC of fresh smoothies was 41.36 ± 0.11 mg ChAE 100 mL−1 within the range of fresh tomato juice (26.8 and 52.3 mg 100 mL−1) (Podsedek et al. 2003). This initial TPC increased slightly after heating and during the initial 30 days of storage (Fig. 2). Immediately after pasteurization, the maximum TPC value was reached in MW smoothies without significant differences among MWP treatments. These results were in agreement with those reported by Dewanto et al. (2002) who showed thermal processing elevated the TPC concentration in tomatoes. This could be attributed to disruptions of the cell wall by thermal processing (Martínez-Hernández et al. 2013), with it being higher when using high-power MW such as 3600 W compared to low-power MW such as 260 W or CP. Additionally, thermal processing is able to inactivate the polyphenol oxidase enzyme, thus preventing polyphenol degradation (Chuah et al. 2008). For this reason, the TPC was better preserved in heated samples. At the end of the storage period, a slight decrease of TPC was found, with MWP smoothies treated with high power/short time being the ones that had the highest TPC.
Fig. 2.
Total phenolic content (mg chlorogenic acid 100 mL−1) in unheated (control), conventional (CP) and microwave (MWP; high power/short time and low power/long time) pasteurized smoothies throughout storage up to 45 days at 5 °C. Means (n = 3) ± standard error. Different letters above the columns indicate significant differences among mean values (p < 0.05)
Total antioxidant capacity
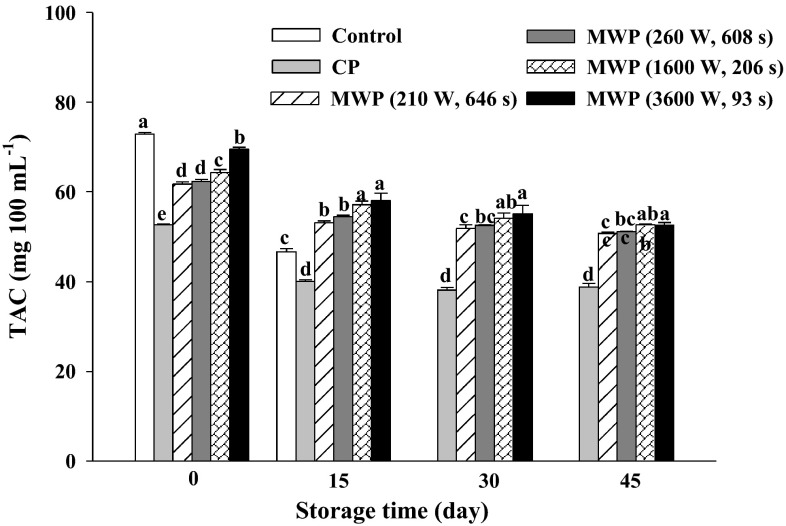
The TAC value for unheated smoothie was 72.86 ± 0.38 mg AAE 100 mL−1 and it decreased after both heating techniques, although MWP-treated smoothies maintained higher levels (Fig. 3). Just after treatment the gradation of total antioxidant was only 5 % under the combination of high power/short time whereas, this amount was 28 % in CP smoothies. Podşedek (2007) reported that antioxidant levels of conventionally-heated vegetables were lower than the corresponding fresh samples. Our results are also confirmed by Zhang and Hamauzu (2004) who indicated that both the florets and stems of broccoli retained about 35 % of total antioxidant activity after conventional and MW cooking for 5 min. Crozier et al. (1997) showed that boiling reduced the antioxidants content in vegetables by 80 %, while MW cooking only by 65 %. In this study, after CP, the TAC decreased to 72 % from its initial value as compared to unheated samples, whereas the MW treatment using low power/long time (210 W-646 s) and high power/short time (3600 W-93 s), retained 84 and 95 %, respectively. The results showed that in continuous MWP, the antioxidant value was strongly influenced by the power/time provided. Jacob et al. (2010) reported that the TAC of tomato samples depended on the extract and the thermal treatment intensity. Recent research showed that pasteurization significantly influenced antioxidant capacity of vegetables and the effects were not consistent in different foods. In this case, the TAC for unheated samples dropped remarkably (32 %) after 15 days, probably due to the action of microbes as they consumed nutrients for growth. In all thermal treatments, TAC losses during storage were found (Fig. 3). Similar to our data on days 28 and 35, Keenan et al. (2010) reported TAC (by FRAP) decreases of 19 % in heat-treated (70 °C for 10 min) fruit smoothies after 30 days at 4 °C. The TAC of treated samples progressively decreased during storage at 5 and 20 °C, resulting in TAC levels that were 19–23 and 8–11 % lower after 58 and 40 days, respectively (Rodríguez-Verásteguil et al. 2015). High power/short time MWP treatments resulted in the lowest TAC reduction at the end of the storage period (21 %), while the combination of low power/long time MWP and CP treatments registered the highest TAC reduction, around 27 and 28 % respectively, compared to their initial values. In this study, total vitamin C played a major role in the antioxidant capacity of the orange-colored smoothie.
Fig. 3.
Total antioxidant capacity (mg ascorbic acid equivalent 100 mL−1) in unheated (control), conventional (CP) and microwave (MWP; high power/short time and low power/long time) pasteurized smoothies throughout storage up to 45 days at 5 °C. Means (n = 3) ± standard error. Different letters above the columns indicate significant differences among mean values (p < 0.05)
Vitamin C content
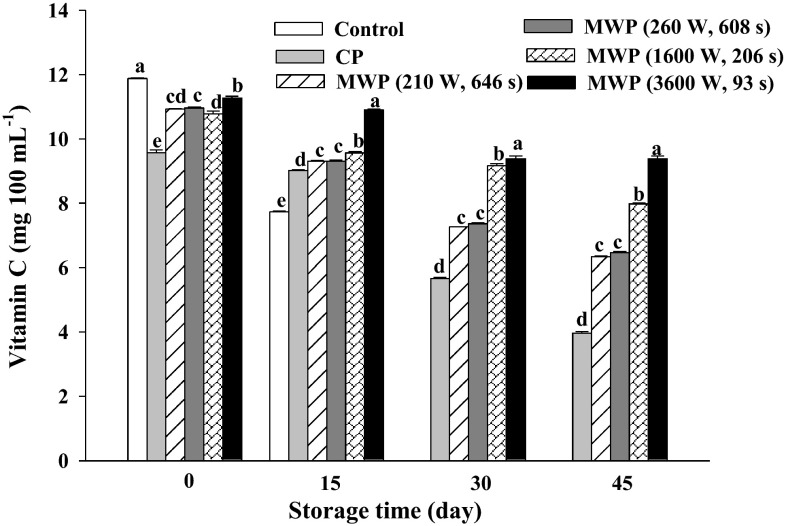
Vitamin C followed a similar pattern to the TAC. Immediately after processing, the vitamin C for unheated smoothie was 11.72 ± 0.02 mg 100 mL−1 (Fig. 4). This amount degratated 6 % under the MWP combination of high power/short time whereas, this amount was 20 % in CP smoothies. With the same final temperature reached in the treatments (90 °C for 35 s), changes in vitamin C content of the thermal treatments were statistically different (p < 0.05) and were also influenced by storage time. At the end of the storage, the vitamin C content in CP-treated smoothies declined to the lowest level obtained (3.9 ± 0.05 mg 100 mL−1) with a 59 % reduction of vitamin C content. As for the rest of the thermal treatments, smoothies treated under low power/long time MWP obtained 6.3 ± 0.01 mg 100 mL−1 of vitamin C (a reduction of 43 %). In contrast, smoothies under high power/short time MWP had the highest vitamin C content (9.3 ± 0.08 mg 100 mL−1) with lower reduction (17 % of the initial value). Decreasing vitamin C content of tomato following heat processing has been previously reported (Leoni 2002). The current results showed that thermal processing led to degradation of vitamin C soon after due to oxidative processes. These results are in agreement with findings by Leoni (2002) who found that vitamin C is a heat-sensitive compound in the presence of oxygen. According to our results, the use of high power/short time MWP led to smoothies having more vitamin C retention than both low power/long time MWP and CP treatments. The use of short time MWP provided the lowest vitamin C degradation due to heat, even when high-power MWP was used. Teixeira (2012) reported that high power/short time MW treatments reduced the adverse thermal degradation on food quality while ensuring food safety because the nutritional characteristics of the product were more sensitive to time than to temperature. The vitamin C degradation by storage time agrees with previous results found for different juices (Klimczak et al. 2007). The changes observed in the vitamin C concentration of the samples stored under refrigeration could be influenced by the persistence of oxidative degradation reactions of ascorbic acid to other oxidised forms such as dehydroascorbic acid, which also has biological activity such as vitamin C (Rusell 2004). Avoiding vitamin C losses during storage is a qualitative factor for the shelf life of juices (Plaza et al. 2006) which demonstrates that the use of high power/short time MWP treatments could be used as a tool to help keep the qualitative factors of refrigerated smoothies.
Fig. 4.
Vitamin C content (mg ascorbic acid 100 mL−1) in unheated (control), conventional (CP) and microwave (MWP; high power/short time and low power/long time) pasteurized smoothies throughout storage up to 45 days at 5 °C. Means (n = 3) ± standard error. Different letters above the columns indicate significant differences among mean values (p < 0.05)
Carotenoids content
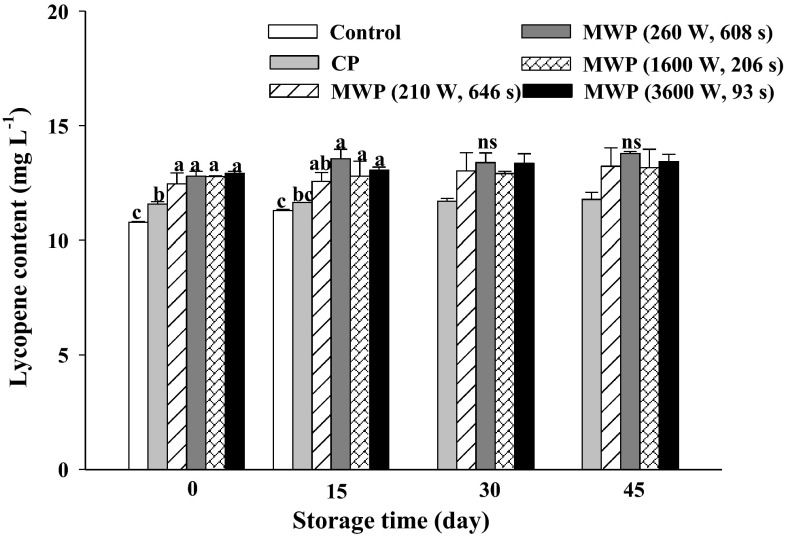
The initial lycopene content in this fresh, orange-colored smoothie was 10.78 ± 0.02 mg L−1 (Fig. 5), with this amount increasing slightly after all the different heat treatments, in particular under high power/short time MW treatment (12.93 ± 0.09 mg L−1). Dewanto et al. (2002) and Shi et al. (2008) reported that thermal processing enhanced the nutritional value of tomatoes by increasing the lycopene content. The main form of lycopene found in tomato is its trans-form (84 %) and its concentration is responsible for the intense redness of tomato. The trans-form is more stable than the cis-form, although changes in the structure of lycopene (isomerization and degradation) occur upon exposure to light, heat and oxygen (Shi et al. 2008). Lycopene is more stable within a matrix than when extracted (D’Evoli et al. 2013). The heat processing could lead to isomerization of lycopene from its trans-form to its cis-form and could also lead to a more efficient extraction of lycopene from the matrix by breaking down cell walls, making it more accessible (Azizah et al. 2009). It would also appear from our results that MWP had a greater effect on cell-wall disruption, possibly aiding in the extraction of other bioactive compounds, as compared to CP treatments. Generally, the thermal treatment of fruits and vegetables leads to the break down of the cellulose structure of the plant cell wall, thus improving the bioavailability of carotenoids (Van het Hof et al. 2000). On the contrary, Sharma and Le Maguer (1996) reported that heating tomato pulp to produce paste, ketchup and juice could cause lycopene degradation. In watermelon juice, pasteurization at 87.7 °C for 20 s significantly reduced the red color and bioactive compounds such as lycopene (Tarazona-Díaz and Aguayo 2013). Contrasting results may depend on the cultivar, ripeness stage, cultivation and environmental conditions (Capanoglu et al. 2010). Throughout storage time the lycopene concentration values increased slightly, without significant differences among the different heat-treatments.
Fig. 5.
Lycopene content (mg 100 L−1) in unheated (control), conventional (CP) and microwave (MWP; high power/short time and low power/long time) pasteurized smoothies throughout storage up to 45 days at 5 °C. Means (n = 3) ± standard error. Different letters above the columns indicate significant differences among mean values (p < 0.05), and “ns” means there were no significant differences
The β-carotene content increased after both methods of heat treatment, but no significant differences among them were found (data not shown). The initial amount of β-carotenes in unheated smoothie was 5.89 ± 0.07 mg 100 L−1. This amount was incremented by all heat treatment methods and achieved the maximum value in the combination of highest power and shortest duration of MWP (6.88 ± 0.09 mg 100 L−1). These results confirmed those found by Stahl and Sies (1992) and Azizah et al. (2009) who individually reported that heating treatments enhanced lycopene and β-carotene content in cooked tomato, carrot, spinach and pumpkin as compared to fresh products. This enhancement could be attributed to cell membrane and wall disruption produced by thermal processing, making β-carotene more accessible for extraction (Van het Hof et al. 2000). As with lycopene, the storage time maintained the β-carotene content, without significant differences among heated samples. The oxidation of carotenoids depends on different type of factors such asperoxidase activity during storage time (Vámos-Vigyázó 1981). Then, since thermal pasteurization reduced enzyme activity, the carotenoid content was quite stable during the storage time.
Conclusion
All heat treatments increased the TPC and carotenoid content while strongly reducing the microbial counts. Based on the overall sensory and microbial quality, the shelf life of those heated samples was set at 45 days at 5 °C. MWP treatments, in particular the treatments using highest power and shortest time, provided the best levels of antioxidant capacity and vitamin C. For those reasons, such industrial pasteurization method could be recommended as a tool for keeping the quality of smoothies.
Acknowledgments
This work was financially supported by MINECO-FEDER (AGL2013-48830-C2-1-R). Thanks are due to Instituto de Biotecnología Vegetal (IBV-UPCT) for providing some of the equipment.
References
- Ahmed J, Ramaswamy HS. Handbook of Food Preservation. Microwave pasteurization and sterilization of foods, Chap 28. 2. São Paulo: Taylor & Francis Group, The University of São Paulo; 2007. pp. 708–710. [Google Scholar]
- AOAC . Official methods of analysis Association of Official Agricultural Chemists. 14. Virginia: AOAC; 1984. pp. 414–420. [Google Scholar]
- Arjmandi A, Oton M, Artés F, Artés-Hernandez F, Gómez P, Aguayo E. Continuous microwave pasteurization of a vegetable smoothie improves its physical quality and hinders detrimental enzyme activity. Food Sci Technol Int. 2016 doi: 10.1177/1082013216654414. [DOI] [PubMed] [Google Scholar]
- ASTM . Physical requirements guidelines for sensory evaluation. Philadelphia: American Society for Testing and Materials Publications, Publication 913; 1986. [Google Scholar]
- Azizah AH, Wee KC, Azizah O, Azizah M. Effect of boiling and stir frying on total phenolics, carotenoids and radical scavenging activity of pumpkin (Cucurbita moschato) Int Food Res J. 2009;16:45–51. [Google Scholar]
- Canumir JA, Celis JE, Bruijn DJ, Vidal LV. Pasteurization of apple juice by using microwave. Lebensm Wiss Technol. 2002;35:389–392. doi: 10.1006/fstl.2001.0865. [DOI] [Google Scholar]
- Capanoglu E, Beekwilder J, Boyacioglu D, Vos RCH, Hall RD. The Effect of industrial food processing on potentially health-beneficial tomato antioxidants. Int J Food Sci Technol. 2010;50:919–930. doi: 10.1080/10408390903001503. [DOI] [PubMed] [Google Scholar]
- Chuah AM, Lee YC, Yamaguchi T, Takamura H, Yin LJ, Matoba T. Effect of cooking on the antioxidant properties of colored peppers. Food Chem. 2008;111:20–28. doi: 10.1016/j.foodchem.2008.03.022. [DOI] [Google Scholar]
- Clare DA, Bang WS, Cartwright G, Drake MA, Corone P, Simunovic J. Comparison of sensory, microbiological, and biochemical parameters of microwave versus indirect UHT fluid skim milk during storage. J Dairy Sci. 2005;88:4172–4182. doi: 10.3168/jds.S0022-0302(05)73103-9. [DOI] [PubMed] [Google Scholar]
- Crozier A, Lean MEJ, McDonald MS, Black C. Quantitative analysis of the flavonoid content of commercial tomatoes, onions, lettuce and celery. J Agric Food Chem. 1997;45:590–595. doi: 10.1021/jf960339y. [DOI] [Google Scholar]
- D’Evoli L, Lombardi-Boccia G, Lucarini M. Influence of heat treatments on carotenoid content of cherry tomatoes. Foods. 2013;2:352–363. doi: 10.3390/foods2030352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewanto V, Wu X, Adom KK, Liu RH. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem. 2002;50:3010–3014. doi: 10.1021/jf0115589. [DOI] [PubMed] [Google Scholar]
- Falagán N, Artés F, Aguayo E. Natural additives to preserve quality and improve nutritional value of fresh-cut nectarin. Food Sci Technol Int. 2015 doi: 10.1177/1082013215621816. [DOI] [PubMed] [Google Scholar]
- Favell DJ. A comparison of the vitamin C content of fresh and frozen vegetables. Food Chem. 1998;62:59–64. doi: 10.1016/S0308-8146(97)00165-9. [DOI] [Google Scholar]
- Fratianni A, Cinquanta L, Panfili G. Degradation of carotenoids in orange juice during microwave heating. J Food Sci Technol. 2010;43:867–871. [Google Scholar]
- Gentry TS, Roberts JS. Design and evaluation of a continuous flow microwave pasteurization system for apple cider. J Food Sci. 2005;38:227–238. [Google Scholar]
- Igual M, García-Martínez E, Camacho MM, Martínez-Navarrete N. Effect of thermal treatment and storage on the stability of organic acids and the functional value of grapefruit juice. Food Chem. 2010;118:291–299. doi: 10.1016/j.foodchem.2009.04.118. [DOI] [Google Scholar]
- Jacob K, García-Alonso FJ, Ros G, Periago MJ (2010) Stability of carotenoids, phenolic compounds, ascorbic acid and antioxidant capacity of tomatoes during thermal processing. Archivos Latinoamericanos de Nutrición versión impresa. ISSN: 0004-0622. ALAN v.60 n.2 Caracas [PubMed]
- Keenan DF, Brunton NP, Gormley TR, Butler F, Tiwari BK, Patras A. Effect of thermal and high hydrostatic pressure processing on antioxidant activity and colour of fruit smoothies. Innov Food Sci Emerg Technol. 2010;11:551–556. doi: 10.1016/j.ifset.2010.07.003. [DOI] [Google Scholar]
- KlimczaK I, Malecka M, Szlachta M, Gliszcynska A. Effect of storage on the content of polyphenols, vitamin C and the antioxidant activity of orange juices. J Food Compos Anal. 2007;20:313–322. doi: 10.1016/j.jfca.2006.02.012. [DOI] [Google Scholar]
- Knoerzer K, Regier M, Hardy EH, Schuchmann HP, Schubert H. Simultaneous microwave heating and three-dimensional MRI temperature mapping. Innov Food Sci Emerg Technol. 2009;10:537–544. doi: 10.1016/j.ifset.2009.05.013. [DOI] [Google Scholar]
- Leoni C. Improving the nutritional quality of processed fruits and vegetables: the case of tomatoes. In: Jongen W, editor. Fruit and vegetable processing: improving quality. Cambridge: Woodhead Publishing and CRC Press; 2002. pp. 83–122. [Google Scholar]
- Macheix JJ, Fleuriet A, Billot J. Fruit phenolics. Boca Raton: CRC Press; 1990. [Google Scholar]
- Martínez-Hernández GB, Francisco Artés-Hernández F, Perla A, Gómez PA, Artés F. Quality changes after vacuum-based and conventional industrial cooking of kailan-hybrid broccoli throughout retail cold storage. Food Sci Technol Int. 2013;50:707–714. [Google Scholar]
- Math R, Nagender A, Nayani S, Satyanarayana A. Continuous microwave processing and preservation of acidic and non acidic juice blends. IJAFST. 2014;2:81–90. [Google Scholar]
- Nagata M, Yamashita I. Simple method for simultaneous determination of chlorophyll and carotenoids in tomato fruit. J Jpn Soc Food Sci. 1992;39:926–928. [Google Scholar]
- Navarro JM, Pérez-Pérez JG, Romero P, Botía P. Analysis of the changes in quality in mandarin fruit, produced by deficit irrigation treatments. Food Chem. 2010;119:1591–1596. doi: 10.1016/j.foodchem.2009.09.048. [DOI] [Google Scholar]
- Park SH, Lamsal BP, Balasubramaniam VM. Food processing: principles and applications. 2. New York: Wiley; 2014. [Google Scholar]
- Picouet PA, Landl A, Abadias M, Castellari M, Viñas I. Minimal processing of a Granny Smith apple purée by microwave heating. Innov Food Sci Emerg Technol. 2009;10:545–550. doi: 10.1016/j.ifset.2009.05.007. [DOI] [Google Scholar]
- Plaza L, Sánchez-Moreno C, Elez-Martínez P, de Ancos B, Martín-Belloso O, Cano MP. Effect of refrigerated storage on vitamin C and antioxidant activity of orange juice processed by high-pressure or pulsed electric fields with regard to low pasteurization. Eur Food Res Technol. 2006;223:487–493. doi: 10.1007/s00217-005-0228-2. [DOI] [Google Scholar]
- Podşedek A. Natural antioxidants and antioxidant capacity of Brasicca vegetables: a review. Lebensm Wiss Technol. 2007;40:1–11. doi: 10.1016/j.lwt.2005.07.023. [DOI] [Google Scholar]
- Podşedek A, Sosnowska D, Anders B. Antioxidative capacity of tomato products. Eur Food Res Technol. 2003;217:296–300. doi: 10.1007/s00217-003-0751-y. [DOI] [Google Scholar]
- Quartey EK, Amoatey HM, Achel DG, Klu GYP, Mba RA. Induced mutations for improved lycopene, total antioxidant properties and other quality factors in wild tomato (Solanum pimpinellifolium L.) J Food Sci Technol. 2012;4:182–188. [Google Scholar]
- Regulation EC 1441/2007 (2007) Commission regulation on microbiological criteria for food stuffs. Off J Eur Union L322:12–29
- Robinson JP, Kingman SW, Snape CE, Shang H, Barranco R, Saeid A. Separation of polyaromatic hydrocarbons from contaminated soils using microwave heating. Sep Purif Technol. 2009;69:249–254. doi: 10.1016/j.seppur.2009.07.024. [DOI] [Google Scholar]
- Rodríguez-Verásteguil LL, Ginés Benito Martínez-Hernández GB, Castillejo N, Gómez PA, Artés F, Artés-Hernández F. Bioactive compounds and enzymatic activity of red vegetable smoothies during storage. Food Bioprocess Technol. 2015;9:137–146. doi: 10.1007/s11947-015-1609-6. [DOI] [Google Scholar]
- Rusell LF. Handbook of food analysis. Physical characterisation and nutrient analysis. New York: Marcel Dekker; 2004. pp. 487–571. [Google Scholar]
- Sharma SK, Le Maguer M. Kinetics of lycopene degradation in tomato pulp solids under different processing and storage conditions. Food Res Int. 1996;29:309–315. doi: 10.1016/0963-9969(96)00029-4. [DOI] [Google Scholar]
- Shi J, Dai Y, Kakuda Y, Mittal G, Xue SJ. Effect of heating and exposure to light on the stability of lycopene in tomato puree. Food Control. 2008;19:514–520. doi: 10.1016/j.foodcont.2007.06.002. [DOI] [Google Scholar]
- Stahl W, Sies H. Uptake of lycopene and its geometrical isomers is greater from heat-processed than from unprocessed tomato juice in humans. J Nutr. 1992;122:2161–2166. doi: 10.1093/jn/122.11.2161. [DOI] [PubMed] [Google Scholar]
- Tajchakavit S, Ramaswamy HS. Continuous-flow microwave heating of orange juice: evidence of nonthermal effects. J Microw Power Electromagn Energy. 1995;30:141–148. doi: 10.1080/08327823.1995.11688270. [DOI] [Google Scholar]
- Tarazona-Díaz MP, Aguayo E. Influence of acidification, pasteurization, centrifugation and storage time and temperature on watermelon juice quality. J Sci Food Agric. 2013;93:3863–3869. doi: 10.1002/jsfa.6332. [DOI] [PubMed] [Google Scholar]
- Teixeira AA. Simulating thermal food processes using deterministic models. In: Wen-Sun D, editor. Thermal food processing: new technologies and quality issues. Boca Raton: CRC Press; 2012. p. 98. [Google Scholar]
- Vámos-Vigyázó L. Polyphenoloxidase and peroxidase in fruits and vegetables. Crit Rev Food Sci Nutr. 1981;15:49–127. doi: 10.1080/10408398109527312. [DOI] [PubMed] [Google Scholar]
- Van het Hof KH, de Boer BCJ, Tijburg LBM, Lucius BRHM, Zijp I, West CE, Hautvast JGAJ, Weststrate JA. Carotenoid bioavailability in humans from tomatoes processed in different ways determined from the carotenoid response in the triglyceride-rich lipoprotein fraction of plasma after a single consumption and in plasma after four days of consumption. J Nutr. 2000;130:1189–1196. doi: 10.1093/jn/130.5.1189. [DOI] [PubMed] [Google Scholar]
- Zhang D, Hamauzu Y. Phenolics, ascorbic acid, carotenoids and antioxidant activity of broccoli and their changes during conventional and microwave cooking. Food Chem. 2004;88:503–509. doi: 10.1016/j.foodchem.2004.01.065. [DOI] [Google Scholar]