Abstract
Casein nano particles and casein-silver conjugated nano composite containing edible bilayer pouch was developed from a heat sealable casein layer laminated with sodium alginate–pectin layer. The physicochemical, mechanical, biodegradability and the toxicity of the film were evaluated. The synthesized casein nano particle was incorporated in the casein layer (inner layer) of the film, however, the casein-silver conjugated nano composite were incorporated in the sodium alginate–pectin (outer) layer of the film. The mechanical, barrier, physical and antimicrobial properties of the film were investigated. Addition of nano composite/nano particle reduced the water solubility (from 67 to 46 % at 80 °C temperature) of the film improved the water barrier properties, light barrier properties, tensile strength and thermal properties of the film. The light barrier properties of the film increased and transparency reduced with increase in silver content in the conjugated nano composite film. The nano particles/nano composite containing film showed antibacterial activity against E. coli. The films were recorded safe for red blood cells as % haemolysis for all the tested samples were found to be well below the safe level.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-016-2343-4) contains supplementary material, which is available to authorized users.
Keywords: Bilayer edible film, Casein, Casein-silver, Nanoparticles, Pectin, Sodium alginate
Introduction
Now-a-days use of biodegradable packaging materials are gaining tremendous attention world-wide as disposal of plastic films has become a big challenge to the whole world. Edible packaging films developed from natural sources are also gaining popularity as a better alternative to plastic films. But, the poor barrier (mechanical, moisture and oxygen) properties of the edible films limited their scope in the food sector. Composite edible films are generally designed to achieve a synergistic effect of combined features of pure components and mechanical and barrier properties of composite bio-films strongly depend on characteristics of constituting polymers and their compatibility (Garcia et al. 2004). Pectin and alginate both belong to the group of polyuronates and are the characteristic examples of natural ionic polysaccharides undergoing chain–chain association and forming hydrogels upon addition of divalent cations (e.g. Ca2+) (Fang et al. 2008). Polysaccharides, proteins and lipids are the widely used polymeric ingredients to develop biodegradable packaging material. These polymeric ingredients may be used as a single or in combinations to develop the edible films. Films based on polysaccharides (carragenan, pectin, chitosan) are hydrophilic and provides strong hydrogen bonding and so have strong oxygen barrier properties. Being thermoplastic heteropolymers proteins are able to form numerous intermolecular linkages (Chinabhark et al. 2007; Nagarajan et al. 2015). Amongst all protein, casein based films have received tremendous attention due to their good film forming properties and oxygen barrier properties. Casein/glycerol containing films possess good tensile and moderate barrier properties (Ghosh et al. 2009; Dangaram et al. 2006). However, there are some limitations to the use of casein-based edible films as a packaging material due to their poor barrier and mechanical properties. Therefore, the single layer edible films are still not widely accepted. Combination of sodium alginate–pectin and casein with some functional ingredients may overcome the disadvantages of single layer edible films. Biomineralization is a natural process where biomolecules are directly involved in the synthesis of functional inorganic materials. BSA conjugated gold nanoparticle improved the mechanical properties of zein protein film (Bakshi et al. 2011). Incorporation of small amounts of nanoparticles to edible films improves their mechanical, thermal, and barrier properties, which can expand the use of the polymers in food packaging (Dean and Yu 2005; Jafarzadeh et al. 2016). So the barrier and mechanical properties of casein film can be improved through incorporation of casein-silver conjugate nanopraticles. In the present investigation, an attempt has been made to develop an edible bilayer film (pectin–sodium alginate/casein) of having good barrier and antimicrobial properties as well as with improved functional properties by incorporating casein nanoparticles in the casein inner layer and casein-silver conjugated nanocomposite in the pectin–sodium alginate outer layer. The effect of incorporation of casein-silver conjugated nanocomposite (in outer layer) and casein nanoparticle (in casein layer) on physicochemical, mechanical properties of the bilayer films were investigated along with the biodegradability and toxicity studies.
Materials and methods
Solvents and reagents
All the solvents, reagents and strain used for the present study were of high quality and were procured from, Merck India Pvt. Limited and Himedia, India.
Synthesis of casein nano particle
Casein nanoparticles were prepared using the desolvation techniques as described by the Sailaja and Amareshwar (2012). In brief 10 g of casein was dispersed in 100 mL to double distilled water and subsequently the pH of the mixture was adjusted to 9 and 11 separately by using 1 M NaOH solution. The solutions were allowed to stir continuously under magnetic stirrer for 8 h at 500 rpm for complete hydration. Then ethanol was added in a drop wise manner to the casein solution at a rate of 1 mL/min until the solution became turbid. 0.1 mL of 4 % calcium chloride–ethanol solution was added to the casein solution to induce intra-particle cross linking followed by stirring of the sample for 3 h. The casein solution was purified by two cycles of centrifugation (Sigma 3–18 K Germany) at 6000 rpm for 30 min. After centrifugation the pellets were redispersed to the original volume of absolute ethanol using an ultrasonicator and then stored at 4 °C for further analysis.
Synthesis of casein-silver conjugated nano particles
Casein-silver nano composite was prepared as per the method of Ashraf et al. (2013). 50 mL of a warm suspension (at 37 °C) of casein (1 %) in tris-buffer was prepared and the pH of the casein solution was adjusted to a predetermined level by using 5 M NaOH. The casein solution was added into the 125 mL of boiling aqueous solution of AgNO3 (1 mM) under vigorous stirring. The colour of the reaction mixture was gradually changed from colourless to light yellow and yellow to brown depending on the reaction conditions. The mixture is refluxed for 1 h to ensure complete reaction resulting in the formation of a brownish silver nanocomposite suspension. The nanocomposite suspension were filtered using 0.45 µm filter paper to remove excess casein proteins and other impurities and then stored at room temperature for further analysis and use in subsequent experiments. The casein and silver nitrate concentration ratio and pH of the solutions were varied which is given below.
Then abbreviations used for the synthesized nanoparticles/nanocomposite are given below:
S1—Casein nanoparticles synthesized at pH 9.
S2—Casein nanoparticles synthesized at pH 11.
S3—Conjugated casein-silver nanocomposite synthesized at pH 13, 24:1 (casein: silver).
S4—Conjugated casein-silver nanocomposite synthesized at pH 13, 23:2 (casein: silver).
S5—Conjugated casein-silver nanocomposite synthesized at pH 13, 22:3 (casein: silver).
S6—Conjugated casein-silver nanocomposite synthesized at pH 11, 24:1 (casein: silver).
S7—Conjugated casein-silver nanocomposite synthesized at pH 11, 23:2 (casein: silver).
S8—Conjugated casein-silver nanocomposite synthesized at pH 11, 22:3 (casein: silver).
Preparation of edible film
Bilayer film was prepared by casting pectin sodium alginate composite film on the casein film. In this method, 5 % casein is added in 100 mL distilled water solution and the pH of the solution was adjusted to 7 with the help of 0.1 M NaOH. The solution was stirred under magnetic stirrer at 35 °C for 30 min. 1.5 % (v/v basis) of glycerol was added into the solution and stirred further under magnetic stirrer at 80 °C for 1 h. The solution was then cooled at room temperature and was casted on petriplates and further dried at 45 °C for 5–6 h.
The pectin–sodium alginate composite layer was prepared as per the method of Galus and Lenart (2013). In brief 2.5 % pectin and 1.25 % sodium alginate were dissolved in water and the mixture was stirred under magnetic stirrer for 1 h. After 15 min of the mixing under magnetic stirrer 0.01 % (of whole solution) of CaCl2 solution and glycerol (50 % w/w of total polysaccharide) as plasticizer was added slowly without discontinuing the stirring. This solution was then poured over the casein film and dried for 1 h at 65 °C and then further dried at 50 °C for 12 h. Dried films were peeled off and conditioned for 2 days at 53 ± 2 % RH and 25 °C for further analysis. The different formulation of the films developed in the present investigations is shown in Table 1.
Table 1.
Different film formulations with their compositions
| S. no. | Particulars | Formulations |
|---|---|---|
| 1. | Film 1 (control) | Casein: sodium alginate–pectin edible bilayer film |
| 2. | Film 2 (S1–S3) | S1 (0.02 g/10 mL) incorporated in the inner (casein) layer, S3 (0.02 g/10 mL) incorporated in the outer (Na-Alginate/pectin) layer |
| 3. | Film 3 (S1–S4) | S1 (0.02 g/10 mL) incorporated in the inner (casein) layer, S4 (0.02 g/10 mL) incorporated in the outer (Na-Alginate/pectin) layer |
| 4. | Film 4 (S1–S5) | S1 (0.02 g/10 mL) incorporated in the inner (casein) layer, S5 (0.02 g/10 mL) incorporated in the outer (Na-Alginate/pectin) layer |
| 5. | Film 5 (S1–S6) | S1 (0.02 g/10 mL) incorporated in the inner (casein) layer, S6 (0.02 g/10 mL) incorporated in the outer (Na-Alginate/pectin)layer |
| 6. | Film 6 (S1–S7) | S1 (0.02 g/10 mL) incorporated in the inner (casein) layer, S7 (0.02 g/10 mL) incorporated in the outer (Na-Alginate/pectin) layer |
| 7. | Film 7 (S1–S8) | S1 (0.02 g/10 mL)incorporated in the inner (casein) layer, S8 (0.02 g/10 mL) incorporated in the outer (Na-Alginate/pectin) layer |
| 8. | Film 8 (S2–S3) | S2 (0.02 g/10 mL) incorporated in the inner (casein) layer, S3 (0.02 g/10 mL) incorporated in the outer (Na-Alginate/pectin) layer |
| 9. | Film 9 (S2–S4) | S2 (0.02 g/10 mL) incorporated in the inner casein layer, S4 (0.02 g/10 mL) incorporated in the outer (Na-Alginate/pectin) layer |
| 10. | Film 10 (S2–S5) | S2 (0.02 g/10 mL) incorporated in the inner (casein) layer, S5 (0.02 g/10 mL) incorporated in the outer (Na-Alginate/pectin) layer |
| 11. | Film 11 (S2–S6) | S2 (0.02 g/10 mL) incorporated in the inner (casein) layer, S6 (0.02 g/10 mL) incorporated in the outer (Na-Alginate/pectin) layer |
| 12. | Film 12 (S2–S7) | S2 (0.02 g/10 mL) incorporated in the inner (casein) layer, S7 (0.02 g/10 mL) incorporated in the outer (Na-Alginate/pectin) layer |
| 13. | Film 13 (S2–S8) | S2 (0.02 g/10 mL) incorporated in the inner (casein) layer, S8 (0.02 g/10 mL) incorporated in the outer (Na-Alginate/pectin) layer |
Physicochemical properties of the film
Film thickness, moisture content, water solubility
The thickness of the film was measured with a micrometer (with accuracy of 2 µm). Moisture content of the film was estimated as per the AOAC method (2010, 18th edition) however solubility of the film in water was determined as per the method of Norajit et al. (2010).
Water vapour permeability and transmission
The water vapour transmission (WVT) of the films was determined as per the method of ASTM E96-80 (ASTM 2013) with slight modifications. In brief a measured amount of anhydrous CaCl2 was taken in a petridish. The petridish along-with the desiccants (CaCl2) were sealed with aluminium foil. Film template of the size 2 cm × 2 cm were cut from the films. A window of the same area was cut from the sealed aluminium foil and the film was attached to the window on the aluminium foil. The sealed petridish was placed in desiccators at RH of 52 ± 1 % RH and 25 °C. The weight of sealed petridish was measured at the interval of 24 h and the WVP was calculated as per the equation given below:
where WVP is water vapour transmission (g H2O mm/cm2); W is the weight gain; X is the thickness of the film; A is the permeation area.
The WVTR was calculated as per the formula given below:
where WVTR is the vapour transmission rate (g H2O mm/h cm2), and t is the time in h.
Tensile strength
Tensile strength of the film was measured as per the method of Hamann et al. (2006) with little modifications. The film samples of 0.5 cm × 7 cm was mounted and griped in Texture analyser (Texture expert exceed TA HD Plus, USA). The grip separation was set at 50 mm and the bilayer film was stretched at the constant rate of 1 mm/s until the sample break.
The overall stress required to break the film is the tensile strength of the film which was calculated by the formula given below:
where TS is the tensile strength of the film; F is the force normal to the film cross section and A is the initial area of the film sample.
The elongation at break of films was determined as per the formula given below:
where EB is elongation at break and Ho & H is the initial and final height of the film before and after deformation respectively.
Antimicrobial activities
To check the antibacterial property of nanoparticle containing bilayer film disk diffusion method was used. In brief nutrient agar was used as culture media. The pathogenic culture of E. coli is grown on nutrient agar broth and incubated for 24 h. Nutrient agar plates were prepared and overnight nutrient broth solution containing microorganism (100 µL) culture is poured over the nutrient agar plates. A small portion of the film as per the size of antibiotic disc and placed over the solid media. Plates were incubated for 24 h and zone of inhibition were measured.
X-ray diffraction (XRD) analysis
Crystalinity of the bilayer film was analyzed by using X-Ray diffractometer (Miniflex, Japan). The film samples were exposed to X-Ray beam at 15 mA and 30 kV. Diffraction angle were recorded over a range of 10°–50° with a step angle of 0.05°. Percent crystalinity of the bilayer film was determined by calculating the ratio of diffraction peak area to the total diffraction area and converted into percentage.
Thermal gravimetric analysis (TGA)
Thermal gravimetric analysis was carried out by a thermo gravimetric analyser (TG 50, Schimadzu, Japan). Non isothermal experiment was performed in the temperature range of 30–600 °C/min. The average sample size to be taken was 2 mg and nitrogen flow rate was kept constant at 30 mL/min. The percentage weight loss using the heating cycle was estimated from TG curves.
Film transparency
Film transparency was measured by exposing the film to light of different wavelengths ranging from 450 to 850 nm using a spectrophotometer (Evolution 600, Thermofisher USA). The film sample of 5 × 25 cm2 was placed on the internal side of plastic cuvette (Han and Floros 1997) and the transparency of the film was calculated as per the formula given below :
where y is the wavelength at which absorbance was taken and x is the thickness of the film.
Tristimulus color
Tristimulus colour values of the bilayer film was calculated by the formula (a2 + b2)1/2 as per the method of Mishra et al. (2014).
Scanning electron microscopy
Microscopic images of edible bilayer films were obtained by using Scanning electron microscope (JSM-6390 LV, Japan, PN junction type semi conducting detector). The film samples were cut into a strip (1 mm × 10 mm) and the film strips were attached to a double sided adhesive tape on SEM stubs, coated with 3-5 mA palladium under vacuum. The SEM equipment was operated at 20 kV (Cho et al. 2010).
Effect of film on % haemolysis
The haemolytic activity assay was performed according to the method described by Purkayastha et al. (2014) with slight modification. Fresh human blood sample from pathology of Tezpur University, India was collected in a centrifuge tube containing anticoagulant, trisodium citrate (3.2 %), and was centrifuged at 3000 rpm for 15 min at 4 °C. The supernatant was discarded, and the red blood corpuscles (RBCs or erythrocytes) were collected. RBCs were further washed three times with phosphate buffer saline (PBS, pH 7.4). A 10 % (v/v) suspension of erythrocytes in PBS was prepared, and 1.9 mL of the erythrocyte solution was placed in a 2 mL centrifuge tube and 1 cm2 films were placed in the corresponding tubes. The tubes were incubated at 37 °C for 1 h. 1 % Triton X-100 and PBS were taken as positive and negative controls, respectively. After completion of incubation, the tubes were subjected to centrifuge at 3500 rpm for 15 min, and absorbance of the supernatant was taken at 450 nm in the UV–visible spectrophotometer. Haemolysis percentages were calculated using the following formula.
Statistical analysis
All the observations were taken in triplicates and the statistical difference were calculated using MS Excel at p < 0.05.
Results and discussion
Thickness, moisture content, solubility and swelling ratio of bilayer film
Casein-silver conjugated nanoparticles were incorporated into sodium alginate–pectin (outer layer) layer of the film however casein nanoparticle was incorporated in casein layer (inner layer of film). Ghodake et al. (2014) reported that the production of silver nanoparticle in presence of casein and NaOH were highly controlled and regulated. The reducing capability of casein was likely to be controlled by amine groups and carboxylic groups in presence of NaOH. The deprotonated form of carboxyl and hydroxyl groups are facilitated by silver ions and subsequently silver ions oxidized the carboxyl group to carbonyl group. The silver ions thus reduced simultaneously into silver nanoparticles. Addition of NaOH ensures that the amino acids of casein are deprotonated, providing the mono anion form in an aqueous solution making it an stronger complexing agent (Ghodake et al. 2010). Ashraf et al. (2013) explained that the tyrosine and histidine residues of casein may be responsible for the reduction of ionic silver in silver nanoparticle. The reduction potential of carboxyl groups increased at higher pH (Ashraf et al. 2013) and so pH 11 and 13 was used to synthesis of casein-silver conjugated nanoparticle in the present investigation. Present finding supports the findings of Ghodake et al. (2010) that the presence of NaOH as an additives effectively reduced the silver ions into silver nanoparticles. The thickness, moisture content, solubility and swelling ratio are the important attributes of packaging material which affect the mechanical and barrier properties of the film. The effect of incorporation of nanoparticle on thickness, moisture content and solubility property of the films are depicted in Table 2. For all films the same amount of the film forming solution was casted. The thickness of the control film was found to be comparatively high than to the other film tested except in the case of film 3, 9 and 10. The thickness of the film was observed in the range of 0.14–0.20 mm. Thickness of film containing S4 or S5 nanocomposite was higher than to other nanocomposite containing film. As per the Galus and Lenart (2013) the final thickness of the films mainly depends on the drying conditions and preparation methods. If the volumes of film forming solution are constant then only the composition and interaction between components affect the thickness of the film and also the formation of film structure during the drying of the film affects the thickness of the film (Basiak et al. 2016).
Table 2.
Thickness, moisture content, water solubility and swelling ratio of bilayer edible films of having different formulations
| Particulars | Thickness (mm) | Moisture content (%) | Water solubility (80 °C) |
|---|---|---|---|
| Film 1 (control) | 0.186 ± 0.008a | 44.03 ± 0.09a | 67.64 ± 0.42a |
| Film 2 (S1–S3) | 0.167 ± 0.014b | 41.31 ± 0.14b | 57.28 ± 0.61b |
| Film 3 (S1–S4) | 0.206 ± 0.069c | 40.57 ± 0.66b,d | 50.48 ± 0.47c |
| Film 4 (S1–S5) | 0.181 ± 0.015a | 41.27 ± 0.19b | 56.11 ± 0.28b |
| Film 5 (S1–S6) | 0.143 ± 0.001d | 41.12 ± 0.15b | 57.69 ± 0.35b |
| Film 6 (S1–S7) | 0.151 ± 0.008d | 42.80 ± 0.71c | 47.39 ± 0.64d |
| Film 7 (S1–S8) | 0.128 ± 0.0001e | 40.16 ± 0.21b,d | 53.49 ± 0.21e |
| Film 8 (S2–S3) | 0.145 ± 0.018d | 39.49 ± 0.09b,d | 46.25 ± 0.49d |
| Film 9 (S2–S4) | 0.202 ± 0.101c | 42.08 ± 0.07c | 47.95 ± 0.78d |
| Film 10 (S2–S5) | 0.195 ± 0.094c | 38.31 ± 0.14e | 44.9 ± 0.98f |
| Film 11 (S2–S6) | 0.155 ± 0.016d | 40.31 ± 0.27b,d | 49.19 ± 0.35g |
| Film 12 (S2–S7) | 0.134 ± 0.011e | 38.16 ± 0.08e | 47.49 ± 0.11d |
Values with different superscript in different columns differ significantly
The water solubility of the nanocomposite containing bilayer had significantly lower than to the control film (Table 2). When synthesize casein nanoparticle at pH 11 were incorporated into the casein layer decreased water solubility of the film were observed however the incorporation of casein-silver conjugated nanocomposites showed no any specific trend. Addition of casein nanoparticle (synthesized at pH 11) increased the interaction between components so reduced the water solubility (Basiak et al. 2016). The dissolution speed of the bilayer film accelerated as the water temperature increased for each film sample. The relatively lower water solubility of the bilayer film in the present study could be attributed due to the less hydrophilic nature of nanocomposite containing film.
Colour values, water vapour permeability, mechanical and light barrier properties
The developed bilayer film was light yellow in colour. The effect of incorporation of nanocomposite on the lightness of the film (L) are given in Table 3. The lightness of the control film was comparatively higher than any other film tested. The lightness of the films was observed in the range of 68.26–74.73. Conjugated nanocomposite (casein-silver) synthesized at pH 13 was darker in colour than to the nanocomposite synthesized at pH 11. The darkness increased with the increase of the silver ratio in synthesized casein-silver conjugated nanocomposite. The probable reason of the darker colour of the synthesized conjugated nano composite may be due to the higher rate of the reaction between casein and silver at pH 13 than to the pH 11 (Ashraf et al. 2013). Ratio of the silver and pH was found to be effective to increase the darkness of the film however the incorporation of casein nano composite in the inner layer showed no any specific effect on the lightness of the film. Incorporation of casein-silver conjugated nanoparticles increased the redness of the film as the a/b value of the bilayer film was significantly high (ranging from 3.52 to 4.66) than to the control film (where a/b value of the film was 3.44). The increased redness in bilayer was due to the dark brown character of casein-silver conjugated nanocomposite. Overall colour difference between the control film and nanoparticle containing bilayer film were detected but specific trend of incorporation casein and casein-silver conjugated nanocomposite could not be established (Table 3) in the present investigation. The transparency of the developed bilayer film is depicted in Table 3. The transparency of the nanocomposite containing film was comparatively less than to the control film. It was found that as the concentration of silver is increased the transparency of the film was decreased, a similar tendency has also been widely observed for the lightness of the films which indicated the incorporation of nanocomposite is effective to increase the light barrier properties of the film. Nemet et al. (2010) reported that the excellent barrier properties in the UV range is due to the presence of high amount of aromatic amino acids in protein based structure, which is capable to absorb UV-light.
Table 3.
Effect of nanoparticles and conjugated nanocomposites on the barrier and mechanical property and color values of films
| Particulars | WVP = (W × X)/A (g H2O mm/cm2) | WVTR = (W × X)/(A × T) (g H2O mm/h cm2) | Tensile strength (MPa) | Elongation at break E (%) | L | a | b | ∆E | Transparency |
|---|---|---|---|---|---|---|---|---|---|
| Film (control) | 0.021 ± 0.004 | (4.73 ± 0.27) E−05 | 1.20 ± 0.03 | 52.69 ± 6.65 | 74.73 ± 1.21 | 10.83 ± 1.35 | 3.15 ± 1.81 | 5.45 ± 0.4 | |
| Film 2 (S1–S3) | 0.020 ± 0.003 | (3.51 ± 0.27) E−05 | 1.50 ± 0.01 | 49.75 ± 5.42 | 71.26 ± 0.68 | 3.64 ± 1.95 | 0.84 ± 0.45 | 8.31 ± 0.73 | 3.09 ± 0.4 |
| Film 3 (S1–S4) | 0.011 ± 0.013 | (3.30 ± 0.07) E−05 | 1.80 ± 0.01 | 64.32 ± 6.93 | 70.67 ± 0.02 | 3.84 ± 0.02 | 0.90 ± 0.01 | 8.39 ± 0.45 | 2.74 ± 0.5 |
| Film 4 (S1–S5) | 0.019 ± 0.004 | (3.94 ± 0.49) E−05 | 1.60 ± 0.03 | 45.13 ± 5.02 | 68.26 ± 0.04 | 6.11 ± 0.07 | 1.50 ± 0.02 | 5.58 ± 0.28 | 2.41 ± 0.2 |
| Film 5 (S1–S6) | 0.018 ± 0.001 | (3.83 ± 0.83) E−05 | 1.40 ± 0.001 | 41.58 ± 3.43 | 72.97 ± 0.64 | 4.98 ± 1.71 | 1.18 ± 0.43 | 6.84 ± 0.37 | 4.78 ± 0.4 |
| Film 6 (S1–S7) | 0.015 ± 0.001 | (3.20 ± 0.14) E−05 | 1.40 ± 0.04 | 43.66 ± 9.13 | 71.20 ± 1.59 | 7.61 ± 1.05 | 2.13 ± 1.39 | 3.71 ± 0.67 | 4.01 ± 0.3 |
| Film 7 (S1–S8) | 0.015 ± 0.001 | (3.16 ± 0.07) E−05 | 1.30 ± 0.004 | 45.94 ± 5.51 | 70.41 ± 1.16 | 3.88 ± 3.02 | 0.94 ± 0.77 | 8.01 ± 0.26 | 3.55 ± 0.2 |
| Film 8 (S2–S3) | 0.014 ± 0.001 | (3.11 ± 0.01) E−05 | 2.60 ± 0.07 | 56.50 ± 6.22 | 73.04 ± 1.76 | 11.13 ± 1.45 | 3.16 ± 1.51 | 0.3 ± 0.49 | 4.74 ± 0.6 |
| Film 9 (S2–S4) | 0.014 ± 0.006 | (3.13 ± 0.79) E−05 | 1.00 ± 0.03 | 37.20 ± 6.85 | 72.58 ± 0.34 | 11.03 ± 0.62 | 3.06 ± 0.23 | 0.26 ± 0.27 | 3.60 ± 0.5 |
| Film10 (S2–S5) | 0.020 ± 0.011 | (3.59 ± 0.65) E−05 | 1.90 ± 0.01 | 52.58 ± 1.09 | 70.46 ± 0.42 | 1.35 ± 1.17 | 0.3 ± 0.28 | 10.78 ± 0.14 | 3.17 ± 0.6 |
| Film 11 (S2–S6) | 0.014 ± 0.001 | (2.93 ± 0.44) E−05 | 1.30 ± 0.01 | 47.41 ± 4.81 | 73.23 ± 0.25 | 0.7 ± 0.73 | 0.15 ± 0.17 | 11.48 ± 0.31 | 4.22 ± 0.8 |
| Film 12 (S2–S7) | 0.013 ± 0.001 | (2.78 ± 0.62) E−05 | 1.70 ± 0.01 | 46.00 ± 1.02 | 72.59 ± 0.75 | 10.42 ± 1.44 | 3.07 ± 0.49 | 0.44 ± 0.54 | 4.12 ± 0.7 |
| Film 13 (S2–S8) | 0.011 ± 0.001 | (2.12 ± 0.01) E−05 | 2.00 ± 0.10 | 52.23 ± 7.03 | 69.09 ± 0.81 | 3.12 ± 2.28 | 0.76 ± 0.59 | 8.85 ± 0.26 | 3.73 ± 0.5 |
Water vapour permeability of film as function of incorporation of nanocomposite/nanoparticles is presented in Table 3. The addition of nanocomposite/casein nanoparticle in film improved the water barrier properties by increasing the cross linking in the film structure and so reduced the rate of water diffusion through the film. The values of WVTR ranged from 2.78 × 10−5 to 3.94 × 10−5 g H2O mm/h cm2 for nano particle containing films. As per the Galus and Kadzinska (2016) the water vapour transfer through edible films is strongly depends upon the particle size distribution, which is directly correlated with the method of preparation and homogenization. Due to the smaller in size nanoparticles could be distributed uniformly and so improved the water barrier properties. Film containing casein nanoparticle (synthesized at pH 11) showed higher water barrier property than to the film incorporated the casein nanoparticles which is synthesized at pH 9.0. The reason may be that at pH 11.0 the particle size of the nanoparticle was comparatively less than the pH 9.0 (Jun et al. 2011) so the casein synthesized at 11.0 pH was uniformly distributed into the film forming solution and improved the water barrier property significantly.
Table 3 shows tensile strength and elongation at break of the films. Addition of conjugated silver-casein in pectin–sodium alginate layer and casein in casein layer improved the tensile strength of the film when compared with control. Addition of casein nanoparticle (synthesized at pH 11) improved the structure of the film and improved the mechanical properties of the film. Casein-silver conjugation synthesized at 11 pH and pH 13 were found to be effective to improve the barrier properties of the film except in the case of film 9, which showed the lowest tensile properties than any other film tested.
Combination of sodium alginate and pectin gave films of higher tensile strength and elongation at break due to compatibility and chemical synergetic interaction between components (Hambleton et al. 2012). Combination of sodium alginate-pectin/casein bilayer film was found to be effective to develop an edible film with good moisture and light barrier properties.
Antibacterial property of the films
The inhibitory activity of the bilayer films against E coli were measured on the basis of clear zone inhibition. Table 4 presents the ability of bilayer films against the growth of E. coli. In terms of the clear zone inhibition control film showed no any inhibitory activity which suggested that the sodium alginate–pectin/casein bilayer film had no antibacterial properties against E. coli, however, all the nanoparticles containing film showed clear zone of inhibition ranging from 5.5 to 8.5 mm. Film 13 showed clear inhibitory zone of 8.5 mm. When the ratio of silver was increased during the preparation of casein-silver conjugated nano composites than the antibacterial properties the films were also increased, however there was no any specific effect of casein nanoparticle on antibacterial property could be established. Kanmani and Rhim (2014) observed the same findings during the development of gelatin/silver nanoparticle containing composite film. Gram negative bacteria is usually suppressed by the silver nanoparticle since the gram negative bacteria are coated by the negatively charged coated membrane and thin peptidoglycan layer which can be easily penetrated by silver nanoparticle (Duncan 2011; Kanmani and Rhim 2014).
Table 4.
Zone of inhibition against E. coli strain was observed for the films
| Edible bilayer films incorporated with nanoparticles & nanocomposites | Zone of inhibition (mm) | % Hemolysis |
|---|---|---|
| Film 1 (control) | No zone of inhibition | 0.18 ± 0.02 |
| Film 2 (S1–S3) | 6 | ND |
| Film 3 (S1–S4) | 7.5 | 0.10 ± 0.03 |
| Film 4 (S1–S5) | 8.0 | ND |
| Film 5 (S1–S6) | 5.5 | ND |
| Film 6 (S1–S7) | 6.5 | ND |
| Film 7 (S1–S8) | 7.5 | ND |
| Film 8 (S2–S3) | 6 | 0.15 ± 0.01 |
| Film 9 (S2–S4) | 7 | ND |
| Film 10 (S2–S5) | 8 | ND |
| Film 11 (S2–S6) | 6 | ND |
| Film 12 (S2–S7) | 8 | 0.09 ± 0.04 |
| Film 13 (S2–S8) | 8.5 | ND |
| Tetracyclin | 17 | NA |
| Ampicilin | 19 | NA |
| Control (−) | NA | 0 |
| Control (+) | NA | 100 |
ND not detected, NA not applicable
X-ray diffraction
On the basis of mechanical, light barrier and water barrier properties, film 3, 8 and 12 were selected for further studies. The crystalline structure of all the developed films was confirmed by XRD graph (Fig. S1a). XRD analysis indicated a clear difference between control and nano composite containing bilayer films. The % crystallinity of the control film was higher than to the nanoparticle containing film which confirms that the addition of nano particle reduced the crystallinity of the film. Su et al. (2008) observed the same finding while developing the soya protein based edible film. They reported that the polyvinyl alcohol and glycerol affected the crystalline structure of film as it may insert into crystalline structure of the film and can modify the microstructure of the film. Similarly the synthesized nano composite modified the microstructure of the film and so affected the crystalline structure.
Thermogravimetric analysis
The thermal properties of the film 1, 3, 8 and 12 are shown in Fig. S1b. The films incorporated with casein nanoparticles and conjugated casein-silver nanocomposite showed almost similar pattern however control film showed a slight different pattern of mass loss. In Film1 (control), first loss was observed at 95 °C and second loss at 180 °C where protein gets degraded however in the case of film 3, 8 and 12, the first weight loss occurred at about 100 °C, second weight loss was to be around at 200 °C and third loss of the weight was observed in the range of 350–450 °C. These weight losses may be due to the water loss, release of volatiles or decomposition of the material. It may be possible that at a high temperature there may be breakage of inter and intra molecular hydrogen bond which are responsible for the maintenance of the polymer chain order (Ogale et al. 2000). The nanocomposite containing films showed comparatively more stability against temperature than to the film1. Mu et al. (2012) observed maximum loss of mass at three different stages they reported that the first loss of mass was due to the free water present in film at the temperature of 53–125 °C, second degradation was due to the glycerol at 250 °C and third was degradation of gelatin-DCMC chain linked, at 265–460 °C.
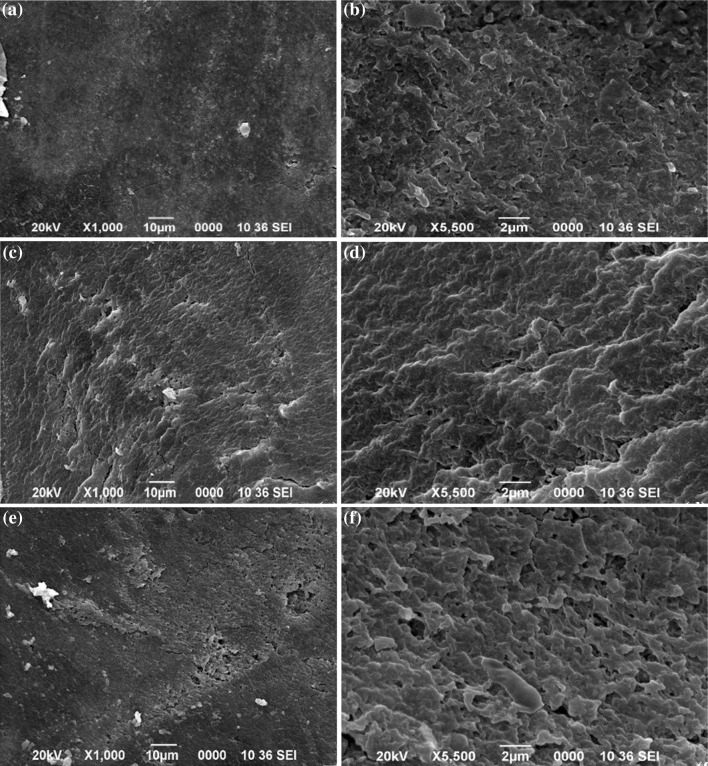
Morphological analysis of films
Film homogeneity and microscopic structure of the films was examined by using SEM. The results of microscopic evaluation of film3, film 12 and film1 are shown in Fig. 1. The results indicated that all the nanocomposite containing film were of smooth surface, compact structure, and had structural integrity without any cracks and cavities similar to the other composite films. It is worth noting from the Fig. 1 that there is no interfaces were observed in the sodium-pectin/casein bilayer film, indicating a high compatibility between the selected components for the development of the bilayer film, yielding a relatively smooth and compact structure film (Sionkowska et al. 2004). Incorporation of casein nanoparticle in the layer of casein and the inclusion of casein-silver conjugated nanocomposite in the pectin layer also provided the compact and smooth structure to the film. It was assumed that at the interface of the sodium alginate–pectin and casein, interaction took place between the two layers which increased the bonding strength between the layers and prevent the manual peeling of sodium alginate–pectin layer from casein layer (Taravel and Domard 1993).
Fig. 1.
Scanning electron micrograph a Control film at ×1000, b control film at ×5500, c bilayer film (S1–S4) at ×1000, d bilayer film (S1–S4) at ×5500, e bilayer film 12 (S2–S7) at ×1000, f bilayer film at ×5500
Effect on haemolysis
A haemolysis test was conducted to determine the toxicity of synthesized nanoparticles on mammalian red blood cells because there are concerns about the health implications of nanoparticles. Occurrence of haemolysis mainly depends on the following impact such as net surface charge of the particle and chemical composition of the used material/excipient. It may also be greatly affected by the surface to volume ratio (macro, micro, and nano). As described above that on the basis of mechanical and barrier properties film 3, 8 and 12 were selected for analysis of biocompatibility of nanocomposite containing bilayer films and film 1 was taken as a control for comparative analysis. Images showing precipitated RBCs at the end of the haemolysis experiment are given in Fig. S2. Effect of casein-silver conjugated and casein nano particle containing films on the % haemolysis were carried out against the positive control. As per the method of ASTM E2524-08 standard (standard test method for analysis of haemolytic products of NPs), haemolysis >5 % indicates that the test material causes damage to RBCs (Purkayastha et al. 2014). The control film (without nanocomposite) showed 0.18 % of haemolysis however the film 3, 8 and 12 showed only 0.10, 0.15 and 0.09 % (Table 4) of haemolysis which showed that addition of casein/casein-silver conjugated nanoparticle at the present level showed no any marked changes in terms of % haemolysis when compared to control film and so having no any adverse impact on the red blood cells. Further we can conclude that the level of casein nanoparticles and casein-silver conjugated nanoparticles used in the film safe and were biocompatible with no detectable hemotoxic effects.
Conclusion
The present investigation casein nano particle and casein-silver conjugated nanocomposite were synthesized by desolvation techniques. The synthesized casein nanoparticle and casein-silver conjugated nanocomposite were incorporated in inner layer (casein layer) and outer layer (sodium alginate–pectin layer) respectively of the bilayer edible film. Addition of casein-silver conjugated nanocomposite in outer layer of film gave antibacterial property to the film and also improved the light barrier properties of the film. The mechanical and WVTR of the film was also improved by the addition of nanoparticle/nano composite. The level of silver used for the present study is safe for the red blood cells as % haemolysis of all the tested films were well below the safe level. The addition of casein nanoparticle in the inner layer of the film may be useful for loading of antioxidant in the future studies can be helpful for the packaging of light sensitive products.
Electronic supplementary material
Below is the link to the electronic supplementary material.
References
- AOAC . Officials methods of analysis of Association of Analytic Chemists. 18. Washington, DC: AOAC International; 2010. [Google Scholar]
- Ashraf S, Abbasi AZ, Pfeiffer C, Hussain SZ, Khalid ZM, Gil PR, Parak WJ, Hussain I. Protein-mediated synthesis, pH-induced reversible agglomeration, toxicity and cellular interaction of silver nanoparticles. Colloids Surf B. 2013;102:511–518. doi: 10.1016/j.colsurfb.2012.09.032. [DOI] [PubMed] [Google Scholar]
- ASTM . Standard test method for analysis of haemolytic properties of nanoparticles: E2524 – 08. Annual book of ASTM standards. 18. Philadelphia, PA: American Society for Testing and Materials; 2013. [Google Scholar]
- Bakshi MS, Kaur H, Khullar P, Banipal TS, Kaur G, Singh N. Protein films of bovine serum albumen conjugated gold nanoparticles: a synthetic route from bioconjugated nanoparticles to biodegradable protein films. J Phys Chem. 2011;115:2982–2992. [Google Scholar]
- Basiak E, Debeaufort F, Lenart A. Effects of carbohydrate/protein ratio on the microstructure and the barrier and sorption properties of wheat starch-whey protein blend edible films. J Sci Food Agric. 2016 doi: 10.1002/jsfa.7807. [DOI] [PubMed] [Google Scholar]
- Chinabhark K, Benjakul S, Prodpran T. Effect of pH on the properties of protein-based film from bigeye snapper (Priacanthus tayenus) surimi. Bioresour Technol. 2007;98:221–225. doi: 10.1016/j.biortech.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Cho YS, Lee YS, Rhee C. Edible oxygen barrier bilayer film pouches from corn zein and soy protein isolates for olive oil packaging. LWT Food Sci Technol. 2010;43:1234–1239. doi: 10.1016/j.lwt.2010.03.014. [DOI] [Google Scholar]
- Dangaram KL, Cooke P, Tomasula PM. The effect of protein size particle reduction on the physical properties of CO2 precipitated casein films. J Food Sci. 2006;71:E196–E201. doi: 10.1111/j.1750-3841.2006.00012.x. [DOI] [Google Scholar]
- Dean K, Yu L. Biodegradable protein-nanocomposites. In: Smith R, editor. Biodegradable polymers for industrial application. Boca Raton: CRC Press; 2005. pp. 289–309. [Google Scholar]
- Duncan TV. Applications of nanotechnology in food packaging and food safety: Barrier materials, antimicrobials and sensors. J Colloid Interface Sci. 2011;363:1–24. doi: 10.1016/j.jcis.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Al-Assaf S, Phillips GO, Nishinari K, Funami T, Williams PA. Binding behaviour of calcium to polyuronates: comparison of pectin with alginate. Carbohydr Polym. 2008;72:334–341. doi: 10.1016/j.carbpol.2007.08.021. [DOI] [Google Scholar]
- Galus S, Kadzinska J. Moisture sensitivity, optical, mechanical and structural properties of whey protein-based edible films incorporated with rapeseed oil. Food Tech Biotech. 2016;54(1):78–89. doi: 10.17113/ftb.54.01.16.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galus S, Lenart A. Development and characterization of composite edible films based on sodium alginate and pectin. J Food Eng. 2013;115(4):459–465. doi: 10.1016/j.jfoodeng.2012.03.006. [DOI] [Google Scholar]
- Garcia MA, Pinotti A, Martino MN, Zaritzky NE. Characterization of composite hydrocolloid films. Carbohydr Polym. 2004;56:339–345. doi: 10.1016/j.carbpol.2004.03.003. [DOI] [Google Scholar]
- Ghodake GS, Deshpande NG, Lee ES. Jin Pear fruit extract-assisted room-temperature biosynthesis of gold nanoplates. Colloids Surf B Biointerfaces. 2010;75:584–589. doi: 10.1016/j.colsurfb.2009.09.040. [DOI] [PubMed] [Google Scholar]
- Ghodake GS, Deshpande NG, Lee YP. Casein hydrolytic peptides mediated green synthesis of antibacterial silver nanoparticles. Colloids Surf. 2014;108:147–151. doi: 10.1016/j.colsurfb.2013.02.044. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Ali MA, Dias GJ. Effect of cross linking on microstructure and physical performance of casein protein. Biomacromol. 2009;10:1681–1688. doi: 10.1021/bm801341x. [DOI] [PubMed] [Google Scholar]
- Hamann DD, Zhang J, Daubert CR, Foegeding EA, Diehl KCJ. Analysis of compression, tension and torsion for testing food gel fracture properties. J Textural Stud. 2006;37:620–639. doi: 10.1111/j.1745-4603.2006.00074.x. [DOI] [Google Scholar]
- Hambleton A, Perpinaz-Sainz M, Fabra MJ, Voilley A, Debeaufort F. The Schroeder paradox or how the state of water affects the moisture transfer through edible films. Food Chem. 2012;132:1671–1678. doi: 10.1016/j.foodchem.2011.03.009. [DOI] [Google Scholar]
- Han JH, Floros JD. Casting antimicrobial packaging films and measuring their physical properties and antimicrobial activity. J Plast Film Sheeting. 1997;13(4):287–298. [Google Scholar]
- Jafarzadeh S, Alias KA, Ariffin F, Mahmud S, Najafi A. Preparation and characterization of bionanocomposite films reinforced with nano kaolin. J Food Sci Technol. 2016;53(2):1111–1119. doi: 10.1007/s13197-015-2017-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun JY, Nguyen HH, Paik SYR, Chun HS, Kang BC, Ko S. Preparation of size-controlled bovine serum albumin (BSA) nanoparticles by a modified desolvation method. Food Chem. 2011;127:1892–1898. doi: 10.1016/j.foodchem.2011.02.040. [DOI] [Google Scholar]
- Kanmani P, Rhim WJ. Physicochemical properties of gelatin/silver nanoparticle antimicrobial composite film. Food Chem. 2014;148:162–169. doi: 10.1016/j.foodchem.2013.10.047. [DOI] [PubMed] [Google Scholar]
- Mishra P, Rai GK, Mahanta CL. Process standardization for development of spray dried lemon juice powder and optimization of amla–lemon based RTS (ready to serve drink) using response surface methodology. J Food Process Preserv. 2014 [Google Scholar]
- Mu C, Guo J, Li X, Lin W, Li D. Preparation and properties of dialdehyde carboxymethyl cellulose crosslinked gelatin edible films. Food Hydrocolloids. 2012;27(1):22–29. doi: 10.1016/j.foodhyd.2011.09.005. [DOI] [Google Scholar]
- Nagarajan M, Benjalul S, Prodpran T, Songtipya P. Properties and characteristics of nanocomposite films from tilapia skin gelatin incorporated with ethanolic extract from coconut husk. J Food Sci Technol. 2015;52(12):76690–76698. doi: 10.1007/s13197-015-1905-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemet NT, Šošo VM, Lazić VL. Effect of glycerol content and pH value of film-forming solution on the functional properties of protein-based edible films. BIBLID. 2010;1450–7188(41):57–67. [Google Scholar]
- Norajit K, Kim MK, Ryu HG. Comparative studies on the characterization and antioxidant properties of biodegradable films containing ginseng extract. J Food Eng. 2010;98:377–384. doi: 10.1016/j.jfoodeng.2010.01.015. [DOI] [Google Scholar]
- Ogale AA, Cunningham P, Dawson PL, Acton JC. Viscoelastic, thermal and microstructural characterization of soy protein isolate films. J Food Sci. 2000;65(4):672–679. doi: 10.1111/j.1365-2621.2000.tb16071.x. [DOI] [Google Scholar]
- Purkayastha MD, Manhar AK, Das VK, Borah A, Mandal M, Thakur AJ, Mahnata CL. Antioxidative, hemocompatible, fluorescent carbon nano dots from “an end of pipe” agriculture waste: exploring its new horizon in food packaging. J Agric Food Chem. 2014;62(20):4509–4520. doi: 10.1021/jf500138f. [DOI] [PubMed] [Google Scholar]
- Sailaja AK, Amareshwar P. Using acetone as desolving agent. Int J Pharm Sci Nanotechnol. 2012;5:1643–1647. [Google Scholar]
- Sionkowska A, Wisniewski M, Skopinska J, Kennedy CJ, Wess TJ. Molecular interactions in collagen and chitosan blends. Biomaterials. 2004;25(795e):801. doi: 10.1016/s0142-9612(03)00595-7. [DOI] [PubMed] [Google Scholar]
- Su JF, Huang Z, Yang CM, Yuan XY. Properties of soy protein isolate/poly(vinyl) alcohol blend :green’ films: compatibility, mechanical properties, and thermal stability. J Appl Polymer Sci. 2008;115(3):1901–1911. doi: 10.1002/app.31150. [DOI] [Google Scholar]
- Taravel MN, Domard A. Relation between the physicochemical characteristics of collagen and its interactions with chitosan: I. Biomaterials. 1993;14:930–938. doi: 10.1016/0142-9612(93)90135-O. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.