Abstract
The control potential of seven plant essential oils was evaluated against Fusarium proliferatum (Matsushima) Nirenberg and Fusarium verticillioides Sheldon. The fungicidal activity was assessed through microtiter plate assay to determine the minimum inhibitory and fungicidal concentration of essential oils. The essential oil of Mentha arvensis was adjudged as best for inhibiting the fungal growth, while oil of Thymus vulgaris and Anethum graveolens showed high efficacy in terms of fungicidal activity. The oil of M. arvensis and T. vulgaris also showed good inhibition activity in agar disc diffusion assay. M. arvensis essential oil was analysed for its composition using gas chromatography/mass spectrometry revealing menthol (63.18 %), menthone (15.08 %), isomenthyl acetate (5.50 %) and limonene (4.31 %) as major components. Significant activity of M. arvensis essential oil against F. proliferatum and F. verticillioides isolates obtained, pave the way for its use as antifungal control agents.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-016-2347-0) contains supplementary material, which is available to authorized users.
Keywords: Fusarium proliferatum, Fusarium verticillioides, Essential oils, Antifungal activity
Introduction
Fusarium sp., Fusarium proliferatum (Matsushima) Nirenberg (FP) and F. verticillioides Sheldon (FV) are considered to be a major pathogen of the plants belonging to Gramineae family, in tropical and subtropical regions (Kushiro et al. 2012; Thippeswamy et al. 2013). Moreover, these Fusarium species are characterized by high intraspecies and interspecies variability in morphological, physiological and genetic properties, which has led to variation in their pathogenecity as well as host plants preference (Jurado et al. 2010; Palacios et al. 2015). According to recent reports, the host range of FP include wide varieties of wild and cultivated plants (Tancic et al. 2012; Guo et al. 2014), while FV has shown pathogenic potential on maize, rice, sugarcane, pine, rye, wheat, asparagus, corn, and sorghum etc. (Chen et al. 2012; Tancic et al. 2012; Kushiro et al. 2012). These fungi by being pathogens, opportunistic colonizers, and saprophytes on plants and agricultural commodities; cause significant economic losses escalating up to 50 % resulting from decreased yield and diminished quality of final products (Logrieco et al. 2003; Thippeswamy et al. 2013).
Fusarium infection affect spikelet and kernel of the plants, which loses its chlorophyll to become straw colored or pinkish-red due to overgrowth of fungus mycelium, and shrivel to assume white, pink, or light-brown scaly appearance, respectively (Logrieco et al. 2003; Guo et al. 2014). Further, mycotoxins produced by FP and FV (fumonisins B1 or FB1) may lead to several disease in plants (black point symptoms in maize, asparagus, pine and palm trees), animals (equine leukoencephalomalacia in horses, porcine pulmonary edema and hydrothorax in swine, and hepatic and immune dysfunction in cattles) and humans (chronic and acute mycotoxicosis) (Bennett and Klich 2003; Busman et al. 2012; Kushiro et al. 2012; Tancic et al. 2012; Guo et al. 2014; Palacios et al. 2015). From the above discussion, it becomes apparent that fungal strains of FP and FV on account of being most diverse and widespread plant-infecting fungi along with varied mycotoxins synthesis capacity, have significant impact on agro-economical and human health (Jurado et al. 2010; Busman et al. 2012). In particular, the Indian subcontinent with favorable environmental condition of relatively high temperature and humidity present optimum settings for Fusarium infection, requiring attentiveness towards suitable control measures. A great deal of efforts has been underway towards searching for newer antifungal agents. Apart from conventional chemical preservatives (benzimidazoles, aromatic hydrocarbons, sterol biosynthesis inhibitors), antioxidants, propionates, parabéns, and phenolic compounds have been used for control of Fusarium spp. (Sreenivasa et al. 2011; Ivanova et al. 2013). However, awareness towards the possible negative effects of synthetic preservatives have escalated interest and research on the antifungal application of plant based products (Sreenivasa et al. 2011; Ivanova et al. 2013; Xing et al. 2014; Panda et al. 2015). In this regard, the present study aimed to investigate control efficacy of seven plant essential oils against F. proliferatum (FP) and F. verticillioides (FV). The efficacy of essential oils was evaluated through microtiter plate assay and agar disc diffusion assay to determine their growth inhibiting and fungicidal potential.
Materials and methodology
Essential oils
Essential oil of Dill seed (Anethum graveolens L.), Grapefruit (Citrus paradisi Macfad), Corn mint (Mentha arvensis L.), Spearmint (Mentha spicata L.), Black pepper (Piper nigrum L.), Rosemary (Rosmarinus officinalis L.) and Thyme (Thymus Vulgaris L.) were purchased from Kanta Chemicals Pvt. Ltd, Khari Bowli, New Delhi, India. The essential oil was stored in plastic bottles at 4 °C.
A 270 mg of resazurin powder (Sigma-Aldrich GmbH, Germany) was dissolved in 40 mL sterile distilled water. The solution was then vortexed till the powder was completely dissolved and homogenous to prepare the resazurin solution.
Fungus and spore suspension
The fungal isolates; Fusarium proliferatum KT855215 (FP1), F. proliferatum KT855218 (FP2), F. verticillioides KT855216 (FV1), F. verticillioides KT855217 (FV2) used in the present study were isolated in our laboratory from wheat samples collected from field and grain storage center of North-West Plain Zone of India (Data unpublished). The fungal isolates were grown on Potato Dextrose Agar (PDA) slants at 25 °C and maintained at 4 °C.
For the preparation of spore suspensions, spores were harvested from fresh slants (cultured for 5 days at 25 ± 2 °C) by addition of 10 mL of distilled water containing 0.1 % sterile Tween 80 to the slants, vortexed for 5 min, and the resulting suspension filtered through a sterilized 8 µm membrane filter disk. The spore concentration of filtered suspensions was enumerated using hemocytometer chamber.
Microtiter plate assay
The antifungal activity of essential oils was assessed through microdilution techniques performed using a sterile 96-well plate (Ivanova et al. 2013). The first four rows of microtiter plate were used for evaluation of essential oil activity, while the last two rows served as positive and negative control. A 50 μL of sterile Potato Dextrose Broth (PDB) was dispensed into each well, followed by addition of 50 μL of essential oil to the first well of the first row of the plate. The content of this well was mixed using sterile pipette tips and 50 µL of the solution was transferred to the second well of the same row. This process of serial dilution was carried out for all the wells of first four rows. After the completion of the serial dilution, all the wells of first four rows (except last well of fourth row) contained 50 µL of essential oil solution in descending concentrations. Then, a 5 µL resazurin solution was added to each well, followed by the addition of 5 µL of fungal suspension (1.86 × 106–2.15 × 106 spore/mL). Resazurin was used as an indicator of fungal growth. Positive control (used to confirm the viability of fungal culture) included 50 µL of PDB, 5 µL resazurin and 5 µL of fungal suspension, while the negative control (for verification of the sterility of the working conditions and solutions) contained 50 µL of PDB and 5 µL of resazurin. Then, the microtiter plates were wrapped in sterile foil in order to prevent contamination and incubated at 25 ± 2 °C for 5 days. A blue colored solution indicated fungal growth inhibition in the test wells, while pale pink to colorless solution indicated microbial growth or absence of inhibition. The minimal inhibitory concentration (MIC) was defined as the lowest concentration of the compounds to inhibit the growth of microorganisms. The values of minimal fungicidal concentration (MFC) were determined by subculturing the inhibited fungal mass at MICs on PDA medium. Observations were recorded after 10 days of incubation at 25 ± 2 °C. Fungal growth on the 10th day was indicative of fungistatic control activity, while the absence of fungal growth denoted fungicidal activity of essential oils. The MFC was regarded as the lowest concentration that did not yield any fungal growth. All the assays were carried out under aseptic conditions and performed in triplicate.
Agar disc diffusion assay
For the disc diffusion assay, 100 μL spore suspension (1.86 × 106–2.15 × 106 spore/mL) of fungal isolates were inoculated on to PDA Petri plates (diameter, 9 cm). The inoculation was done using a sterile glass spreader to get a uniform fungal growth. Then, the different concentrations of essential oils (1–13 ppm) were prepared by diluting it with acetone, and applied (10 μL) to sterile paper discs (diameter, 0.6 cm) placed in the center of the agar medium. The disc of control plates were applied with 10 μL of acetone. Three replicates of each oil treatment were performed. The Petri plates were incubated at 25 ± 2 °C for 10 days. After the observation period, the antifungal activity of essential oils were estimated by measuring the diameter of the inhibition zone, also called the zone of clearance.
Gas chromatography–mass spectrometry (GC–MS) of essential oil
The essential oil showing best control activity against tested fungi was evaluated to quantify its chemical components. The chemical composition of oil was analyzed using GC–MS, carried out on a Shimadzu QP-2010 Plus gas chromatograph equipped with Thermal Desorption System TD 20 (AB-Innowax 7031428, Japan). A flame ionization detector (FID) fitted with a 60 m × 0.25 mm × 0.25 µm WCOT column and coated with diethylene glycol was used. Carrier gas was helium with a flow rate of 3 mL/min. Both injector and detector temperature were maintained at 260 °C. The oven temperature was programmed from 60 to 260 °C at 3 °C/min and then held at 260 °C for 10 min, with a total run time of 50 min. Samples (0.2 µL) were injected into the column with a split ratio of 80:1. The relative percentage of the oil constituents was expressed as percentages by FID peak area normalization. The individual components were identified by matching their mass spectral data from National Institute of Standards and Technology (NIST12 or NIST62) and Wiley 229 mass spectrometry libraries.
Statistical analysis
The data obtained for different assays were subjected to descriptive analysis to determine standard deviation, Chi square, 95 %-confidence intervals for regression coefficient and other statistical parameters (SPSS version 17.0 2008). The Chi square value was calculated for essential oil application against fungal strains to statistically validate test of independence. This was computed by summing the difference between the expected and observed frequencies for MIC, MFC or ZOC obtained with a particular essential oil, and dividing this by respective expected frequencies. One-way analysis of variance (ANOVA) was used to analyze the effect of varying doses of essential oil on ZOC obtained in agar disc diffusion assay. The effect of essential oils on fungal strains was presented in terms of statistical values of R Square, Sum of Squares of Regression, regression coefficient, odds ratio etc. The probit analysis to predict the effect of EOs against fungi was also performed.
Results and discussion
The phytopathogenic fungi, FP and FV are significant pathogens of wide variety of agricultural and non-agricultural plants. Moreover, due to their role in several diseases of human and animals; utmost attention toward their control strategies were needed. In this regards, essential oils and their components presented a lucrative possibility, owing to their ephemeral and biodegradable nature (Hassanien et al. 2015). Subsequently, several studies investigating control efficacy of essential oils against Fusarium sp. were reported (Velluti et al. 2004; Sreenivasa et al. 2011; Xing et al. 2014). The current study describes essential oils efficacy against isolates of FP and FV.
Microtiter plate assay
Microtiter plate assay was used to determine the activity of essential oils in term of MIC. The MIC of essential oil is the minimum concentration required for inhibition of fungal growth, while MFC is the minimum concentration required to kill that particular fungus. The values of MIC and MFC for different essential oils against FP and FV are presented in Table 1. The results revealed high efficacy T. vulgaris, A. graveolens and M. arvensis essential oils. For the control of FP1, T. vulgaris essential oil (MIC = 7.45 × 10−8 µL/103 fungal spores) showed best efficacy, followed by oil of A. graveolens (3.81 × 10−5 µL/103 spore). However, similar high efficacy of T. vulgaris could not be obtained against FP2, FV1 and FV2. Thus, the essential oil of M. arvensis with a MIC (µL/103 spore) of 1.49 × 10−7 (FP2), 2.33 × 10−9 (FV1), and 1.5 × 10−4 (FV2) was adjudged as best for inhibiting fungal growth. Sufficiently high growth inhibiting activity was also observed with the essential oils of A. graveolens and M. spicata. The essential oil of P. nigrum showed no antifungal activity, while the oil of C. paradisi was largely ineffective for Fusarium growth inhibition. Zabka et al. (2009) reported mycelial growth inhibition of Fusarium oxysporum and F. verticillioides using Pimenta dioica and T. vulgaris essential oils. In their study, essential oil of Pimenta dioica showed a MIC of 0.5 and 0.6 µL/mL against F. oxysporum and F. verticillioides, respectively, while a MIC of 1.2 µL/mL (F. oxysporum) and 1.8 µL/mL (F. verticillioides) was observed with the application of T. vulgaris essential oil. Similarly, significant inhibition in the radial growth of Fusarium solani was reported using essential oils of peppermint (MIC, 0.3 %), spearmint (MIC, 0.5 %), and lemongrass (MIC, 0.6 %) (Omyma et al. 2008). The use of cinnamon oil, natural cinnamaldehyde, and synthetic cinnamaldehyde against F. verticillioides showed a MIC of 60, 50, and 45 mL/L, respectively (Xing et al. 2014), while Fandohan et al. (2004) reported complete growth inhibition of F. verticillioides (for over 21 days) with the application of Cymbopogon citratus (8 µL/g), Ocimum basilicum (6.4 µL/g), and O. gratissimum (4.8 µL/g) essential oils.
Table 1.
MIC and MFC (both, µL/103spore) of essential oils against isolates of Fusarium proliferatum and F. verticillioides
| Essential oils | MIC | MFC | ||||||
|---|---|---|---|---|---|---|---|---|
| F. proliferatum KT855215 | F. proliferatum KT855218 | F. verticillioides KT855216 | F. verticillioides KT855217 | F. proliferatum KT855215 | F. proliferatum KT855218 | F. verticillioides KT855216 | F. verticillioides KT855217 | |
| Anethum graveolens | 3.81 × 10−5 | 0.04 | 1.91 × 10−5 | 2.44 × 10−3 | 1.22 × 10−3 | 0.04 | 4.89 × 10−3 | 0.16 |
| Citrus paradisi | 5 | 5 | 1.25 | 2.5 | 5 | 5 | 1.25 | 2.50 |
| Mentha arvensis | 6.10 × 10−4 | 1.49 × 10−7 | 2.33 × 10−9 | 1.53 × 10−4 | 0.16 | 2.44 × 10−3 | 6.10 × 10−4 | 0.16 |
| Mentha spicata | 0.04 | 9.77 × 10−3 | 9.54 × 10−6 | 0.02 | 0.04 | 9.77 × 10−3 | 2.44 × 10−3 | 1.25 |
| Piper nigrum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rosmarinus officinalis | 0.08 | 0.31 | 0.63 | 0.31 | 1.25 | 1.25 | 0.63 | 0.31 |
| Thymus Vulgaris | 7.45 × 10−8 | 1.53 × 10−4 | 3.05 × 10−4 | 1.91 × 10−5 | 3.05 × 10−4 | 1.53 × 10−4 | 9.77 × 10−3 | 1.91 × 10−5 |
In terms of MFC, essential oils of T. vulgaris and A. graveolens showed high efficacy against Fusarium isolates (Table 1). Interestingly, remarkable activity in inhibiting fungal growth observed with M. arvensis oil was not reflected in its killing activity against FP and FV. This indicate fungistatic attribute of M. arvensis essential oil against fungal isolates. In contrast, MFC and MIC values of T. vulgaris oil against FP2 and FV2 were identical, indicating that a range of fungal growth inhibition concentrations were also sufficient for killing of fungal strains. Similar to the present study, Kohiyama et al. (2015) reported similar values of MIC and MFC (250 µg/mL) of T. vulgaris essential oil against Aspergillus flavus.
The values of inhibition concentrations of essential oils obtained from microtiter plate assay were plotted as 0–1 sheet; where 1 represent positive inhibition while 0 stands for negative or no inhibition. This data was subjected to descriptive analysis for better representation of results (Suppl. Table 1). Similarly, descriptive analysis of fungicidal concentrations of essential oils is depicted in Suppl. Table 2. The essential oil of M. arvensis showed fungal growth inhibition with 14 (FP1), 26 (FP2), 32 (FV1), and 16 (FV2) oil concentrations (Suppl. Table 1). However, only 6 (FP1), 12 (FP2), 14 (FV1), and 6 (FV2) oil concentrations showed fungicidal activity (Suppl. Table 2). The Chi square value of essential oils obtained through this analysis confirms the efficacy of essential oils discussed above. The relationship between activity of essential oils against FP1, FP2, FV1 and FV2 was depicted through dendrograms (Suppl. Fig. 1). The dendrogram based on growth inhibition activity of essential oils showed a close similarity between essential oils of M. arvensis & A. graveolens and P. nigerum & C. paradisi, indicating similar efficacy of these oils towards control of Fusarium isolates (Suppl. Fig. 1-d). However, no apparent relationship between essential oils could be deduced based on their fungicidal activity (Suppl. Fig. 1-h).
The reports from literature studies as well as findings of the present investigation confirms the efficacy of essential oils in controlling spore germination and growth of mycotoxigenic fungi of Fusarium sp. However, fungal growth inhibition by essential oils may not result in inhibition of their toxin production. The application of cinnamon oil (500 mg/g) against Fusarium culmorum and F. graminearum showed significant growth inhibition, but increase in toxin production (Hope et al. 2005). The reason for this was inferred to be stress induction resulting from application of sub optimal concentrations of fungicidal agent. Nevertheless, application of essential oils at its fungicidal concentrations was shown to inhibit both fungal growth as well as toxin production. However, before being considered for practical and commercial application, the possible toxic effect of essential oil on grains and food products need to be ascertained.
Agar disc diffusion assay
The growth inhibition of Fusarium isolates in diffusion assay are represented in term of ZOC (Fig. 1). The results revealed maximum antifungal efficacy of M. arvensis essential oils against FP1 (ZOC, 17.8–37.8 %; Fig. 1a), and T. vulgaris oil against FP2 (ZOC, 18.9–28.3 %; Fig. 1b), FV1 (ZOC, 21.1–30.6 %; Fig. 1c), and FV2 (ZOC, 20.0–36.7 %; Fig. 1d). Significant growth inhibition efficacy was also observed with essential oils of R. officinalis and M. spicata against FP2 and FV1, respectively. Interestingly, P. nigrum essential oil, which showed no activity in microtiter assay, depicted significant efficacy against both the strains of FV (ZOC, FV1 = 13.9–31.7 %; FV2 = 15.6–25.0 %) in agar disc diffusion assay.
Fig. 1.
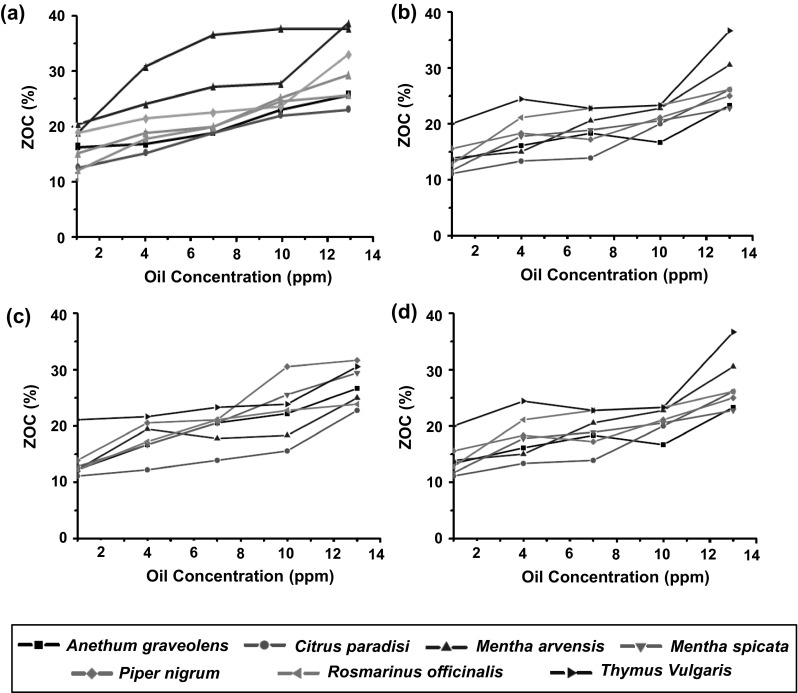
Zone of clearance (ZOC, %; n = 3) obtained by the application of different concentrations of essential oils against Fusarium isolates in agar disc diffusion assay, a Fusarium proliferatum 1 (KT855215), b F. proliferatum 2 (KT855218), c F. verticillioides 1 (KT855216), d F. verticillioides 2 (KT855217)
A descriptive statistical analysis of ZOC of Fusarium isolates obtained at different concentrations of essential oils is represented in Suppl. Table 3. Further, the ZOC values were subjected to probit analysis to determine a relationship between essential oil concentration and fungal growth inhibition (Table 2). This analysis was used to determine essential oil concentrations required for 50 % ZOC (ZOC50). Again, M. arvensis essential oil showed requirement of lowest oil concentration for ZOC50 against FP1 (17.51 ppm), FP2 (26.97 ppm), and FV2 (24.19 ppm) (Table 2). The ZOC values of Fusarium isolates were also subjected to analysis of variance (ANOVA) for fitted linear model equation (Table 3). From the regression model, R2 for FP1 (0.761–0.975), FP2 (0.971–0.999), FV1 (0.719–0.998) and FV2 (0.617–0.995) with different essential oils, indicated that the linear regression model was able to explains more than 61.7 % variability of the response data around its mean. The smaller values for standard error of the estimate (<0.5) indicates that the observations were closer to the fitted line. Similarly, low values of residual sum of squares (RSS) indicates a tight fit of the model to the data. This also supported a high correlation between observed and predicted values.
Table 2.
Probit analysis to predict effect different concentrations of essential oils against isolates of Fusarium proliferatum and F. verticillioides in agar disc diffusion assay
| Fungal isolates | Statistical parameters | Essential oils | ||||||
|---|---|---|---|---|---|---|---|---|
| Anethum graveolens | Citrus paradisi | Mentha arvensis | Mentha spicata | Piper nigrum | Rosmarinus officinalis | Thymus vulgaris | ||
| F. proliferatum KT855215 | EOC* | 0.033 | 0.039 | 0.045 | 0.043 | 0.035 | 0.045 | 0.043 |
| SE | 0.036 | 0.037 | 0.033 | 0.036 | 0.035 | 0.037 | 0.034 | |
| Intercept | −1.112 | −1.233 | −0.789 | −1.130 | −0.991 | −1.213 | −0.923 | |
| Chi square | 0.151 | 0.170 | 0.888 | 0.093 | 0.273 | 0.333 | 0.239 | |
| df a | 8 | 8 | 8 | 8 | 8 | 8 | 8 | |
| Sig. | 1.000b | 1.000b | 0.999b | 1.000b | 1.000b | 1.000b | 1.000b | |
| ** | 33.910 | 31.460 | 17.517 | 26.161 | 28.038 | 26.662 | 21.286 | |
| F. proliferatum KT855218 | EOC | 0.024 | 0.040 | 0.040 | 0.028 | 0.044 | 0.024 | 0.026 |
| SE | 0.038 | 0.038 | 0.036 | 0.036 | 0.036 | 0.035 | 0.034 | |
| Intercept | −1.187 | −1.249 | −1.084 | −1.101 | −1.164 | −0.974 | −0.895 | |
| Chi square | 0.138 | 0.138 | 0.712 | 0.037 | 0.234 | 0.187 | 0.206 | |
| df a | 8 | 8 | 8 | 8 | 8 | 8 | 8 | |
| Sig. | 1.000b | 1.000b | 0.999b | 1.000b | 1.000b | 1.000b | 1.000b | |
| 49.169 | 31.582 | 26.975 | 39.277 | 26.170 | 39.941 | 34.674 | ||
| F. verticillioides KT855216 | EOC | 0.042 | 0.038 | 0.031 | 0.050 | 0.050 | 0.036 | 0.023 |
| SE | 0.036 | 0.038 | 0.036 | 0.036 | 0.035 | 0.036 | 0.034 | |
| Intercept | −1.163 | −1.311 | −1.117 | −1.173 | −1.090 | −1.121 | −0.864 | |
| Chi square | 0.106 | 0.173 | 0.894 | 0.106 | 0.268 | 0.190 | 0.218 | |
| df a | 8 | 8 | 8 | 8 | 8 | 8 | 8 | |
| Sig. | 1.000b | 1.000b | 0.999b | 1.000b | 1.000b | 1.000b | 1.000b | |
| 27.560 | 34.462 | 36.347 | 23.540 | 21.624 | 31.429 | 38.335 | ||
| F. verticillioides KT855217 | EOC | 0.027 | 0.049 | 0.049 | 0.032 | 0.026 | 0.034 | 0.033 |
| SE | 0.037 | 0.038 | 0.036 | 0.037 | 0.036 | 0.036 | 0.034 | |
| Intercept | −1.125 | −1.323 | −1.181 | −1.134 | −1.051 | −1.044 | −0.901 | |
| Chi square | 0.208 | 0.453 | 0.729 | 0.153 | 0.483 | 0.429 | 0.604 | |
| df a | 8 | 8 | 8 | 8 | 8 | 8 | 8 | |
| Sig. | 1.000b | 1.000b | 0.999b | 1.000b | 1.000b | 1.000b | 1.000b | |
| 42.236 | 26.954 | 24.199 | 35.556 | 40.009 | 30.848 | 26.950 | ||
* EOC, estimated oil concentration (in ppm)
** , Essential oil concentration required for 50 % ZOC (in ppm)
a, bValues followed by same uppercase letter in a row are not significantly different (p < 0.05)
Table 3.
Analysis of variance (ANOVA) for fitted linear regression model depicting effect of essential oils against isolates of Fusarium proliferatum and F. verticillioides in an agar disc diffusion assay
| Fungal isolates | Statistical parameters* | Essential oils | ||||||
|---|---|---|---|---|---|---|---|---|
| Anethum graveolens | Citrus paradisi | Mentha arvensis | Mentha spicata | Piper nigrum | Rosmarinus officinalis | Thymus vulgaris | ||
| F. proliferatum KT855215 | R square | 0.940 | 0.975 | 0.761 | 0.965 | 0.795 | 0.954 | 0.863 |
| Adjusted R2 | 0.920 | 0.967 | 0.682 | 0.954 | 0.727 | 0.939 | 0.818 | |
| Regression coefficient | 0.969 | 0.988 | 0.873 | 0.983 | 0.892 | 0.977 | 0.929 | |
| E | 0.111 | 0.077 | 0.434 | 0.114 | 0.269 | 0.130 | 0.280 | |
| Odd ratio | 1.160 | 0.932 | 1.898 | 1.100 | 1.403 | 0.952 | 1.542 | |
| RES | 0.576 | 0.702 | 1.806 | 1.089 | 0.841 | 1.056 | 1.482 | |
| RSS | 0.037 | 0.018 | 0.566 | 0.039 | 0.217 | 0.051 | 0.235 | |
| Regression df | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Residual df | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |
| F | 46.703 | 118.690 | 9.578 | 83.769 | 11.627 | 62.438 | 18.942 | |
| Sig. | 0.006 | 0.002 | 0.054 | 0.003 | 0.042 | 0.004 | 0.022 | |
| F. proliferatum KT855218 | R square | 0.981 | 0.999 | 0.935 | 0.995 | 0.971 | 0.945 | 0.864 |
| Adjusted R2 | 0.974 | 0.999 | 0.913 | 0.993 | 0.961 | 0.927 | 0.819 | |
| Regression coefficient | 0.990 | 0.985 | 0.967 | 0.998 | 0.999 | 0.972 | 0.930 | |
| E | 0.040 | 0.016 | 0.149 | 0.026 | 0.105 | 0.084 | 0.156 | |
| Odd ratio | 1.038 | 1.040 | 1.207 | 1.193 | 0.902 | 1.467 | 1.648 | |
| RES | 0.240 | 0.702 | 0.961 | 0.400 | 1.089 | 0.361 | 0.462 | |
| RSS | 0.005 | 0.001 | 0.067 | 0.002 | 0.033 | 0.021 | 0.073 | |
| Regression df | 1.038 | 1.040 | 1.207 | 1.193 | 0.902 | 1.467 | 1.648 | |
| Residual df | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |
| F | 151.737 | 2809.00 | 43.030 | 600.00 | 99.000 | 51.571 | 19.062 | |
| Sig. | 0.001 | 0.000 | 0.007 | 0.000 | 0.002 | 0.006 | 0.022 | |
| F. verticillioides KT855216 | R square | 0.983 | 0.838 | 0.719 | 0.998 | 0.930 | 0.920 | 0.780 |
| Adjusted R2 | 0.977 | 0.785 | 0.626 | 0.997 | 0.906 | 0.893 | 0.706 | |
| Regression coefficient | 0.991 | 0.916 | 0.848 | 0.999 | 0.964 | 0.959 | 0.883 | |
| E | 0.075 | 0.192 | 0.251 | 0.032 | 0.206 | 0.140 | 0.184 | |
| Odd ratio | 1.047 | 0.800 | 1.157 | 1.003 | 1.163 | 1.143 | 1.727 | |
| RES | 0.961 | 0.576 | 0.484 | 1.444 | 1.681 | 0.676 | 0.361 | |
| RSS | 0.017 | 0.111 | 0.189 | 0.003 | 0.127 | 0.059 | 0.102 | |
| Regression df | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Residual df | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |
| F | 169.588 | 15.568 | 7.683 | 1444.00 | 39.709 | 34.373 | 10.618 | |
| Sig. | 0.001 | 0.029 | 0.069 | 0.000 | 0.008 | 0.010 | 0.047 | |
| F. verticillioides KT855217 | R square | 0.773 | 0.898 | 0.938 | 0.896 | 0.854 | 0.817 | 0.617 |
| Adjusted R2 | 0.697 | 0.864 | 0.917 | 0.861 | 0.806 | 0.757 | 0.490 | |
| Regression coefficient | 0.879 | 0.948 | 0.968 | 0.947 | 0.924 | 0.904 | 0.786 | |
| E | 0.183 | 0.203 | 0.174 | 0.140 | 0.147 | 0.224 | 0.417 | |
| Odd ratio | 1.148 | 0.750 | 0.987 | 1.125 | 1.295 | 1.303 | 1.613 | |
| RES | 0.342 | 1.089 | 1.369 | 0.506 | 0.380 | 0.676 | 0.841 | |
| RSS | 0.101 | 0.124 | 0.091 | 0.059 | 0.065 | 0.151 | 0.521 | |
| Regression df | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Residual df | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |
| F | 10.191 | 26.347 | 45.132 | 25.851 | 17.618 | 13.430 | 4.843 | |
| Sig. | 0.050 | 0.014 | 0.007 | 0.015 | 0.025 | 0.035 | 0.115 | |
* SE of the estimate (E); regression sum of squares (RES); residual sum of squares (RSS)
Growth reduction of F. proliferatum mycelium have been reported using essential oils of Citrus aurantifolia (91.5 %), C. sinensis (59.5 %), C. reticulata (50.9 %) and C. grandis (63.0 %) (Van et al. 2013). Amri et al. (2013) reported growth inhibition (57.9 %) of F. verticilloides with the application of Juniperus oxycedrus (4 μL/mL) essential oil, while use of clove (92 %) and cinnamon oils (80 %) showed significant reduction in the colony growth of F. verticillioides, F. proliferatum and F. graminearum (Velluti et al. 2004). López et al. (2004) reported inhibition in mycelial development of F. verticillioides using essential oils of Origanum vulgare, Aloysia triphylla, A. polystachya and Mentha piperita. Sreenivasa et al. (2011) observed a proportionality between essential oil concentrations and inhibition activity of clove, peppermint, lemongrass and citronella against Fusarium species. The observations was in agreement with the results of present investigation which depicted increase in ZOC with increased oil concentrations. However, radial growth inhibition of Fusarium isolates in the present study was obtained at much lower oil (1–13 ppm) concentrations than the reported findings. For instance, in their study to evaluate control efficacy of 5 essential oils against 9 Fusarium spp., Sreenivasa et al. (2011) could attain antifungal activity only at the oil concentrations higher than 500 ppm.
GC–MS of essential oil
The efficacy of essential oils is influenced by the types and content of their terpenes constituents, which warrants investigation of chemical composition of oil to discern its bio-efficacy (Kumar et al. 2012). M. arvensis essential oil on account of their high control efficacy [in both microtiter plate assay and agar disc diffusion assay] against fungal strains of FP and FV, was selected for their qualitative and quantitative analysis using GC–MS. The chemical composition of M. arvensis essential oil identified using above process is listed in Suppl. Table 4. The M. arvensis essential oil was characterized by the presence of 38 compounds which formed 99.85 % of the total oil components. The major component identified in the oil was menthol (63.18 %), menthone (15.08 %), isomenthyl acetate (5.50 %) and limonene (4.31 %). All the other components were present at less than 2 %. Padalia et al. (2013) reported menthol (73.7–85.8 %), menthone (1.5–11.0 %) and menthyl acetate (0.5–5.3 %) to be major constituents of M. arvensis in their investigation of 9 cultivars from western Himalayan region of India, while Singh et al. (2005) observed menthol (77.5–89.3 %), menthone (0.3–7.9 %) and isomenthone (3.7–6.1 %) as the key components of six different cultivars of M. arvensis. Plant of Mentha species have been reported to show high variation in their essential oil content and composition, subject to environmental factors, agronomic and genotype conditions of the plant and cultivation practices (Kumar et al. 2011). It is suggested that the activity of essential oils results from synergistic or cumulative effects existing between their major components. In this regard, the significant efficacy of M. arvensis oil obtained in the present study, could be attributed to its principal component, menthol and menthone. Although, the present study did not look into efficacy of oil components, few studies on evaluation of antifungal activity of terpenes against Fusarium isolates, presented conflicting results. For instance, Dambolena et al. (2008) observed no significant antifungal activity of menthol and menthone against F. verticillioides MRC 826 in a semisolid agar susceptibility assay. However, in another study, varying levels of F. verticillioides growth inhibition was reported with menthol stereoisomers (Dambolena et al. 2010). Nevertheless, several other reports asserts higher activity of essential oils than its individual components (Paster et al. 1994). This may be due to variation in experimental condition or fungal strains or any other unaccounted factor. It will be interesting to investigate this aspect of research in future studies.
The exact mechanism of action of essential oils and its components on fungi remain unclear. Literature reports suggests significant morphological changes to the fungal hyphae resulting from loss of rigidity and integrity, plasma membrane disruption leading to leakage of radicals, cytochrome C, proteins and ions, and disruption of mitochondrial structure causing cell death by apoptosis and necrosis (Bakkali et al. 2008; Amri et al. 2013). Daferera et al. (2003) attributed fungitoxic activity of essential oils to the increased concentration of lipidic peroxides such as hydroxyl, alkoxyl, and alkoperoxyl radicals resulting from formation of hydrogen bonds between -OH of essential oil phenols and active sites of fungal enzyme. Nevertheless, significant antifungal efficacy of essential oils and their constituents provide opportunity to investigate their control activity in view of commercial applicability.
Conclusion
The present study reports antifungal efficacy of plant essential oils through microtiter plate and agar disc diffusion assays. The results showed significant growth inhibition activity of M. arvensis and T. vulgaris essential oils against FP and FV. Antifungal activity of tested essential oils utilized fungistatic and fungicidal action. However, control by M. arvensis was prominently fungistatic, while T. vulgaris essential oils maneuvered fungal control mostly through fungicidal action. The disc diffusion assay depicted antifungal activity of essential oils at very low concentrations. The requirement of low concentrations of essential oils to control Fusarium sp. may find application in control of fungi in grains or processed food industry. The results also open up the possibility of developing essential oils into an ecofriendly, economical, and acceptable fungicidal products.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by Science and Engineering Research Board, Department of Science and Technology (DST), India (SERB/LS-994/2013). The support provided by Mr. Omkar Sharma (IARI, New Delhi, India) for his help in experimental work is greatly acknowledged. The authors also acknowledge the technical support provided by Mr. Ajai Kumar (AIRF JNU, India) for GC–MS analysis.
References
- Amri I, Hamrouni L, Gargouri S, Hanana M, Jamoussi B. Chemical composition and antifungal activity of essential oils isolated from Juniperus oxycedrus L. Int J Appl Biol Pharm Technol. 2013;4:227–233. [Google Scholar]
- Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils, a review. Food Chem Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- Bennett JW, Klich M. Mycotoxins. Clin Microbiol Rev. 2003;16(3):497–516. doi: 10.1128/CMR.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busman M, Desjardins AE, Proctor RH. Analysis of fumonisin contamination and the presence of Fusarium in wheat with kernel black point disease in the United States. Food Addit Contam A. 2012;29:1092–1100. doi: 10.1080/19440049.2012.671787. [DOI] [PubMed] [Google Scholar]
- Chen Y, Huang TT, Chen CJ, Hou YP, Zhang AF, Wang WX, Gao TC, Zhou MG. Sensitivity of Fusarium verticillioides isolates from rice to a novel cyanoacrylate fungicide. Crop Prot. 2012;39:106–109. doi: 10.1016/j.cropro.2012.03.016. [DOI] [Google Scholar]
- Daferera DJ, Ziogas BN, Polissiou MG. The effectiveness of plant essential oils in the growth of Botrytis cinerea, Fusarium sp. and Clavibacter michiganensis subsp. Michiganensis. Crop Prot. 2003;22:39–44. doi: 10.1016/S0261-2194(02)00095-9. [DOI] [Google Scholar]
- Dambolena JS, López AG, Cánepa MC, Theumer MG, Zygadlo JA, Rubinstein HR. Inhibitory effect of cyclic terpenes (limonene, menthol, menthone and thymol) on Fusarium verticillioides MRC 826 growth and fumonisin B1 biosynthesis. Toxicon. 2008;51:37–44. doi: 10.1016/j.toxicon.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Dambolena JS, López AG, Rubinstein HR, Zygadlo JA. Effects of menthol stereoisomers on the growth, sporulation and fumonisin B1 production of Fusarium verticillioides. Food Chem. 2010;123:165–170. doi: 10.1016/j.foodchem.2010.04.024. [DOI] [Google Scholar]
- Fandohan P, Gbenou JD, Gnonlonfin B, Hell K, Marasas WFO, Wingfield MJ. Effect of essential oils on the growth of Fusarium verticillioides and fumonisin contamination in corn. J Agric Food Chem. 2004;52:6824–6829. doi: 10.1021/jf040043p. [DOI] [PubMed] [Google Scholar]
- Guo Z, Döll K, Dastjerdi R, Karlovsky P, Dehne HW, Altincicek B. Effect of fungal colonization of wheat grains with Fusarium spp. on food choice weight gain and mortality of meal beetle larvae (Tenebrio molitor) PLoS ONE. 2014;9:100–112. doi: 10.1371/journal.pone.0100112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassanien MFR, Assiri AMA, Alzohairy AM, Oraby HF. Health-promoting value and food applications of black cumin essential oil: an overview. J Food Sci Technol. 2015;52(10):6136–6142. doi: 10.1007/s13197-015-1785-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope R, Aldred D, Magan N. Comparison of environmental profiles for growth and deoxynivalenol production by Fusarium culmorum and F. graminearum on wheat grain. Lett Appl Microbiol. 2005;40:295–300. doi: 10.1111/j.1472-765X.2005.01674.x. [DOI] [PubMed] [Google Scholar]
- Ivanova E, Pancevska NA, Dz Kungulovski. Antimicrobial activities of laboratory produced essential oil solutions against five selected fungal strains. Proc Natural Sci Matica srpska (Novi Sad Serbia) 2013;124:171–185. [Google Scholar]
- Jurado M, Marin P, Callejas C, Moretti A, Vazquez C, Gonzalez-Jaen MT. Genetic variability and fumonisin production by Fusarium proliferatum. Food Microbiol. 2010;27:50–57. doi: 10.1016/j.fm.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Kohiyama CY, Ribeiro MMY, Mossini SAG, Bando E, da Silva Bomfim N, Nerilo SB, Machinski M. Antifungal properties and inhibitory effects upon aflatoxin production of Thymus vulgaris L. by Aspergillus flavus Link. Food Chem. 2015;173:1006–1010. doi: 10.1016/j.foodchem.2014.10.135. [DOI] [PubMed] [Google Scholar]
- Kumar P, Mishra S, Malik A, Satya S. Insecticidal properties of Mentha species: a review. Ind Crops Prod. 2011;34:802–817. doi: 10.1016/j.indcrop.2011.02.019. [DOI] [Google Scholar]
- Kumar P, Mishra S, Malik A, Satya S. Efficacy of Mentha × piperita and Mentha citrata essential oils against housefly, Musca domestica L. Ind Crops Prod. 2012;39:106–112. doi: 10.1016/j.indcrop.2012.02.021. [DOI] [Google Scholar]
- Kushiro M, Saitoh H, Sugiura Y, Aoki T, Kawamoto S, Sato T. Experimental infection of Fusarium proliferatum in Oryza sativa plants; fumonisin B1 production and survival rate in grains. Int J Food Microbiol. 2012;156:204–208. doi: 10.1016/j.ijfoodmicro.2012.03.021. [DOI] [PubMed] [Google Scholar]
- Logrieco A, Bottalico A, Mulé G, Moretti A, Perrone G. Epidemiology of toxigenic fungi and their associated mycotoxins for some Mediterranean crops. Eur J Plant Pathol. 2003;109:645–667. doi: 10.1023/A:1026033021542. [DOI] [Google Scholar]
- López AG, Theumer MG, Zygadlo JA, Rubinstein HR. Aromatic plants essential oils activity on Fusarium verticillioides Fumonisin B1 production in corn grain. Mycopathologia. 2004;158(3):343–349. doi: 10.1007/s11046-005-3969-3. [DOI] [PubMed] [Google Scholar]
- Omyma A, Abu ASM, El-Souod SMW, El-Debaky ASA. Control of dry and soft rot diseases of potato tubers using essential oils fungicides and antagonistic fungi. Egypt J Exp Biol. 2008;4:1–11. [Google Scholar]
- Padalia RC, Verma RS, Chauhan A, Sundaresan V, Chanotiya CS. Essential oil composition of sixteen elite cultivars of Mentha from western Himalayan region, India. Maejo Int J Sci Technol. 2013;7:83–93. [Google Scholar]
- Palacios SA, Susca A, Haidukowski M, Stea G, Cendoya E, Ramírez ML, Chulze SN, Farnochi MC, Moretti A, Torres AM. Genetic variability and fumonisin production by Fusarium proliferatum isolated from durum wheat grains in Argentina. Int J Food Microbiol. 2015;201:35–41. doi: 10.1016/j.ijfoodmicro.2015.02.011. [DOI] [PubMed] [Google Scholar]
- Panda P, Aiko V, Mehta A. Effect of aqueous extracts of Mentha arvensis (mint) and Piper betle (betel) on growth and citrinin production from toxigenic Penicillium citrinum. J Food Sci Technol. 2015;52(6):3466–3474. doi: 10.1007/s13197-014-1390-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paster N, Menasherov M, Ravid U, Juven B. Antifungal activity of oregano and thyme essential oils applied as fumigants against fungi attacking stored grain. J Food Prot. 1994;58:81–85. doi: 10.4315/0362-028X-58.1.81. [DOI] [PubMed] [Google Scholar]
- Singh AK, Raina VK, Naqvi AA, Patra NK, Kumar B, Ram P, Khanuja SPS. Essential oil composition and chemoarrays of menthol mint (Mentha arvensis L. f. piperascens Malinvaud ex. Holmes) cultivars. Flavour Fragr J. 2005;20:302–305. doi: 10.1002/ffj.1417. [DOI] [Google Scholar]
- SPSS . Statistical product and service solution statistics for Windows version 175 170. Chicago: SPSS Inc; 2008. [Google Scholar]
- Sreenivasa MY, Dass RS, Raj APC, Prasad MNN, Achar PN, Janardhana GR. Assessment of the growth inhibiting effect of some plant essential oils on different Fusarium species isolated from sorghum and maize grains. J Plant Dis Prot. 2011;118:208–213. doi: 10.1007/BF03356405. [DOI] [Google Scholar]
- Tancic S, Stanković S, Lević J, Krnjaja V, Vukojević J. Diversity of the Fusarium verticillioides and F. proliferatum isolates according to their fumonisin B1 production potential and origin. Genetika. 2012;44:163–176. doi: 10.2298/GENSR1201163T. [DOI] [Google Scholar]
- Thippeswamy S, Mohana DC, Abhishek RU, Manjunath K. Effect of plant extracts on inhibition of Fusarium verticillioides growth and its toxin fumonisin B1 production. J Agric Sci Technol. 2013;9(4):889–900. [Google Scholar]
- Van HP, Chi PTL, Phi NTL. Comparison of antifungal activities of Vietnamese citrus essential oils. Nat Prod Res. 2013;27:506–508. doi: 10.1080/14786419.2012.706293. [DOI] [PubMed] [Google Scholar]
- Velluti A, Marín S, Gonzalez P, Ramos AJ, Sanchis V. Initial screening for inhibitory activity of essential oils on growth of Fusarium verticillioides, F. proliferatum and F. graminearum on maize-based agar media. Food Microbiol. 2004;21:649–656. doi: 10.1016/j.fm.2004.03.009. [DOI] [Google Scholar]
- Xing F, Hua H, Selvaraj JN, Zhao Y, Zhou L, Liu X, Liu Y. Growth inhibition and morphological alterations of Fusarium verticillioides by cinnamon oil and cinnamaldehyde. Food Control. 2014;46:343–350. doi: 10.1016/j.foodcont.2014.04.037. [DOI] [Google Scholar]
- Zabka M, Pavela R, Slezakova L. Antifungal effect of Pimenta dioica essential oil against dangerous pathogenic and toxinogenic fungi. Ind Crops Prod. 2009;30:250–253. doi: 10.1016/j.indcrop.2009.04.002. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


