Abstract
This study evaluated the effect of conventional maceration and ultrasound assisted extraction techniques on the extraction yield, chemical composition and sensory characteristics of nutmeg oleoresins. Extraction was performed using material: absolute ethanol ratio 1:4, at room temperature. The volatile components of the oleoresin were identified by gas chromatography–mass spectrometry. The results of the study showed that the yield ranged from 4.55 to 9.63 %. Fifty-three compounds in the nutmeg oleoresin have been identified to account for >90 % of the total oil content. Sabinene, myristicin, elemicin, α-pinene, β-pinene, limonene, terpinen-4-ol and myristic acid were found as major compounds of all the nutmeg oleoresins obtained by different techniques. The sensory characteristics of the oleoresin were strongly influenced by the ultrasonic intensity and duration of extraction. The experimental results showed that ultrasound assisted extraction technique at 40 % of the maximal output power and 10 min produced superior quality nutmeg oleoresin with a remarkable yield.
Keywords: Nutmeg, Oleoresin, Ultrasonic, Maceration, β-Pinene, Myristicin
Introduction
Nutmeg (Myristica fragrans Houtt.) is one of the most popular spices, which belongs to the family Myristicaceae (Periasamy et al. 2016). A dried kernel of broadly ovoid seed of the nutmeg is known for its aromatic properties (Tajuddin et al. 2003). It is a tropical tree and commonly available in Malaysia, India, Indonesia and South East of Asia (Al-Rawi et al. 2011). Nutmeg’s flavor varies depending on its origin. Its flavor can vary from a sweetly spicy to a heavier taste (Charles 2013).
Oleoresins are truer to the spice or herb in flavor character and tend to withstand high temperature processing to a greater extent than comparable essential oils. This phenomenon can be attributed to the influence of the nonvolatile constituents of oleoresin (Eiserle and Rogers 1972). Oleoresin is a naturally occurring mixture of essential oil and a resin extracted from various spices with organic solvents (Moyler 1991). The type of solvent affects the quality and quantity of oleoresin obtained (Said et al. 2015). Alcohol meets the definition of a green solvent (Chemat et al. 2015). Oleoresin has characteristic flavor and aroma of spices, which are the same as the original (Weiss 2002). The seeds of nutmeg are used as a culinary spice due to its flavor and food preservation properties (López et al. 2015). Nutmeg oleoresins are often used in flavoring soft drinks, canned foods, and cosmetics (Lim 2012). Nutmeg oleoresin is used in the preparation of meat and vegetable dishes (Charles 2013). Pharmacological research revealed various activities of nutmeg such as antioxidant, antibacterial, antidiabetic, hypolipidemic, hepatoprotective and analgesic (Gupta et al. 2013). Ethanolic extract of M. fragrans seeds showed low antiproliferative activity (≤53 % growth inhibitory effect) against lung, colon, prostate, head and neck and breast human cancer cell lines (Pandey et al. 2016). The flavor and therapeutic action are due to the volatile oil (Leela 2008).
The novel alternatives to conventional techniques for the extraction of target compounds from various matrices include those assisted with ultrasound or microwave and pre-treatments that include high-pressure processing and pulsed electric field. These novel techniques eliminate the use of toxic solvents, improve process efficiency and enhance extraction yields in a shorter time with a less thermal degradation and a high quality flavor of the extract. The application of ultrasound-assisted extraction increased recently due to the disadvantages associated with conventional and some other novel extraction techniques, such as high capital investment, high-energy consumption and the residues of toxic organic solvents in the extract (Tiwari 2015; Morsy 2015). Food components such as aromas, pigments, antioxidants, and natural bioactive compounds have been extracted, from a variety of matrices by ultrasound-assisted extraction (Chemat et al. 2017).
Ultrasonication generated cavitation bubbles that collapse. This collapse gives rise to micro-jets to release the essential oil from its oil glands. This cavitation effect is dependent on ultrasonic frequency and intensity, temperature, treatment time, which are crucial to the operation of ultrasonic (Li et al. 2014).
The present study was carried out to compare the effect of conventional extraction technique (based on maceration) and ultrasound-assisted extraction (UAE) techniques on the yield, chemical composition and sensory properties of the nutmeg oleoresin.
Materials and methods
Plant material
The fully matured dried seeds of nutmeg were purchased from Harraz Company for Medicinal Plants, Cairo, Egypt. Dried seeds were ground into a fine powder and then kept in an airtight container at 4 °C until use.
Preparation of oleoresins
Maceration
The nutmeg dried seeds powder was extracted using maceration technique (technique 1) with absolute ethanol for 3 days at room temperature according to Handa et al. (2008). The extraction was carried out using nutmeg powder to solvent ratio of 1:4 (w/v) and, subsequently, the mixture was filtered and concentrated under vacuum using a rotatory evaporator at 40 °C.
Ultrasound assisted extraction (UAE)
The oleoresin was obtained by extracting 10 g of the nutmeg fine powder with 40 mL of absolute ethanol. The mixture was subjected to ultrasonic radiation in an ultrasound generator (Fisher Sonic Dismemberator, Model 300, 50 Hz, USA), equipped with a 19 mm diameter tip. The ultrasound probe was immersed to a depth of approximately 5 mm. The ultrasound assisted extraction was carried out at 20 % of the maximal output power (60 W) for 10 and 25 min (techniques 2 and 3, respectively) and at 40 % of the maximal output power (120 W) for 10 and 25 min (techniques 4 and 5, respectively), at room temperature. After ultrasonic step, the extracts were filtered, and the filtrates were evaporated to dryness at 40 °C under reduced pressure. The oleoresins obtained by different techniques were filled in dark glass bottles and stored in a refrigerator at 4 °C until use. Each extraction process was performed three times and the mean value of the extraction yield was recorded.
Gas chromatography–mass spectrometry (GC–MS)
GC analysis was performed on a Shimadzu GC MS-QP 2010 Ultra gas chromatograph (Kyoto, Japan) fitted with flame ionization detector (FID). The column used was a 30 m × 0.25 mm i.d. × 0.25 μm film thickness RTX-5 column. Flow rate of helium carrier gas was set at 1.33 mL/min. The inlet was adjusted at 210 °C and the detector temperature was kept at 230 °C. The split ratio was 1:10. The ionization voltage maintained at 70 eV. One μL sample was injected. The GC oven was maintained at 40 °C for 2 min after injection and then programmed at 5 °C/min to 210 °C, at which the column was maintained for 5 min. Identification of volatile compounds was carried out using GC retention data, Kovats retention indices calculated by using n-hydrocarbons (C9–C22) and mass spectra by computerized matching of volatile compounds with NIST 2008 and Wiley library.
Sensory evaluation
Sensory evaluation of the randomly coded oleoresin samples was conducted with ten trained panelists. Three descriptive adjectives clove-like, sweet, and spicy were submitted to these panelists. Scores were given from 0 to 10 for each adjective; 0 indicated that oleoresin was not in accordance with the adjective and 10 that the oleoresin was in perfect accordance with the descriptive adjective.
Statistical analysis
The nutmeg oleoresin yields obtained by the investigated techniques are reported as mean ± standard deviation (SD). Data were analyzed using one-way analysis of variance (ANOVA). Duncan’s multiple range test was used to compare the significance of differences at P < 0.05.
Results and discussion
Yield of nutmeg oleoresins
The average of the yields of the different extraction techniques is presented in Table 1. The oleoresin yield of maceration technique was significantly higher as compared with that of the other extraction techniques. UAE at 40 % of the maximal output power for 10 min resulted in oleoresin recovery similar to that obtained within 3 days of maceration. Extraction rates of oleoresin for UAE were more rapid than the maceration technique. Ji et al. (2006) reported that ultrasound could enhance the diffusion of the solvent in a substance, due to the influence of the ultrasound cavity.
Table 1.
Chemical composition (% relative area percentage) of the nutmeg oleoresin obtained by maceration and ultrasonic assisted extraction techniques
| Compounds | RIa | Techniquesb | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Monoterpene Hydrocarbons | ||||||
| α-Thujene | 938 | 1.79 | 1.38 | 1.08 | 0.99 | 1.27 |
| α-Pinene | 939 | 9.64 | 7.87 | 7.21 | 6.03 | 5.9 |
| Camphene | 953 | 0.19 | 0.17 | nd | 0.12 | 0.14 |
| Sabinene | 972 | 17.22 | 16.38 | 18.81 | 15.65 | 20.39 |
| β-Pinene | 981 | 6.43 | 4.93 | 6.42 | 3.05 | 2.98 |
| β-Myrcene | 992 | 2.43 | 2.06 | 1.91 | 1.6 | 2.09 |
| L-Phellandrene | 1007 | 0.6 | 0.53 | 0.44 | 0.33 | 0.53 |
| Δ3-Carene | 1009 | 1.06 | 0.93 | 0.8 | 0.39 | 1 |
| α-Terpinene | 1012 | 1.58 | 1.08 | 0.88 | 0.83 | 0.94 |
| ρ-Cymene | 1027 | 1.2 | 0.75 | 0.73 | 0.54 | 0.9 |
| Limonene | 1030 | 4.93 | 4.23 | 4.01 | 3.44 | 4.49 |
| γ -Terpinene | 1074 | 2.52 | 1.86 | 1.63 | 1.44 | 1.58 |
| (E)-Sabinene hydrate | 1073 | 1.34 | 1.08 | 1.36 | 0.84 | 1.13 |
| α-Terpinolene | 1081 | 1.21 | 1.03 | 0.94 | 0.59 | 0.98 |
| (Z)-trans Sabinene hydrate | 1110 | 1.37 | 1.04 | 1.27 | 0.79 | 1.03 |
| Oxides | ||||||
| 1,8-Cineole | 1035 | 0.21 | nd | nd | nd | 0.17 |
| Sesquiterpene Hydrocarbons | ||||||
| α-Copaene | 1377 | 0.56 | 1.02 | 1.04 | nd | 0.53 |
| Trans-β-Caryophyllene | 1415 | 0.25 | 0.25 | nd | 0.06 | 0.08 |
| Trans-α-Bergamotene | 1431 | 0.3 | nd | nd | 0.07 | 0.17 |
| β-Sesquiphellandrene | 1560 | nd | nd | nd | nd | 0.08 |
| Germacrene-D | 1487 | 0.52 | 0.96 | 0.93 | 0.37 | 0.44 |
| Bicyclogermacrene | 1496 | 0.15 | 0.26 | nd | 0.1 | 0.16 |
| β-Bisabolene | 1498 | 0.15 | 0.2 | nd | 0.07 | 0.18 |
| Aromatics | ||||||
| Safrole | 1280 | 2.82 | 1.98 | 2.14 | 0.83 | 1 |
| Eugenol | 1364 | 0.46 | 0.33 | 0.36 | 0.1 | 0.13 |
| Methyl eugenol | 1407 | 0.83 | 1.41 | 1.63 | 0.85 | 0.68 |
| Chavibetol (trans-isoeugenol) | 1447 | 0.95 | 0.63 | nd | 0.3 | 0.38 |
| Methyl isoeugenol | 1454 | 0.33 | 0.26 | nd | 0.42 | 0.37 |
| Myristicin | 1532 | 10.25 | 6.23 | 7.21 | 3.24 | 3.42 |
| Elemicin | 1551 | 5.7 | 9.53 | 11.53 | 7.06 | 6.28 |
| Methoxyeugenol | 1558 | 1.4 | 0.97 | 0.43 | 0.36 | 0.46 |
| Trans-isoelemicin | 1570 | 0.43 | 0.41 | 0.47 | 1.34 | 1.03 |
| Monoterpene Alcohols | ||||||
| Linalool | 1100 | 0.25 | 0.18 | nd | 0.15 | 0.19 |
| ρ-Menth-2-en-1-ol | 1124 | 0.21 | 0.18 | nd | 0.15 | 0.15 |
| Cis ρ-2- menthen-1-ol | 1142 | 0.45 | nd | nd | 0.1 | 0.1 |
| Terpinen-4-ol | 1175 | 3.21 | 2.8 | 3.52 | 2.97 | 2.9 |
| α-Terpineol | 1195 | 0.49 | 0.36 | 0.46 | 0.26 | 0.34 |
| Piperitol isomer | 1207 | nd | nd | nd | 0.07 | nd |
| Geranyl linalool isomer | 2198 | 0.24 | 0.24 | 1.17 | nd | 0.33 |
| Sesquiterpene Alcohols | ||||||
| Guaiol | 1599 | 0.13 | 0.24 | nd | 0.15 | nd |
| Esters | ||||||
| Trans-Sabinene hydrate acetate | 1180 | 0.12 | nd | nd | 0.18 | 0.18 |
| Cis-Sabinene hydrate acetate | 1184 | 0.19 | nd | 0.37 | 0.11 | 0.14 |
| Endobornyl acetate | 1285 | 0.13 | nd | 3 | nd | 0.11 |
| α-Terpinyl acetate | 1353 | 0.38 | 0.49 | 0.54 | 0.22 | 0.27 |
| Citronellyl acetate | 1358 | 0.17 | 0.2 | nd | 0.08 | – |
| Neryl acetate | 1381 | 0.3 | 0.37 | 0.69 | 0.15 | 0.21 |
| Tetradecanoic acid, ethyl ester | 1796 | 0.37 | nd | nd | 0.06 | 0.08 |
| 9-Octadecenoic acid, ethyl ester | 2193 | 0.38 | 0.5 | 0.47 | 0.9 | 0.75 |
| Ketones | ||||||
| α-Ionone | 1437 | nd | 0.8 | nd | 0.16 | nd |
| Acids | ||||||
| Dodecanoic acid | 1595 | 0.44 | 0.69 | nd | 0.39 | 0.49 |
| Myristic acid | 1767 | 8.13 | 13.02 | 7.29 | 19.38 | 17.48 |
| Pentadecanoic acid | 1857 | 0.95 | 1.29 | 0.64 | 9.82 | 6.48 |
| Oleic acid | 2173 | 1.08 | 1.64 | 1.18 | 3.33 | 3.28 |
| Total identified compounds (%) | 96.44 | 92.76 | 92.56 | 90.43 | 94.39 | |
| Total monoterpene hydrocarbons (%) | 53.51 | 45.32 | 47.49 | 36.63 | 45.35 | |
| Total oxygenated compounds (%) | 41 | 44.75 | 43.10 | 53.13 | 47.4 | |
| Extraction time (min) | 4320 | 10 | 25 | 10 | 25 | |
| β-pinene/(Myristicin + Safrole) | 0.49 | 0.60 | 0.69 | 0.75 | 0.67 | |
| Oleoresin yield (%)c | 9.63 ± 0.01a | 4.55 ± 0.01e | 5.15 ± 0.03d | 8.26 ± 0.01b | 7.98 ± 0.01c | |
nd not detected
aRetention indices using a RTX-5 column and n-alkanes (C9–C22) as references
bExtraction techniques: (1) Maceration, (2) Ultrasound assisted extraction at 20 % of the maximal power for 10 min, (3) Ultrasound assisted extraction at 20 % of the maximal power for 25 min, (4) Ultrasound assisted extraction at 40 % of the maximal power for 10 min, (5) Ultrasound assisted extraction at 40 % of the maximal power for 25 min
cMean values ± SD of triplicates followed by different superscript letters are different at P < 0.05 according to Duncan’s test
The expansion of ultrasonic waves creates cavities in the solvent that collapse near the cell walls and produce high-speed jets of solvent. These jets increased the solvent penetration into the cell, caused an enlargement in the pores of the cell wall, improving the diffusion process and accelerated mass transfer (Vardanega et al. 2014). Yield increased significantly with the extending of UAE time at lower intensity. Extending ultrasound assisted extraction time to 25 min at higher intensity caused a significant decrease in the yield of nutmeg oleoresin. Wang et al. (1997) reported that ultrasonic power was able to degrade compounds for long periods and this effect was related to ultrasound power, stability of compounds and the medium. In addition, Kowalski and Wawrzykowski (2009) as well as Da Porto and Decorti (2009), Pingret et al. (2012) and Pingret et al. (2013) demonstrated that composition of oil distilled from plant material treated with ultrasounds differed from that of oil obtained with the use of the classical methods. This difference may result from the effective liberation of certain chemical constituents from the secretory cells and structural transformations of unstable chemical compounds (degradation, oxidation, isomerization, etc.). This could explain the variation in the percentage of some components in nutmeg oleoresin due to UAE conditions.
Constituents of nutmeg oleoresin
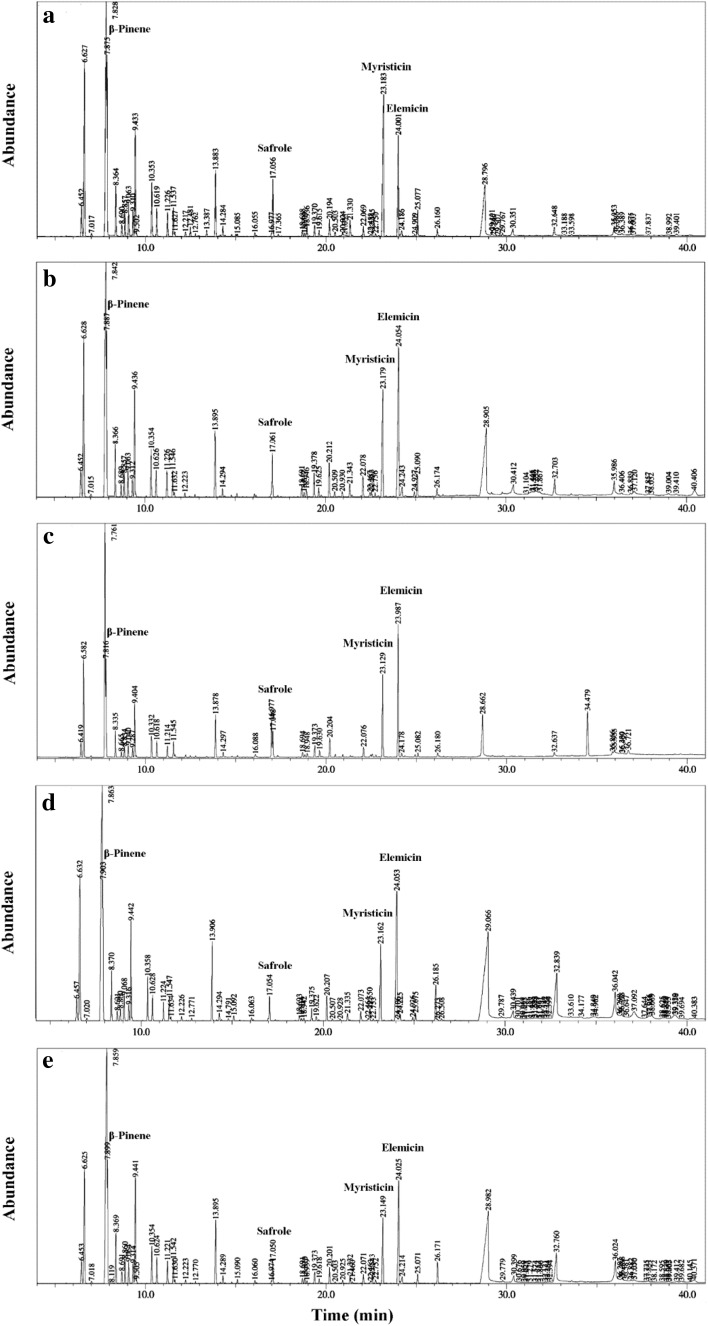
Figure 1a–e illustrates the GC chromatograms of the nutmeg oleoresins obtained by different techniques. Table 1 lists the composition of the obtained nutmeg oleoresins according to the results of GC–MS.
Fig. 1.
GC–MS chromatograms of nutmeg oleoresin extracted by a maceration (technique 1), b ultrasonic assisted extraction at 20 % of the maximal power for 10 min (technique 2), c ultrasonic assisted extraction at 20 % of maximal power for 25 min (technique 3), d ultrasonic assisted extraction at 40 % of the maximal power for 10 min (technique 4), e ultrasonic assisted extraction at 40 % of maximal power for 25 min (technique 5)
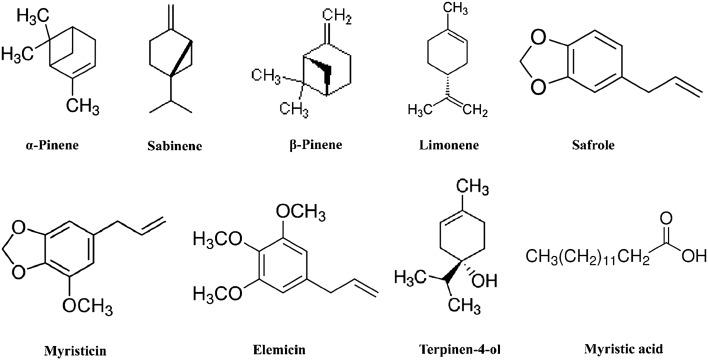
The chemical structure of the major volatile compounds are depicted in Fig. 2.
Fig. 2.
Chemical structure of major compounds of nutmeg oleoresins
GC–MS analysis led to the identification and quantification of 53 different compounds representing >90 % of oleoresins volatile constituents. In total, 15 monoterpene hydrocarbons, 7 sesquiterpene hydrocarbons, 9 aromatics, 7 monoterpene alcohols, one oxide, one sesquiterpene alcohol, 8 esters, one ketone and 4 acids were identified. The monoterpene hydrocarbons showed the highest mean percentage followed in decreasing order by aromatic compounds, acids, monoterpene alcohols and esters in oleoresin samples obtained by maceration or UAE techniques 2 and 3, whereas acids exceeded aromatics in oleoresins obtained by UAE techniques 4 and 5. The main components in nutmeg’s oleoresin were hydrocarbon monoterpenes, monoterpenes acid and aromatic ether (De Guzman and Siemonsma 1999). Oxygenated compounds are highly odoriferous (most valuable) whereas the monoterpene hydrocarbons contribute only little to fragrance (less valuable) (Lucchesi et al. 2004). The highest level of oxygenated compounds was recorded in the oleoresin obtained by UAE technique 4. Sabinene, myristicin, elemicin, α-pinene, β-pinene, limonene, terpinen-4-ol and myristic acid were found as major compounds. This result is in accordance with that found by Ding (2015).
The GC–MS analysis of nutmeg alcoholic extract showed the presence of four main compounds: myristicin, myristic acid, terpinen-4-ol and methoxyeugenol (López et al. 2015).
Sanford and Heinz (1971) concluded that the myristic acid content might serve as an indicator of the age of ground nutmeg. The UAE techniques influenced the chemical composition of nutmeg’s oleoresin. Several new components, such as β-sesquiphellandrene, piperitol isomer and α-ionone were not identified in nutmeg oleoresin obtained by maceration technique.
Increase of UAE intensity from 20 to 40 % of the maximal output power caused: (1) a remarkable increase of acids (myristic acid, pentadecanoic acid and oleic acid) on the account of aromatics, (2) extraction of tetradecanoic acid ethyl ester, trans-sabinene hydrate, cis-ρ-2-menthen-1-ol, endobornyl acetate, trans-α-bergamotene and trans-sabinene hydrate acetate regardless extraction time used and (3) remarkable decrease in myrsiticin, α-pinene, β-pinene and safrole. α-Ionone, citronellyl acetate, guaiol did not stand long time (25 min) of UAE at both investigated intensities. On the other hand, short UAE time (10 min) at lower intensity was not efficient in the extraction of cis-sabinene hydrate acetate and endobornyl acetate. Oleoresin obtained by the high UAE intensity for 25 min was characterized by its content of β-sesquiphellandrene. Its content of 1,8-cineole was similar to that obtained by maceration.
Salzer (1977) recommended the ratio of 0.7 for β-pinene to myristicin and safrole as a characterizing value of nutmeg oil flavor. This ratio reached 0.49 in the maceration oleoresin. Meanwhile, it ranged from 0.60 to 0.69 in the oleoresins obtained by UAE at low intensity and exceeded 0.75 in the oleoresin obtained at high UAE intensity for short time. This indicates that the oleoresins obtained by UAE had the characteristic flavor of nutmeg.
Sensory analysis
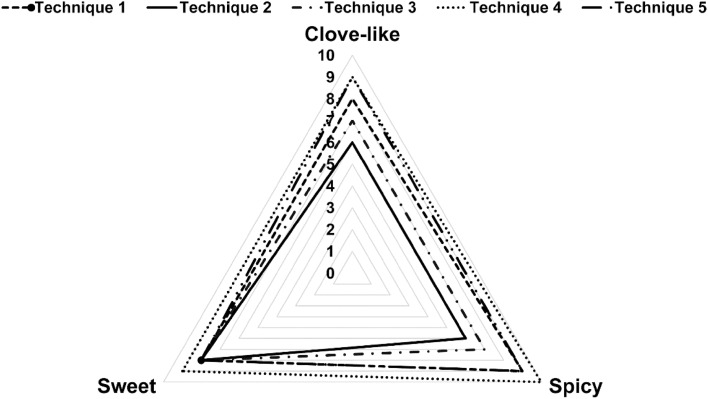
Nutmeg oleoresin has a clove-like, sweet and spicy aroma (Charles 2013). The sensory characteristics of nutmeg oleoresin samples varied depend on the extraction technique. The scores of all investigated features ranged from 6 to 10 on the 10 point scale. Results in Fig. 3 show that nutmeg oleoresin obtained by UAE, technique 4, possess the highest scores. This result is consistent with the chemical composition of the oleoresin as shown in Table 1 and Fig. 1d (shows a high ratio of β-pinene to myristicin and safrole) indicating its superior quality. Oleoresin obtained by technique 5 registered nearly similar acceptance. The highest score of the clove-like attribute was recorded for nutmeg oleoresins obtained by UAE (techniques 4 and 5). The highest score of spicy aroma was noted for nutmeg oleoresins obtained by UAE (techniques 4 followed by 5) and maceration technique. On the other hand, the sweetest aroma was recorded for oleoresin obtained by UAE (techniques 4). The lowest evaluation was determined in the oleoresin sample obtained by technique 2.
Fig. 3.
Flavor profile intensity for nutmeg oleoresin per extraction technique
Conclusions
In this study, conventional maceration and ultrasound-assisted extraction were used to extract the oleoresin from nutmeg seeds. GC/MS results indicate that there were significant differences in the percentage of components detected in the oleoresin extracted by various techniques. UAE showed substantial advantages over conventional maceration. A similar extraction yield was achieved at a shorter time when using UAE at 40 % of the maximal output power for 10 min instead of maceration for 3 days. Oleoresin obtained by this UAE technique characterized by a high ratio of β-pinene to myristicin and safrole and the highest scores of the clove-like attribute, spicy aroma and sweet aroma indicating its superior quality.
References
- Al-Rawi SS, Ibrahim AH, Rahman NNNA, Nama MMB, Abdul Majid AMS, Kadir MOA. The effect of supercritical fluid extraction parameters on the nutmeg oil extraction and its cytotoxic and antiangiogenic properties. Procedia Food Sci. 2011;1:1946–1952. doi: 10.1016/j.profoo.2011.09.286. [DOI] [Google Scholar]
- Charles DJ. Antioxidant Properties of Spices. Frontier Natural Products Co-op Norway, IA, USA: Herbs and Other Sources; 2013. [Google Scholar]
- Chemat F, Rombaut N, Fabiano-Tixier A-S, Pierson JT, Bily A. Green extraction: from concepts to research, education, and economical opportunities. In: Chemat F, Strube J, editors. Green extraction of natural products. Theory and practice. Germany: Wiley; 2015. pp. 1–30. [Google Scholar]
- Chemat F, Rombaut N, Sicaire A-G, Meullemiestre A, Fabiano-Tixier A-S, Abert-Vian M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason Sonochem. 2017;34:540–560. doi: 10.1016/j.ultsonch.2016.06.035. [DOI] [PubMed] [Google Scholar]
- Da Porto C, Decorti D. Ultrasound-assisted extraction coupled with under vacuum distillation of flavour compounds from spearmint (carvone-rich) plants: comparison with conventional hydrodistillation. Ultrason Sonochem. 2009;16:795–799. doi: 10.1016/j.ultsonch.2009.03.010. [DOI] [PubMed] [Google Scholar]
- De Guzman CC, Siemonsma JS. Plant resources of South-East Asia No 13: Spices. Bogor: Indonesia Prosea Fundation; 1999. [Google Scholar]
- Ding P. Myristica fragrans Houtt. (Roudoukou, Nutmeg) In: Liu Y, Wang Z, Zhang J, editors. Dietary Chinese herbs chemistry, pharmacology and clinical evidence. Heidelberg: Springer; 2015. pp. 439–446. [Google Scholar]
- Eiserle RJ, Rogers JA. The composition of volatile oils derived from oleoresins. J Am Oil Chem Soc. 1972;49:573–577. doi: 10.1007/BF02609229. [DOI] [Google Scholar]
- Gupta AD, Bansal VK, Babu V, Maithil N. Chemistry, antioxidant and antimicrobial potential of nutmeg (Myristica fragrans Houtt.) J Genet Eng Biotechnol. 2013;11:25–31. doi: 10.1016/j.jgeb.2012.12.001. [DOI] [Google Scholar]
- Handa SS, Khanuja SPS, Longo G, Rakesh DD. Extraction technologies for medicinal and aromatic plants. Trieste: International Centre for Science and High Technology; 2008. pp. 21–25. [Google Scholar]
- Ji JB, Lu XH, Cai MQ, Xu ZC. Improvement of leaching process of Geniposide with ultrasound. Ultrason Sonochem. 2006;13:455–462. doi: 10.1016/j.ultsonch.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Kowalski R, Wawrzykowski J. Effect of ultrasound-assisted maceration on the quality of oil from the leaves of thyme Thymus vulgaris L. Flavour Fragr J. 2009;24:69–74. doi: 10.1002/ffj.1918. [DOI] [Google Scholar]
- Leela NK. Nutmeg and mace. In: Parthasarathy VA, Chempakam B, Zachariah TJ, editors. Chemistry of spices. Cambridge: CAB International; 2008. pp. 165–189. [Google Scholar]
- Li Y, Fabiano-Tixier A-S, Chemat F. Essential oils as reagents in green chemistry. Heidelberg: Springer; 2014. [Google Scholar]
- Lim TK. Edible medicinal and non-medicinal plants. Volume 3, fruits. Netherlands: Springer; 2012. [Google Scholar]
- López V, Gerique J, Langa E, Berzosa C, Valero MS, Gómez-Rincón C. Antihelmintic effects of nutmeg (Myristica fragans) on Anisakis simplex L3 larvae obtained from Micromesistius potassou. Res Vet Sci. 2015;100:148–152. doi: 10.1016/j.rvsc.2015.03.033. [DOI] [PubMed] [Google Scholar]
- Lucchesi ME, Chemat F, Smadja J. Solvent-free microwave extraction of essential oil from aromatic herbs: comparison with conventional hydro-distillation. J Chromatogr A. 2004;1043:323–327. doi: 10.1016/j.chroma.2004.05.083. [DOI] [PubMed] [Google Scholar]
- Morsy NFS. A short extraction time of high quality hydrodistilled cardamom (Elettaria cardamomum L. Maton) essential oil using ultrasound as a pretreatment. Ind Crop Prod. 2015;65:287–292. doi: 10.1016/j.indcrop.2014.12.012. [DOI] [Google Scholar]
- Moyler DA. Oleoresins, tinctures and extracts. In: Ashurst PR, editor. Food flavourings. Glasgow: Blackie; 1991. [Google Scholar]
- Pandey R, Mahar R, Hasanain M, Shukla SK, Sarkar J, Rameshkumar KB, Kumar B. Rapid screening and quantitative determination of bioactive compounds from fruit extracts of Myristica species and their in vitro antiproliferative activity. Food Chem. 2016;211:483–493. doi: 10.1016/j.foodchem.2016.05.065. [DOI] [PubMed] [Google Scholar]
- Periasamy G, Karim A, Gibrelibanos M, Gebremedhin G, Gilani AUH (2016) Nutmeg (Myristica fragrans Houtt.) oils. In: Preedy VR (ed) Essential oils in food preservation, flavor and safety. Academic Press, Elsevier, London, pp 607–615
- Pingret D, Durand G, Fabiano-Tixier AS, Rockenbauer A, Ginies C, Chemat F. Degradation of edible oil during food processing by ultrasound: electron paramagnetic resonance, physicochemical, and sensory appreciation. J Agric Food Chem. 2012;60:7761–7768. doi: 10.1021/jf301286f. [DOI] [PubMed] [Google Scholar]
- Pingret D, Fabiano-Tixier AS, Chemat F. Degradation during application of ultrasound in food processing: a review. Food Control. 2013;31:593–606. doi: 10.1016/j.foodcont.2012.11.039. [DOI] [Google Scholar]
- Said PP, Arya OP, Pradhan RC, Singh RS, Rai BN. Separation of oleoresin from ginger rhizome powder using green processing technologies. J Food Process Eng. 2015;38:107–114. doi: 10.1111/jfpe.12127. [DOI] [Google Scholar]
- Salzer UJ. The analysis of essential oils and extracts (oleoresins) from seasonings—a critical review. CRC Crit Rev Food Sci Nutr. 1977;9:345–373. doi: 10.1080/10408397709527239. [DOI] [PubMed] [Google Scholar]
- Sanford JK, Heinz DE. Effects of storage on the volatile composition of nutmeg. Phytochem. 1971;10:1245–1250. doi: 10.1016/S0031-9422(00)84325-3. [DOI] [Google Scholar]
- Tajuddin Ahmad S, Latif A, Qasmi IA. Aphrodisiac activity of 50% ethanolic extracts of Myristica fragrans Houtt. (nutmeg) and Syzygium aromaticum (L) Merr. & Perry. (clove) in male mice: a comparative study. BMC Complement Altern Med. 2003;3:6–10. doi: 10.1186/1472-6882-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari BK. Ultrasound: a clean, green extraction technology. Trends Anal Chem. 2015;71:100–109. doi: 10.1016/j.trac.2015.04.013. [DOI] [Google Scholar]
- Vardanega R, Santos DT, De Almeida MA. Intensification of bioactive compounds extraction from medicinal plants using ultrasonic irradiation. Pharmacogn Rev. 2014;8:88–95. doi: 10.4103/0973-7847.134231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YJ, Pan MH, Cheng AL, Lin LI, Ho YS, Hsieh CY, Lin JK. Stability of curcumin in buffer solutions and characterization if its degradation products. J Pharm Biomed Anal. 1997;15:1867–1876. doi: 10.1016/S0731-7085(96)02024-9. [DOI] [PubMed] [Google Scholar]
- Weiss EA. Spice crops. New York: CAB International; 2002. [Google Scholar]