Abstract
Effects of three salting treatments (Formulation II: 80 % NaCl + 20 % KCl; Formulation III: 60 % NaCl + 40 % KCl and Formulation IV: 40 % NaCl + 60 % KCl) on physicochemical properties, residual nitrite, N-nitrosamines and biogenic amines were compared with those of control bacons (Formulation I: 100 % NaCl) during processing and storage. Results showed that there were no significant differences among treatments for moisture, pH, and total volatile basic nitrogen (TVBN) content in dry-cured bacons during processing. The replacement of 40 % or less NaCl by KCl had no negative effects on the sensory quality of bacons during processing. Formulation III significantly reduced putrescine, cadaverine and histamine contents and enhanced nitrite residues compared with the control. After 12-day ripening and during storage, the substitution of NaCl by 60 % KCl significantly increased the N-nitrosodimethylamine (NDMA) content than the control. Principal component analysis showed that there were positive correlations between TVBN, biogenic amines (putrescine, cadaverine, histamine and tyramine) and NDMA, and negative correlation between NDMA and nitrite. These findings suggested the partial substitution of NaCl by KCl could be utilized for producing reduced-sodium dry-cured bacons to improve safety of finished products.
Keywords: Dry-cured bacon, Sodium chloride, Potassium chloride, Physicochemical property, Biogenic amines, Nitrosodimethylamine
Introduction
Sodium chloride (NaCl) is an essential ingredient in processed meat products. It enhances meat water-holding capacity, reduces water activity, and prevents microbes from growth (Martínez-Alvarez and Gómez-Guillén 2013). Moreover, NaCl influences some chemical and biochemical reactions occurred during processing of cured meat products, such as proteolysis, lipolysis and lipid oxidation, and thus contributes to the formation of typical flavors of finished products (Armenteros et al. 2012). However, considerable studies have shown that excessive sodium intake may be responsible for the development of hypertension and cardiovascular diseases in modern society (Doyle and Glass 2010). Therefore, WHO/FAO (2003) recommend that dietary NaCl intake should be restricted to less than 5 g per day for the general public. This has initiated increased interest of the consumers in consuming reduced-sodium products, especially processed meat.
Different strategies have been attempted to reduce the sodium levels in the processed meat products, mainly by adding less salt or replacing NaCl with other salts (i.e. KCl, MgCl2, and CaCl2) (Lorenzo et al. 2015a, b). However, adding less NaCl may cause increased proteolysis due to the intense action of exopeptidase enzymes or increased formation of nitrosamines due to the enhanced activity of microorganisms. Compared with other salt substitutes, KCl is probably the most common one used in reduced-sodium processed meat products due to its similarity in properties to NaCl and no link between its intake and the development of cardiovascular and hypertension (Castro and Raij 2013). Therefore, numerous attempts have been made in recent decades to produce acceptable reduced-sodium meat products by partially replacing NaCl with KCl. For example, substitution of sodium with 50 % KCl had no significant effects on sensory properties of dry-cured loins (Fuentes et al. 2011). Studies from fermented sausages indicate that the substitution of NaCl with 50 % KCl did not affect sausage texture, flavor, and color as well as microbiological characteristics (Gelabert et al. 2003). However, to our knowledge, there are few investigations focusing on effects of partial replacement of NaCl by KCl on the quality of dry-cured bacons.
Biogenic amines (BAs) occur naturally in plants, animal and microorganisms, and in low concentrations, they play important roles in many physiological activities. However, excessive consumption of BAs will expose health risks due to their adverse effects on blood pressure, neurotransmitters, and perception (Kalac and Krausova 2005). In fermented meat products, high concentration of biogenic amines is easily produced by decarboxylation of amino acid by microbes. N-nitrosamines are usually also formed in meat products by reduction of nitrates to nitrites and subsequent reaction with amines from degradation of proteins. A variety of N-nitrosamines have been classified as probable or possible carcinogens by International Agency for Research on Cancer (IARC 1978). However, there is little scientific information about the effect of the partial replacement of NaCl by KCl on biogenic amines and N-nitrosamine contents in dry-cured bacon during ripening and storage.
The purpose of this study was to evaluate the effects of partial substitution of NaCl by KCl on physicochemical properties, with special emphasis on BAs and N-nitrosamines formation in dry-cured bacons during ripening and storage. Furthermore, principal component analysis (PCA) was performed to analyze the possible relationship among these parameters.
Materials and methods
Chemicals and reagents
Standard solution of five volatile nitrosamines including N-nitrosodiethylamine (NDEA), N-nitrosopyrrolidine (NPYR), N-nitrosodimethylamine (NDMA), N-nitrosopiperidine (NPIP) and N-nitrosodibutylamine (NDBA) (2000 μg/mL in dichloromethane) were purchased from Sigma-Aldrich Chemical Co. (Milwaukee, WI, USA). Sodium chloride (NaCl), potassium chloride (KCl) and sodium nitrite were obtained from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Eight BAs standards including putrescine, tryptamine, spermidine, histamine, tyramine, 2-phenylethylamine, cadaverine and spermine were obtained from Sigma (St. Louis, MO, USA). All other chemicals were of analytical grade.
Preparation of bacon samples
Eighty-four green pork belly pieces with an average weight of 1.2 ± 0.3 kg were sampled from postmortem porcine carcasses. Immediately after purchase, all the pieces were randomly divided into four groups (21 pieces each) according to salt formulations used during salting process. Group I was used as the control, which contained 100 % NaCl salt. Other three groups contained different combinations of NaCl and KCl salts (w/w), including 80 % NaCl–20 % KCl (Group II), 60 % NaCl–40 % KCl (Group III), and 40 % NaCl–60 % KCl (Group IV). The amount of salt formulation was 2 % of the total weight of raw bacon (w/w). In addition, sodium nitrite was weighted accurately at a ratio of 150 mg per kilogram raw bacon, and then it Afterwards, the sodium nitrite was mixed with each salt formulation as curing agents. The salting process was initiated by rubbing and kneading the four salt mixtures containing sodium nitrite on the surface of the muscle. Then, the pieces treated with the same salt formulation were piled in a plastic box and stored at 4 °C and 85–90 % relative humidity (RH) for 3 days. Pile-turning treatment was taken every other day to inhibit the formation of the brine during curing. After salting process, all the samples were dry-cured and ripened in a dry-curing room according to the following drying program: the temperature was increased from 13 to 31 °C at 1.5 °C/day, RH was decreased from 85 to 79 % at 0.5 %/day, air speed was 1.5 m/s, and dry-curing lasted for 12 days (Jin et al. 2010). The bacons were then vacuum-packed with polyamide/polyethylene composite film and stored for 3 weeks at 4 °C. In all the treatments, the sampling was carried out at following process points: raw material, end of salting, ripening 6 days, ripening 12 days, storage 1, 2 and 3 weeks. At each sampling point, three bacon pieces from each treatment (three replicates) were randomly sampled, and each index was measured three times (three parallels) for each bacon muscle. Visible connective tissues and subcutaneous fat were removed from bacons, and then the muscles were minced and stored at −20 °C until further analysis.
Determination of physicochemical parameters
The moisture content was assessed by drying the samples at 103 ± 2 °C to constant weight as per AOAC Official Method 925.04 (AOAC 1995). pH values were determined using a SevenEasy S20 pH meter (Mettler Toledo, Zurich, Switzerland). The content of residual sodium nitrite was measured by colorimetric method according to AOAC Official Method 973.31 (AOAC 1995). Total volatile basic nitrogen (TVBN) content was analyzed according to the method described by Roseiro et al. (2010).
Sensory evaluation
Sensory analysis was conducted at the end of ripening stage following the guidance of International Standard Organization (ISO6658 2005). Ten assessors (from both sexes within the age range of 30–45 years) were chosen from the staff members of Department of Food Science and Engineering in Shandong Agricultural University, China. They had received general training to gain consensus on the attributes and the use of the scale prior to the test. Sensory test was performed in separate rooms with controlled temperature, free from noise and odor, with normal white light. Bacons were heated in a microwave oven for 2 min, and then cut into 0.5 cm thick slices. The samples were randomly assigned to each assessor with a three digit number, and tap water was provided to neutralize the palate between samples. Each panelist assessed three replicates of all formulas in a randomized order and was asked to give a score on five-point intensity scale for following attributes: color, aroma, hardness, saltiness, bitterness and overall acceptability where one denotes extremely weak intensity and five denotes extremely high intensity, respectively.
Extraction, purification and analysis of BAs
Biogenic amines content was determined by an Alliance 2690 HPLC system (Waters, Milford, MA) equipped with a Zorbax Eclipse XDB-C18 column (4.6 × 250 mm, 5 µm particle size) (Agilent Technologies, Santa Clara, CA) and a photodiode array detector. BAs were extracted from 5.0 g of the samples with 20 mL of 0.4 M perchloric acid. The mixture was homogenized, centrifuged at 3000×g and 4 °C for 10 min, and filtered twice. The filtrates were collected in a 50 mL volumetric flask and then subjected to derivatization. Briefly, 1 mL of the filtrate or standard solution was mixed with 200 µL of 2 M NaOH, 300 µL of saturated NaHCO3 solutions and 2 mL of dansylchloride solution (10 mg/mL in acetone), and the mixture was reacted for 45 min at 40 °C. Afterwards, 100 µL of 25 % ammonia solution was added to remove the excessive dansylchloride. After filtration with a 0.45 µm nylon membrane filter, a sample of exactly 20 µL aliquot was used for HPLC analysis.
The separation was achieved using a linear gradient of mobile phase A (acetonitrile) and B (H2O) at a flow rate of 1 mL/min. The solvent gradient was as follows: 65–100 % A (0–24 min), 100–65 % A (25–30 min). Detective wavelength was set at 254 nm. Each biogenic amine in the samples was quantified by comparing with a calibration curve generating by analyzing standard BAs solution.
Determination of N-nitrosamines by GC–MS
N-nitrosamines were analyzed by an Agilent 7890A gas chromatograph equipped with a 5975C quadrupole mass spectrometer (Agilent Technologies, Palo Alto, CA, USA). Briefly, N-nitrosamines were extracted by adding 10 mL of 0.1 M NaOH, 20 mL of methanol to 10 g of grated sample in a 50 mL test tube. After sonication for 15 min at 25 °C, the mixture was homogenized and centrifuged at 10,000×g and 4 °C for 10 min. The homogenate was filtered through a glass microfiber filter, and mixed with 5 mL of 20 % NaCl solution prior to purification on a ChemElut column with dichloromethane as eluent. The collected eluent was concentrated under a gentle stream of nitrogen to a final volume of 100 μL.
Separation of N-nitrosamines was achieved using an Agilent DB-5MS (30 m × 0.25 mm, 0.25 μm film thickness) fused-polyethylene glycol capillary column (Agilent Technologies, Palo Alto, CA, USA). The GC oven temperature was maintained at 50 °C for 2 min, and then ramped to 150 °C at 8 °C/min and held for 5 min, to 250 °C at 20 °C/min, and lastly ramped to 280 °C at 20 °C/min. The injection was performed at 230 °C and the electronic pressure constant flow mode of 1 mL/min. Mass spectrometry was performed in the electron-impact mode and 70 eV of ionization energy was used. Ion source temperature was set 230 °C. The analytes were quantitated and qualitated by external calibration and comparing with the retention times and quantifier ion/qualifier ion ratios obtained from N-nitrosamines standard solutions, respectively. Our results showed that only NDMA content in the samples was above the detection limit among five volatile N-nitrosamines. Thus, NDMA content was analyzed in the present study.
Statistical analysis
All statistical analysis was performed using SAS 8.2 statistical software (SAS Institute Inc., Cary, NC). Significant differences were determined by one-way analysis of variance (ANOVA), and a least significant difference (LSD) test was used to compare the mean values for processing time and salting treatments at p < 0.05. The relationship among the variables determined during ripening and storage of dry-cured bacons was evaluated by principal component analysis (PCA) using IBM SPSS Statistics 16.0 software (IBM, Chicago, IL).
Results and discussion
Physicochemical analysis
As shown in Table 1, the moisture content in the bacons decreased gradually (p < 0.05) from 74 % to approximate 42 % during dry-curing processing, and then leveled off during 3-week storage. These moisture values were lower than those previously reported for other dry-cured meat products (Martín et al. 2001). The difference might be related to large specific surface area of bacons due to their smaller size and thickness, which accelerated the dehydration during processing. The decline in moisture content might be attributed to high drying temperature and low relative humidity during dry-curing. There were no significant (p > 0.05) differences between the bacons salted with formulation II, III and IV at the same processing stage. Similar results were reported by Aliño et al. (2009), who did not observe significant differences among salting formulations on moisture content of dry-cured loin. However, formulation IV had significantly (p < 0.05) higher moisture content after 6-day ripening than the control. Our finding is in agreement with previous report where replacement of 70 % NaCl with KCl resulted in higher moisture content in dry-cured bacon as compared with the control (Wu et al. 2014). This might be explained by the fact that the salt mixtures containing KCl has the quicker penetration that would hinder the exit of water from the inside of the meat (Aliño et al. 2009).
Table 1.
Moisture content, pH, and TVBN values during dry-curing and storage of bacons treated with four types of salt formulations
| Index | Treatment | Processing step | ||||||
|---|---|---|---|---|---|---|---|---|
| Raw material | End of salting | Ripening 6 days | Ripening 12 days | Storage 1 week | Storage 2 weeks | Storage 3 weeks | ||
| Moisture (%) | I | 74.07 ± 0.04aA | 71.28 ± 0.79aA | 57.19 ± 1.88bB | 41.63 ± 1.15cA | 41.44 ± 1.66cA | 41.40 ± 3.89cA | 43.60 ± 0.36cA |
| II | 74.09 ± 1.14aA | 73.05 ± 0.76aA | 58.79 ± 1.67bAB | 42.15 ± 3.73cA | 41.42 ± 0.56cA | 41.32 ± 0.02cA | 41.47 ± 1.25cA | |
| III | 73.99 ± 1.44aA | 73.20 ± 0.56aA | 59.24 ± 1.96bAB | 43.03 ± 2.09cA | 43.31 ± 1.04cA | 43.75 ± 1.02cA | 42.31 ± 1.60cA | |
| IV | 74.14 ± 0.37aA | 73.57 ± 0.13aA | 61.67 ± 2.88bA | 45.01 ± 0.18cA | 44.32 ± 2.70cA | 43.40 ± 0.78cA | 43.69 ± 1.02cA | |
| pH | I | 5.73 ± 0.10cA | 5.89 ± 0.08bA | 6.18 ± 0.04aA | 6.23 ± 0.04aA | 6.21 ± 0.05aA | 6.18 ± 0.01aA | 6.14 ± 0.01aA |
| II | 5.70 ± 0.15cA | 5.88 ± 0.12bA | 6.15 ± 0.04aA | 6.19 ± 0.07aA | 6.14 ± 0.09aAB | 6.11 ± 0.05aA | 6.09 ± 0.06aA | |
| III | 5.69 ± 0.04cA | 5.87 ± 0.14bA | 6.14 ± 0.12aA | 6.17 ± 0.05aA | 6.13 ± 0.11aAB | 6.09 ± 0.07aA | 6.07 ± 0.08aA | |
| IV | 5.69 ± 0.05dA | 5.85 ± 0.18cdA | 6.10 ± 0.07aA | 6.13 ± 0.07aA | 6.05 ± 0.02abB | 5.92 ± 0.11bcB | 5.80 ± 0.12cdB | |
| TVBN (mg/kg) | I | 71.2 ± 7.8eA | 123.8 ± 1.6deA | 158.1 ± 10.3cdA | 190.9 ± 1.8cA | 273.5 ± 7.5bA | 317.7 ± 15.6abA | 352.4 ± 1.4aA |
| II | 71.2 ± 0.7eA | 125.2 ± 3.0dA | 153.7 ± 5.7cdA | 190.0 ± 7.1cA | 260.3 ± 8.9bA | 288.7 ± 8.6bA | 344.7 ± 18.1aA | |
| III | 71.5 ± 0.8gA | 123.9 ± 2.4fA | 152.8 ± 3.1eA | 186.4 ± 4.8dA | 258.5 ± 12.3cA | 282.6 ± 10.8bAB | 338.6 ± 1.7aA | |
| IV | 71.8 ± 0.6gA | 123.3 ± 8.1fA | 150.8 ± 4.0eA | 179.3 ± 14.6dA | 225.0 ± 10.8cA | 248.5 ± 4.7bB | 293.1 ± 6.9aB | |
Formulation I: control, 100 % NaCl; Formulation II: 80 % NaCl + 20 % KCl; Formulation III: 60 % NaCl + 40 % KCl; Formulation IV: 40 % NaCl + 60 % KCl
Data are presented as means of triplicates ± standard deviations
Values with no common superscript in the same row (a, b, c…) or in the same column (A, B, C…) are statistically different (p < 0.05)
Bacons treated with different salt formulations showed a similar trend in pH changes during processing and storage, where pH value significantly (p < 0.05) increased from initial 5.69 (raw material) to 6.18 (ripening 6 days), and afterwards remained stable. The change of pH during processing could be related to the accumulation of non-protein nitrogen and amino compounds produced during proteolysis in dry-cured bacons (Flores and Toldrá 2011). No significant differences (p > 0.05) were found with respect to pH among all treatments until 1 week of storage. It is noteworthy that the pH values in the bacons salted with formulation IV were consistently lower (p < 0.05) than the control during storage period. Similarly, Campagnol et al. (2011) found that the fermented cooked sausages with 50 % replacement of NaCl by KCl had significantly lower pH value than the control (100 % NaCl). These results might be attributed to the inhibitory action of NaCl substitution by KCl towards the growth of coliforms, which metabolized basic nitrogen compounds leading to pH changes in dry-cured bacon (Aliño et al. 2010).
TVBN concentration in the raw material was about 71.5 mg/kg, and significantly (p < 0.05) increased during dry-ripening and storage in all treatments. The increase in TVBN contents was associated with the activity of spoilage bacteria and endogenous enzymes. At the end of storage period, the highest TVBN (352.4 ± 1.4 mg/kg) was detected in the control, while the lowest (293.1 ± 6.9 mg/kg) in group IV. Compared to the control, group IV significantly (p < 0.05) delayed the accumulation of TVBN after being stored for 2 weeks. This is possibly owing to the replacement of NaCl by KCl, since the replacement was shown to reduce the quantity of salt-tolerant flora and suppress the growth of coliforms (Aliño et al. 2010). A similar TVBN change was observed in smoked sea bass (Fuentes et al. 2011) and salted black carp fillets when stored at 4 °C for 16 days (Fan et al. 2014). A limit of 450 mg/kg TVBN has been set by the Ministry of Agriculture of the People’s Republic of China for cured meat products. The values obtained in this study were under the upper allowable limit, indicating that any formula tested can be used to process dry-cured bacon without negative effect on quality.
Sensory quality
Sensory scores of the bacons treated with four types of salt formulations are shown in Table 2. There were no significant differences in color and aroma scores between the control and any of other treatments. The dry-cured bacon treated formulation IV received significantly (p < 0.05) lower scores in terms of hardness, saltiness and overall acceptability, and higher bitterness score than other groups, while there were no significant differences between other formulations and the control. Similar reduced hardness was also observed in dry-cured loins with 70 % KCl substitutes (Aliño et al. 2009). Some studies showed that there was a negative relationship between hardness and moisture content of dry-cured meat products (Aliño et al. 2009). During dry-curing of meat product, there is product shrinkage proportional to water loss, which promotes a closer contact between proteins and the formation of new interactions, and finally leads to the increased hardness (Randall and Bratzler 1970). In this study, the reduced hardness in treatment IV might be partly attributed to its high moisture content at the end of ripening. Besides, NaCl reduction in some cured meat products was able to cause an excess of proteolysis because of the intense action of tissue proteases, leading to softness texture (Toldrá 1998). Saltiness is an essential sensory attribute in dry-cured bacon, which is defined as the taste occasioned by NaCl. Due to the fact that the saltiness of other salts is not as pure as that of NaCl (Doyle et al. 2010), the replacement of NaCl with KCl led to a less salty taste in dry-cured bacon. The bitterness was the most notable defect in dry-cured bacon manufactured with 60 % substitutions of NaCl with KCl. Our results are in agreement with those previously reported by other workers (Wu et al. 2014), who found that the maximum level of NaCl that could be substituted in dry-cured bacons without imparting undesirable flavor was 40 %. However, Gelabert et al. (2003) suggested that replacement of up to 50 % of NaCl by KCl in fermented sausages did not affect the flavor attribute. These differences in NaCl replacement level might depend on the type of food product. Taken together, our results suggested that the replacement of 40 % or less NaCl by KCl did not significantly affect the sensory quality of the bacons during processing compared with the control. However, substitution of NaCl with up to 60 % KCl had negative effects on sensory properties and overall acceptability of bacons.
Table 2.
Sensory analysis of bacons treated with four types of salt formulations
| Attributes | Treatment | |||
|---|---|---|---|---|
| I | II | III | IV | |
| Color | 3.12 ± 0.50a | 3.29 ± 0.14a | 3.28 ± 0.34a | 3.33 ± 0.39a |
| Aroma | 3.27 ± 0.20a | 3.24 ± 0.18a | 3.40 ± 0.28a | 3.29 ± 0.23a |
| Hardness | 3.58 ± 0.55a | 3.40 ± 0.23a | 3.52 ± 0.28a | 3.06 ± 0.30b |
| Saltiness | 3.97 ± 0.49a | 3.65 ± 0.33ab | 3.37 ± 0.29b | 2.87 ± 0.57c |
| Bitterness | 1.26 ± 0.52b | 1.44 ± 0.29b | 1.51 ± 0.21b | 4.54 ± 0.25a |
| Overall acceptability | 3.27 ± 0.39ab | 3.23 ± 0.90ab | 3.82 ± 0.31a | 2.81 ± 0.92b |
Formulation I: control, 100 % NaCl; Formulation II: 80 % NaCl + 20 % KCl; Formulation III: 60 % NaCl + 40 % KCl; Formulation IV: 40 % NaCl + 60 % KCl
Data are presented as means of triplicates ± standard deviations
Values with no common superscript in the same row (a, b, c…) are statistically different (p < 0.05)
Residual nitrites
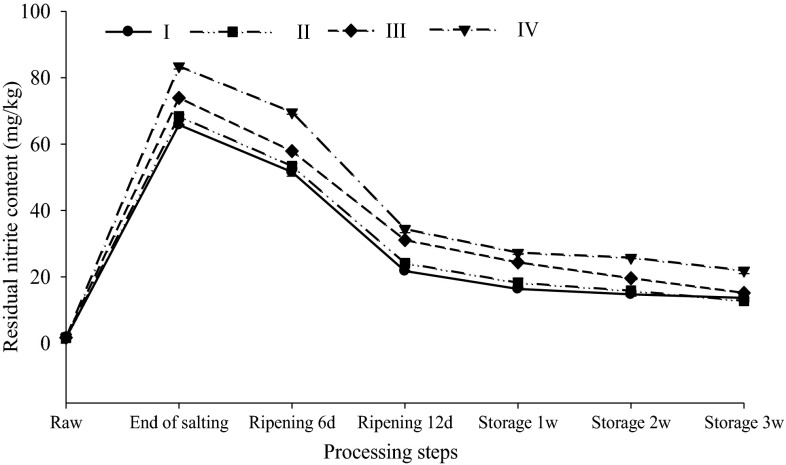
There were significant (p < 0.05) differences in residual nitrites of dry-cured bacon during either dry-ripening or storage regardless of treatment, and also between the KCl treatments (formulations III and IV) and the control (100 % NaCl) at each sampling point (Fig. 1). Formulation IV presented the highest residual nitrite content (83.47 mg/kg) at the end of salting, and the nitrite content was dropped sharply to 34.41 mg/kg after 12-day ripening, and afterwards decreased gradually. This result might be attributed to dynamic transformation of nitrogen compounds in muscle matrix. A similar trend in nitrite change during processing was also reported for dry-cured hams, indicating that nitrite penetration from the surface to the inner muscles was independent of sodium salt during curing, and the penetration variations resulted from the differences in salting formulations (Armenteros et al. 2012). Metal ion K+ penetrated more easily than Na+ in the muscle because potassium has a relative lower charge density (0.026 units of charge/molecular weight) compared to Na+ (0.043 units) (Aliño et al. 2009). In addition, the pH values in the bacons salted with formulation IV were significantly (p < 0.05) lower than the control, which might lead to a quicker penetration of K+ in the muscle. Moreover, when both nitrite and salts diffuse in muscle, there might exist a synergistic effect, so the replacement of Na+ by K+ in curing ingredients enhances the penetration of nitrite in bacon muscle (Bergstrom and Bramer 2008).
Fig. 1.
The residual nitrite contents during processing and storage of bacons treated with four types of salt. Formulation I: control, 100 % NaCl; Formulation II: 80 % NaCl, 20 % KCl; Formulation III: 60 % NaCl, 40 % KCl; Formulation IV: 40 % NaCl, 60 % KCl. Error bars indicate standard deviations (n = 3)
Another interesting result is that nitrite residues in KCl-treated samples were much higher (p < 0.05) than those in the control (100 % NaCl) throughout the storage. Salt formulations could affect the microbial communities and proteinase activities during the salting process of dry-cured ham (Blesa et al. 2008). These microbes and enzymes, which naturally occur in the tissue or are released by various microorganisms, can affect the dynamic transformation of nitrogen compounds and consequently contribute to the significant differences in nitrite content among different salting formulations. But other studies show that the diffusion and penetration of nitrite are independent of the formulations of chloride salts used, suggesting that nitrite depletion in processed meat can be affected by many factors, such as addition amount of nitrite, processing temperatures, raw meat type, and the presence of reductants (Honikel 2008).
BAs contents
At the end of the experiment, tyramine was the predominant biogenic amine, followed by cadaverine, putrescine, spermidine, histamine, and spermine (Table 3). Formulation IV caused a considerable delay in the formation of tyramine during dry-curing and storage of bacons, showing a significant reduction of 24.97 % after 12-day ripening compared to the control, while no significant differences were detected between other formulations and the control after 12-day ripening. These results indicated that the variation of tyramine content during processing was dependent on the type of salt used, with the replacement of 40 % or less NaCl by KCl having no influence on tyramine content. After storage for 3 weeks, the tyramine contents increased and reached 59.50 and 44.43 mg/kg in control and group IV, respectively. However, no significant difference was observed between the control and group IV throughout the storage period. In general, the tyramine level allowed in foods is 100–800 mg/kg, while 1080 mg/kg will lead to toxic effect (Latorre-Moratalla et al. 2008). Herein, the levels of tyramine determined in all groups were below the threshold value.
Table 3.
Biogenic amines contents during dry-curing and storage of bacons treated with four types of salt formulations
| Biogenic amine (mg/kg) | Treatment | Processing step | ||||||
|---|---|---|---|---|---|---|---|---|
| Raw material | End of salting | Ripening 6 days | Ripening 12 days | Storage 1 week | Storage 2 weeks | Storage 3 weeks | ||
| Putrescine | I | 1.47 ± 0.16eA | 3.05 ± 0.15eA | 12.92 ± 1.36dA | 14.55 ± 1.52dA | 20.03 ± 1.87cA | 28.09 ± 2.96bA | 31.58 ± 1.21aA |
| II | 1.49 ± 0.26gA | 3.04 ± 0.70fA | 12.87 ± 0.84eA | 14.32 ± 0.64dA | 19.06 ± 0.20cA | 27.30 ± 1.40bA | 30.37 ± 0.77aA | |
| III | 1.47 ± 0.14eA | 2.76 ± 1.16eB | 10.79 ± 0.80 dB | 12.03 ± 0.62 dB | 15.48 ± 1.60cB | 19.06 ± 0.06bB | 23.29 ± 1.72aB | |
| IV | 1.45 ± 0.26fA | 2.40 ± 0.83fC | 9.21 ± 0.95eB | 10.97 ± 2.80 dB | 13.02 ± 1.13cB | 18.98 ± 0.82bB | 21.17 ± 1.71aB | |
| Cadaverine | I | nd | 8.46 ± 1.09eA | 10.62 ± 2.47deA | 12.79 ± 1.77dA | 22.65 ± 1.14cA | 48.12 ± 1.28bA | 56.85 ± 2.70aA |
| II | nd | 7.83 ± 1.00dA | 8.56 ± 1.56dAB | 11.49 ± 3.49dAB | 21.57 ± 6.12cA | 45.00 ± 4.23bA | 53.56 ± 2.67aA | |
| III | nd | 6.73 ± 1.98bA | 7.14 ± 0.70bB | 9.28 ± 2.49bAB | 19.45 ± 1.89abA | 32.22 ± 3.69abA | 44.31 ± 6.21aB | |
| IV | nd | 5.78 ± 1.43cA | 6.24 ± 0.59cB | 8.00 ± 1.88cB | 14.95 ± 1.34bcA | 24.65 ± 6.29abA | 35.81 ± 3.46aB | |
| Histamine | I | nd | 1.27 ± 0.54cA | 4.64 ± 1.24bA | 15.65 ± 2.10aA | 16.90 ± 1.86aA | 16.22 ± 1.82aA | 17.20 ± 0.99aA |
| II | nd | nd | 2.61 ± 0.91cA | 10.46 ± 1.64bB | 13.67 ± 1.51abB | 13.76 ± 2.35abAB | 14.07 ± 2.80aA | |
| III | nd | nd | nd | 7.67 ± 0.47aBC | 8.77 ± 1.63aC | 8.82 ± 1.52aBC | 8.63 ± 2.77aB | |
| IV | nd | nd | nd | 5.59 ± 0.65aC | 5.93 ± 1.01aC | 6.19 ± 1.51aC | 6.39 ± 1.95aB | |
| Tyramine | I | nd | 6.29 ± 0.62dA | 14.72 ± 3.70cdA | 18.22 ± 2.82cdA | 33.05 ± 3.27bcA | 45.61 ± 2.25abA | 59.50 ± 5.34aA |
| II | nd | 6.26 ± 0.64eA | 14.65 ± 4.01deA | 17.63 ± 0.41dAB | 32.97 ± 5.52cA | 45.06 ± 8.96bA | 58.78 ± 3.04aA | |
| III | nd | 5.77 ± 0.52cA | 12.91 ± 3.30cA | 14.30 ± 2.15cAB | 27.79 ± 3.11bA | 34.92 ± 2.40bA | 50.58 ± 1.51aA | |
| IV | nd | 5.76 ± 0.92eA | 11.95 ± 1.48dA | 13.67 ± 2.77 dB | 26.42 ± 5.37cA | 34.13 ± 2.88bA | 44.43 ± 4.75aA | |
| Spermine | I | 5.39 ± 0.73cA | 6.52 ± 1.30bcA | 9.38 ± 1.32abcA | 12.14 ± 3.27aA | 11.99 ± 2.00aA | 10.77 ± 1.69abA | 10.54 ± 2.17abcA |
| II | 5.53 ± 0.72dA | 6.37 ± 0.18cdA | 6.93 ± 1.20bcdA | 9.78 ± 2.05aA | 9.29 ± 1.51aA | 8.36 ± 0.49abA | 8.27 ± 0.38abcA | |
| III | 5.56 ± 0.54dA | 7.19 ± 0.44cdA | 7.79 ± 1.32bcA | 10.25 ± 1.60aA | 9.09 ± 1.17abA | 8.72 ± 0.23abcA | 8.70 ± 0.23abcA | |
| IV | 5.38 ± 0.70dA | 6.56 ± 1.09cdA | 7.52 ± 0.20bcA | 10.45 ± 0.34aA | 9.87 ± 1.59aA | 9.07 ± 1.20abA | 8.82 ± 0.88abA | |
| Spermidine | I | 25.32 ± 2.37aA | 26.79 ± 1.27aA | 25.66 ± 1.12aA | 25.02 ± 3.75aA | 23.22 ± 0.20aA | 22.31 ± 1.41aA | 20.83 ± 3.68aA |
| II | 24.00 ± 1.19aA | 24.47 ± 0.66aA | 25.83 ± 5.22aA | 23.29 ± 1.36abA | 22.08 ± 2.69abA | 21.48 ± 2.16abA | 19.03 ± 1.93bA | |
| III | 24.43 ± 1.76aA | 23.14 ± 0.81aA | 23.46 ± 1.39aA | 21.49 ± 1.23aA | 20.49 ± 2.03aA | 20.20 ± 3.21aA | 19.87 ± 2.70aA | |
| IV | 24.43 ± 0.56abA | 23.01 ± 1.45bcA | 26.88 ± 2.65aA | 24.74 ± 0.84abA | 22.95 ± 0.94bcA | 22.76 ± 0.61bcA | 20.75 ± 3.43cA | |
Formulation I: control, 100 % NaCl; Formulation II: 80 % NaCl + 20 % KCl; Formulation III: 60 % NaCl + 40 % KCl; Formulation IV: 40 % NaCl + 60 % KCl
Data are presented as means of triplicates ± standard deviations
Values with no common superscript in the same row (a, b, c…) or in the same column (A, B, C…) are statistically different (p < 0.05)
nd Not detected
Histamine levels were significantly affected by the salt mixture and dry-cured processing (p < 0.05). At the end of ripening, their values reached 15.65, 10.46, 7.67, and 5.59 mg/kg in the bacons salted with formulation I, II, III and IV, respectively. It is noteworthy that treatment IV presented the lowest histamine levels among treatments, suggesting that the partial replacement of NaCl by KCl had an inhibitory effect on histamine formation. Similar reduced histamine content was also detected in smoked sea bass treated with the salt mixture as compared to the individual salt (Fuentes et al. 2010). To our knowledge, there is limited information available regarding the effect of different types of salts on histamine formation in dry-cured bacons. However, it is evidenced that the levels of histamine-forming bacteria can vary depending on the raw material, processing, handling, salt content, or storage conditions (Fuentes et al. 2011). Herein, we speculated that the lower histamine content in KCl-treated samples might be attributed to the reduced aw value by the substitution (Fuentes et al. 2010), since available water is a critical factor affecting microbial growth in foods.
The raw material did not detect cadaverine. During ripening, cadaverine contents increased gradually to 12.79, 11.49, 9.28 and 8.00 mg/kg and reached 56.85, 53.56, 44.31 and 35.81 mg/kg after 3 weeks of storage for the control and treatment II, III and IV, respectively. The levels of cadaverine in processed meat were significantly affected by salt types, and they were generally lower in bacons with sodium replacement. The formation of cadaverine is usually related to the activity of decarboxylase-positive contaminant microbiota, which might be restrained by the replacement of NaCl with KCl during dry-curing processing.
Putrescine content in the dry-cured bacons increased gradually during processing and storage. The control bacon presented the highest putrescine concentration, while treatments IV and III had the lowest level of putrescine, suggesting they were more effective in inhibiting accumulation of putrescine than other treatments. Similar putrescine evolution has been observed in other studies (Fuentes et al. 2011).
The evolution of spermine and spermidine showed fluctuated throughout the experiment. A slight decrease in both amines was found in all bacons during storage at 4 °C. The lack of increase (even a slight reduction during storage) in spermine and spermidine contents may be attributed to that these amines naturally occur in food, and their formation is not related to bacterial spoilage (Fan et al. 2014). No significant differences on spermine and spermidine contents were observed among treatments, and their contents were within the range reported for dry-fermented ripened sausages (Fuentes et al. 2011).
BAs can cause a range of toxicological effects on human health when ingested in high concentrations, such as headaches, nausea, migraine, respiratory distress, gastric and intestinal problems, and pseudoallergic responses, mainly due to the toxic action of histamine and tyramine (Kalac and Krausova 2005). Moreover, histamine toxicity seems to be enhanced by the presence of other amines (putrescine, cadaverine and tyramine), which act as potentiators of its toxicity (Tofalo et al. 2016). Polyamines such as putrescine, cadaverine, spermine and spermidine have no adverse effects on health by themselves, but when subjected to heat, they can give rise to the formation of secondary amines, and these subsequently react with nitrite to form carcinogenic nitrosamines (Kalac and Krausova 2005). In meat products, biogenic amines are mainly produced from decarboxylation of amino acid by microbes that have amino acid decarboxylases. Inhibition of the biogenic amines by partial replacement of NaCl with KCl varied widely, with formulation III and IV being more effective in delaying the formation of histamine, putrescine, and cadaverine than other treatments. However, some authors did not observe significant influences of NaCl partial replacement by KCl on the microbial load of various pork meat products (Aliño et al. 2010; Blesa et al. 2008). Therefore, it appeared that the effect of NaCl substitution on BAs accumulation was not due to their inhibition towards BAs-forming bacteria, but related to their enhancing penetration of nitrite in bacon muscle (Bergstrom and Bramer 2008).
N-nitrosodimethylamine (NDMA) content
As shown in Table 4, regardless of treatment, the NDMA level gradually increased during the experiment, and the highest NDMA content was observed in treatment IV and the lowest in the control. After storage for 3 weeks, their concentrations reached 2.34, 2.47, 2.60 and 2.97 μg/kg in dry-cured bacons salted with formulation I, II, III, and IV, respectively. Our results are in agreement with previous reports from Yurchenko and Mölder (2007), who observed an increased NDMA in Estonian meat products during storage at 5 °C. It was reported that the accumulation of NDMA during storage was associated with chemical reactions between precursors of NDMA present or formed in meat products (Drabik-Markiewicz et al. 2011). Regardless of KCl content and storage time, presence of KCl resulted in higher NDMA contents in dry-cured bacons than those treated with NaCl alone.
Table 4.
NDMA content during dry-curing and storage of bacons treated with four types of salt formulations
| Processing step | NDMA content (μg/kg) | |||
|---|---|---|---|---|
| I | II | III | IV | |
| Raw material | nd | nd | nd | nd |
| End of salting | nd | nd | nd | nd |
| Ripening 6 days | 0.50 ± 0.03dA | 0.50 ± 0.07dA | 0.61 ± 0.10dA | 0.68 ± 0.13dA |
| Ripening 12 days | 0.61 ± 0.08dB | 0.61 ± 0.06dB | 0.76 ± 0.12dAB | 0.84 ± 0.05dA |
| Storage 1 week | 1.19 ± 0.10cB | 1.21 ± 0.18cAB | 1.27 ± 0.06cAB | 1.42 ± 0.10cA |
| Storage 2 weeks | 1.54 ± 0.06bB | 1.56 ± 0.19bB | 1.81 ± 0.13bAB | 2.18 ± 0.19bA |
| Storage 3 weeks | 2.34 ± 0.05aB | 2.47 ± 0.26aB | 2.60 ± 0.34aAB | 2.97 ± 0.10aA |
Formulation I: control, 100 % NaCl; Formulation II: 80 % NaCl + 20 % KCl; Formulation III: 60 % NaCl + 40 % KCl; Formulation IV: 40 % NaCl + 60 % KCl
Data are presented as means of triplicates ± standard deviations
Values with no common superscript in the same column (a, b, c…) or in the same row (A, B, C…) are statistically different (p < 0.05)
nd Not detected
It is well-known that N-nitrosamines have been classified as probable or possible carcinogens (IARC 1978). During nitrosamine metabolism, several intermediates are formed, that may be directly bind with DNA, or react with another compound to form alkylating agents that may cause mutation and cancer (Veena and Rashmi 2014). The NDMA contents in bacon salted with formulation IV was significantly (p < 0.05) higher than the control regardless of sampling point except for day 6 of ripening. To our knowledge, there are no published studies on the effect of salt types on NDMA formation in meat products, while there were many reports on the effect of nitrite on the formation of N-nitrosamine. Nitrite is the main nitrosation agent and directly leads to the formation of NDMA in dry-cured meat. As analyzed above, adding KCl enhanced the diffusion of nitrite and effectively maintained the nitrite content in cured meat. Therefore, it can be deduced that the NDMA level would have been higher in the bacon treated with NaCl/KCl mixtures than those treated with 100 % NaCl. However, it has been concluded that in dry fermented sausages, the residual nitrite levels were not high enough to pose a risk in N-nitrosamine formation regardless of variation (De Mey et al. 2013).
Principal component analysis (PCA)
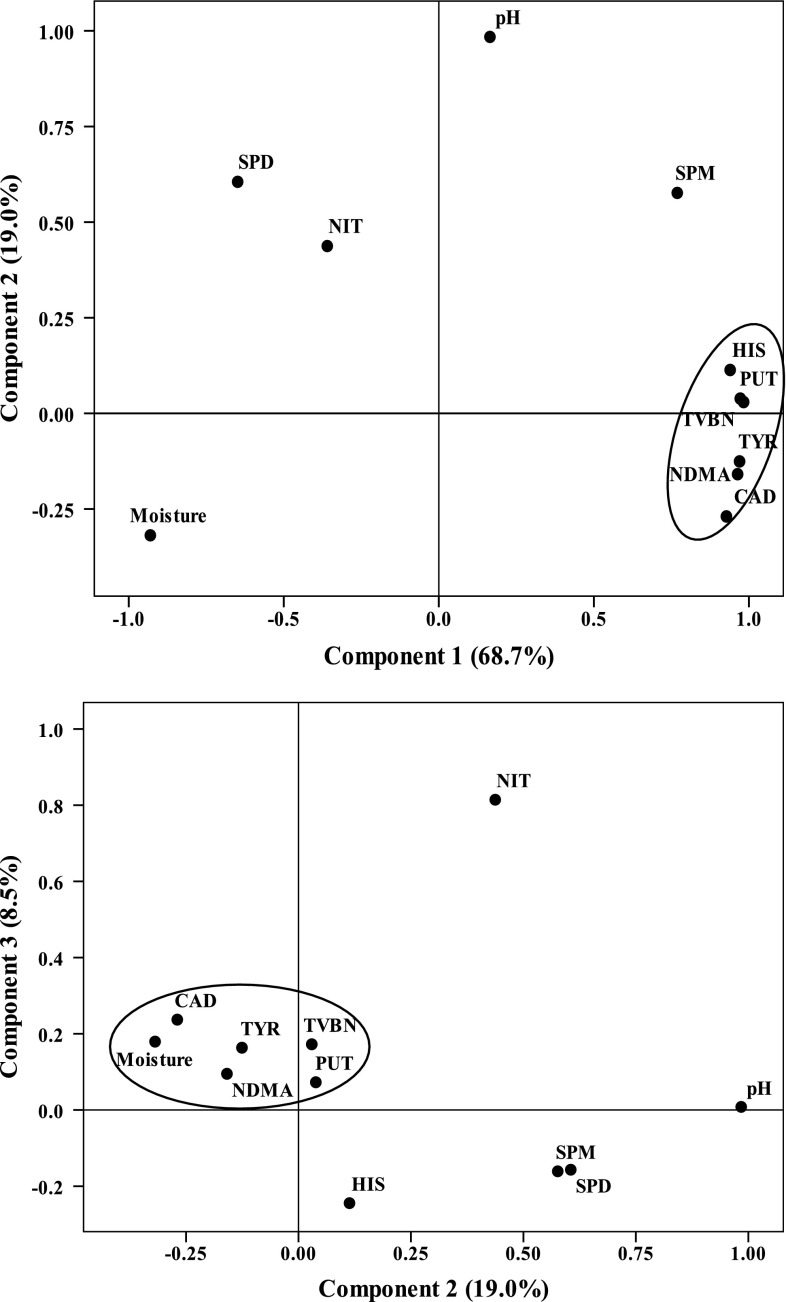
Principal component analysis was performed to analyze the relationship among the physicochemical parameters, TVBN, NDMA, residual nitrite and biogenic amines contents of processed bacons during ripening and storage. The first three principal components (PCs) that collectively explained 96.2 % of total variances were extracted for regression. The loadings of different variables on the scatter plots of PC1–PC2 and PC2–PC3 were presented in Fig. 2.
Fig. 2.
PCA loading plots in the plane of a PC1–PC2, and b PC2–PC3. PUT Putrescine, CAD cadaverine, HIS histamine, TYR tyramine, SPM spermine, SPD spermidine, NDMA N-nitrosodimethylamine, NIT sodium nitrite
PC1 explained 68.7 % of total variance, which was characterized by moisture, TVBN, BAs (histamine, putrescine, cadaverine, and tyramine and histamine) and NDMA content. These variables were placed in the loading biplot far from the origin of PC1. PC2 explained 19.0 % of total variance, in which pH value was the predominant factor. PC3 explained the 8.5 % of total variance, and sodium nitrite content was the most important in defining PC3.
As shown in Fig. 2a, TVBN, BAs and NDMA contents were clustered in the same group in PC1, suggesting that both TVBN and BAs were positively correlated with NDMA contents during ripening and storage of bacons. The correlation coefficients between NDMA and BAs content were 0.977 (putrescine), 0.978 (cadaverine), 0.990 (tyramine) and 0.825 (histamine), respectively. It is evidenced that some BAs result in the accumulation of NDMA when nitrite is present, due to their roles as precursors of carcinogenic N-nitrosamines. For example, both putrescine and cadaverine were able to lead to the formation of NDMA when exposed to certain microorganisms and enzymes (Rywotycki 2007). TVBN was positively correlated with NDMA level (0.964), as well as with the BAs contents, including putrescine (0.973), cadaverine (0.946), tyramine (0.984) and histamine (0.877). This could be explained by the fact that, on the one hand, the increase of TVBN content in dry-cured bacons usually depended on the activity of spoilage bacteria. On the other hand, these bacteria with higher amino acid decarboxylases activity will be conducive to the formation of biogenic amine, and subsequently the accumulation of more NDMA in fermented meats. The moisture content was located far in the PC1, opposite to the NDMA and BAs, indicating that they were negatively correlated. This might be explained considering the relationship between water activity inside the dry-cured bacons and enzymes activity of some spoilage microorganisms (Toldrá 2006).
The sodium nitrite content was placed nearly 180° from the NDMA in the loading plots (Fig. 2b), indicating the negative correlation between them (−0.362). It is reported that the N-nitrosamines can be formed by reacting of biogenic amines with the nitrosating agent NO+, which was from the conversion of nitrite under low pH condition (Honikel 2008). In addition, sodium nitrite content was negatively correlated with putrescine (0.972), cadaverine (0.928) and histamine (0.940) since they were located far in the different quadrants. This suggests that sodium nitrite may have an inhibitory effect on the accumulation of biogenic amines in dry-cured bacons. Similar results were reported by De Mey et al. (2013), who found that addition of sodium nitrite during processing of fermented sausages decreased the contents of cadaverine and putrescine. However, the addition of sodium nitrite had a promoting effect on the formation of N-nitrosamine.
Conclusion
Partial replacement of NaCl by KCl did not markedly influence moisture, pH and TVBN content compared with the control in dry-cured bacon during processing, but had significant effects on residual nitrite, BAs and N-nitrosamine contents. The replacement of 40 % or less NaCl by KCl had no negative effects on the sensory quality of the bacons during processing. The replacement of 40 % NaCl by KCl effectively inhibited putrescine, cadaverine, and histamine formation and enhanced nitrite residues in comparison with the control (p < 0.05) during processing. After 12-day ripening and during storage, the substitution of 60 % NaCl by KCl significantly increased the NDMA content. PCA showed that both NDMA and BAs contents (putrescine, cadaverine, histamine, and tyramine) positively correlated with TVBN, but negatively correlated with sodium nitrite contents. These findings suggest that the partial substitution of NaCl by KCl may produce healthier dry-cured bacon without negative effects on quality.
Acknowledgments
This study was supported by the National Key Technology R&D Program in the 12th Five Year Plan of China (2012BAD28B01) and Science and Technology Foundation in Higher Education of Shandong Province (J16LE18).
References
- Aliño M, Grau R, Toldrá F, Blesa E, Pagán MJ, Barat JM. Influence of sodium replacement on physicochemical properties of dry-cured loin. Meat Sci. 2009;83:423–430. doi: 10.1016/j.meatsci.2009.06.022. [DOI] [PubMed] [Google Scholar]
- Aliño M, Grau R, Toldrá F, Blesa E, Pagán MJ, Barat JM. Physicochemical properties and microbiology of dry-cured loins obtained by partial sodium replacement with potassium, calcium and magnesium. Meat Sci. 2010;85:580–588. doi: 10.1016/j.meatsci.2010.03.009. [DOI] [PubMed] [Google Scholar]
- AOAC . Official methods of analysis of AOAC international. 16. Arlington: Association of Official Analytical Chemists; 1995. [Google Scholar]
- Armenteros M, Aristoy MC, Toldrá F. Evolution of nitrate and nitrite during the processing of dry-cured ham with partial replacement of NaCl by other chloride salts. Meat Sci. 2012;91:378–381. doi: 10.1016/j.meatsci.2012.02.017. [DOI] [PubMed] [Google Scholar]
- Bergstrom LM, Bramer T. Synergistic effects in mixtures of oppositely charged surfactants as calculated from the Poisson-Boltzmann theory: a comparison between theoretical predictions and experiments. J Colloid Interf Sci. 2008;322:589–595. doi: 10.1016/j.jcis.2008.02.065. [DOI] [PubMed] [Google Scholar]
- Blesa E, Alino M, Barat JM, Grau R, Toldra F, Pagan MJ. Microbiology and physicochemical changes of dry-cured ham during the post-salting stage as affected by partial replacement of NaCl by other salts. Meat Sci. 2008;78:135–142. doi: 10.1016/j.meatsci.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Campagnol PC, dos Santos BA, Morgano MA, Terra NN, Pollonio MA. Application of lysine, taurine, disodium inosinate and disodium guanylate in fermented cooked sausages with 50 % replacement of NaCl by KCl. Meat Sci. 2011;87:239–243. doi: 10.1016/j.meatsci.2010.10.018. [DOI] [PubMed] [Google Scholar]
- Castro H, Raij L. Potassium in hypertension and cardiovascular disease. Semin Nephrol. 2013;33:277–289. doi: 10.1016/j.semnephrol.2013.04.008. [DOI] [PubMed] [Google Scholar]
- De Mey E, De Maere H, Goemaere O, Steen L, Peeters MC, Derdelinckx G, Paelinck H, Fraeye I. Evaluation of N-nitrosopiperidine formation from biogenic amines during the production of dry fermented sausages. Food Bioprocess Technol. 2013;7:1269–1280. doi: 10.1007/s11947-013-1125-5. [DOI] [Google Scholar]
- Doyle ME, Glass KA. Sodium reduction and its effect on food safety, food quality, and human health. Compr Rev Food Sci Food Saf. 2010;9:44–56. doi: 10.1111/j.1541-4337.2009.00096.x. [DOI] [PubMed] [Google Scholar]
- Drabik-Markiewicz G, Dejaegher B, De Mey E, Kowalska T, Paelinck H, Vander Heyden Y. Influence of putrescine, cadaverine, spermidine or spermine on the formation of N-nitrosamine in heated cured pork meat. Food Chem. 2011;126:1539–1545. doi: 10.1016/j.foodchem.2010.11.149. [DOI] [PubMed] [Google Scholar]
- Fan H, Luo Y, Yin X, Bao Y, Feng L. Biogenic amine and quality changes in lightly salt- and sugar-salted black carp (Mylopharyngodon piceus) fillets stored at 4 & #xB0;C. Food Chem. 2014;159:20–28. doi: 10.1016/j.foodchem.2014.02.158. [DOI] [PubMed] [Google Scholar]
- Flores M, Toldrá F. Microbial enzymatic activities for improved fermented meats. Trends Food Sci Technol. 2011;22:81–90. doi: 10.1016/j.tifs.2010.09.007. [DOI] [Google Scholar]
- Fuentes A, Fernández-Segovia I, Serra JA, Barat JM. Development of a smoked sea bass product with partial sodium replacement. LWT—Food Sci Technol. 2010;43:1426–1433. doi: 10.1177/1082013211415156. [DOI] [PubMed] [Google Scholar]
- Fuentes A, Fernández-Segovia I, Barat JM, Serra JA. Influence of sodium replacement and packaging on quality and shelf life of smoked sea bass (Dicentrarchus labrax L.) LWT—Food Sci Technol. 2011;44:917–923. [Google Scholar]
- Gelabert J, Gou P, Guerrero L, Arnau J. Effect of sodium chloride replacement on some characteristics of fermented sausages. Meat Sci. 2003;65:833–839. doi: 10.1016/S0309-1740(02)00288-7. [DOI] [PubMed] [Google Scholar]
- Honikel KO. The use and control of nitrate and nitrite for the processing of meat products. Meat Sci. 2008;78:68–76. doi: 10.1016/j.meatsci.2007.05.030. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer . Some N-nitroso compounds. In: IARC, editor. IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans. Lyon: IARC; 1978. pp. 257–327. [Google Scholar]
- ISO . Sensory analysis-methodology-general guidance. Geneva: International Organization for Standardization; 2005. [Google Scholar]
- Jin G, Zhang J, Yu X, Zhang Y, Lei Y, Wang J. Lipolysis and lipid oxidation in bacon during curing and drying-ripening. Food Chem. 2010;123:465–471. doi: 10.1016/j.foodchem.2010.05.031. [DOI] [Google Scholar]
- Kalac P, Krausova A. A review of dietary polyamines: formation, implications for growth and health and occurrence in foods. Food Chem. 2005;90:219–230. doi: 10.1016/j.foodchem.2004.03.044. [DOI] [Google Scholar]
- Latorre-Moratalla ML, Veciana-Nogués T, Bover-Cid S, Garriga M, Aymerich T, Zanardi E, Ianieri A, Fraqueza MJ, Patarata L, Drosinos EH, Lauková A, Talon R, Vidal-Carou MC. Biogenic amines in traditional fermented sausages produced in selected European countries. Food Chem. 2008;107:912–921. doi: 10.1016/j.foodchem.2007.08.046. [DOI] [Google Scholar]
- Lorenzo JM, Bermúdez R, Domínguez R, Guiotto A, Franco D, Purriños L. Physicochemical and microbial changes during the manufacturing process of dry-cured lacón salted with potassium, calcium and magnesium chloride as a partial replacement for sodium chloride. Food Control. 2015;50:763–769. doi: 10.1016/j.foodcont.2014.10.019. [DOI] [Google Scholar]
- Lorenzo JM, Cittadini A, Bermúdez R, Munekata PE, Domínguez R. Influence of partial replacement of NaCl with KCl, CaCl2 and MgCl2 on proteolysis, lipolysis and sensory properties during the manufacture of dry-cured lacón. Food Control. 2015;55:90–96. doi: 10.1016/j.foodcont.2015.02.035. [DOI] [Google Scholar]
- Martín L, Antequera T, Ventanas J, Benítez-Donoso R, Córdoba JJ. Free amino acids and other non-volatile compounds formed during processing of Iberian ham. Meat Sci. 2001;59:363–368. doi: 10.1016/S0309-1740(01)00088-2. [DOI] [PubMed] [Google Scholar]
- Martínez-Alvarez O, Gómez-Guillén C. Influence of mono- and divalent salts on water loss and properties of dry salted cod fillets. LWT—Food Sci Technol. 2013;53:387–394. [Google Scholar]
- Randall C, Bratzler L. Changes in various protein properties of pork muscle during smoking process. J Food Sci. 1970;45:248–249. doi: 10.1111/j.1365-2621.1970.tb12149.x. [DOI] [Google Scholar]
- Roseiro LC, Gomes A, Goncalves H, Sol M, Cercas R, Santos C. Effect of processing on proteolysis and biogenic amines formation in a Portuguese traditional dry-fermented ripened sausage “Chourico Grosso de Estremoz e Borba PGI”. Meat Sci. 2010;84:172–179. doi: 10.1016/j.meatsci.2009.08.044. [DOI] [PubMed] [Google Scholar]
- Rywotycki R. The effect of baking of various kinds of raw meat from different animal species and meat with functional additives on nitrosamine contamination level. Food Chem. 2007;101:540–548. doi: 10.1016/j.foodchem.2006.02.012. [DOI] [Google Scholar]
- Tofalo R, Perpetuini G, Schirone M, Suzzi G. Biogenic amines: toxicology and health effect. In: Caballero B, Finglas PM, Toldrá F, editors. Encyclopedia of food and health. Oxford: Oxford University Press; 2016. pp. 424–429. [Google Scholar]
- Toldrá F. Proteolysis and lipolysis in flavour development of dry-cured meat products. Meat Sci. 1998;49:101–110. doi: 10.1016/S0309-1740(98)90041-9. [DOI] [PubMed] [Google Scholar]
- Toldrá F. The role of muscle enzymes in dry-cured meat products with different drying conditions. Trends Food Sci Technol. 2006;17:164–168. doi: 10.1016/j.tifs.2005.08.007. [DOI] [Google Scholar]
- Veena S, Rashmi S. A review on mechanism of nitrosamine formation, metabolism and toxicity in in vivo. Int J Toxicol Pharmacol Res. 2014;6:86–96. [Google Scholar]
- WHO/FAO (2003) Diet, nutrition and the prevention of chronic diseases. WHO technical report series 916. World Health Organization, Geneva [PubMed]
- Wu H, Zhang Y, Long M, Tang J, Yu X, Wang J, Zhang J. Proteolysis and sensory properties of dry-cured bacon as affected by the partial substitution of sodium chloride with potassium chloride. Meat Sci. 2014;96:1325–1331. doi: 10.1016/j.meatsci.2013.10.037. [DOI] [PubMed] [Google Scholar]
- Yurchenko S, Mölder U. The occurrence of volatile N-nitrosamines in Estonian meat products. Food Chem. 2007;100:1713–1721. doi: 10.1016/j.foodchem.2005.10.017. [DOI] [Google Scholar]