Abstract
Ultrasound-assisted extraction (UAE) was used to extract the anthocyanins from pericarp and lipids from the seeds of mangosteen. The optimum time for extraction of anthocyanin by maceration method and shaking water bath was 6 and 4 h, respectively, whereas, it was 5 min only for ultrasonic assisted extraction method. The anthocyanin content, extracted by UAE, was 23.54 mg Cyn-3-Glu/100 g. The regression equation derived by response surface methodology (RSM), was used to predict the anthocyanin content extracted by using UAE. The gas chromatography-flame ionized detector analysis showed that mangosteen seed contained both saturated (palmitic acid, 4.66 g/100 g; stearic acid, 47.64 g/100 g) and unsaturated fatty acids (oleic acid, 28.62 g/100 g; linoleic acid, 14.68 g/100 g). The mangosteen ice-cream exhibited up to 83.6 and 75.1 % DPPH inhibition, on addition of 2 and 1 % mangosteen pericarp extract respectively, where as control only 52.6 %.
Keywords: Mangosteen pericarp, Anthocyanins, Response surface methodology (RSM), Ultrasound- assisted extraction, Antioxidant activity
Introduction
Mangosteen (Garcinia mangostana L.) is one of the praised tropical fruit that belongs to family Guttiferae. Mangosteen is famous for its unique taste and named as “The queen of fruits”. Color of rind develops from yellowish white to reddish purple depending on levels of the maturity. Bioactive compounds in mangosteen pericarp are mostly polyphenolic compounds including; xanthones, anthocyanin, proanthocyanidins and catechins. Mangosteen pericarp has been reported for containing high amount of anthocyanins. External mangosteen pericarp has the highest anthocyanin contents (Chaovanalikit and Mingmuang 2007). Moreover, the anthocyanins in the external pericarp of mangosteen are generally composed of cyaniding-sophoroside, cyaniding-glucoside-pentoside, cyaniding-glucoside, and cyaniding-glucoside-X (Palapol et al. 2008). These bioactive compounds exhibited biological activities such as antioxidant, antibacterial, antifungal, anti-inflammatory, anti-allergy, anti-cancer, anti-tumor, immunomodulation and diarrheal treatments (Arunrattiyakorn et al. 2011).
The extraction is widely used in separating the bioactive compounds from the agricultural and biological materials, especially from plants, such as herbs, fruits and vegetables. Soxhlet extraction method spends needs a longer time and large amount of solvent in each extraction. Ultrasound assisted extraction improves the yield of desired compounds, decreasing the volume of solvents and the time of extraction. Plants are good sources of antioxidants and can be used as natural additives in food and feed formulations (Taghvaei and Jafari 2015).
Several ingredient related products, such as plain ice-cream, reduced fat, low fat, nonfat, fruit, and nut ice-creams, sherbet, frozen yoghurt, besides other frozen products are available commercially (Marshall et al. 2003). The plant and plant products are added in ice-cream to improve the properties of ice-cream such as color, viscosity or antioxidant activities. Hwang et al. (2008) reported the grape wine lees were added in ice-cream to improve the rheological and antioxidant properties of ice-cream. The grape wine lees decreased pH, lightness, firmness but increased yellowness and viscosity of ice-cream, where percentage of grape wine lees increased.In this study, three methods namely maceration (ME), shaking water bath (SWE) and ultrasound-assisted extraction (UAE) were used to study the optimized conditions for anthocyanin extraction from mangosteen pericarp. The extracts from mangosteen pericarp were added to improve the antioxidant properties of ice cream in order to determine the potential of using external mangosteen pericarp as a health ingredient.
Materials and methods
Plant Materials
The mature mangosteen were purchased from the mangosteen plantation in Chanthaburi, Thailand. The pericarp of mangosteens were used as raw materials for extraction. The pericarp of fruits were cut into uniform sizes (~0.5 cm3). The hull was dried in hot air oven at 55 °C for 48 h until the moisture content reached below 20 % (w/w). The dried plant parts were grounded by mechanical grinder (Philips Co. Ltd., China) into the fine powder and stored in polyethylene bags cover with aluminum foil at −18 °C for further experiments.
Full factorial design for ultrasonic assisted extraction
Three-level and three-factor full factorial experiments were determined using Central Composite Design (CCD) to cover the ranges for factors; time of extraction (X1), sample to solvent ratio (X2) and amplitude (X3) as independent variables. Total anthocyanin content (TAC) was the response of the design with 20 different experimental conditions. Some of the recent reports published for optimizing the work of extraction from other plant sources (Çam and Içyer 2015; Mahmoodani et al. 2014; Chakraborty et al. 2011).
Ultrasound-assisted extractor (UP200S, Hielscher, Germany) was used for the extraction of anthocyanins from mangosteen pericarp powder by using ethanol (50 % v/v) as solvent. For the experimental design, the extraction parameters were optimized using CCD followed by Response Surface Design (RSM). Samples and solvents (1:6.6, 1:10, 1:15, 1:20 and 1:23.5 w/v) were added in a 200 ml beaker and soaked with the probe maintaining a gap of one cm between probe (2.54 cm diameter cylindrical titanium alloy head) and the bottom of the beaker. Sample solutions were extracted at different amplitudes (30, 45, 65, 85 and 100 %) and time (1.5, 5, 10, 15 and 18 min). Each experiment was conducted in triplicates.
The full factorial experimental design for each response with each extraction solvent was analyzed using CCD.The second order response surface model was implemented by considering R-square (R2 = 0.86) value. The selected model was used to determine the coefficients of three independent variables.
Model
where Y = Anthocyanin content (mg/100 g fresh sample), X1 = Extraction Times (T), X2 = Ratio sample and solvent (R), X3 = Amplitude of Ultrasound (A).
Maceration and Shaking Water Bath method
Fresh and dried mangosteen pericarps were extracted to measure the anthocyanin content by using the conventional method as maceration and shaking water bath. For maceration, samples and solvents were mixed on incubator shaker (100 rpm, Gallenkamp, UK) at 25 °C. Temperature of water was controlled at 55 °C. The sample to solvent ratio used was same as optimized in conditions from UAE.
Determination of total anthocyanin content (TAC)
The TAC of the crude mangosteen extract was determined using the pH differential method (Lee et al. 2005) with slight modifications. The crude anthocyanin extract (1 ml) was placed into a 10 ml of volumetric flask with the final volume adjusted by 0.025 M potassium chloride buffer (pH 1.0). Similarly, another 10 ml of volumetric flask was filled with one mL of extract and 9 mL of 0.4 M sodium acetate buffer (pH 4.5). The absorbance of samples was measured (within 20–50 min of preparation) at 510 and 700 nm, diluted with pH 1 and pH 4.5 buffers respectively, by using UV- visible spectrophotometer (UNICAM UV/Vis Spectrophotometer, UK). The TAC was expressed as mg of cyanindin-3-glucoside equivalents per 100 g of dry sample and calculated by using the following equation.
where Abs (Absorbance) = [(A510−A700) pH1.0]−[(A510−A700) pH4.5]; ε (cyanindin-3-glucoside molar absorbance) = 26,900 L mol−1 cm−1; L (the cell path length) = 1 cm; MW (the molecular weight of anthocyanin) = 449.2 D and DF = the dilution factor.
Determination of total phenolic content (TPC)
The total phenolic content of extracts was determined by Folin-Ciocalteu reagent following the method described by Ainsworth and Gillespie (2007) with slight modifications. The extracts optimized by RSM for the highest concentration of anthocyanins were selected for TPC estimation. The stock solution (1 mg/ml) of each extract was diluted to prepare 100 µg/ml. For each extract, 0.5 ml (100 µg/ml) was mixed with 2 ml of freshly prepared Folin-Ciocalteu reagent (1:10 diluted with de-ionized water) and further neutralized with 4 ml of sodium carbonate solution (7.5 % w/v). The reaction mixture was incubated at room temperature for 30 min and subjected to shaking intermittently. The absorbance was then measured at 765 nm using UV- visible spectrophotometer (UNICAM UV/Vis Spectrophotometer, UK). The results were expressed as Gallic acid equivalents (GAE) per 100 g of sample.
Extraction of lipids from mangosteen seed
Total lipid content of mangosteen seed was estimated by using Maceration Extraction (ME), Soxhlet extraction (SE) and Ultrasound-assisted extraction (UAE). Soxhlet was considered as the control method. Seed samples were dried at 105 °C in hot air oven until weight remains constant. The seed samples (2 g) were were soaked in 150 ml of petroleum ether. Samples were extracted for 2 h (control time) in case of SE and at varied times (30, 60 and 90 min) for UAE to determine the best condition for high lipid yield. Solvent from the extract was removed by heating at 105 °C within a controlled fuming hood chamber to completely evaporate petroleum ether. The lipid content was calculated from the measured weight after extraction.
Quantification of fatty acids in mangosteen seed
Mangosteen seed oil samples were converted to corresponding (fatty acid methyl esters) FAMEs by trans-esterification with sodium hydroxide (ISO 5509, 2000).
Fatty acid content analysis was carried out by using Shimadzu GC-2014 system (Gas chromatography system) coupled with FID (flame ionized detector). A stabilwax-DA (30 m, 0.25 mm ID, 0.25 µm) column was used for separation. Helium gas (16.5 kPa, 1.98 ml/min column flow) was used as carrier gas. Methyl heptacanoate and n-heptane were used as internal standard (ISTD) and solvent, respectively. The sample (0.5 μl) was injected into the GC-FID system by auto sampler in split mode and the injector temperature was maintained at 250 °C. The quantification of fatty acids was performed by external standard calibration method by using saturated fatty acid (Palmitic and stearic acids) and unsaturated fatty acid (Oleic and linoleic acids) as standards.
Ice-cream processing
Mangosteen ice-cream was added with the extracts from mangosteen pericarp to improve the antioxidant activity of ice-cream. The ice cream formula consisted of mangosteen fruit puree (50 %), whipping low fat cream (12.5 %), sugar (12.5 %) and milk (25 %). Ice cream was prepared using modified standard method mentioned by Schmidt (2004). The ice cream mixture was divided into three parts containing different amount of anthocyanins (0, 1, 2 % w/w) mangosteen pericarp extract respectively). The obtained mixtures were blended using an ice-cream mixer (Model FR-F2, Fry King) for 20 min and stored at −18 °C for 24 h before determination of antioxidant activity, pH, Brix and color (Sun-Waterhouse et al. 2013).
Chemical and physical properties
Total soluble solid (TSS) content was measured by using a hand-held refractometer (Atago, Tokyo Japan) and expressed in °Brix. The pH of the ice cream was measured using a digital pH-meter (microprocessor pH meter, United States). Texture profile analysis of ice-cream samples was carried out by texture analyzer (TA-XT plus model, Texture Technologies, Scarsdale, NY) as described by Hwang et al. (2008).
Determination of DPPH radical scavenging activity
The antioxidant activity of plant mangosteen pericarp ice-cream (1, 2 % and control) was determined in terms of DPPH (1, 1diphenyl-2-picryl hydrazyl) radical-scavenging activity following the method as described by Sadiq et al. (2015) with slight modifications. Different samples (1, 2 % and control) of ice-cream containing mangosteen pericarp extract were prepared for determination of % DPPH inhibition. Ascorbic acid (1 mg/ml) was used as a positive control. Each sample (50 μm) was added to 5 ml of freshly prepared 40 ppm methanol solution of DPPH (Sigma-Aldrich, USA) and the reaction mixture was kept at 25 °C for 30 min at a dark place. After incubation the absorbance was read against blank at 517 nm. DPPH solution without sample was used as control. The assay was carried out in triplicates for each sample and percentage inhibition was determined by the following equation:
where AC = Absorbance Control, AS = Absorbance test sample.
Statistical analysis
All experiments were carried out in triplicates and results are expressed as mean values with standard deviation (±SD) of three replicates. One-way analysis of variance (ANOVA) was carried out to determine significant group differences (p < 0.05) between means by using SPSS statistical software package (SPSS, version 16.0).
Results and discussion
Ultrasound-assisted anthocyanin extraction
Ultrasound-assisted extraction was used to extract the anthocyanins from mangosteen pericarp because this method can reduce extraction time and improve the extraction efficiency. Analyses of variance (ANOVA) for the response was performed to determine the degree of effects of the independent variables. The variance was analyzed at 95 % significant level (p < 0.05). Thus, all three variables had significant effect at 5 % level of significance (p < 0.05) on the response (anthocyanin content). While the combined effects of any two among the three variables were significant at 5 % level of significance (p < 0.05) except for the combined effects between ratio sample to solvent and time of extraction. This combined effect was not significant (p > 0.05). It was concluded that the effects of the variables was not depended on only individual parameter.
The regression equation following the second order polynomial regression model was used to determine anthocyanin content as
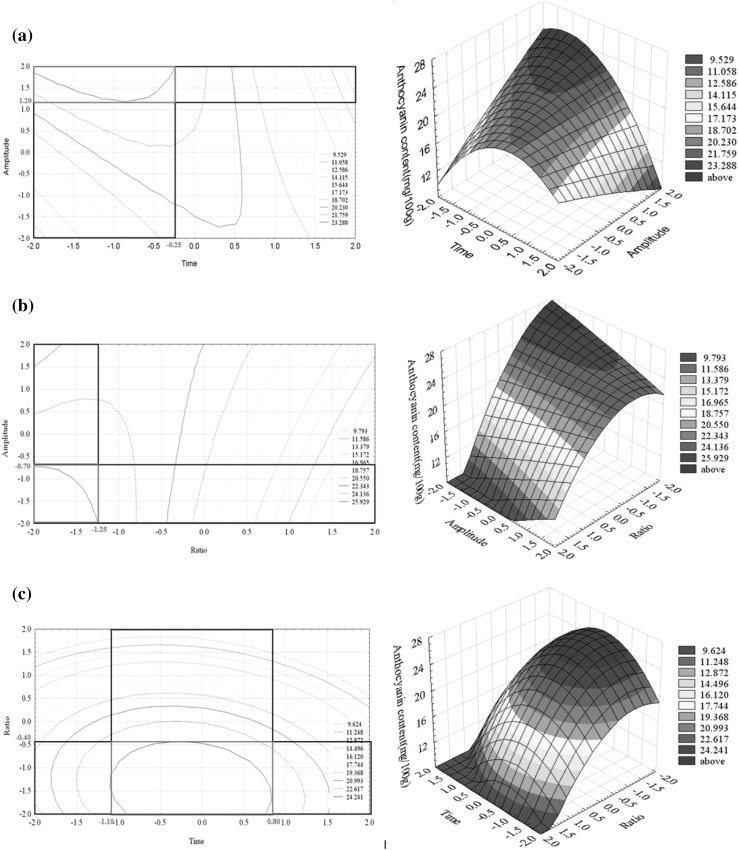
Figure 1 illustrates the contour graphs to receive three different optimum regions. Three contour graphs were generated at different combination of variable parameters as (1) solute: solvent and time (2) solute: solvent and amplitude of ultrasound and (3) extraction time and amplitude of ultrasound. The optimum regions were area which presented the highest amount of anthocyanin content. The optimum point is the middle point of the optimum ranges.
Fig. 1.
The optimum region of contour plot a amplitude of ultrasound and time b amplitude of ultrasound and sample ratio to solvent c sample ratio to solvent and time
The time of extraction, ratio sample to solvent and amplitude of ultrasound were 5 min, 1:8.5 sample to solvent at 94 % of amplitude. Linear regression equation was used to predict the anthocyanin content at optimum condition (1:8.5, 94 % amplitude at 5 min) and compared with the experimental values. The trend of value was similar, predicted value (25.29 mg Cyn-3-Glu/100 g) was near to experimental value (23.54 ± 0.31 mg Cyn-3-Glu/100 g).
This aim of this experiment was to determine the optimum condition of three methods as maceration, shaking water bath and ultrasound-assisted extraction. The most common anthocyanin is the Cy-3-glucoside (Kong et al. 2003). Table 1 shows amount of anthocyanins which were extracted from both fresh and dried mangosteen pericarp. The optimum times for extraction of anthocyanins by maceration, SWE and UAE were 6, 4 h and 5 min, respectively (same ratio of sample to solvent). Extraction time of UAE was much lesser than other two methods. For fresh mangosteen pericarp, anthocyanin content values from UAE and ME were not significantly different (p > 0.05) but were significantly different from SWE (p < 0.05). The anthocyanin content that was extracted by UAE for fresh and dried mangosteen pericarp was 23.54 ± 0.31 and 20.83 ± 0.96 mg Cyn-3-Glu/100 g, respectively. Compared to conventional extraction methods, UAE is one of the most simple, inexpensive extraction technique and can be applied to wide range of solvent systems for industrial scale preparations (Lee and Lin 2007). Apart from increasing the extraction rate, UAE may reduce the dependence on a particular solvent and enables the use of alternative solvents which may provide more benefits in terms of economic, environmental and health safety (Vilkhu et al. 2008). UAE preserve the structural and molecular characteristics of bioactive compounds.
Table 1.
Total phenolic and anthocyanin contents from mangosteen pericarp at optimum condition of each extraction method
| Method | Optimum Time | Total phenolic content (mg GAE/100 g) | Anthocyanin content (mg Cyn-3-Glu/100 g) | ||
|---|---|---|---|---|---|
| Fresh MP | Dried MP | Fresh MP | Dried MP | ||
| ME | 6 h | 1826.16 ± 60.79c | 1796.16 ± 81.50b | 23.36 ± 0.41a | 21.19 ± 0.49a |
| SWE | 4 h | 2493.56 ± 40.84a | 2172.61 ± 30.02a | 19.07 ± 1.94b | 19.26 ± 1.34b |
| UAE | 5 min | 1975.00 ± 74.34b | 2170.32 ± 71.76a | 23.54 ± 0.31a | 20.83 ± 0.96ab |
The values are mean ± standard deviation
ME, SWE and UAE present maceration, shaking water bath and ultrasonic assisted extraction respectively, where as, MP mangosteen pericarp
a,bThe values in the same column followed by different superscripts were significantly different (p < 0.05)
At industrial scale, UAE gains worthwhile economic benefits due to improved extraction efficiency and extraction rate. The equipment used for UAE is commercially viable and scalable at industrial level (Vilkhu et al. 2008). UAE provides the improved extraction of heat sensitive bioactive compounds and food components at low processing temperature. UAE leads to reproducible food processes that can be completed in seconds or minutes with high reproducibility reduced processing cost, improved purity of final product and consuming only a fraction of energy compared to conventional extraction methods (Chemat and Khan 2011).
Total phenolic content
For total phenolic content, the linear regression equation was determined from the relationship between absorbance at 760 nm and standard solution (gallic acid). The total phenolic content was expressed as mg of GAE/100 g of extract.
Table 1 shows the values of total phenolic content, extracted together with anthocyanins at optimum condition of all extraction methods. For total phenolic content, the highest values were extracted by SWE. Total phenolic values of fresh and dried mangosteen pericarp were 2493.56 ± 40.84 and 2172.61 ± 30.02 mg GAE/100 g. However, the TPC from fresh samples was significantly different by all methods (p < 0.05) but TPC from SWE and UAE was not significantly different in dried mangosteen pericarp extract (p > 0.05). In this case, maceration was the method that extracted the least total phenolic content, 1826.16 ± 60.79 GAE/100 g in fresh sample while dried sample yield 1796.16 ± 81.50 mg GAC/100 g. The amount TPC in this study was similar to the the finidnings of Chaovanalikit and Mingmuang (2007) who studied, anthocyanin and total phenolic content of mangosteen and its juice. TPC of dried samples was greater than fresh mangosteen pericarp as 2170.32 ± 71.76 and 1975.00 ± 74.34 mg GAE/100 g respectively, when extracted by UAE. Dried mangosteen pericarp had high TPC value because UAE ensured well mixing between the sample particles and solvent, moreover the small particles exposed increased surface area to solvent. So, the dried sample was exhausted well by solvent and yield high TPC.
Total lipid content and fatty acids analysis in mangosteen seed
The value of total lipid by soxhlet extraction was 47.71 ± 0.26 % wb which was significantly different from other methods (p < 0.05) and by UAE at 60 min (43.22 ± 0.91 % wb) was the best condition. Similar results were observed for lipid extraction (44 min at 49 °C) from Isatis indigotica seed by ultrasound-assisted extraction (Li et al. 2012). The lipid contents by maceration and UAE (30 and 90 min) were 40.15 ± 0.5 %, 39.67 ± 0.49 % and 39.36 ± 0.26 % respectively.
Mangosteen seed contains both saturated (palmitic acid, 4.66 g/100 g; stearic acid, 47.64 g/100 g) and unsaturated fatty acids (oleic acid, 28.62 g/100 g; linoleic acid, 14.68 g/100 g). The lipids from mangosteen seed showed waxy appearance due to high concentration of stearic acid. Moreover, high contents of monounsaturated fatty acids (MUFA), in seed oil suggest mangosteen seed as a good source of dietary lipids. Oleic acid is associated with the low incidence of coronary heart disease (CHD) as it lowers the total cholesterol (Dennys et al. 2006). In comparison to coconut oil the current study presented that mangosteen seed oil contained high contents of stearic acid, linoleic acid and oleic acid, but palmitic acid was found in high content in coconut oil (Dauqan et al. 2011).
Properties of mangosteen ice-cream
Chemical and physical properties of ice-cream
Mangosteen is known as “queen of fruits” because of its pleasant taste compared to other fruits. The edible portion is milky white and pericarp is dark red and constitute about two times of the edible portion. In food processing industry fruit aril is separated and peel is considered as waste. The mangosteen pericarp is important source of natural phenolic compounds (Suttirak and Manurakchinakorn 2014). The aim of developing mangosteen pericarp extract ice-cream was to propose waste valorization for value addition and formulation of phenolic compounds enriched ice-cream. The chemical and physical characteristics of mangosteen ice-cream sample (control, 1 % MP and 2 % MP) are shown in Table 2.
Table 2.
Characteristics and texture of ice-cream prepared from mangosteen pericarp extracts
| Properties | Control | 1 % MP | 2 % MP |
|---|---|---|---|
| pH | 4.29 ± 0.01a | 4.26 ± 0.01a | 4.27 ± 0.01a |
| Brix | 22.33 ± 0.58b | 24.33 ± 0.58a | 25.00 ± 0.01a |
| Colour | |||
| L* | 79.86 ± 0.19a | 63.79 ± 0.29b | 57.62 ± 0.45c |
| a* | 2.34 ± 0.03c | 21.56 ± 0.16b | 26.02 ± 0.09a |
| b* | 13.95 ± 0.14a | 9.52 ± 0.10b | 9.47 ± 0.16b |
| Firmness | 87.45 ± 53.00ns | 90.22 ± 41.69ns | 89.23 ± 44.36ns |
| Cohesiveness | 0.05 ± 0.02ns | 0.06 ± 0.02ns | 0.06 ± 0.02ns |
The values are mean ± standard deviation. The color meter parameters were L * for lightness, a * for redness or greenness, and b * for yellowness or blueness, respectively
MP mangosteen pericarp
a,b,c,dThe values in the same row followed by different superscripts were significantly different (p < 0.05)
nsNot significantly
The samples were analyzed for pH and total soluble solid (TSS). Form the results, pH of three sample including; control, 1 % MP and 2 % MP, were not significantly different. The range of pH was 4.26–4.29 while pH of fresh mangosteen juice was 3.84. Moreover, total soluble solid content of ice-cream was greater than fresh pulp mangosteen also. Total soluble solid of control, 1 % and 2 % MP mangosteen ice-cream were 22.33 ± 0.58, 24.33 ± 0.58 and 25.00 ± 0.01, respectively which were significantly different from fresh pulp of mangosteen. These results demonstrated about the taste of ice-cream, as mangosteen ice-cream was sweeter and less sour as compared to fresh mangosteen.
The addition of mangosteen pericarp increased redness of ice-cream and decreased lightness and yellowness. These were similar trends with experiment of Hwang et al. (2008) who reported that color of ice-cream changed with different amount of grape wine lees due to anthocyanins. L* increased while a* and b* decreased slightly. The mangosteen ice-cream samples after adding pericarp were mainly pink color due to the presence of anthocyanins.
Firmness and cohesiveness values of control, 1 % MP and 2 % MP mangosteen ice-cream were not significantly different (p < 0.05). So, mangosteen pericarp did not affect the texture profiles of ice-cream. The textures of mangosteen ice-cream had similar result with the 15 % grape wine ice-cream having the firmness and cohesiveness of 113 N and 0.16, respectively (Hwang et al. 2008).
Antioxidant activity of mangosteen ice-cream
There was considerable variation in antioxidant potential among the mangosteen pericarp ice-cream samples. The highest DPPH inhibition (83.6 ± 0.54 %) was found in the ice-cream containing 2 % of mangosteen pericarp extract, whereas it was 75.1 ± 1.09 % and 52.6 ± 0.23 % for 1 % pericarp extract ice-cream and control (without mangostene pericarp extract), respectively. The positive control, L- ascorbic acid showed DPPH inhibition (96.50 ± 0.36 % at 1000 µg/ml). The increase in % DPPH inhibition was found with the increase in concentration of mangosteen pericarp extract. Anthocyanin content in ice-cream containing 2 and 1 % pericarp extract were 70.36 ± 2.15 and 35.18 ± 1.58 mg/L, respectively. TPC was higher for ice cream containing 2 % pericarp extract (1.53 ± 0.02 mg/ml), compared to 1 % pericarp extract (1.14 ± 0.10 mg/ml) and control (0.80 ± 0.03 mg/ml) ice-cream samples.
The modern trend has been shifted from utilization of synthetic food antioxidants to natural ones that has fostered the research on finding the new antioxidants from plant derived raw materials. In food and agriculture industry the growing interest is to focus on inexpensive and residual sources. The fruits peels are good source of antioxidants and often disposed of by food industry as waste (Suttirak and Manurakchinakorn 2014). The mangosteen peel contains various bioactive substances such as phenolic acids and flavonoids that are mainly responsible for antioxidant activities. Therefore it is essential to identify the bioactive potential of mangosteen waste for food and pharmaceutical potentials.
All samples of mangosteen pericarp in this study were capable of scavenging DPPH free radicals. The DPPH radical scavenging activity decreased in order among the ice-cream samples containing pericarp extract as, 2 % pericarp extract > 1 % pericarp extract > control (without pericarp extract).The free radical inhibition capacity of 2 % MP mangosteen ice-cream was the highest due to the highest values of total phenolic and anthocyanin content within ice-cream, as compared with 1 % MP and control, respectively. Thus, the antioxidant activity of samples was in direct relation to the amount of TPC. The antioxidant activity of mangosteen juice was high because of high total phenolic content (Chaovanalikit and Mingmuang, 2007). Hwang et al. (2008) indicated total phenolic content and total anthocyanin content increased when percentage of grape wine lees increased from 5 to 15 %. However, if mangosteen pericarp is added more than 2 %, ice-cream was having bitter taste.
Conclusion
Mangosteen pericarp is considered as processing waste by the food industries. The current study suggested utilization of pericarp as a source of natural antioxidants that can be used in food and pharmaceutical industries. Moreover a functional ice-cream was developed by incorporating the pericarp extract that presented high antioxidant potential compared to control. The extraction rate of UAE depends on two major factors as efficiency of cell disruption and mass transfer. However, the sonication time and amplitude were considered critical because, excess of sonication can damage the quality of the extracts and their bioactivity properties. The optimum extraction conditions for UAE were determined by RSM design. The overall optimum point of extraction time, sample to solvent ratio and amplitude of ultrasound were 5 min, 1:8.5 and 94 % respectively. Moreover, the seeds of mangosteen were found rich in lipid content, both saturated and unsaturated fatty acids. Hence the mangosteen can be a potential source of unsaturated lipids, antioxidants and phenolic compounds in commercial preparation of food and pharmaceutical products.
The mangosteen ice-cream developed by the addition of pericarp extract showed higher TPC and DPPH inhibition compared to the ice-cream without pericarp extract.
Acknowledgments
The authors acknowledged Asian Institute of Technology, Thailand to conduct this work.
References
- Ainsworth EA, Gillespie KM. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat Protoc. 2007;2(4):875–877. doi: 10.1038/nprot.2007.102. [DOI] [PubMed] [Google Scholar]
- Arunrattiyakorn P, Suksamrarn S, Suwannasai N, Kanzaki H. Microbial metabolism of α-mangosin isolated from Garcinia mangostana L. Phytochemistry. 2011;72(2011):730–734. doi: 10.1016/j.phytochem.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Çam M, İçyer NC. Phenolics of pomegranate peels: extraction optimization by central composite design and alpha glucosidase inhibition potentials. J Food Sci Technol. 2015;52(3):1489–1497. doi: 10.1007/s13197-013-1148-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty SK, Kumbhar BK, Chakraborty S, Yadav P. Influence of processing parameters on textural characteristics and overall acceptability of millet enriched biscuits using response surface methodology. J Food Sci Technol. 2011;48(2):167–174. doi: 10.1007/s13197-010-0164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaovanalikit A, Mingmuang A. Anthocyanin and total phenolic content of mangosteen and its juices. SWU Sci J. 2007;23(1):68–78. [Google Scholar]
- Chemat F, Khan MK. Applications of ultrasound in food technology: processing, preservation and extraction. Ultrason Sonochem. 2011;18(4):813–835. doi: 10.1016/j.ultsonch.2010.11.023. [DOI] [PubMed] [Google Scholar]
- Dauqan EMA, Sani HA, Abdullah A, Kasim ZM (2011) Fatty acids composition of four different vegetable oil (Red palm olein, Palm olein, Corn oil and Coconut oil) by GC. IPBEE vol 14 [DOI] [PubMed]
- Dennys ECC, Andre GVC, Maria CGP, Sergio Matta DS, Marco TCS, Neuza MBC. Lipid profile of rats fed high-fat diets based on flaxseed, peanut, trout, or chicken skin. J Nutr. 2006;22:197–205. doi: 10.1016/j.nut.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Hwang JY, Shyu YS, Hsu CK. Grape wine lees improve the rheological and adds antioxidant properties to ice cream. J Food Sci Technol. 2008;42:312–318. [Google Scholar]
- Kong JM, Chia LS, Goh NK, Chia TF, Brouillard R. Analysis and Biological activities of anthocyanins. Phytochemistry. 2003;64(5):923–933. doi: 10.1016/S0031-9422(03)00438-2. [DOI] [PubMed] [Google Scholar]
- Lee MH, Lin CC. Comparison of techniques for extraction of isoflavones from the root of Radix Puerariae: ultrasonic and pressurized solvent extractions. Food Chem. 2007;105(1):223–228. doi: 10.1016/j.foodchem.2006.11.009. [DOI] [Google Scholar]
- Lee J, Durst RW, Wrosltad RE. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: collaborative study. J AOAC Int. 2005;88:1269–1278. [PubMed] [Google Scholar]
- Li T, Qu XY, Zhang QA, Wang ZZ. Ultrasound-assisted extraction and profile characteristics of seed oil from Isatis indigotica Fort. Ind Crop Prod. 2012;35(1):98–104. doi: 10.1016/j.indcrop.2011.06.013. [DOI] [Google Scholar]
- Mahmoodani F, Ardekani VS, See SF, Yusop SM, Babji AS. Optimization and physical properties of gelatin extracted from pangasius catfish (Pangasius sutchi) bone. J Food Sci Technol. 2014;51(11):3104–3113. doi: 10.1007/s13197-012-0816-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall RT, Goff HD, Hartel RW. Ice cream. New York: Springer; 2003. p. 357. [Google Scholar]
- Palapol Y, Ketsa S, Stavenson D, Cooney JM, Allan AC, Ferguson IB. Colour development and quality of mangosteen (Garinia mangostana L.) fruit during ripening and after harvest. J Postharvest Biol Technol. 2008;51:349–353. doi: 10.1016/j.postharvbio.2008.08.003. [DOI] [Google Scholar]
- Sadiq MB, Hanpithakpong W, Tarning J, Anal AK. Screening of phytochemicals and in vitro evaluation of antibacterial and antioxidant activities of leaves, pods and bark extracts of Acacia nilotica (L.) Del. Ind Crop Prod. 2015;77:873–882. doi: 10.1016/j.indcrop.2015.09.067. [DOI] [Google Scholar]
- Sun-Waterhouse D, Edmonds L, Wadhwa SS, Wibisono R. Producing ice-cream using a substantial amount of juice from kiwifruit with green, gold or red flesh. J Food Res Int. 2013;50(2):647–656. doi: 10.1016/j.foodres.2011.05.030. [DOI] [Google Scholar]
- Suttirak W, Manurakchinakorn S. In vitro antioxidant properties of mangosteen peel extract. J Food Sci Technol. 2014;51(12):3546–3558. doi: 10.1007/s13197-012-0887-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghvaei M, Jafari SM. Application and stability of natural antioxidants in edible oils in order to substitute synthetic additives. J Food Sci Technol. 2015;52(3):1272–1282. doi: 10.1007/s13197-013-1080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilkhu K, Mawson R, Simons L, Bates D. Applications and opportunities for ultrasound assisted extraction in the food industry—A review. Innov Food Sci Emerg Technol. 2008;9(2):161–169. doi: 10.1016/j.ifset.2007.04.014. [DOI] [Google Scholar]