Abstract
Spent cumin (SC), generated from Ayurvedic industry, was evaluated for its nutraceutical potential in terms of antioxidant, antidiabetic and anticancer properties, and compared with that of the raw cumin (RC). SC and RC seeds were extracted with ethyl acetate (E) and methanol (M). SCM (methanol extract) were rich in p-coumaric acid, ferulic acid, ellagic acid and cinnamic acid (6.4445, 5.8286, 2.1519, 4.3085 mg/g dry extract). SCM reduced Fe2+ ion (89.68 µM AA/g dry weight), scavenged DPPH radical (IC50-238.6 µg/mL), better α-amylase inhibition (IC50-337.22 µg/mL) and glucose uptake activity in 30.7% of L6 cells. SCM inhibited viability, retarded migration area up to 41.02%, arrested cell cycle at S phase and induced apoptosis in 2.45% of HT29 colon cancer cells. The results indicated that dietary interventions using nutraceutical food formulation made out of SC can play a significant role in the prevention and management of degenerative diseases.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-016-2372-z) contains supplementary material, which is available to authorized users.
Keywords: Spent cumin, Nutraceutical, Polyphenols, Antidiabetic, Anticancer
Introduction
Ayurvedic medicine is one of the world’s oldest medical practices that originated in India and remains the most important traditional health care system in the country. Ayurveda is gaining a lot of importance all over the world as complementary and alternative medicine and the World Health Organization recognizes it as Traditional Medicine. More than 1000 plant materials and valuable spices are used as primary raw materials in Ayurveda (Ballabh and Chaurasia 2007). The processing is being practiced as per guidelines of traditional Ayurvedic texts. The active principles from raw materials are extracted with water, oil, ghee, milk, etc., where recovery of active principles from most of the herbs, into the extracted phase, will not be complete. Hence, most of the residues from the Ayurvedic industries are very rich sources of valuable bioactive phytochemicals.
Cumin (Cumin cyminum), an annual herbaceous plant that belongs to the Umbelliferae family is an important component of many of the Ayurvedic decoctions (Sharma et al. 2016). Cumin seeds are used as a spice for their distinctive flavor and aroma and are valued for its medicinal and therapeutic properties (Thippeswamy and Naidu 2005). Apart from taste and flavour, cumin is being recognized for its medicinal, antioxidant, antimicrobial and food stabilizing properties. Cumin seeds are being used by the spice industries for the extraction of essential oil and oleoresin that is used in many seasoning mix and perfumeries. Apart from spice industry, large quantities of cumin seeds are also used by Ayurvedic industries. Cumin is an important component of most of the Ayurvedic decoctions specifically used for the conditions like bloating, vomiting, diarrhea, dysentery, malabsorption syndrome, fever and skin diseases (Sharma et al. 2016).
Jeerakarishtam, one of the cumin based decoction, is prescribed by Ayurvedic doctors for abdominal distension, discomfort in the chest, respiratory disorders, and all digestive disorders. Cumin seeds used after the preparation of these decoctions were considered as a waste, except in veterinary feeds to a small percentage. The amount of Spent Cumin (SC) generated from spice industry is around 400 tons/year which was calculated from the export figure of cumin oleoresin, and the yield of oleoresin from cumin (Mathew 2004). Almost same amount of SC has generated from Ayurvedic industry also (unpublished data collected from various Ayurvedic industry). Sowbhagya et al. (2007) reported that SC is a potential source of dietary fibre.
The value addition of agro-industrial waste is a matter of great interest for research, as it is a potential source of bioactive phytochemicals (Balasundram et al. 2006). Many of the spices are reported to possess significant health benefits and are widely used in Ayurvedic industries for the preparation of decoctions. The spent cumin seeds generated from the Ayurvedic industry are chosen for the current study based on the commercial potential as it undergoes minimum thermal processing during preparation and thereby ascertaining the retention of bio-actives after processing. Value addition to the SC is important in economic terms as well as on environmental protection grounds. Most of the studies on cumin are based on the antimicrobial and antioxidant activity of fresh cumin. Moreover, antidiabetic and anticancer potential of cumin is never explored. As cumin is reported to have potential antioxidant properties, it will be worth to assess the potential of cumin in the prevention and management of many of the chronic diseases. Therefore, present study mainly emphasizes on the effective utilization of SC residue as a repository of biologically active ingredients for the prevention and management of diabetes and colorectal cancer and compares it with that of fresh cumin. The study is of great significant to the Ayurvedic industry, spice and flavour industry as a large volume of SC is generated from these industries.
Materials and methods
Chemicals
2,2-diphenyl-1-picrylhydrazyl (DPPH), α-glucosidase, α-amylase, acarbose, bovine serum albuminb(BSA), propidium iodide, crystal violet, formaldehyde, Trolox, curcumin and polyphenol standards were procured from Sigma-Aldrich Chemicals, Bangalore, India. 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose (2-NBDG) was procured from Invitrogen Bioservices Pvt.Ltd., Bangalore, India. Copper chloride and neocuproine were purchased from Alfa Aesar, Heysham, England. Ascorbic acid and Folin–Ciocalteau reagent were purchased from Sisco Research Laboratories Pvt. Ltd., Mumbai, India. All other reagents used were of analytical grade.
Sample preparation
The work was done in collaboration with M/s Kottakkal Arya Vaidya Sala, Kottakkal, Kerala, which is one of the leading manufacturers of Ayurvedic formulations in India. Authenticated samples of raw (RC) and spent cumin (SC) seeds of the same batch were supplied by the collaborating industry. SC provided was the residue from the preparations of Ayurvedic decoctions where it was boiled with other ingredients used for the preparation of Ayurvedic formulations. Cumin seeds were then freeze-dried using lyophilizer (VirTis Genesis, USA), powdered (40–60 mesh) and stored under refrigeration until further use. RC and SC seed powder were defatted with hexane and extracted using ethyl acetate (E) and methanol (M) (1:10, w/v) at ambient temperatures (30 ± 2 °C). The extracts (RCE, RCM, SCE and SCM) were concentrated under reduced pressure in rotavapor (BUCHI R215, Switzerland), filtered through Whatman No.1 filter paper followed by lyophilization (VirTis Genesis, USA). The lyophilized extracts were stored at 4 °C until further biochemical analysis.
Determination of phytochemicals
Total phenolic and flavonoid content (TPC and TFC)
TPC was determined using Folin-ciocalteu reagent with Gallic acid as standard. The results were calculated as Gallic acid equivalents (mg GAE/g dry weight). TFC was determined according to AlCl3 colorimetric method with quercetin as standard. The results were expressed as milligram quercetin equivalents (mg QE/g dry weight). The TPC and TFC experiments were performed as described in Carciochi et al. (2015).
HPLC profiling and quantification of phenolic compounds
The active extracts and the reference compound (1 mg/mL) solutions were prepared in methanol and filtered through 0.45 µm PTFE filter; 20 µL was injected into the HPLC system. The analysis was performed on a Prominence UFLC system (Shimadzu, Japan) containing LC-20AD system controller, Phenomenex Gemini C18 column (250 × 4.6 mm, 5 µm), a column oven (CTO-20A), a Rheodyne injector (USA) with a loop of 20 µL volume and a diode array detector (SPD-M20A).
The HPLC analysis was performed by the method of Rodriguez-Delgado et al. (2001) with some modifications. The mobile phase used was—solvent A: methanol-acetic acid–water (10:2:88, v/v) and solvent B: methanol-acetic acid–water (90:2:8, v/v) with the gradient program 0–15 min 15% B, 16–20 min 50% B, 21–35 min 70% B, 36–50 min 100% B and finally the column was regenerated in 10 min. The flow rate was 1 mL/min; the injection volume was 20 µL and column was at room temperature. The fractions were monitored at 280 nm. Sample peaks were identified by comparing with retention times of standard peaks. LC LabSolutions software was used for data acquisition and analysis.
Determination of antioxidant activity
Scavenging activity of extracts was analyzed using DPPH radical scavenging assay. The method of Brand-Williams et al. (1995) was followed for DPPH scavenging activity with Gallic acid as standard.
Determination of antidiabetic activity
Inhibition of α-amylase, α-glucosidase and glycation process
α-amylase enzyme inhibition assay was carried out based on the starch-iodine test (Xiao et al. 2006). The absorbance was read at 580 nm with Acarbose as the positive control. α-glucosidase enzyme inhibition assay was done as described by Adisakwattana et al. (2004) with acarbose as the positive control. The reaction was monitored by increase an in absorbance at 405 nm and compared with the enzyme reaction without the extract. Antiglycation assay was performed as previously described by Arom (2005). The relative amount of glycated BSA was quantified based on fluorescence intensity at 370 nm (excitation) and 440 nm (emission). Ascorbic acid was used as the positive control.
Glucose uptake study
Cell lines, cell culture, and cytotoxicity determination
L6 rat skeletal muscle cell line obtained from National Centre for Cell Sciences, Pune, India. L6 cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic–antimycotic mix. Cells were maintained at 37 °C in a humidified 5% CO2 environment. Cytotoxicity of the cumin extract was evaluated against L6 after 24 h incubation by MTT assay (Wilson 2000).
Glucose uptake assay using 2-NBDG
Glucose uptake assay was performed in differentiated L6 cell lines following the method of Chen et al. (2010). Cells were pretreated with different concentration of extracts for 24 h. Media was removed, and cells were washed twice with precooled phosphate buffered saline (PBS) replaced with the fluorescent analogue of glucose, 2-NBDG (100 µM) and incubated for 30 min. The 2-NBDG uptake reaction was stopped by removing the incubation medium and washing the cells twice with pre-cold PBS. Cells in each well were trypsinized and subsequently resuspended in 1 mL buffer for flow cytometry analysis. For each measurement, data from 10,000 single cell events were collected using Fluorescence Activated Cell Sorting (BD FACS Aria II, USA). Rosiglitazone (100 nM) was used as positive control.
Determination of anticancer activity
Cell lines, cell culture and cytotoxicity determination
HT29 colon cancer cell line was obtained from National Centre for Cell Sciences, Pune, India. The growth conditions were similar to that of L6 cell lines described earlier. Cytotoxicity of the cumin extract was evaluated against HT29 after 24 h incubation by MTT assay (Wilson 2000).
Colony forming assay
Colony formation assay was done according to Wang et al. (2008), with slight modifications. The cells at the exponential growth phase were harvested and counted using a hemocytometer. Following this, cells were diluted and seeded at about 500 cells per well of a six-well plate. After 12 h incubation, cells were treated for 24 h with RCM and SCM, compared with untreated control. Every 3 days, the medium was replaced with raw medium containing the compound. After 10 days of treatment, the medium was removed, and the cell colonies were washed with PBS and fixed with 3.7% formaldehyde for 10 min. Wells were rinsed once again with PBS and colonies were stained with 0.2% crystal violet in 10% ethanol for 10 min. Excess stain was removed by washing with PBS. Images were obtained using Nikon Eclipse TS100 microscope (Nikon Instruments Inc., Melville, USA).
Cell cycle analysis
After the treatment with extracts for 24 h, cells were trypsinized and suspended in PBS. The nuclei were stained with propidium iodide (Lim et al. 2005) and subjected to fluorescence-activated cell sorting analysis utilizing the Fluorescence Activated Cell Sorting (BD FACS Aria II, USA). The data were analyzed using BD FACSDivaTM Software v6.1.2.
Apoptosis detection
Early and late apoptosis was detected using Annexin V-FITC Apoptosis Detection kit (Cayman Chemical Company, USA). The phosphatidyl serine exposed on the membranes of apoptotic cells has a strong affinity towards Annexin V, which is measured using flow cytometry (BD FACS Aria II, USA). Camptothecin (50 μM) was used as the positive control.
Scratch wound assay
The scratch wound assay was determined as described by Cory (2011). Cells were grown to confluence, and a thin wound was introduced by scratching with a pipette tip. After scratching cells were rinsed with buffer to remove detached cells from the well followed by the addition of media containing different concentrations of extracts. Images were obtained using Nikon Eclipse TS100 microscope and analyzed by NIS-Elements D (Nikon Instruments Inc., USA) digitally measuring the distance of the gap between the wound edges. The cell migration rate was determined by
where R = regeneration percentage, Tt = gap width after 24 h and T0 = initial gap width.
Statistics
The experimental results are expressed as the mean ± standard deviation of triplicate measurements. The data were analyzed by one-way ANOVA with one factor using SPSS software version 11.5. The level of significance was set at p < 0.05.
Results and discussion
Determination of phytochemicals
Plant foods contain an array of bioactive and polyphenols that are multifunctional and can act as free radical terminators. Consumption of foods and beverages rich in phenolic content is correlated with reduced incidence of lifestyle associated diseases/metabolic disorders (Rana and Bhushan 2016). Thus, the determination of phenolic compounds plays a very important role in perceiving the antioxidant activity.
Estimation of polyphenols and flavonoids
The TPC and TFC of the ethyl acetate and methanol fractions of RC and SC were evaluated. It was found that both SC and RC are potential sources of phenolic compounds. It was interesting to note that the SCM retained 15.89 mg GAE/g dry weight of total phenolics and 7.88 mg QE/g dry weight of total flavonoids, even after the processing it had undergone during the preparation of Ayurvedic formulation (Supplementary Table 1). According to Thippeswamy and Naidu (2005), the TPC of methanol extracts of Cuminum cyminum was 5.2 mg/g. However, TPC of both RC and SC in the present study were higher than the reported values for methanol extracts of Cuminum cyminum. This may attribute to the difference in the variety, geographical area and the season in which Cuminum cyminum was collected.
HPLC profiling and quantification of polyphenols
The phenolic acids in the ethyl acetate and methanol extracts of RC and SC were determined and quantified by high-performance liquid chromatography. Thirteen standard polyphenolic compounds were initially analyzed-(1) Gallic acid, (2) Catechol, (3) Chlorogenic acid, (4) Caffeic acid, (5) Syringic acid, (6) p-coumaric acid, (7) Ferulic acid, (8) Ellagic acid, (9) Myricetin, (10) Cinnamic acid, (11) Quercetin, (12) Kaempferol and (13) Apigenin in 1 mg/mL concentration. The retention times are 7.724, 12.773, 23.807, 25.349, 26.298, 27.567, 28.629, 31.155, 31.814, 33.329, 34.531, 37.586 and 38.322 respectively. Among RC extracts five polyphenols were identified in the RCE (4, 5, 6, 8 and 11) and nine polyphenols were identified RCM (3, 4, 5, 6, 7, 8, 9, 10 and 11) (Table 1). In the case of SC, seven polyphenols were identified in the SCE (1, 5, 6, 7, 8, 10 and 11) and eight in SCM (4, 5, 6, 7, 8, 10, 11 and12) (Table 1). Gallic acid, ferulic acid, and cinnamic acid were the additional polyphenols present in SCE. Chlorogenic acid and myricetin were not present in SCM when compared to the RCM. Kaempferol was present only in SCM. The quantification studies suggest that both RC and SC are rich sources of polyphenols. The chromatogram of ethyl acetate and methanol extracts of RC and SC were shown in Supplementary Figs. 1 and 2 respectively.
Table 1.
Quantification of polyphenols in different extracts of Raw and Spent Cumin
| Polyphenols (Yield in mg/g dry weight of extract) | ||||
|---|---|---|---|---|
| Raw cumin | Spent cumin | |||
| Ethyl acetate | Methanol | Ethyl acetate | Methanol | |
| Gallic acid | – | – | 0.0306 ± 0.002 | – |
| Catechol | – | – | – | – |
| Chlorogenic acid | – | 1.2940 ± 0.420 | – | – |
| Caffeic acid | 0.2799 ± 0.020a | 0.3124 ± 0.012b | – | 0.1964 ± 0.052c |
| Syringic acid | 0.3691 ± 0.034a | 0.1666 ± 0.015a | 0.1804 ± 0.080a | 1.1877 ± 0.408b |
| p-Coumaric acid | 1.2049 ± 0.190a | 8.0591 ± 0.587b | 0.6068 ± 0.044c | 6.4445 ± 1.020d |
| Ferulic acid | – | 5.1587 ± 0.674a | 0.0574 ± 0.019b | 5.8286 ± 0.891c |
| Elagic acid | 0.9419 ± 0.105a | 0.4216 ± 0.075b | 0.6182 ± 0.089ab | 2.1519 ± 0.775c |
| Myrcetin | – | 2.1638 ± 0.238 | – | – |
| Cinnamic acid | – | 1.3221 ± 0.159a | 2.4974 ± 0.921b | 4.3085 ± 0.753c |
| Quercetin | 2.1556 ± 0.817a | 0.7248 ± 0.088 cd | 1.1389 ± 0.452c | 0.2478 ± 0.188d |
| Kaempferol | – | – | – | 0.3461 ± 0.099 |
| Apigenin | – | – | – | – |
Each value represents mean ± SD (standard deviation) from triplicate measurements
Values in a row with different superscript are significantly different and with same superscript are not significantly different (p < 0.05)
Determination of antioxidant efficacy
Polyphenols are gaining a lot of importance as natural antioxidants that can scavenge free radicals and inactivate other pro-oxidants, and can also interact with a number of biologically relevant molecules. Evidence suggests that additive and synergistic interactions of natural antioxidants significantly strengthen the protective effects against oxidative damage in the body (Sudha et al. 2016). The potential of SC as a functional food/nutraceutical ingredient was assessed in terms of DPPH free radical scavenging activity.
The free radical production plays an important role in the development and progression of diabetes and cancer (Rana and Bhushan 2016). Hyperglycemia of diabetes mellitus accelerates free radical generation and attenuates the antioxidant defense system creating oxidative stress, which plays a key role in the development of diabetic complications, especially cardiovascular problems. Free radicals and their derivatives have the potential to modulate gene expression leading to carcinogenesis. So scientists are always in search for identifying new sources of antioxidants that can scavenge free radicals for the management of these diseases prophylactically or curatively.
In the present study, the free radical scavenging efficiency of the extracts was explained by the DPPH scavenging assay. It is an electron transfer based assay measuring the capacity of an antioxidant to reduce an oxidant, which decreases the intensity of colour upon reduction. In our study, it was found that the ethyl acetate extract did not exhibit any scavenging activity. However, the methanol extract of both raw and spent cumin exhibited DPPH radical scavenging activity in a dose-dependent manner. SCM exhibited comparatively higher DPPH radical scavenging activity (238.6 μg/mL) than that of RCM (276.66 μg/mL). The IC50 values obtained from DPPH scavenging assay for extracts and standards are given in Supplementary Table 2.
Determination of antidiabetic activity
Dietary antioxidants play a vital role in managing the oxidative stress and diabetes. They also act on the carbohydrate digestive enzymes, thus influencing the absorption and further metabolism. The antidiabetic potential of the extracts was assessed using α-amylase and α-glucosidase inhibition, antiglycation properties and whether it enhances the glucose uptake ability of the cells.
Inhibition α-amylase, α-glucosidase and glycation process
α-amylase and α-glucosidase were two important enzymes in carbohydrate metabolism. Inhibitors of these enzymes delay the breakdown of carbohydrates in the small intestine decreasing the absorption of glucose from starch and thereby diminishing the postprandial blood glucose excursion. These enzymes are important therapeutic targets for the modulation of postprandial hyperglycemia that is the earliest metabolic abnormality associated with type 2 diabetes mellitus (Ruiz-Ruiz et al. 2015).
The ability of extracts from RC and SC to inhibit α-amylase and α-glucosidase enzymes was analyzed. The IC50 values were shown in Supplementary Table 2. It was found that the SCM (337.22 µg/mL) exhibited more activity than RCM (413.06 µg/mL) in inhibiting the α-amylase enzyme. In the case of α-glucosidase, RCM exhibited better activity than SCM. It may be due to the presence of myricetin in RCM (2.1638 mg/g dry extract) as myricetin is reported to have α-glucosidase inhibition activity (Tadera et al. 2006). However, as we used the crude extracts; it is likely that the synergistic effect of various compounds in the extracts also might have contributed to the above activity of SCM and RCM. It can be seen that the RCE and SCE did not exhibit any activity.
Advanced glycation end products (AGEs) are modifications of proteins or lipids that become nonenzymatically glycated and oxidized after contact with sugars especially glucose. AGEs formed in vivo in hyperglycemic environments contributes to the pathophysiology of vascular disease in diabetes (Suantawee et al. 2015). It was found that SCM (376.22 µg/mL) was displayed antiglycation activity similar to that of RCM (373.31 µg/mL) (Supplementary Table 2). However, both RCE and SCE did not show antiglycation activity.
Glucose uptake assay
Cell homeostasis is maintained primarily by the metabolism of glucose. The glucose is absorbed by muscle and liver cells to store it in the form of glycogen. The glucose uptake process is strictly regulated by the combined effect of hormones, growth factors and transporters. It is well established that diabetes mellitus is characterized by the elevated level of glucose in the blood that mainly arise due to the inefficiency of insulin due to which cells may not be able to uptake glucose. Studies are focused on isolating compounds that enhance the glucose uptake efficiency of cells for the management and treatment of diabetes.
Before the assay, cytotoxicity of the extracts was assessed by MTT assay. The methanolic extracts were tested for cytotoxic effects against L6 cells. Toxicity increased in a concentration dependent manner. The results showed that concentration below 266.59 and 122.22 µg/mL was 50% toxic to L6 cells at 24 h incubation, for RCM and SCM respectively. SCM is more toxic to cells at less concentration than RMC (Supplementary Fig. 3). The increased cytotoxicity of SCM against L6 may be due to the presence of kaempferol (present only in SCM) and higher concentration of syringic acid, ellagic acid, and cinnamic acid as evident from HPLC analysis.
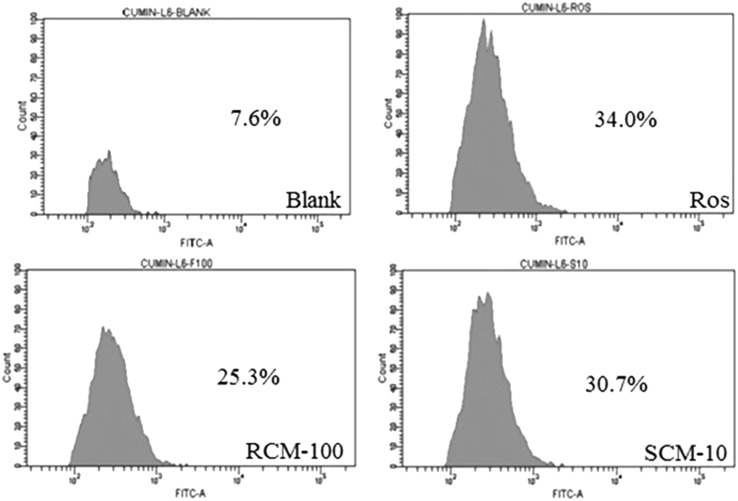
Skeletal muscle is the principal target for insulin action for maintaining glucose homeostasis. Due to the insulin resistance in type 2 diabetes, skeletal muscles fail to uptake glucose. The extracts ability to enhance glucose uptake was recorded by 2NBDG uptake by the L6 cells using flow cytometry. The data analysis showed 34, 25.3 and 30.7 of 2-NBDG uptake in cells treated with Rosiglitazone (100 nM), RCM (100 µg/mL) and SCM (10 µg/mL), respectively. The histogram representing the glucose uptake is shown in Fig. 1. At 10 µg/mL concentration, the SCM enhanced 30.7% of L6 cells to uptake glucose and exhibited potential activity than that of RCM.
Fig. 1.
Flow cytometry analysis of 2-NBDG uptake. FACS analysis of 2-NBDG uptake in differentiated L6 cells by plotting cell count against FITC. The groups contained untreated cells (Blank), Rosiglitazone (Positive control, 100 nM), cells treated with methanol extract of Raw (RCM-100 µg/mL) and Spent (SCM-10 µg/mL) cumin
The glucose uptake study revealed that crude extracts of cumin enhance the glucose uptake by the cells. Cinnamic acid, ferulic acid, ellagic acid and quercetin have been associated with increased glucose uptake activity (Lakshmi et al. 2009; Prabhakar and Doble 2009; Poulose et al. 2011; Dhanya et al. 2014). The higher content of polyphenols like cinnamic acid, ferulic acid, ellagic acid, quercetin, etc. (Table 1) in SCM would have played a significant role in glucose uptake activity compared with RCM. It is reported that the methanolic extract of cumin seeds reduced the blood glucose, glycosylated hemoglobin and improved serum insulin and glycogen in alloxan and streptozotocin induced in diabetic rats (Jagtap and Patil 2010). The blood glucose reduction mentioned in the above study can be linked to the property of extracts of cumin to enhance the cells to uptake glucose as obtained from our studies.
The study indicates RC and SC are a good resource of bioactive molecules which is reported to have good antidiabetic potential. Extracts from RC and SC exhibited promising antidiabetic potential though the activities of the extracts were less than the standards used. The activity of the extracts from SC was comparable to that of RC indicating that it could be exploited for further value addition.
Determination of anticancer activity
The present study confirmed the presence of many biologically active phytochemicals in both spent and raw cumin. It is a well-known fact that the major part of the phytochemicals in plants is bound to dietary fibre. Reports indicate that dietary fibre and bioactive compounds associated with it play a major role in the prevention and management of colorectal cancer (Zeng et al. 2014). Based on this fact, the anticancer activity of cumin extracts was investigated by MTT assay, colony formation assay, cell cycle analysis and scratch wound assay against HT29 colon cancer cells.
Cytotoxicity
The inhibitory effect of methanol extracts on HT29 cells was detected by MTT assay. The results showed that the cells when treated with RCM (100, 200, 300, 400 and 500 µg/mL) and SCM (200, 400, 600, 800 and 1000 µg/mL) for 24 h, inhibited the HT29 cells viability in a dose-dependent manner. According to the MTT assay results (Supplementary Fig. 3), the half maximal inhibitory concentration (IC50) values of RCM and SCM treatment for 24 h were found to be 281.39 ± 4.84 and 452.67 ± 6.62. Hence, working concentration below IC50 values was used in subsequent experiments. According to available research, there are no reports stating the effect of Cuminum cyminum extract against HT29 colon cancer cell lines. As can be observed, the toxicity in terms of the IC50 values of the extracts against normal cells (L6) and in cancer cells were in the similar range for RCM whereas SCM was less toxic to colon cancer cells as compared to L6. Cancer cells have the ability to with stand more chemical stress as compared to normal cells, however the difference in the toxicity of RCM and SCM against HT29 needs to be investigated further.
Colony formation assay
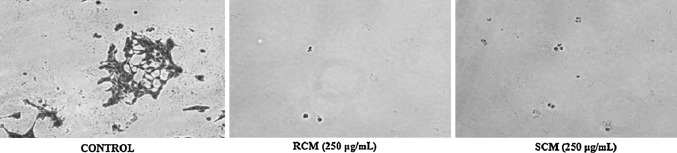
Colony formation assay is an in vitro cell survival assay based on the ability of a single cell to grow into a colony. This assay can detect the cytotoxic effect of a compound or extract, despite mechanism, provided that the agent affects the cell’s reproductive ability to form progenies. It is one of the primary screening techniques for the development of anticancer drugs. Colony formation assay was done to investigate the long-term effect of RCM and SCM on proliferation and colony formation of HT29 cells. As can be seen in Fig. 2, the colony-forming ability of the cell lines was reduced after exposure to the extracts when compared to untreated control. The activity of SCM is comparable to that of RCM. These findings suggest that the components of extracts can disturb the cell proliferation mechanisms and there by inhibits the colony formation. This data thus provide the preliminary idea about the anticancer effect from Cumin. The antiproliferation effect may be due to its role either in inhibiting cell cycle machinery/initiates apoptosis or due to the combined action which is confirmed in further experiments.
Fig. 2.
Colony formation assay. Representative images obtained after 24 h treatment with 250 μg/mL of RCM and SCM
Cell cycle analysis
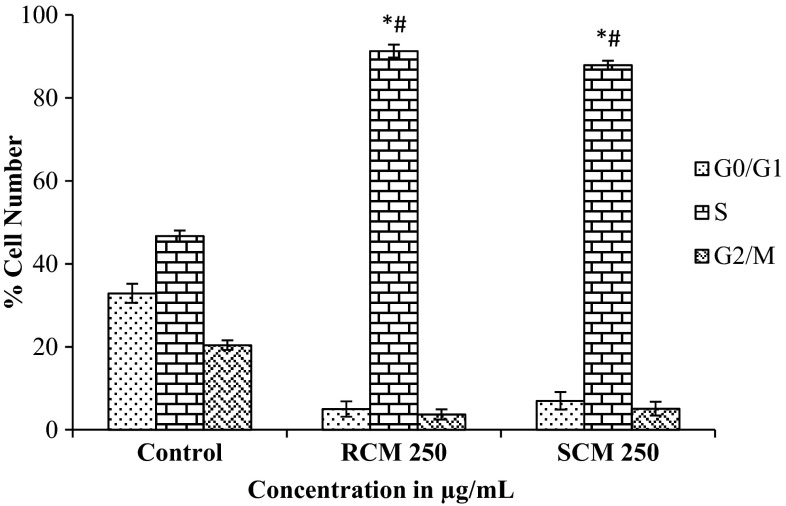
Cell cycle can be described as the process of DNA replication followed by cell division. The cell cycle is strictly monitored by different factors and checkpoints that ensure proper cell division. Cell cycle machinery controls cell proliferation, and cancer is a disease of improper cell proliferation. Hence cancer research is also focusing on the identification of compounds which specifically inhibits cell cycle machinery so that progression of the cancer cell can be prevented. HT29 cells were treated with 250 µg/mL of SCM and RCM for 24 h compared with untreated control. Flow cytometry was performed, and the results showed a significant increase in the percentage of S-phase cells in a dose-dependent manner (Fig. 3). The methanol extract of spent cumin, at higher concentration, efficiently inhibited the HT29 cells at S phase of the cell cycle. The results of inhibition of cells at S phase of the cell cycle were comparable between the SC (89.5%) and RC (91.3%). Together, these results suggest cumin extracts may exert its anti-cancer effect through cell cycle arrest.
Fig. 3.
Cell cycle analysis. After 24 h treatment with 250 μg/mL concentration of RCM and SCM, cell cycle analysis was performed by FACS. Percentages of cells in G0/G1, S and G2/M phases are shown in this figure. Each value represents mean ± SD (standard deviation) from triplicate measurements. *Values of S phase of RCM and SCM are significantly different from value of control S phase (p < 0.05). #Values of S phase of RCM and SCM are significantly different (p < 0.05)
Different spices have been reported to exhibit anticancer effect by inhibiting cell cycle, and this potential is usually attributed to phytochemicals present in them. Polyphenols identified in cumin extracts have been contributed significantly to the inhibition of cell cycle. It is a well-known fact that cell cycle machinery regulates cell proliferation, and cancer is a disease that occurs due to improper cell proliferation. Thus, the link between the cell cycle and cancer is apparently evident. Mutations occurring in protooncogenes and tumor suppressor genes play a significant role in cell cycle malfunction that leads to cancer development (Collins et al. 1997). The results from cell cycle analysis showed that Cumin extracts are capable of arresting the cell cycle and inhibiting cell proliferation after that. The extracts may have interfered with the formation of Cdk1 and Cyclin B complex that prevents cells from entering the G2/M phase of cell cycle. Further studies are required for confirmation.
Detection of apoptosis
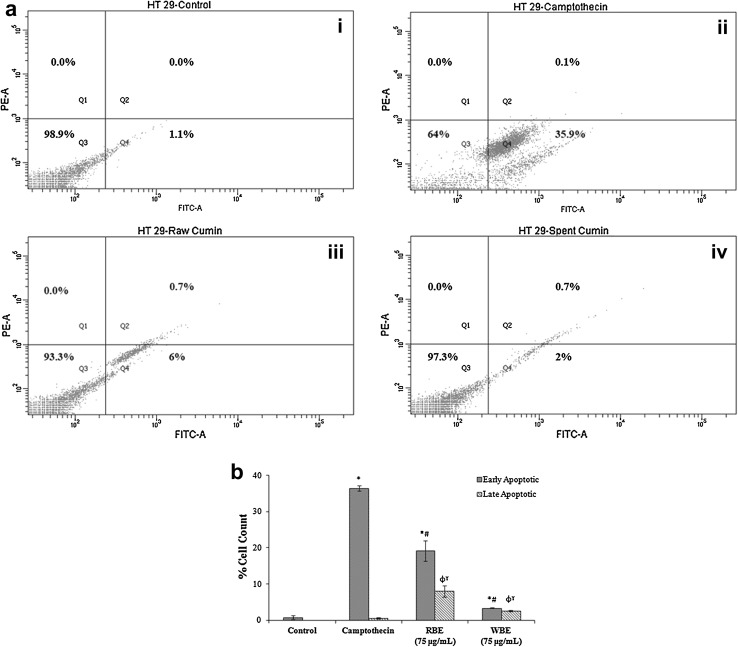
Apoptosis is a process by which a sequence of programmed events leads to the death of aged cells in our body. Cancer research has advanced much better in understanding in the role of oncogenic mutation which disrupts the apoptotic pathway that leads to tumour initiation, progression or metastasis. Phytochemicals especially polyphenols are known to skip out this oncogenic mutation and induce apoptosis in cancer cells (Chen and Kong 2005). Hence, we would like to know the effect of cumin extracts on apoptosis for which we have performed the Annexin V-FITC and PI combination staining method. The flow cytometry results depicted that extracts of SC can induce apoptosis in HT29 colon cancer cells, though not significant. About, 2.45% of cells were in the early stage of apoptosis after treating with SCM whereas RCM induced apoptosis in 5.65% cells (Fig. 4a, b). Further studies may identify specific compounds from cumin extracts which can be used as a treatment strategy to induce apoptosis in cancer cells.
Fig. 4.
Apoptosis detection. The four quadrants of rectangular gate—Q1, Q2, Q3 and Q4 represents necrotic, late apoptotic, live and early apoptotic cells respectively. a Representative dot plot graph of cells: i Untreated control, ii Positive control—Camptothecin, iii Raw Cumin (250 μg/mL) and iv Spent Cumin (250 μg/mL); b Percentage of cells in early and late apoptotic stage. Each value represents mean ± SD (standard deviation) from triplicate measurements. *ϕ significantly different from control (early stage and late apoptosis respectively; p < 0.05). #ϒ significantly different from Camptothecin (early stage and late stage apoptosis respectively; p < 0.05)
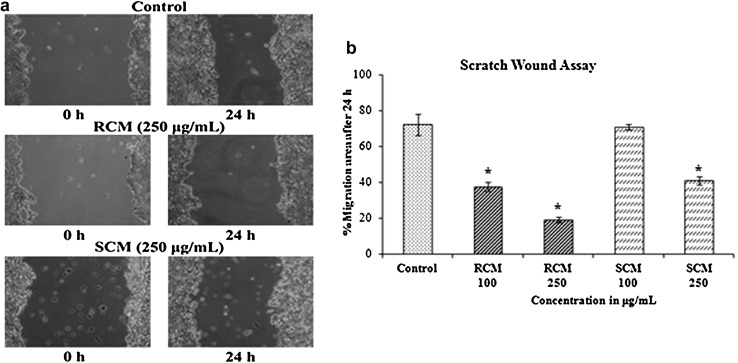
Scratch wound assay
The ability of extracts of RCM and SCM (100 and 250 μg/mL) to inhibit the metastatic potential of tumour cells to migrate was assessed with the scratch wound assay, and the representative photomicrographs are shown in Fig. 5. The percentage of migration area covered after 24 h was 72.46 ± 5.9% for control cells; 37.56 ± 2.4 and 19.28 ± 1.4% for RCM (100 and 250 µg/mL, respectively) treated cells; and 71.07 ± 1.5 and 41.02 ± 2.2% for SCM (100 and 250 µg/mL, respectively) treated cells. The anti-metastatic effect of RCM and SCM on HT29 cells was confirmed to be dose-dependent. The results are promising and give an idea of the effect of cumin extracts in inhibiting the migration of cancer cells from tumour border to the blood vessels. Further studies are required to analyze the mechanism behind this property.
Fig. 5.
Scratch Wound Assay. a Representative images for scratch wound assay at 250 μg/mL concentration of RC and SC. b Average percentage of cell migration of three independent experiments ± standard deviation. *Significantly different from control (p < 0.05)
Even though the toxicity of the extracts against the cells under study were less, further studies demonstrated that phenolic compounds extracted from spent cumin beneficially modulated all three distinct phases of carcinogenesis (initiation, promotion, and progression) by altering pathways involved in the control of cell division and growth, apoptosis and metastasis and the activity was comparable to that of raw cumin. The RCM is rich in p-coumaric acid, chlorogenic acid and myricetin whereas p-coumaric acid, syringic acid, ellagic acid, cinnamic acid and ferulic acid were the major compounds in the SCM. All these phenolic compounds are reported to possess anticancer properties (Jaganathan et al. 2013; Xu et al. 2013; Mohamed Salah et al. 2011; Zhang et al. 2014; Niero and Machado-Santelli 2013; Kim et al. 2013). The activities of the extracts of SC and RC may be attributed to the presence of these compounds. Furthermore there are no previous reports stating the anticancer effect of Cumin cyminum against colon cancer.
Conclusion
The in vitro studies in terms of inhibition of α-amylase, α-glucosidase and glycation process and glucose uptake have shown very positive results for the treatment of diabetes mellitus. One of the reasons for the onset of cancer is the accumulation of free radicals that initiates DNA damage and enhances the conversion of procarcinogens to carcinogens. The radical scavenging activity of cumin extracts helps in quenching the free radicals that may initiate carcinogenesis. The extracts were able to inhibit cell cycle and enhance apoptosis in HT29 cancer cells that prevents further promotion of colon cancer. Apart from this the scratch wound assay revealed that the cumin extracts inhibited metastasis. Thus the results indicated that Spent Cumin residue from the Ayurvedic industry is a potential source of bioactive compounds with antidiabetic and anticancer activity. The availability of large quantities of the spent cumin residue at little or no cost suggests their use in functional food and nutraceutical formulation for the prevention and management of lifestyle associated diseases. The results are of great significant to Ayurvedic/spices processing industries in terms of better utilization of the by-product utilization for profit generation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This research supported by Council of Scientific and Industrial Research (CSIR) and Indian Council of Medical Research (ICMR). Authors gratefully acknowledge Arya Vaidya Sala, Kottakkal (AVS: Kerala, India) for the active scientific collaboration, identification of the thrust areas for value addition of the processed waste in addition to the supply of spent and raw material samples. Authors are also thankful to Department of Science and Technology (DST), Government of India for providing the financial support for the collaborative research activity.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest in this work.
References
- Adisakwattana S, Sookkongwaree K, Roengsumran S, Petsom A, Ngamrojnavanich N, Chavasiri W, Deesamer S, Yibchok-Anun S. Structure-activity relationships of trans-cinnamic acid derivatives on α-glucosidase inhibition. Bioorg Med Chem Lett. 2004;14:2893–2896. doi: 10.1016/j.bmcl.2004.03.037. [DOI] [PubMed] [Google Scholar]
- Arom J. In vitro antiglycation activity of Arbutin. Naresuan Univ J. 2005;13(2):35–41. [Google Scholar]
- Balasundram N, Sundram K, Samman S. Phenolic compounds in plants and agri-industrial by-products: antioxidant activity, occurrence, and potential uses. Food Chem. 2006;99:191–203. doi: 10.1016/j.foodchem.2005.07.042. [DOI] [Google Scholar]
- Ballabh B, Chaurasia OP. Traditional medicinal plants of cold desert Ladakh- used in treatment of cold, cough and fever. J Ethnopharmacol. 2007;112(2):341–349. doi: 10.1016/j.jep.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Carciochi RA, Manrique DE, Dimitrov K. Optimization of antioxidant phenolic compounds extraction from quinoa (Chenopodium quinoa) seeds. J Food Sci Technol. 2015;52(7):4396–4404. doi: 10.1007/s13197-014-1514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Kong AN. Dietary cancer-chemopreventive compounds from signalling and gene expression to pharmacological effects. Trends Pharmacol Sci. 2005;26(6):318–326. doi: 10.1016/j.tips.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Chen QC, Zhang WY, Jin W, Lee IS, Min BS, Jung HJ, Na M, Lee S, Bae K. Flavonoids and isoflavonoids from Sophorae Flos improve glucose uptake in vitro. Planta Med. 2010;76(1):79–81. doi: 10.1055/s-0029-1185944. [DOI] [PubMed] [Google Scholar]
- Collins K, Jacks T, Pavletich NP. The cell cycle and cancer. PNAS. 1997;94(7):2776–2778. doi: 10.1073/pnas.94.7.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory G. Scratch wound assay. Methods Mol Biol. 2011;769:25–30. doi: 10.1007/978-1-61779-207-6_2. [DOI] [PubMed] [Google Scholar]
- Dhanya R, Arun KB, Syama HP, Nisha P, Sundaresan A, Santhosh Kumar TR, Jayamurthy P. Rutin and quercetin enhance glucose uptake in L6 myotubes under oxidative stress induced by tertiary butyl hydrogen peroxide. Food Chem. 2014;158:546–554. doi: 10.1016/j.foodchem.2014.02.151. [DOI] [PubMed] [Google Scholar]
- Jaganathan SK, Supriyanto E, Mandal M. Events associated with apoptotic effect of p-Coumaric acid in HCT-15 colon cancer cells. World J Gastointest Oncol. 2013;19(43):7726–7734. doi: 10.3748/wjg.v19.i43.7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagtap AG, Patil PB. Antihyperglycemic activity and inhibition of advanced glycation end product formation by Cuminum cyminum in streptozotocin induced diabetic rats. Food Chem Toxicol. 2010;48(8–9):2030–2036. doi: 10.1016/j.fct.2010.04.048. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Ryu K, Kang JH, Choi AJ, Kim T, Oh JM. Anticancer activity of ferulic acid-inorganic nanohybrids synthesized via two different hybridization routes, reconstruction and exfoliation-reassembly. Sci World J. 2013 doi: 10.1155/2013/421967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmi BS, Sujatha S, Anand S, Sangeetha KN, Narayanan RB, Katiyar C, Kanaujia A, Duggar R, Singh Y, Srinivas K, Bansal V, Sarin S, Tandon R, Sharma S, Singh S. Cinnamic acid, from the bark of Cinnamomum cassia, regulates glucose transport via activation of GLUT4 on L6 myotubes in a phosphatidyl inositol 3-kinase-independent manner. J Diabetes. 2009;1(2):99–106. doi: 10.1111/j.1753-0407.2009.00022.x. [DOI] [PubMed] [Google Scholar]
- Lim DY, Tyner AL, Park JB, Lee JY, Choi YH, Park JH. Inhibition of colon cancer cell proliferation by the dietary compound conjugated linoleic acid is mediated by the CDK inhibitor p21 (CIP1/WAF1) J Cell Physiol. 2005;205(1):107–113. doi: 10.1002/jcp.20380. [DOI] [PubMed] [Google Scholar]
- Mathew AG. Future of spice and floral extract. Indian Perfum. 2004;48:35–40. [Google Scholar]
- Mohamed Salah IA, Mohamed A, Rajaa A, Radhika B, Ghanim A, Mathew K. Anti-mitogenic and chemo-sensitizing activities of syringic acid in human colorectal cancer cells: potential molecular mechanisms of action. FASEB J. 2011;25(621):2. [Google Scholar]
- Niero EL, Machado-Santelli GM. Cinnamic acid induces apoptotic cell death and cytoskeleton disruption in human melanoma cells. J Exp Clin Can Res. 2013;32:31. doi: 10.1186/1756-9966-32-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulose N, Vishnu Prasad CN, Nidhina Haridas PA, Anilkumar G. Ellagic acid stimulates glucose transport in adipocytes and muscles through AMPK mediated pathway. Diabetes Metab J. 2011;2:149. doi: 10.4172/2155-6156.1000149. [DOI] [Google Scholar]
- Prabhakar PK, Doble M. Synergistic effect of phytochemicals in combination with hypoglycemic drugs on glucose uptake in myotubes. Phytomedicine. 2009;16(12):1119–1126. doi: 10.1016/j.phymed.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Rana S, Bhushan S. Apple phenolics as nutraceuticals: assessment, analysis and application. J Food Sci Technol. 2016;53:1727–1738. doi: 10.1007/s13197-015-2093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Delgado MA, Malovana S, Perez JP, Borges T, Montelongo FJG. Separation of phenolic compounds by high performance liquid chromatography with absorbance and fluorimetric detection. J Chromatogr A. 2001;912:249–257. doi: 10.1016/S0021-9673(01)00598-2. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ruiz JC, Moguel-Ordoñez YB, Matus-Basto AJ, Segura-Campos MR. Antidiabetic and antioxidant activity of Stevia rebaudiana extracts (Var. Morita) and their incorporation into a potential functional bread. J Food Sci Technol. 2015;52(12):7894–7903. doi: 10.1007/s13197-015-1883-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma LK, Agarwal D, Rathore SS, Malhotra SK, Saxena SN. Effect of cryogenic grinding on volatile and fatty oil constituents of cumin (Cuminum cyminum L.) genotypes. J Food Sci Technol. 2016;53:2827–2834. doi: 10.1007/s13197-016-2258-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowbhagya HB, Florence Suma P, Mahadevamma S, Tharanathan RN. Spent residue from cumin—a potential source of dietary fiber. Food Chem. 2007;104:1220–1225. doi: 10.1016/j.foodchem.2007.01.066. [DOI] [Google Scholar]
- Suantawee T, Wesarachanon K, Anantsuphasak K, Daenphetploy T, Thien-Ngern S, Thilavech T, Pasukamonset P, Ngamukote S, Adisakwattana S. Protein glycation inhibitory activity and antioxidant capacity of clove extract. J Food Sci Technol. 2015;52(6):3843–3850. doi: 10.1007/s13197-014-1452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudha ML, Dharmesh SM, Pyanam H, Bhimangouder SV, Eipson SW, Somasundaram R, Nanjarajurs SM. Antioxidant and cyto/DNA protective properties of apple pomace enriched bakery products. J Food Sci Technol. 2016;53(4):1909–1918. doi: 10.1007/s13197-015-2151-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadera K, Minami Y, Takamatsu K, Matsuoka T. Inhibition of alpha-glucosidase and alpha-amylase by flavonoids. J Nutr Sci Vitaminol. 2006;52(2):149–153. doi: 10.3177/jnsv.52.149. [DOI] [PubMed] [Google Scholar]
- Thippeswamy NB, Naidu KA. Antioxidant potency of cumin varieties (cumin, black cumin and bitter cumin) on antioxidant systems. Eur Food Res Technol. 2005;220:472–476. doi: 10.1007/s00217-004-1087-y. [DOI] [Google Scholar]
- Wang X, Hawk N, Yue P, Kauh J, Ramalingam SS, Fu H, Khuri FR, Sun S. Overcoming mTOR inhibition-induced paradoxical activation of survival signalling pathways enhances mTOR inhibitors’ anticancer efficacy. Cancer Biol Ther. 2008;7(12):1952–1958. doi: 10.4161/cbt.7.12.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AP. Cytotoxicity and viability assay in animal cell culture: a practical approach. 3. Oxford: Oxford University Press; 2000. [Google Scholar]
- Xiao Z, Storms R, Tsang AA. Quantitative starch-iodine method for measuring alpha-amylase and glucoamylase. Anal Biochem. 2006;351(1):146–148. doi: 10.1016/j.ab.2006.01.036. [DOI] [PubMed] [Google Scholar]
- Xu R, Kang Q, Ren J, Li Z, Xu X. Antitumor molecular mechanism of chlorogenic acid on inducting genes GSK-3β and APC and inhibiting gene β-Catenin. J Anal Methods Chem. 2013 doi: 10.1155/2013/951319. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zeng H, Lazarova DL, Bordonaro M. Mechanisms linking dietary fiber gut microbiota and colon cancer prevention. World J Gastointest Oncol. 2014;6(2):41–51. doi: 10.4251/wjgo.v6.i2.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HM, Zhao L, Li H, Xu H, Chen WW, Tao L. Research progress on the anticarcinogenic actions and mechanisms of ellagic acid. Cancer Biol Med. 2014;11(2):92–100. doi: 10.7497/j.issn.2095-3941.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.