Abstract
Although slugs and snails play important roles in terrestrial ecosystems and cause considerable damage on a variety of crop plants, knowledge about the mechanisms of plant immunity to mollusks is limited. We found slugs to be natural herbivores of Arabidopsis thaliana and therefore investigated possible resistance mechanisms of this species against several molluskan herbivores. Treating wounded leaves with the mucus residue (“slime trail”) of the Spanish slug Arion lusitanicus increased wound-induced jasmonate levels, suggesting the presence of defense elicitors in the mucus. Plants deficient in jasmonate biosynthesis and signaling suffered more damage by molluskan herbivores in the laboratory and in the field, demonstrating that JA-mediated defenses protect A. thaliana against slugs and snails. Furthermore, experiments using A. thaliana mutants with altered levels of specific glucosinolate classes revealed the importance of aliphatic glucosinolates in defending leaves and reproductive structures against mollusks. The presence in mollusk feces of known and novel metabolites arising from glutathione conjugation with glucosinolate hydrolysis products suggests that molluskan herbivores actively detoxify glucosinolates. Higher levels of aliphatic glucosinolates were found in plants during the night compared to the day, which correlated well with the nocturnal activity rhythms of slugs and snails. Our data highlight the function of well-known anti-herbivore defense pathways in resistance against slugs and snails and suggest an important role for the diurnal regulation of defense metabolites against nocturnal molluskan herbivores.
Keywords: mollusk, slug, snail, plant defense, jasmonates, glucosinolates
Introduction
Plants interact with complex communities of herbivores. While interactions of plants with arthropods have been studied in detail (Erb et al. 2012; Howe& Jander 2008; Wu& Baldwin 2010), the molecular and chemical cues important for plant-mollusk interactions are not well described, despite the fact that molluskan herbivores can have large effects on plant performance and biodiversity (Allan& Crawley 2011) and cause considerable damage in agriculture. In cabbage, sunflowers and maize for example, slug feeding can be the dominant type of herbivory during the crop establishment phase (Barker 2002).
In general, plants show strong defensive responses upon perceiving an herbivore. Specificity in defense induction is mediated by the perception of herbivore-associated molecular patterns (HAMPS) that vary across herbivore species. HAMPS include low molecular weight compounds or enzymes present in insect oral secretions that are perceived during feeding and amplify wound induced plant responses (Mithofer& Boland 2008). A common pathway that is activated in reaction to both wounding and herbivory is an increase in the biosynthesis of oxylipins, including jasmonic acid (JA) and its isoleucine conjugate (JA-Ile). In several plant species, such as thale cress (Arabidopsis thaliana), wild tobacco (Nicotiana attenuata), tomato (Solanum leucopersicum), pea (Pisum sativum) and maize (Zea mays), HAMP perception specifically triggers JA biosynthesis (Halitschke et al. 2003; Schäfer et al. 2011; Schmelz et al. 2009), which in turn regulates the biosynthesis of defense metabolites (De Geyter et al. 2012). Consequently, plants with diminished JA production or perception are generally more susceptible to a wide range of herbivores in the laboratory and the field (Farmer& Dubugnon 2009; Kessler 2004). Many insect herbivores are active during the day and recent experiments in A. thaliana show that JA biosynthesis and signaling can be synchronized with the behavior of an insect herbivore (Goodspeed et al. 2012).
Due to its short life cycle, small genome, and ease of transformation, A. thaliana has served as a valuable model to decipher JA signaling and defense. A. thaliana plants maintain within their leaves and inflorescences a diverse cache of JA-regulated secondary metabolites. Glucosinolates are sulfur- and nitrogen-containing natural products found in members of the plant order Capparales. Although biologically inactive in their intact form, glucosinolates can be hydrolyzed through the action of the enzyme myrosinase, which leads to the formation of breakdown products such as isothiocyanates, nitriles and epithionitriles in macerated tissues (Halkier& Gershenzon 2006). Breakdown products of glucosinolates appear to be deterrent to most herbivores although some insects have the ability to detoxify glucosinolates upon ingestion (Falk& Gershenzon 2007; Ratzka et al. 2002; Wittstock et al. 2004). Several mutant lines of A. thaliana have been developed that are altered in glucosinolate metabolism, including tgg1xtgg2 (lacking myrosinase), cyp79B2xB3 (reduced levels of indolic glucosinolates), myb28x29 (reduced levels of aliphatic glucosinolates) and 35S:ESP (overexpression of epithiospecifier protein resulting in higher levels of nitriles vs. isothiocyanates) (Barth& Jander 2006; Burow et al. 2006b; Sonderby et al. 2007; Zhao et al. 2002). These mutant lines have been used to test the effect of altered glucosinolate metabolism on insect herbivory, utilizing many Lepidopteran species (Müller et al. 2010). However, since A. thaliana accessions in temperate climates generally overwinter as seeds or rosettes without flowering until the spring (Shindo et al. 2007), and most caterpillars emerge in the summer, it is unlikely that plant tissue is available for caterpillar herbivory under natural conditions in northern Europe. It has been proposed that the major herbivores of A. thaliana in spring are mollusks rather than insects (Harvey et al. 2007). However, this idea was based on the observation of slime trails around damaged plants rather than actual herbivore attack rates. To date, no information on the identity of the slug or snail species that attack A. thaliana in nature is available.
In this manuscript, we show that mollusks are natural defoliators of A. thaliana. To gain insights into the molecular and chemical basis of plant-mollusk interactions, we conducted a series of experiments to test 1) if A. thaliana responds to mucus of a natural slug herbivore; 2) if the JA pathway protects plants against slugs, 3) if glucosinolates are important for slug and snail resistance, 4) if mollusks metabolize glucosinolates and 5) if diurnal glucosinolate patterns are correlated with the activity of molluskan herbivores. By characterizing the resistance of JA- and glucosinolate deficient A. thaliana plants to slugs and snails in the laboratory and the field, and by profiling JA and glucosinolate accumulation patterns of different genotypes, we provide evidence for the key role of these defenses in the interaction with mollusks.
Materials and Methods
Plants and cultivation
All A. thaliana lines used in this study were derived from the Columbia-0 (Col-0) wild type background. The tgg1xtgg2 double knockout mutant, the ESP over-expressing line 35S:ESP (line 38.1), the myb28x29 double knockout mutant, the cyp79B2xcB3 double knockout, the coi1-16, coi1-1 and aos (allene oxide cyclase) lines were provided by Georg Jander, Ute Wittstock, Dan Kliebenstein, Inga Mewis, Gustavo Bonaventure and Philippe Reymond, respectively. Plants for experiments were grown in a standard growth substrate (Fruhstorfer Nullerde:vermiculite:sand, 8:1:1) in a climate chamber (21°C, 55% relative humidity and 130 µmol m-2s-1 photosynthetically active radiation) with a photoperiod of 10h light/14h dark for pre-bolting plants. To mimic light conditions during seasonal progression, a photoperiod of 16h light/ 8h dark was used for flowering plants.
Mollusk cultivation
Arion lusitanicus, a slug that was found to feed on A. thaliana in nature, was used for choice assays with glucosinolate mutants. A. lusitanicus were maintained in clear plastic cages (38 x 24 x 19cm) lined with moist paper towel, and fed a combination of lettuce, cucumber and cat food diet. The cat food diet was similar to that of Hagnell et al (2006) except that 10g of sucrose was added, agar was reduced to 5g and water was increased to 450ml. Cages were cleaned every 2-4 days.
The snails and slugs used for the other experiments, including species that occur sympatrically with A. thaliana (A. lusitanicus, Cepaea hortensis, Deroceras laeve, Deroceras reticulatum, Helix pomatia, Lehmannia valentiana), and one exotic species (Achatina fulica) were cultivated in a growth chamber (Snijders scientific, Tilburg, The Netherlands), under constant humidity of 80%, a temperature of 16-20° C, and short day conditions (9.5 h light/ 13.5h dark). All slugs and snails were collected in Jena. Achatina fulica is an important pest in many tropical countries and was provided by Dr. Gustavo Bonaventura. Different numbers of snails were separated in bigger plastic boxes (OKT easyfresh, Sternwede, Germany; 26.5 x 13 x 15 cm) dependent on their size and species. Slugs were maintained in smaller boxes (10.5 x 4 x 8 cm) and the number of individuals in one box was dependent on their size and social compatibility. Potatoes, lettuce and cucumber were provided as, with the addition of cuttlebone for calcium (ArtNr.5050, TRIXIE Heimtierbedarf GmbH & Co. KG, Tarp, Germany). The food was changed twice a week and the boxes were cleaned and provided with fresh tissue paper and moistened with tap water at the same time.
Field Experiments
For the field experiments in Michigan (USA), seeds of the Col-0 ecotype and coi1-1 mutants were surface sterilized and sown on 0.5 x MS media and 1% sucrose. For coi1, plates were further supplemented with 50 μM JA to select homozygous coi1 mutants as described previously (Xie et al. 1998). Plates were placed in a cold room without light for 4 days. On March 24 (2008), plates were moved to a growth chamber with 12-h days at 20°C. After 7 days, seedlings were transplanted into plug trays containing a 1.1 mixture of Premier Pro-Mix and Metromix. Seedlings were transplanted on April 23 at the field site at the Michigan State University Southwest Michigan Research and Extension Center (42°5’33.72"N, 86°21’22.76"W). Plants were spaced 1/3 m within rows and 1 m between rows. Leaf damage was recorded for 117 coi1-1 and 115 Col-0 plants on May 5 between 5 and 7 pm. Plants were inspected with naked eyes from above; the only herbivores that were detected feeding on the plants were slugs of the species Deroceras laeve. Slime trails around damaged leaves also indicated that molluskan herbivores have contributed to the observed damage. Slugs were observed on several days before and after recording leaf damage. Statistical analysis was done with R (http://www.r-project.org/) using the Mann-Whitney U test (Wilcoxon test).
Mucus treatments and hormone measurements
To quantify the induction of JA following simulated slug herbivory, four full-size rosette leaves from five week-old or flowering A. thaliana (Col-0) plants were wounded along the entire leaf lamina with a fabric pattern wheel and water or mucus of A. lusitanicus was subsequently applied to the leaves. Unwounded leaves were harvested as controls. The sticky nature of collected A. lusitanicus crawling mucus does not allow uniform leaf treatments. Instead, mucus application was realized by having A. lusitanicus crawl over the wounded leaves. Water-treated leaves were gently struck with a finger (covered with glove) to mimic the physical pressure of crawling slugs. Plants were kept under constant relative humidity (80%) and temperature (16-20° C) under short day conditions (9.5 h light/ 13.5h dark) to mimic conditions under which slug attack is likely to happen. Treated leaves were harvested in liquid nitrogen two and eight hours after treatments, and JA/JA-Ile were quantified as described (Schäfer et al. 2011). Statistical analysis was done with SPSS (http://www-01.ibm.com/software/de/analytics/spss/). The different treatments were analyzed by using ANOVA and post-hoc Tukey HSD. Data from flowering plants were log10 transformed to fulfill the assumption of homogeneity of variance. Details of the hormone measurements can be found in the supplemental material.
Laboratory choice and non-choice bioassays
To test the effect of glucosinolates and JA-dependent defenses on A. lusitanicus slugs, a series of choice and no-choice assays were conducted. First we used several A. thaliana lines (myb28x29, tgg1xtgg2, cyp79b2xb3, 35S:ESP) with altered glucosinolate patterns to test the effect of these secondary metabolites on A. lusticanicus. Individual slugs weighing 2-8 g that had been collected in the wild and raised for a minimum of 8 weeks on diet without glucosinolates, chiefly a mixture of cucumbers and cat food, were starved for 48 hours. Bioassays were carried out in plastic trays with transparent lids (38 x 24 x 19 cm) provided with air holes covered by nylon mesh. Whole plants were placed side by side at one end of the chamber and the starved slug was placed in a pot lying on its side at the other end of the chamber. The position of the control (Col-0) vs. the mutant was switched from left to right in adjacent chambers. Slugs had no trouble reaching the plants in a little as 1 min and were allowed to feed for 48 hours on whole plants. Plants were photographed prior to the addition of the slugs and at the end of the experiment, and damage was estimated visually on a scale of 0 to 5 with 0 = no damage, 1 = 1-5% eaten, 2 = 5-25% eaten, 3 = 25-50% eaten, 4 = 50-75% eaten, and 5 = 75-100% eaten. Bioassays of inflorescences were carried out in the same fashion as for whole plants except that cut stalks were placed in a 15 ml falcon tube containing ½ strength MS medium and sealed with parafilm. Tubes with inflorescences were placed on their sides to fit in the chambers, and care was taken to make sure inflorescences of the two different plants did not touch each other. For all choice assays, data represent the mean ± SE of three experiments with 20 replicates each. Each replicate consisted of a single slug; animals were tested only once and not reused.
In a second experiment, individual 4 month-old A. lusitanicus collected and reared in captivity for over 12 weeks, as described above, were fed either Col-0 inflorescences only or myb28x29 inflorescences only, weighed every 2-3 days for 3 weeks, and provided with fresh inflorescences after each weighing to ensure ad libitum feeding. Each treatment was replicated on six individuals with each slug kept separately in a single chamber.
We also tested if JA and aliphatic glucosinolates improve plant resistance against other slug and snail species, including the slugs D. laeve and A. distinctus and the snails H. pomatia and C. hortensis. For these experiments, slugs and snails were added to a plastic box (OKT easyfresh; 26.5 x 13 x 15 cm, OKT Germany GmbH, Stemwede, Germany) filled with soil (Fruhstorfer Nullerde:vermiculite:sand, 8:1:1) containing one rosette stage A. thaliana wild type plant (Col-0) and one mutant plant, either coi1-16 or aos. Boxes were placed in a growth chamber (Snijders scientific, Tilburg, The Netherlands), under a constant humidity of 80%, a temperature of 16-20° C, and short day conditions (9.5 h light/ 13.5h dark). Five replicate boxes with one slug (two slugs for D. leave since they were smaller) and one snail were used for each comparison. Each molluskan species was tested separately. Percentage of damaged leaf area was estimated at various times from 1-15 days. Statistical differences were analyzed by two way repeated measures ANOVA using SigmaPlot (www.systat.de).
Glucosinolate and glucosinolate product analysis
To quantify glucosinolates in leaves and inflorescences, 20-50 mg freeze-dried (mutants) and 100 mg fresh leaf (diurnal samples) material was extracted and analyzed by HPLC as previously described (Burow et al. 2006a, see also supplemental material). The plants included the Col-0 line and the four transgenic lines used in feeding experiments (Tables 1, 3) and plants used in the diurnal variation experiment (Figs. 5, S7).
Table 1. Glucosinolate content in tissues of Arabidopsis mutants.
| Glucosinolate content in rosette leaves (µmol*g dry weight-1) | |||||
|---|---|---|---|---|---|
| Side chain1 | Col-0 | myb28x29 | tgg1xtgg2 | cyp79b2xb3 | 35S:ESP |
| 3MSOP | 1.24 ± 0.17 | n.d. | 1.36 ± 0.25 | 0.91 ± 0.18 | 1.42 ± 0.25 |
| 4MSOB | 9.90 ± 1.5 | 0.017 ± 0.01 | 10.75 ± 1.79 | 7.17 ± 1.31 | 10.25 ± 1.79 |
| 5MSOP | 0.37 ± 0.04 | n.d. | 0.43 ± 0.04 | 0.28 ± 0.04 | 0.35 ± 0.04 |
| 7MSOH | 0.13 ± 0.03 | n.d. | 0.13 ± 0.04 | 0.13 ± 0.05 | 0.16 ± 0.04 |
| 4MTB | 0.53 ± 0.23 | n.d. | 0.50 ± 0.21 | 0.41 ± 0.19 | 0.47 ± 0.21 |
| 8MSOO | 0.66 ± 0.19 | n.d. | 0.62 ± 0.17 | 0.47 ± 0.13 | 0.73 ± 0.17 |
| I3M | 2.03 ± 0.11 | 3.21 ± 0.32 | 2.13 ± 0.21 | 0.01 ± 0.01 | 1.98 ± 0.21 |
| 4MOI3M | 0.95 ± 0.21 | 0.78 ± 0.09 | 0.95 ± 0.19 | n.d. | 0.66 ± 0.19 |
| 1MOI3M | 0.20 ± 0.08 | 0.18 ± 0.07 | 0.08 ± 0.02 | n.d. | 0.13 ± 0.02 |
| total | 16.00 ± 1.83 | 4.19 ± 0.38 | 16.95 ± 2.19 | 9.38 ± 1.89 | 16.15 ± 2.30 |
| Glucosinolate content in inflorescences (µmol*g dry weight-1) | |||||
| Side chain1 | Col-0 | myb28x29 | tgg1xtgg2 | cyp79b2xb3 | 35S:ESP |
| 3MSOP | 2.82 ± 0.19 | n.d. | 5.58 ±0.58 | 2.09 ± 0.42 | 2.30 ± 0.84 |
| 4MSOB | 25.71 ± 2.68 | n.d. | 58.13 ± 5.32 | 20.39 ± 1.39 | 21.60 ± 5.55 |
| 5MSOP | 0.47 ± 0.22 | n.d. | 1.89 ± 0.18 | 0.61 ±0.03 | 0.55 ± 0.13 |
| 7MSOH | 0.38 ± .04 | n.d. | 0.78 ± 0.05 | 0.30 ±0.04 | 0.33 ± 0.10 |
| 4MTB | 0.31 ± 0.10 | n.d. | 0.57 ± 0.06 | 0.15 ± 0.01 | 0.28 ± 0.03 |
| 8MSOO | 1.65 ± 0.22 | n.d. | 3.95 ± 0.18 | 1.08 ± 0.13 | 2.04 ± 0.74 |
| I3M | 1.22 ± 0.35 | 0.96 ± 0.13 | 3.54 ± 0.23 | n.d. | 1.40 ± 0.56 |
| 4MOI3M | 0.05 ± 0.02 | 0.04 ± 0.01 | 0.08 ± 0.01 | n.d. | 0.03 ± .02 |
| 1MOI3M | 0.07 ± 0.02 | 0.02 ± 0.004 | 0.16 ± 0.04 | 0.04 ± 0.02 | 0.04 ± 0.03 |
| total | 32.68 ± 3.54 | 1.02 ± 0.14 | 74.68 ± 6.52 | 24.66 ± 1.92 | 28.57 ± 7.86 |
abbreviations: 3MSOP: 3-methylsulfinylpropyl-; 4-MSOB: 4-methylsulfinylbutyl-; 5MSOP: 5-methylsulfinylpentyl-; 7MSOH: 7-methylsulfinylheptyl-; 4MTB: 4methylthiobutyl-; 8MSOO: 8-methylsulfinyloctyl-; I3M: indol-3-ylmethyl-; 4OHI3M: 4-hydroxyindol-3-ylmethyl-: 4MOI3M: 4-methoxyindol-3-ylmethyl-; 1MOI3M: 1-methoxyindol-3-ylmethyl-.
n.d.: not detected
Table 3. Slug herbivory-induced changes in glucosinolate content in genotypes studied.
| Total glucosinolates (µmol*g dry weight-1) | |||||
|---|---|---|---|---|---|
| Rosette leaves | Col-0 | myb28x29 | tgg1xtgg2 | cyp79b2xb3 | 35S:ESP |
| no herbivory | 15 ± 3.00a | 6.3 ± 0.7 | 15.4 ± 2.6 | 7.7 ± 1.2 | 14.7 ± 3.5 |
| slug herbivory | 15.1 ± 2.7 | 4.8 ± 0.3 | 14.9 ± 0.8 | 6.7 ± 1.1 | 10.7 ± 0.9 |
| Inflorescences | |||||
| no herbivory | 34.4 ± 3.4 | 2.9 ± 0.4 | 77 ± 6.8 | 27 ± 2 | 31.3 ± 8.1 |
| slug herbivory | 28.4 ± 2.1 | 3.0 ± 0.3 | 59 ± 7.9 | 21.8 ± 2.5 | 14.2 ± 7.8 |
| Total glucosinolate hydrolysis products (nmol*g fresh weight-1) | |||||
| Rosette Leaf | Col-0 | myb28x29 | tgg1xtgg2 | cyp79b2xb3 | 35S:ESP |
| no herbivory | 0.33 ± 0.01 | 0.014 ± 0.004 | 0.089 ± 0.002 | 0.29 ± 0.01 | 0.34 ± 0.1 |
| slug herbivory | 0.33 ± 0.06 | 0.013 ± 0.004 | 0.028 ± 0.006 | 0.34 ± 0.07 | 0.34 ± 0.1 |
| Inflorescences | |||||
| no herbivory | 2.28 ± 0.19 | 0.074 ± 0.01 | 1.70 ± 0.19 | 1.86 ± 0.15 | 3.54 ± 0.8 |
| slug herbivory | 2.45 ± 0.39 | 0.092 ± 0.003 | 1.19 ± 0.28 | 1.98 ± 0.18 | 2.89 ± 1.1 |
HPLC-MS analyses of aqueous feces extracts
In order to determine what metabolic processes might act on glucosinolates and their hydrolysis products in vivo, we analyzed and compared frass extracts obtained from different mollusks after feeding on plant diets that do or do not contain 4-methylsufinylbutyl glucosinolate (4msob-GLS), i.e. Col-0 or myb28x29 A. thaliana leaves, respectively. Individuals of D. laeve, A. lusitanicus and H. pomatia were kept on A. thaliana rosettes (Col-0 or myb28myb29). These species were used since they all fed readily on A. thaliana and their frass could be easily collected. Frass was collected daily and freeze-dried. Twenty mg dry frass was extracted with 1 mL acidified methanol (1:1 v/v, pH 3) in a vortex mixer and centrifuged (10 min at 20,000 xg). The supernatant was then analyzed by HPLC. Frass from individuals fed on an artificial diet (based on instant mashed potatoes with 2.4 % agar) containing 4MSOB-ITC (2 µmol g-1) was extracted and analyzed in the same way. Initial HPLC-MS screenings of frass extracts were carried out as described in Schramm et al., 2012. Further analyses of known derivatives are described in the supplemental material.
Diurnal mollusk activity
Observations were made under short day conditions, 10 hours light (white light: 380-800 nm) and 14 hours dark (infrared light: 725-925 nm). Molluskan behavior was recorded separately for each species using a Logitech Webcam 600 and the program Webcam XP. Mollusks (two-three individuals for each species) were placed in a plastic box (26.5 x 13 x 15 cm) filled with soil, wood, stones and one or two A. thaliana plants. Every ten minutes, the behavior of A. fulica, D. laeve, D. reticulatum, H. pomatia and L. valentiana were recorded by taking a picture of the setup. For A. lusitanicus and C. hortensis, the program Yawcam 0.3.7 was used and pictures were taken every five seconds. Yawcam 0.3.7 was chosen because it is more convenient for taking pictures at smaller time intervals to visualize species that were harder to track with the Webcam XP program. Pictures were edited with Adobe Photoshop CS5 12.0 and converted into time-lapse videos with VirtualDub 1.9.11.0. Slug behavior was analyzed by measuring the distance moved per hour. For the statistical analysis of diurnal mollusk behavior, we used the program CircWave v1.4 (courtesy of Dr. Roelof Hut). This program is an extension of cosinor analysis, which is commonly used with circadian data sets. It produces a Fourier curve with as many additional harmonics as best describe the data. More information is available at http://www.euclock.org/.
Diurnal metabolite experiment
Plants were grown on soil (Fruhstorfer Nullerde:vermiculite:sand, 8:1:1) in a controlled climate chamber (21°C, 55% relative humidity and 130 µmol m-2s-1 photosynthetically active radiation) with a photoperiod of 10h light/14h dark. Samples were taken from four weeks old, rosette stage plants. Five rosette leaves of each of ten plants were harvested every four hours, starting at 8 am from ten plants for each time point. For each time point a new set of plants were taken. Leaves were harvested in liquid nitrogen, ground with a pestle and stored at -80°C until analysis. Statistical differences were analyzed with SPSS by using ANOVA and Tukey’s HSD post-hoc tests. Grubbs tests were used to identify single outliers and data were log10 transformed when homogeneity of variance was violated. If variance homogeneity could not be achieved, then Kruskal-Wallis one-way analysis of variance was used.
Results and Discussion
Slugs attack A. thaliana in nature
Molluskan herbivores were previously speculated to be a major cause of herbivore damage on A. thaliana in the field (Harvey et al. 2007). In line with these observations, we found that A. thaliana plants were frequently attacked by molluskan herbivores in common garden experiments in Europe and mollusks were the only type of herbivores that were detected on plants in North America (Supplemental Figures 1 and 2, see also supplemental information for descriptions of the field sites and herbivore damage observations). The major defoliator in the European experiments was identified as A. lusitanicus, commonly known as the Spanish slug, a native of southern Europe that has expanded its range northward in recent years (Supplemental Figure 2A, B). The scientific name of this species is currently under debate and some studies also use the name A. vulgaris (Welter-Schultes 2012). During field studies in 2008 in Michigan, USA, slugs of the species D. laeve were found to feed on A. thaliana (Supplemental Figure 1 C, D). Since slugs and snails are sympatric with A. thaliana, we hypothesized that the plant may have evolved specific recognition and defense mechanisms to these herbivores.
JA-mediated resistance of A. thaliana against molluskan herbivores
Mollusk-specific cues may be present in the external mucus of these herbivores. To test whether such cues are perceived by plants after mollusk feeding, we wounded leaves and applied either water or mucus by allowing A. lusitanicus to crawl on the leaves and deposit a mucus residue. We measured the induction of JA and its isoleucine conjugate (JA-Ile), which are often used as an indicator for herbivore perception in plants (Halitschke et al. 2003; Schäfer et al. 2011; Schmelz et al. 2009). The results show that two hours after treatment of rosette stage plants, JA levels were approximately thirty percent higher after mucus treatments relative to wounding alone (Figure 1A). Eight hours after treatment, JA levels were more than 6 times higher after mucus treatments compared to wounding and water (Figure 1A). We also found that JA-Ile levels are induced upon mucus treatments (Fig. 1A). Similar results were found for plants at the flowering stage (Supplemental Figure 4). The fact that A. thaliana responds to the mucus of a molluskan herbivore points to the presence of molluskan herbivore-associated molecular patterns (HAMPs). Orrock (2013) recently found that frequent exposure of A. thaliana plants to snail mucus increased their resistance to herbivores. It is tempting to speculate that the increase in resistance is mediated through activation of the JA pathway.
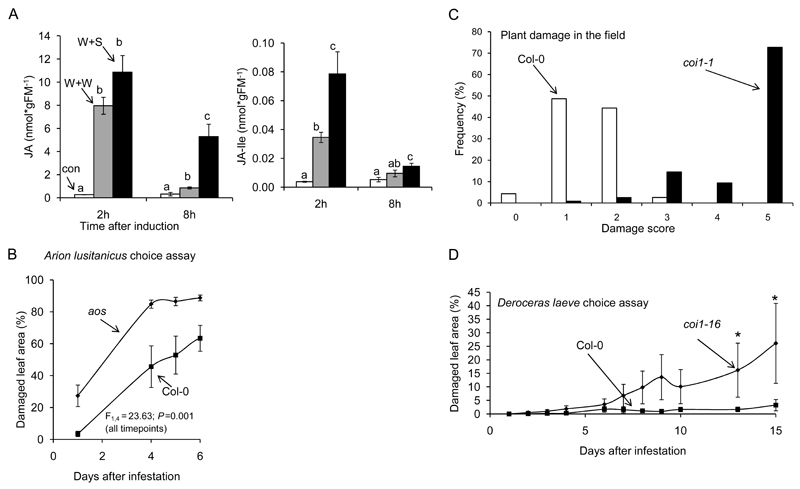
Figure 1. The jasmonate pathway defends Arabidopsis thaliana against slugs and snails.
(A) Wounded A. thaliana leaves treated with the mucus residue (“slime”) of crawling Arion lusitanicus (W+S) accumulate higher levels of jasmonic acid (JA) and its isoleucine conjugate (JA-Ile) when compared to wounded leaves treated with water (W+W) n ≥3, different letters indicate significant differences between treatments for each time point (ANOVA, Turkey HSD, P< 0.05). (B) Arion lusitanicus causes more damage on JA-deficient A. thaliana plants (AOC) when compared to WT (Col-0) (aos: 89 ±2%; Col-0: 63 ± 8%; ANOVA F1,4= 23,63; p= 0.008). (C) JA-perception deficient (coi1-1) A. thaliana plants suffer more damage by slugs than wild type (Col-0) plants in the field (Michigan, USA). Wilcoxon Test W = 13262, p < 2.2e-16. (D) The native slug Deroceras leave causes more damage on coi1-16 plants when compared to Col-0 in the lab (two way repeated measures ANOVA,*P<0.05).
The JA pathway regulates many secondary metabolites that act in defense against herbivores (De Geyter et al. 2012; Meldau et al. 2012). To test if JA-mediated defenses confer resistance to molluskan herbivores, we performed choice assays using A. thaliana aos mutants, which are defective in JA biosynthesis (Farmer& Dubugnon 2009). While wild type (Col-0) plants received almost no damage by A. lusitanicus after 24 hours, thirty percent of the leaf material was removed from aos plants (Figure 1B). After four days, approximately 80 % of the leaf area of aos plants was removed, while Col-0 plants only had 41 percent leaf damage. To further test the role of JA in plant defense against naturally occurring slugs and snails, Col-0 and JA-perception deficient (coi1-1) plants were transferred to field sites in Michigan. The results show that most coi1-1 plants were defoliated, while Col-0 plants received only little damage (Figure 1C). We also performed choice assays using D. laeve collected from German field sites and found that this slug causes more damage on coi1-1 relative to Col-0 (Figure 1D). In addition, we performed choice assays with C. hortensis, A. distinctus and H. pomatia, three other molluskan species that are commonly found in typical habitats of A. thaliana. All species caused higher damage on aoc and coi1-16 mutants when compared to Col-0 (Supplemental Figure 4). Taken together these data provide evidence for an important role of JA-mediated defenses against molluskan herbivores.
Role of glucosinolates in defense against molluskan herbivores
We used several mutant A. thaliana lines with altered levels of different glucosinolates to test their role in defense against molluskan herbivores. To confirm the glucosinolate composition and content in the mutants, intact glucosinolates were extracted and analyzed by HPLC (Table 1). Aliphatic glucosinolates are the major group of glucosinolates in all tissues of wild type A. thaliana Col-0, with 4-methylsulfinylbutyl glucosinolate the most abundant of these species (Brown et al. 2003). Plants of the myb28x29 line had the lowest total content of glucosinolates due to the complete absence of detectable aliphatic glucosinolates. Plants of the cyp79B2xB3 line, while having almost no detectable indolic glucosinolates in the leaves or inflorescences still possessed levels of aliphatic glucosinolates comparable to wild type. The 35S:ESP plants contained comparable levels of glucosinolates in leaves and inflorescences to Col-0. However, the tgg1xtgg2 plants, while having wild-type levels of glucosinolates in their leaves surprisingly contained more than double the amount of glucosinolates in its inflorescences as Col-0. All plants, except for myb28x29, contained at least double the total amount of glucosinolates in their inflorescences as compared to leaves.
Glucosinolate hydrolysis products (GHPs), the active players in most plant-herbivore interactions, are generated by the activity of myrosinase and other attendant proteins upon leaf maceration (Halkier& Gershenzon 2006). Table 2 shows the total amount of GHPs evolved from leaves and inflorescences of Col-0 and the four mutants. The myb28x29 and tgg1xtgg2 lines produced the lowest amounts of GHPs in the leaves. Among inflorescences, only myb28x29 showed a drastic reduction of GHPs while Col-0, tgg1xtgg2, and cyp79B2xB3 produced GHPs at similar levels. Surprisingly, tggx1tgg2 inflorescences contained approximately 60% of the wild type inflorescence myrosinase activity. Barth & Jander (2006) reported that tggx1tgg2 inflorescences possessed only 1% of wild type myrosinase activity, but this activity was measured using an indirect spectrophotometric assay that followed the disappearance of sinigrin added to plant extracts rather than by direct measurement of GHPs released after tissue maceration. The main difference between Col-0 and 35S:ESP is an elevated level of nitriles in the ESP overproducing line, but this line cannot be used to compare the toxicity of isothiocyanates versus nitriles to slugs and snails because of the high residual amount of isothiocyanates.
Table 2. Glucosinolate hydrolysis products in tissues of Arabidopsis mutants.
| Glucosinolate hydrolysis products in rosette leaves (nmol*g fresh weight-1) | |||||
|---|---|---|---|---|---|
| Side chain1 | Col-0 | myb28x29 | tgg1xtgg2 | cyp79b2xb3 | 35S:ESP |
| 3MSOP-CN | 1.05 ± 0.3 | n.d. | 0.4 ± 0.1 | 3.1 ± 2.9 | 32.8 ± 11.4 |
| 3MSOP-ITC | 52.5 ± 4.2 | n.d. | 12.7 ± 5.3 | 55.9 ± 5.1 | 20.6 ± 14.9 |
| 4MSOB-CN | 25.1 ± 5.7 | n.d. | 1.1 ± 0.3 | 24.8 ± 6.9 | 149 ± 39 |
| 4MSOB-ITC | 231 ± 15 | n.d. | 27.3 ± 11 | 204.5 ± 17.2 | 86.1 ± 52.8 |
| 4MTB-CN | 3.7 ± 1.0 | n.d. | 1.5 ± 0.7 | 2.1 ± 0.3 | 1.2 ± 1.2 |
| 4MTB-ITC | 5.3 ± 0.9 | n.d. | 4.9 ± 2.6 | 9.8 ± 0.6 | 30.1 ± 2.8 |
| IndACN | 9.9 ± 1.2 | 9.9 ± 2.5 | 9.8 ± 1.3 | n.d. | 20.5 ± 3.7 |
| 4MOI3M-CN | 0.8 ± 0.2 | 0.4 ± 0.1 | 0.5 ± 0.2 | 0.3 ± 0.02 | 0.4 ± 0.03 |
| Total | 229 ± 28 | 10.3 ± 2.6 | 58 ± 22.5 | 301 ± 34 | 340 ± 126 |
| Glucosinolate hydrolysis products in inflorescences (nmol*g fresh weight-1) | |||||
| Side chain1 | Col-0 | myb28x29 | tgg1xtgg2 | cyp79b2xb3 | 35S:ESP |
| 3MSOP-CN | 91.4 ± 3.5 | n.d. | 15.9 ± 38.9 | 121 ± 9.9 | 161 ± 21 |
| 3MSOP-ITC | 175 ± 24 | n.d. | 158 ± 29 | 124 ± 12.6 | 73 ± 46 |
| 4MSOB-CN | 525 ± 41 | n.d. | 189.0 ± 231 | 649 ± 34 | 1058 ± 195 |
| 4MSOB-ITC | 1511 ± 119 | n.d. | 1026 ± 302 | 969 ± 50 | 1587 ± 292 |
| 4MTB-CN | 2.26 ± 2.26 | n.d. | 9.7 ± 13.8 | 17 ± 2.7 | 63 ± 22 |
| 4MTB-ITC | 17.9 ± 2.4 | n.d. | 17.3 ± 6.9 | 31.8 ± 6.7 | 30 ± 17 |
| IndACN | 38.8 ± 5.1 | 75.2 ± 6.3 | 26.5 ± 54.9 | n.d. | 230 ± 94 |
| 4MOI3M-CN | 4.1 ± 1.92 | 0.6 ± 0.3 | 4.1 ± 3.1 | n.d. | 13.1 ± 6.1 |
| Total | 2365 ± 199 | 76.6 ± 6.6 | 1446 ± 679 | 1911 ± 116 | 3215 ± 693 |
abbreviations: 3MSOP-CN: 3-methylsulfinylpropyl cyanide; 3MSOP-ITC: 3-methylsulfinylpropyl isothiocyanate; 4MSOB-CN: 4-methylsulfinylbutyl cyanide; 4MSOB-ITC: 4-methylsulfinylbutyl isothiocyanate; 4MTB-CN; 4-methylthiobutyl cyanide; 4-MTB-ITC: 4-methylthiobutyl isothiocyanate; IndACN: indole-3-acetonitrile; 4MOI3M-CN: 4-methoxyindole-3-methyl cyanide.
n.d.: not detected
Induction of glucosinolates by slug herbivory
Herbivore feeding is known to result in an induction of glucosinolates and their hydrolysis products on glucosinolate-containing plants. We therefore tested the effect of a period of 24 h of slug herbivory on these compounds in Col-0 and the mutants. A. lusitanicus feeding did not result in glucosinolate induction in Col-0 or any of the mutants and even appeared to cause a reduction in glucosinolate levels in some leaf and inflorescence tissues (Table 3). This is in contrast to previous reports of glucosinolate induction by insect herbivore feeding (Textor& Gershenzon 2009). Since glucosinolate concentrations in A. thaliana are partially controlled by the JA pathway (Mewis et al. 2006), which is induced by A. lusitanicus mucus (Figure 1A, Supplemental Figure 4), it is possible that inductions of JA-mediated defenses in A. thaliana are suppressed by other signaling pathways that are induced by slug herbivory, as has been shown repeatedly for lepidopteran herbivores (Bruessow et al. 2010; Diezel et al. 2009; Musser et al. 2005). A comprehensive study of the response of A. thaliana leaves to slimy secretions of different molluskan herbivores might reveal the induction of other pathways that may interfere with JA-induced defenses and glucosinolate induction.
Role of glucosinolates in defense of leaves against slugs and snails
To determine whether A. thaliana glucosinolates affect the feeding preferences of A. lusitanicus, pairwise bioassays comparing herbivory on wild type Col-0 versus mutants were performed. There was no statistical difference in herbivory on Col-0 compared to any of the glucosinolate mutants, suggesting that the genetically-engineered changes in glucosinolates and glucosinolate hydrolysis products were not dramatic enough to affect feeding choice by A. lusitanicus (Figure 2A). However, when using the Roman snail H. pomatia for choice assays, we found that this species caused more damage on leaves of myb28x29 compared to Col-0 (Supplemental Figure 6). These data show that aliphatic glucosinolates in leaves act as defense metabolites against some mollusks.
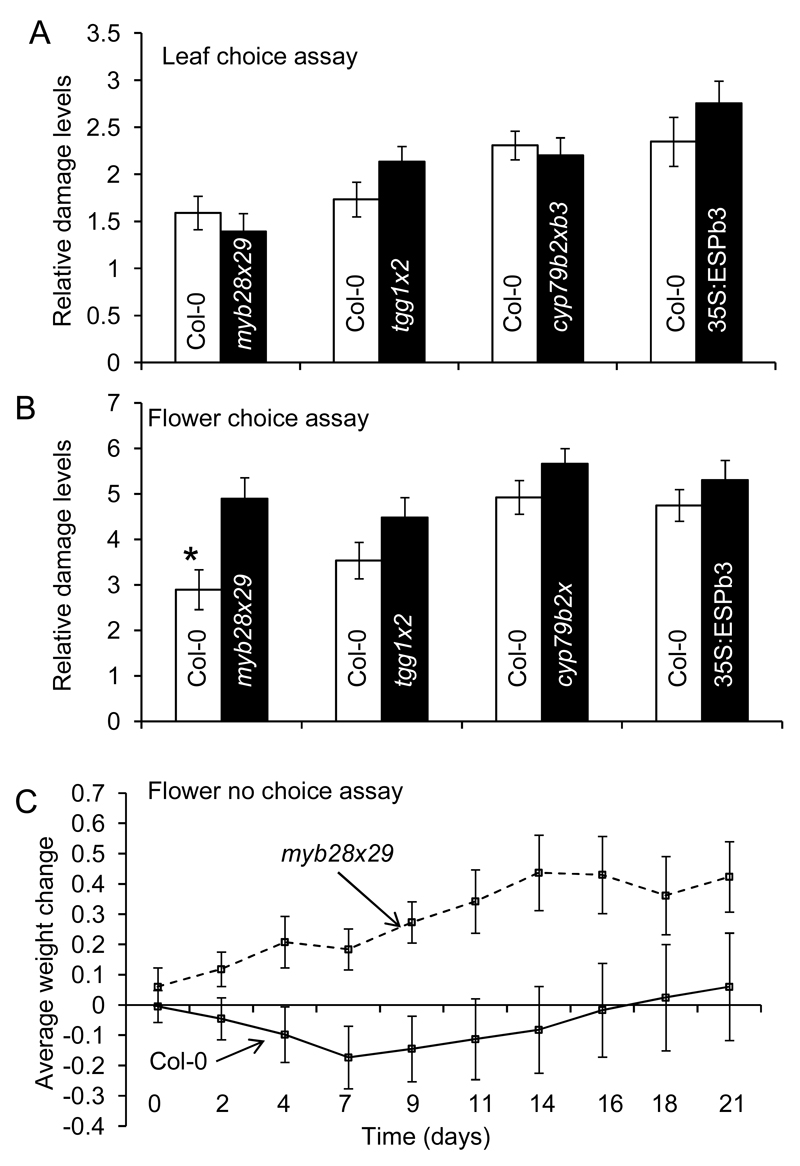
Figure 2. Glucosinolates defend Arabidopsis thaliana against the slug Arion lusitanicus.
A. lusitanicus damage was determined in choice assays with leaves (A) or inflorescences (B) of A. thaliana Col-0 or transgenic plants (myb28x29, tgg1x2, cyp79b2xb3, 35S:ESPb3). Data represent the mean ± SE of three experiments with 20 replicates each. (C) No choice growth experiment. Naïve slugs were fed either only Col-0 flowers or only myb28x29 flowers and weighed every 2-3 days. Data represent the average weight change per slug for 12 slugs per treatment. Data were analyzed using students t-test or via linear mixed effects model in R.
Role of glucosinolates in defense of inflorescences against A. lusitanicus
As described above, inflorescences of A. thaliana, and of most plants in the family Brassicaceae, contain higher concentrations of glucosinolates than rosette leaves. Inflorescences may be better defended against herbivory than leaves because they are much more critical to the fitness of the plant (Meldau et al. 2012). Whereas A. thaliana plants can regrow rosette leaves and mature to flowering after A. lusitanicus consumes the entire rosette (K. Falk, personal observation), consumption of the inflorescences of an individual plant impairs reproduction entirely. To test whether the higher concentration of glucosinolates in inflorescences affected A. lusitanicus herbivory, we performed pairwise bioassays using only the flowering stalks of A. thaliana Col-0 vs. those of the glucosinolate mutants. In all bioassay pairs, the slugs seemed to prefer the mutant inflorescences as compared to the wild type, and the preference for myb28x29 flowers vs. Col-0 was statistically significant (Figure 2). This result suggests that, whereas glucosinolates in leaves may be less important for defense against A. lusticanus than other components of A. thaliana’s defensive chemistry, glucosinolates, especially aliphatic glucosinolates, may be critical for defense of reproductive structures. A. lusitanicus has been observed consuming flowers in nature at the Martinfeld field site, and is capable of shearing a flowering stalk at the base and eating the stalk without actually having to climb it (observed by Kim Falk). When slugs were provided with flowering plants of Col-0 as well as all of the mutants in one arena, they showed a clear preference for myb28x29 flowers, indicating that it is the aliphatic glucosinolates themselves, or more properly their hydrolysis products, that reduce the acceptance of plant by slugs (Supplemental Figure 7). There was over a 30-fold difference in hydrolysis products between the flowers of the Col-0 and the myb28x29 genotypes (Figure 2B, Table 2). Although among hydrolysis products isothiocyanates are thought to have more biological activity than nitriles, there was no indication that A. lusitanicus made any distinction between isothiocyanates and nitriles in feeding choice assays (Figure 2AB, Table 2- comparison between Col-0 and 35S-ESP lines).
Effect of glucosinolates on performance of A. lusitanicus
We next tested whether consumption of aliphatic glucosinolates had physiological consequences for A. lusitanicus. As shown in Figure 2C, the slugs fed only myb28x29 inflorescences continuously gained weight while the slugs eating Col-0 inflorescences lost weight for approximately 9 days, then recovered and started to gain weight. From this result, it can be concluded that glucosinolates are mildly toxic or act as feeding inhibitors to A. lusitanicus, but that these slugs are able to eventually overcome these effects, perhaps by induction of detoxifying enzymes or habituation.
Mollusk glucosinolate metabolism
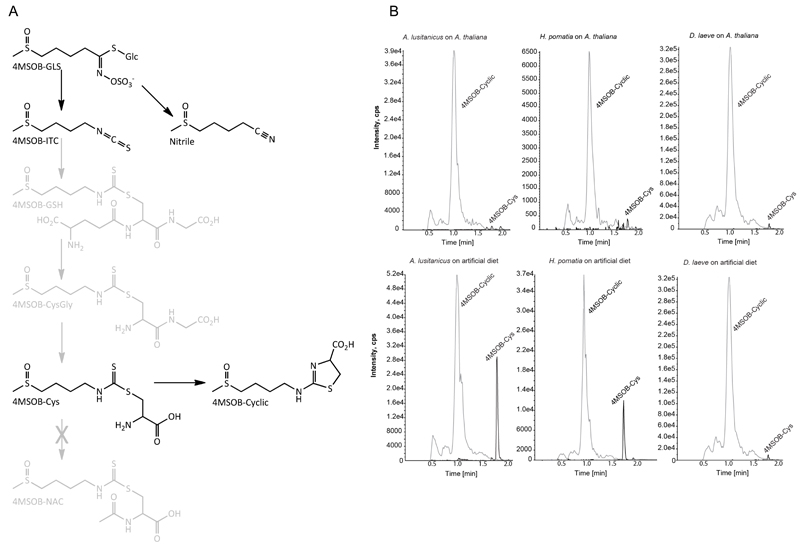
A sulfatase from H. pomatia is used in standard glucosinolate extraction protocols to hydrolyze substances bound to anion exchange columns releasing glucosinolates as desulfo-glucosinolates (Thies 1979). Whether A. lusitanicus or other molluskan herbivores also detoxify glucosinolates by using sulfatases is currently not known. We analyzed A. lusitanicus, H. pomatia and D. laeve frass for potential metabolites of 4-methylsulfinylbutyl (4MSOB) glucosinolate, the main glucosinolate in A. thaliana Col-0, and its derived isothiocyanate 4MSOB-ITC. We did not detect desulfo-glucosinolates as detoxification end-product metabolites in the frass of H. pomatia or A. lusitanicus or in our chromatographic screens. However, in accordance with generalist isothiocyanate detoxification pathways previously reported in humans (e.g. Shapiro et al. 2006) and lepidopteran generalists (Schramm et al. 2012), we could detect the cysteine conjugate of 4MSOB-ITC (4MSOB-Cys, Figure 3B), which results from conjugation of the isothiocyanate to glutathione (GSH) followed by hydrolytic steps via the mercapturic acid pathway. 4MSOB-Cys has previously proved a good marker for the operation of the mercapturic pathway in mammals (Shapiro et al., 2006) and insects (Schramm et al., 2012), and it is unlikely that this conjugate arises from direct reaction between the ITC and Cys or via another route. Unlike in humans, the N-acetylcysteine conjugate (4MSOB-NAC) was not detected. Instead, a novel pathway end-product was observed, and further characterized as the cyclic derivative of 4MSOB-Cys (Figure 3B), a compound not previously described. These compounds were detected in frass of Col-0-fed A. lusitanicus, H. pomatia and D. laeve after feeding on Col-0, as well as after feeding on 4MSOB-ITC-containing artificial diet, but not in the corresponding negative controls (feeding on myb28x29 leaves and on artificial diet without 4MSOB-ITC, data not shown). Similar cyclic derivatives of other isothiocyanates have also been described after in vivo metabolism in other species and also after in vitro conjugation to cysteine (Conaway et al. 2002; Kawakishi& Namiki 1982). Additionally, a compound with molecular mass and fragmentation in agreement with the corresponding cyclic derivative of 8-methylsulfinyloctyl (8MSOO)-ITC was also detected (data not shown), thus further corroborating the participation of the mercapturic acid pathway in isothiocyanate detoxification in these mollusks.
Figure 3. Glucosinolates are metabolized in molluskan herbivores.
(A) Metabolism of 4MSOB glucosinolate in A. lusitanicus, H. pomatia and D. laeve. After hydrolysis of the plant glucosinolate (4MSOB-GLS), the isothiocyanate (4MSOB-ITC) thus formed is metabolized in vivo by conjugation to glutathione and further peptide hydrolysis steps to afford the cysteine conjugate (4MSOB-Cys). The latter can then undergo further metabolism to result in the novel derivative (4MSOB-Cyclic, 2-(4-(methylsulfinyl) butylamino)-4,5-dihydrothiazole-4-carboxylic acid). Compounds shaded in gray were not detected in this study. (B) LC-MS/MS traces (MRMs in positive mode) of 4MSOB-Cys and 4MSOB-Cyclic detected from frass of corresponding mollusk species fed on leaf tissue (upper graphs) or artificial diets (lower graphs).
Diurnal rhythms in plant metabolites and mollusk activity
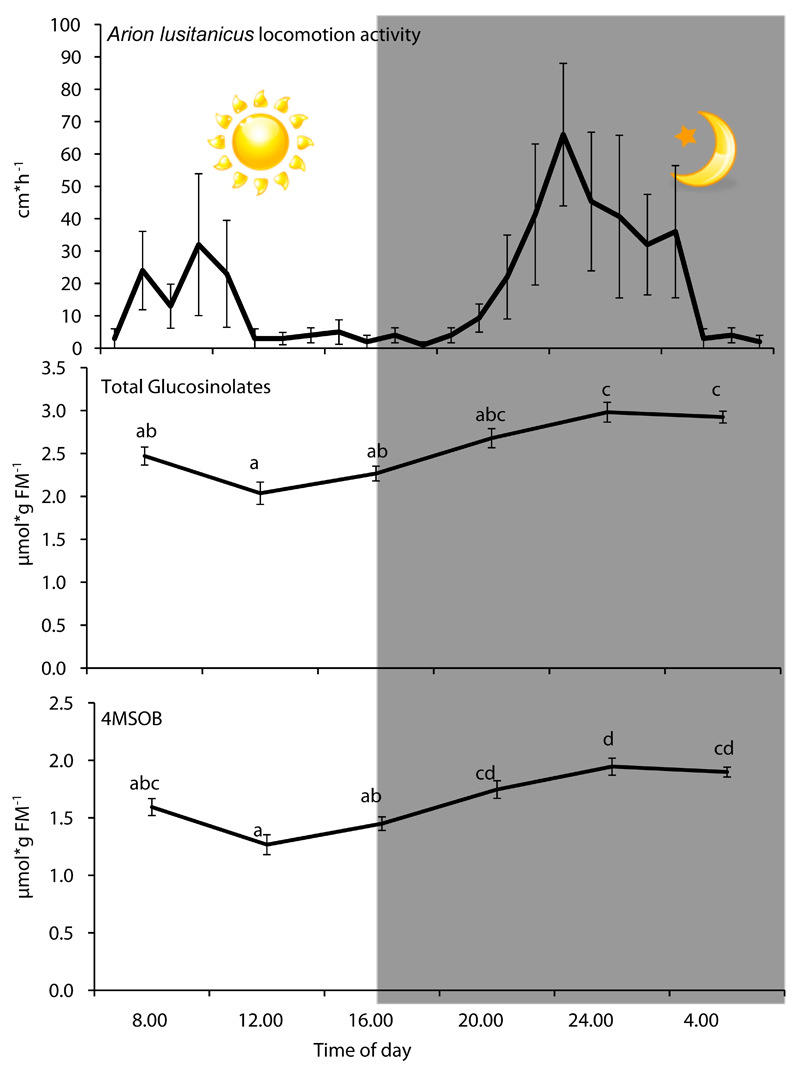
Diurnal differences in the accumulation of JA in A. thaliana were previously shown to be controlled by the circadian clock and the results were interpreted as a way to adjust plant defenses for deployment against day-active herbivores (Goodspeed et al. 2012). However, against mollusks the ecological relevance of defenses that peak during the day is unclear. We observed A. lusitanicus on A. thaliana mainly during night or early morning periods in the field experiments (see supplemental material). When we analyzed the activity of A. lusitanicus under controlled conditions, we found that this slug is significantly nocturnal (F11.94, p<0.001; Figure 4). Additionally, most slugs and snails that we tested displayed nocturnal activity patterns under controlled conditions (Figure S7; Table S1). Since glucosinolates are involved in resistance against molluskan herbivores, we tested whether glucosinolate accumulation correlates with the activity of these herbivores during a diurnal cycle. We measured glucosinolate levels in rosette leaves under short day conditions, since slugs and snails cause most damage early in the season. Interestingly, we found glucosinolates, in particular aliphatic glucosinolates, to be nearly 50% higher during the night than the middle of the day (Figure 4, Supplemetal Figure 9). This is in contrast with a previous study, which reported higher concentrations during daytime (Huseby et al. 2013). The fact that Huseby and colleagues used seedlings grown in petri dishes on Murashige and Skoog medium under long day conditions, rather than in soil under short day conditions may account for this difference. In Brassica oleracea, grown under long day conditions in a sand/peat mixture, glucosinolates in leaves were also higher at night (Rosa 1997). Whether the length of light period or the growth medium alters the peak accumulation of glucosinolates in A. thaliana needs further investigation. A recent study by Chan et al. (2011) demonstrated that natural variation in glucosinolates is partially under the control of the circadian clock (Chan et al. 2011). Whether the clock controls seasonal changes in glucosinolate levels to optimize plant resistance against natural molluskan herbivores is an interesting question for future research.
Figure 4. Diurnal slug activity and variations in glucosinolates in leaves of Arabidopsis thaliana.
Activity of two Arion lusitanicus of two individuals was analyzed for four days and values (mean±SE) for average movements during the diurnal cycle are shown. Total glucosinolates include all compounds presented in Supplemental Figure 7. 4-MSOB: 4-methylsulfinylbutyl-glucosinolate is the most abundant glucosinolate in leaves of Arabidopsis thaliana. Mean±SE, N≥9. Different letters indicate statistical differences between times of day (One-way ANOVA, Turkey HSD).
Conclusions
Despite the ecological and economic relevance of plant interactions with slugs and snails, our current knowledge of defense mechanisms against these herbivores is very limited. Here we provide evidence that the JA pathway and glucosinolates can mediate resistance to molluskan herbivores in A. thaliana. The induction of the JA pathway by the mucus of a slug species indicates that some plants have evolved perception machinery to detect molluskan herbivores. Identifying the metabolites responsible for this immune response will greatly advance our understanding of plant-mollusk interactions. Further experiments are required to evaluate how commonly plants respond to molluskan mucus. The detrimental effect of high aliphatic glucosinolate concentrations on slug feeding choice and growth rate demonstrates that these compounds defend against feeding mollusks. Detection of glucosinolate conjugates in molluskan feces indicates active detoxification of this group of defense compounds. However, it is still unclear how commonly molluskan herbivores feed on A. thaliana. Screens of herbivory on various ecotypes in different native geographic locations will help in evaluating the frequency of A. thaliana-mollusk interactions and in determining the usefulness of this system for analyzing the potential co-evolutionary arms-race between plants and molluskan herbivores.
Supplementary Material
Acknowledgements
The research of Erb, Falk, Gershenzon, Meldau, Paetz, Reichelt, Schramm and Vassao was supported by the Max Planck Society. The work of Meldau was funded by Advanced Grant No 293926 of the European Research Council to Ian Baldwin. The work of Erb was supported by a Marie Curie Intra European Fellowship (grant no. 273107). We thank Himanshu Himanshu for technical support. We are grateful to Alfred and Ursula Sonntag who provided space in their common garden in Martinfeld for field experiments and documented herbivory on A. thaliana. We thank Grit Kunert for help with statistical analysis of Michigan field data. We thank Beate Rothe for help with sample extraction.
Footnotes
Data Accessibility
R script: uploaded as online supporting information
Author Contributions Box
Kästner, Meldau, Knorre and Falk performed field studies in Jena. Meldau performed field studies in Martinfeld. Bodenhausen, Bergelson performed field studies in Michigan. Knorre identified slug and snail species. Kästner, Erb and Meldau performed mucus treatments and plant hormone analyses. Kästner performed choice assays with aos, coi1-16 mutants and myb28/29 mutants with H. pomatia. Kästner and Erb analyzed choice assay data. Falk and Gershenzon designed and performed experiments with glucosinolate mutants and A. lusitanicus. Reichelt analyzed glucosinolate data. Erb, Kästner and Meldau set up diurnal mollusk behavior analysis and Kästner analyzed mollusk activity data. Schramm, Paetz and Vassao performed and analyzed mollusk glucosinolate metabolism experiments. Meldau harvested diurnal kinetic samples. Reichelt and Meldau analyzed metabolite data from diurnal kinetic samples. Meldau, Erb, Falk and Kästner wrote, and all other authors edited the manuscript.
References
- Agerbirk N, Olsen CE. Glucosinolate structures in evolution. Phytochemistry. 2012;77:16–45. doi: 10.1016/j.phytochem.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Allan E, Crawley MJ. Contrasting effects of insect and molluscan herbivores on plant diversity in a long-term field experiment. Ecology Letters. 2011;14:1246–1253. doi: 10.1111/j.1461-0248.2011.01694.x. [DOI] [PubMed] [Google Scholar]
- Barker GM. Molluscs as crop pests. CABI publishing; 2002. ISBN 9780851993201. [Google Scholar]
- Barth C, Jander G. Arabidopsis myrosinases TGG1 and TGG2 have redundant function in glucosinolate breakdown and insect defense. Plant Journal. 2006;46:549–562. doi: 10.1111/j.1365-313X.2006.02716.x. [DOI] [PubMed] [Google Scholar]
- Brown PD, Tokuhisa JG, Reichelt M, Gershenzon J. Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochemistry. 2003;62:471–481. doi: 10.1016/s0031-9422(02)00549-6. [DOI] [PubMed] [Google Scholar]
- Bruessow F, Gouhier-Darimont C, Buchala A, Metraux JP, Reymond P. Insect eggs suppress plant defence against chewing herbivores. Plant Journal. 2010;62:876–885. doi: 10.1111/j.1365-313X.2010.04200.x. [DOI] [PubMed] [Google Scholar]
- Burow M, Markert J, Gershenzon J, Wittstock U. Comparative biochemical characterization of nitrile-forming proteins from plants and insects that alter myrosinase-catalysed hydrolysis of glucosinolates. Febs Journal. 2006a;273:2432–2446. doi: 10.1111/j.1742-4658.2006.05252.x. [DOI] [PubMed] [Google Scholar]
- Burow M, Müller R, Gershenzon J, Wittstock U. Altered glucosinolate hydrolysis in genetically engineered Arabidopsis thaliana and its influence on the larval development of Spodoptera littoralis. Journal of Chemical Ecology. 2006b;32:2333–2349. doi: 10.1007/s10886-006-9149-1. [DOI] [PubMed] [Google Scholar]
- Chan EKF, Rowe HC, Corwin JA, Joseph B, Kliebenstein DJ. Combining genome-wide association mapping and transcriptional networks to identify novel genes controlling glucosinolates in Arabidopsis thaliana. Plos Biology. 2011;9 doi: 10.1371/journal.pbio.1001125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conaway CC, Yang YM, Chung FL. Isothiocyanates as cancer chemopreventive agents: Their biological activities and metabolism in rodents and humans. Current Drug Metabolism. 2002;3:233–255. doi: 10.2174/1389200023337496. [DOI] [PubMed] [Google Scholar]
- De Geyter N, Gholami A, Goormachtig A, Goossens A. Transcriptional machineries in jasmonate-elicited plant secondary metabolism. Trends in Plant Science. 2012;17:1360–1385. doi: 10.1016/j.tplants.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Diezel C, von Dahl CC, Gaquerel E, Baldwin IT. Different lepidopteran elicitors account for cross-talk in herbivory-induced phytohormone signaling. Plant Physiology. 2009;150:1576–1586. doi: 10.1104/pp.109.139550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb M, Meldau S, Howe GA. Role of phytohormones in insect-specific plant reactions. Trends in Plant Science. 2012;17:250–259. doi: 10.1016/j.tplants.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk KL, Gershenzon J. The desert locust, Schistocerca gregaria, detoxifies the glucosinolates of Schouwia purpurea by desulfation. Journal of Chemical Ecology. 2007;33:1542–1555. doi: 10.1007/s10886-007-9331-0. [DOI] [PubMed] [Google Scholar]
- Farmer EE, Dubugnon L. Detritivorous crustaceans become herbivores on jasmonate-deficient plants. Proc Natl Acad Sci U S A. 2009;106:935–940. doi: 10.1073/pnas.0812182106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodspeed D, Chehab EW, Min-Venditti A, Braam J, Covington MF. Arabidopsis synchronizes jasmonate-mediated defense with insect circadian behavior. Proc Natl Acad Sci U S A. 2012;109:4674–4677. doi: 10.1073/pnas.1116368109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagnell F, von Proschwitz T, Schander C. Two notes on the invasive Iberian slug, Arion lusitanicus Mabille, 1868. Journal of Conchology. 2006;39:108–110. [Google Scholar]
- Halitschke R, Gase K, Hui DQ, Schmidt DD, Baldwin IT. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. VI. Microarray analysis reveals that most herbivore-specific transcriptional changes are mediated by fatty acid-amino acid conjugates. Plant Physiology. 2003;131:1894–1902. doi: 10.1104/pp.102.018184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halkier BA, Gershenzon J. Biology and biochemistry of glucosinolates. Annual Review of Plant Biology. 2006;57:303–333. doi: 10.1146/annurev.arplant.57.032905.105228. [DOI] [PubMed] [Google Scholar]
- Harvey JA, Witjes LMA, Benkirane M, Duyts H, Wagenaar R. Nutritional suitability and ecological relevance of Arabidopsis thaliana and Brassica oleracea as foodplants for the cabbage butterfly, Pieris rapae. Plant Ecology. 2007;189:117–126. [Google Scholar]
- Howe GA, Jander G. Plant immunity to insect herbivores. Annual Review of Plant Biology. 2008;59:41–66. doi: 10.1146/annurev.arplant.59.032607.092825. [DOI] [PubMed] [Google Scholar]
- Huseby S, Koprivova A, Lee BR, et al. Diurnal and light regulation of sulphur assimilation and glucosinolate biosynthesis in Arabidopsis. Journal of Experimental Botany. 2013;64:1039–1048. doi: 10.1093/jxb/ers378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakishi S, Namiki M. Oxidative Cleavage of the disulfide bond of cystine by allyl isothiocyanate. J Agric Food Chem. 1982;30:618–620. [Google Scholar]
- Kessler A. Silencing the jasmonate cascade: Induced plant defenses and insect populations. Science. 2004;306:2042–2042. doi: 10.1126/science.1096931. (vol 305, pg 665, 2004) [DOI] [PubMed] [Google Scholar]
- Lambrix V, Reichelt M, Mitchell-Olds T, Kliebenstein DJ, Gershenzon J. The Arabidopsis epithiospecifier protein promotes the hydrolysis of glucosinolates to nitriles and influences Trichoplusia ni herbivory. Plant Cell. 2001;13:2793–2807. doi: 10.1105/tpc.010261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldau S, Erb M, Baldwin IT. Defence on demand: mechanisms behind optimal defence patterns. Ann Bot. 2012;110:1503–1514. doi: 10.1093/aob/mcs212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mewis I, Tokuhisa JG, Schultz JC, et al. Gene expression and glucosinolate accumulation in Arabidopsis thaliana in response to generalist and specialist herbivores of different feeding guilds and the role of defense signaling pathways. Phytochemistry. 2006;67:2450–2462. doi: 10.1016/j.phytochem.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Mithofer A, Boland W. Recognition of herbivory-associated molecular patterns. Plant Physiology. 2008;146:825–831. doi: 10.1104/pp.107.113118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R, de Vos M, Sun JY, et al. Differential effects of indole and aliphatic glucosinolates on lepidopteran herbivores. Journal of Chemical Ecology. 2010;36:905–913. doi: 10.1007/s10886-010-9825-z. [DOI] [PubMed] [Google Scholar]
- Musser RO, Cipollini DF, Hum-Musser SM, et al. Evidence that the caterpillar salivary enzyme glucose oxidase provides herbivore offense in Solanaceous plants. Archives of Insect Biochemistry and Physiology. 2005;58:128–137. doi: 10.1002/arch.20039. [DOI] [PubMed] [Google Scholar]
- Orrock JL. Exposure of unwounded plants to chemical cues associated with herbivores leads to exposure-dependent changes in subsequent herbivore attack. Plos One. 2013 doi: 10.1371/journal.pone.0079900. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratzka A, Vogel H, Kliebenstein DJ, Mitchell-Olds T, Kroymann J. Disarming the mustard oil bomb. Proc Natl Acad Sci U S A. 2002;99:11223–11228. doi: 10.1073/pnas.172112899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa EAS. Daily variation in glucosinolate concentrations in the leaves and roots of cabbage seedlings in two constant temperature regimes. Journal of the Science of Food and Agriculture. 1997;73:364–368. [Google Scholar]
- Schäfer M, Fischer C, Meldau S, et al. Lipase activity in insect oral secretions mediates defense responses in Arabidopsis. Plant Physiology. 2011;156:1520–1534. doi: 10.1104/pp.111.173567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz EA, Engelberth J, Alborn HT, Tumlinson JH, Teal PEA. Phytohormone-based activity mapping of insect herbivore-produced elicitors. Proc Natl Acad Sci U S A. 2009;106:653–657. doi: 10.1073/pnas.0811861106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm K, Vassao DG, Reichelt M, Gershenzon J, Wittstock U. Metabolism of glucosinolate-derived isothiocyanates to glutathione conjugates in generalist lepidopteran herbivores. Insect Biochemistry and Molecular Biology. 2012;42:174–182. doi: 10.1016/j.ibmb.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Shapiro TA, Fahey JW, Dinkova-Kostova AT, et al. Safety, tolerance, and metabolism of broccoli sprout glucosinolates and isothiocyanates: A clinical phase I study. Nutrition and Cancer-an International Journal. 2006;55:53–62. doi: 10.1207/s15327914nc5501_7. [DOI] [PubMed] [Google Scholar]
- Shindo C, Bernasconi G, Hardtke CS. Natural genetic variation in Arabidopsis: Tools, traits and prospects for evolutionary ecology. Ann Bot. 2007;99:1043–1054. doi: 10.1093/aob/mcl281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonderby IE, Hansen BG, Bjarnholt N, et al. A systems biology approach identifies a R2R3 MYB gene subfamily with distinct and overlapping functions in regulation of aliphatic glucosinolates. PLoS One. 2007;2 doi: 10.1371/journal.pone.0001322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Textor S, Gershenzon J. Herbivore induction of the glucosinolate-myrosinase defense system: major trends, biochemical bases and ecological significance. Phytochemistry Reviews. 2009;8:149–170. [Google Scholar]
- Thies W. Detection and utilization of a glucosinolate sulfohydrolase in the edible snail, Helix pomatia. Naturwissenschaften. 1979;66:364–365. [Google Scholar]
- Welter-Schultes F. European non-marine molluscs, a guide for species identification. Planet Poster Editions; 2012. ISBN 987-3-933922-75-5 edn. [Google Scholar]
- Wittstock U, Agerbirk N, Stauber EJ, et al. Successful herbivore attack due to metabolic diversion of a plant chemical defense. Proc Natl Acad Sci U S A. 2004;101:4859–4864. doi: 10.1073/pnas.0308007101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JQ, Baldwin IT. New insights into plant responses to the attack from insect herbivores. Annual Review of Genetics. 2010;44:1–24. doi: 10.1146/annurev-genet-102209-163500. [DOI] [PubMed] [Google Scholar]
- Xie DX, Feys BF, James S, Nieto-Rostro M, Turner JG. COI1: An Arabidopsis gene required for jasmonate-regulated defense and fertility. Science. 1998;280:1091–1094. doi: 10.1126/science.280.5366.1091. [DOI] [PubMed] [Google Scholar]
- Zhao YD, Hull AK, Gupta NR, et al. Trp-dependent auxin biosynthesis in Arabidopsis: involvement of cytochrome P450s CYP79B2 and CYP79B3. Genes Dev. 2002;16:3100–3112. doi: 10.1101/gad.1035402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.