Abstract
The role of the AcrAB-TolC pump in macrolide and ketolide susceptibility in Escherichia coli and Enterobacter aerogenes was studied. Efflux pump inhibitor restored erythromycin, clarithromycin, and telithromycin susceptibilities to multidrug-resistant isolates. No modification of telithromycin accumulation was detected in E. aerogenes acrAB or tolC derivatives compared to that in the parental strain. Two independent efflux pumps, inhibited by phenylalanine arginine β-naphthylamide, expel macrolides and telithromycin in E. aerogenes.
Macrolides and ketolides are protein synthesis inhibitors, and macrolide resistance is mainly due to target ribosome modification or active efflux (1, 2, 8, 17, 18, 23, 24). Ribosome modification is achieved by methylation of specific nucleotides in 23S rRNA, via specific Erm methyltransferase, or by mutations in 23S rRNA and in L4 and L22 ribosomal proteins in some bacterial species (8, 14, 17). Decreased cell membrane permeability and/or multidrug efflux pumps make macrolides ineffective against gram-negative bacteria (9, 24). In Neisseria gonorrhoeae, the first efflux pump to be documented was the tripartite MtrCDE complex, expelling various antibacterial compounds, including macrolides, detergents, and antimicrobial peptides (6, 14, 21). The AcrAB-TolC system has been cited as being responsible for macrolide efflux associated with resistance, and recently, a novel macrolide-specific efflux pump belonging to the ABC transporter family has been described to be involved in Escherichia coli macrolide resistance (7, 15, 19). The acrAB locus has been identified in Haemophilus influenzae, and inactivation of either one of these genes has been reported to increase the susceptibility to some drugs, such as macrolides, as well as to dyes, such as ethidium bromide (19). In addition, the protonophore carbonyl cyanide m-chlorophenylhydrazone inhibits macrolide efflux in several clinical isolates of H. influenzae exhibiting intermediate or high-level macrolide resistance (15). The aim of the present study was to decipher the role of the AcrAB-TolC efflux pump in macrolide and ketolide resistance in Enterobacteriaceae.
The strains and plasmids used in this work were E. coli BW5104 and its acrAB and acrAB tolC derivatives, Enterobacter aerogenes ATCC 13048, and the clinical isolate E. aerogenes EA27, which exhibits energy-dependent norfloxacin and chloramphenicol efflux (13, 16). Strain EAEP289 is a Kans derivative of EA27, strain EAEP294 is an acrA::Kanr derivative of EAEP289, and strain EAEP298 is a tolC::Kanr derivative of EAEP289 (16). The MIC determinations were carried out with or without phenylalanine arginine β-naphthylamide (PAβN) at various concentrations which have no antibacterial effect (4, 10). Antibiotic uptake was determined as previously described (4, 12, 13).
Efflux pumps are involved in macrolide and ketolide resistance.
The MICs of two macrolides, clarithromycin and erythromycin, and of telithromycin were determined for E. coli BW5104 and its acrAB and acrAB tolC derivatives (Table 1). The acrAB tolC mutant was more susceptible than the wild-type strain to both macrolides (32- to 64-fold decreases in MICs). In contrast, the telithromycin susceptibility was weakly affected in the acrAB tolC strain, with a fourfold reduction of the corresponding MIC. In the presence of PAβN, the respective MICs of the three antibiotics were decreased for both wild-type and mutant strains (Table 1), indicating that a PAβN-sensitive efflux mechanism actively participates in macrolide and ketolide resistance in E. coli.
TABLE 1.
Clarithromycin, erythromycin, and telithromycin susceptibilities of E. coli strainsa
| E. coli strain | Inhibitor concn (μg/ml) | MIC (μg/ml)
|
||
|---|---|---|---|---|
| Clarithromycin | Erythromycin | Telithromycin | ||
| BW5104 | 0 | 256 | 512 | 64 |
| 26.3 | 1 | 1 | 0.25 | |
| BW5104 acrAB | 0 | 64 | 64 | 16 |
| 26.3 | 0.5 | 0.5 | 0.125 | |
| BW5104 acrAB tolC | 0 | 8 | 8 | 16 |
| 26.3 | 0.125 | 0.125 | 0.06 | |
The MICs were determined for strains in Mueller-Hinton broth in the absence or presence of PAβN as previously described (4). The values shown are means of three independent determinations.
Concerning E. aerogenes ATCC 13048, we observed a significant dose-dependent effect of the efflux pump inhibitor on macrolides and telithromycin susceptibilities (Table 2). In E. aerogenes EAEP289, which overproduces the AcrAB component, we observed a reduced effect of the efflux pump inhibitor (16). The tolC mutant, strain EAEP298, was much more susceptible to erythromycin and clarithromycin than the parental strain, EAEP289 (16- to 32-fold decreases in MICs). In the presence of PAβN (10.5 μg/ml), a sensitive phenotype was obtained with EAEP298, in contrast to that of the parental resistant strain, EAEP289 (Table 2). The acrA mutant, EAEP294, was much more resistant to macrolides than EAEP298. The four- to eightfold difference in MICs of macrolides for EAEP298 (TolC−) and EAEP294 (AcrA−) suggests that another efflux pump contributes to macrolide efflux in E. aerogenes. In contrast, the acrAB and tolC mutants presented the same increasing level of chloramphenicol susceptibility as the parental resistant strain, and this drug susceptibility was further increased in the presence of PAβN (Table 2). The initial MICs were recovered when the mutants were transformed with plasmids bearing the E. aerogenes tolC gene or the acrAB genes (Table 2).
TABLE 2.
Clarithromycin, erythromycin, telithromycin, and chloramphenicol susceptibilities of E. aerogenes strainsa
| E. aerogenes strain | Inhibitor concn (μg/ml) | MIC (μg/ml)
|
|||
|---|---|---|---|---|---|
| Clarithromycin | Erythromycin | Telithromycin | Chloramphenicol | ||
| ATCC 13048 | 0 | 256 | 512 | 64 | 4 |
| 10.5 | 4 | 16 | 1 | ||
| 26.3 | 1 | 4 | 0.25 | 4 | |
| EAEP289 (AcrAB over producer) | 0 | 512 | 512 | 64 | 1,024 |
| 10.5 | 32 | 32 | 8 | ||
| 26.3 | 4 | 8 | 0.5 | 64 | |
| EAEP294 (AcrA−) | 0 | 128 | 128 | 32 | 32 |
| 10.5 | 8 | 8 | 2 | ||
| 26.3 | 1 | 2 | 0.06 | 4 | |
| EAEP298 (TolC−) | 0 | 32 | 16 | 16 | 32 |
| 10.5 | 2 | 1 | 1 | ||
| 26.3 | 1 | 1 | 0.06 | 4 | |
| EAEP294(pAcrAB) | 0 | 256 | 256 | 64 | nd |
| 10.5 | 16 | 8 | 4 | ||
| 26.3 | 4 | 1 | 0.5 | nd | |
| EAEP298(pTolC) | 0 | 256 | 256 | 64 | nd |
| 10.5 | 16 | 16 | 4 | ||
| 26.3 | 2 | 2 | 1 | nd | |
Antibiotics were tested alone or with various PAβN concentrations as previously described (4). MICs for the E. aerogenes strains with 526 μg of PAβN/ml have been previously determined (3, 12). The MICs were determined for strains in Mueller-Hinton broth as previously described (4). The MIC values shown are results of three independent experiments. nd, not determined.
A weak reduction of the telithromycin resistance level was detected in the mutants compared to that in the parental strain. A 32-fold decrease in the erythromycin MIC for the tolC strain was observed, when this mutation conferred only a 4-fold decrease in telithromycin MIC (Table 2). These results show that the TolC component is capital in chloramphenicol resistance and is involved in macrolide efflux. Conversely, telithromycin resistance is only weakly mediated by a TolC-dependent efflux pump.
An efflux pump modulates the intracellular concentration of telithromycin.
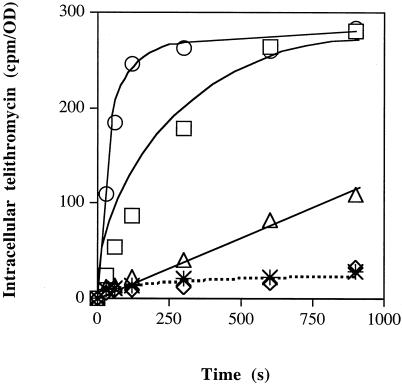
We determined the intracellular concentration of radiolabeled telithromycin in E. aerogenes ATCC 13048 in the presence of PAβN. Increasing amounts of the efflux inhibitor significantly modified the ketolide accumulation (Fig. 1). A high steady-state intracellular concentration was obtained with 52.6 and 105.2 μg of PAβN/ml. The maximal accumulation obtained under these conditions corresponded to about a 10-fold increase in the intracellular telithromycin concentration compared to that in the absence of inhibitor. The effect of PAβN as an inhibitor of telithromycin efflux was quite similar to that observed with carbonyl cyanide m-chlorophenylhydrazone (data not shown), an energy uncoupler which collapses the energy of drug efflux mechanisms (12, 13).
FIG. 1.
Effect of efflux pump inhibitor on intracellular accumulation of telithromycin in E. aerogenes ATCC 13048. Exponential-phase bacteria grown in Luria-Bertani broth were removed, resuspended in sodium phosphate buffer, and incubated with radiolabeled telithromycin for various times. Intracellular telithromycin accumulation was followed in the absence (✴) or presence of PAβN at a final concentration of 5.26 (◊), 26.3 (▵), 52.6 (□), or 105.2 (○) μg/ml. Values (expressed as counts per minute/optical density) were obtained from two independent experiments carried out in duplicate.
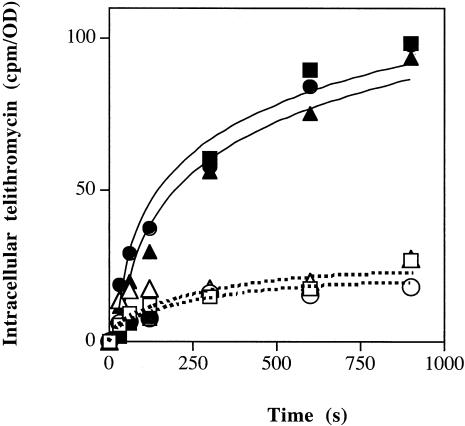
To analyze the role of AcrAB-TolC tripartite complex, we compared the radiolabeled telithromycin accumulation in EAEP289 and its acrA and tolC derivatives in the absence or presence of PAβN. As shown in Fig. 2, the intracellular drug level was quite similar in the parental, acrA, and tolC strains in the absence of efflux inhibitor. In the presence of the efflux inhibitor, a similarly significant increase of telithromycin intracellular concentration was observed with the three strains, indicating the presence of an active efflux mechanism inhibited by PAβN (Fig. 2). These data suggest that the intracellular telithromycin concentration is governed by an active efflux mechanism which is independent of the AcrAB-TolC pump but PAβN sensitive.
FIG. 2.
The AcrAB-TolC efflux pump and telithromycin efflux. Exponential-phase bacteria grown in Luria-Bertani broth were removed, resuspended in sodium phosphate buffer, and incubated with radiolabeled telithromycin for various times. Intracellular telithromycin accumulation was monitored in strains EAEP289 (□, ▪), EAEP298 (○, •), and EAEP294 (▵, ▴). The experiments were carried out in the absence (open symbols) or presence (filled symbols) of PAβN (final concentration, 52.6 μg/ml). Values (expressed as counts per minute/optical density) were obtained from two independent experiments carried out in duplicate.
Telithromycin, the first ketolide approved for clinical use, is a structural macrolide derivative which presents a preserved antibacterial activity against many bacteria that are resistant to macrolides (1, 5, 11, 23). In the present study, we clearly show that an active efflux mechanism confers macrolide and ketolide resistance via a PAβN-sensitive pump in E. coli and E. aerogenes. Clarithromycin and erythromycin susceptibilities were greatly increased in the acrAB and tolC null mutants. This indicates that the intrinsic efflux pump mechanism is the major support of macrolide resistance in E. coli and E. aerogenes. In E. aerogenes, the AcrAB-TolC complex is able to efficiently expel macrolides, such as erythromycin or clarithromycin, while another machinery seems to pump out the ketolide telithromycin. For the tolC mutant, we observed a 32-fold reduction of erythromycin MIC, but only a 4-fold decrease is reached with telithromycin. In contrast, PAβN treatment generates a similar 16-fold decrease in erythromycin and telithromycin MICs. The role of the efflux mechanism in telithromycin resistance is demonstrated by the significant increase in ketolide uptake in the presence of increasing PAβN concentrations. Consequently, these results showed that telithromycin is expelled independently of AcrAB-TolC, since ketolide accumulation is not significantly modified in the acrAB or tolC mutants.
Thus, our results argue that at least two distinct efflux pumps participate in macrolide resistance in E. aerogenes: the AcrAB-TolC machinery and a second mechanism that is PAβN sensitive but AcrAB-TolC independent. The latter also seems to be involved in ketolide efflux. Recent studies concerning the eucaryotic cell transport of this drug family report that telithromycin is less sensitive than other macrolides to P-GP transport expelling molecules from Caco-2 cells (20). Taking into account these data and results concerning the selective bacterial efflux, the difference in the structure of the expelled molecule, telithromycin, clarithromycin, and erythromycin (24) may explain the level of affinity for the drug binding sites located in the respective transporters and efflux pumps (22). Therefore, the preserved antimicrobial activity of this ketolide against gram-negative bacteria, in contrast to that of the macrolides, may result from a weak substrate recognition profile which protects the molecule from AcrAB-TolC efflux machinery.
In conclusion, in E. aerogenes, macrolide molecules can be extruded by the AcrAB-TolC system, but this is not the sole active pump transporting this substrate family. Our results suggest that telithromycin is not a good substrate for this efflux system and that it is efficiently recognized by another PAβN-sensitive pump. This other pump, constitutively expressed in the E. aerogenes ATCC 13048 susceptible strain and in a multidrug-resistant clinical isolate, remains to be identified.
Acknowledgments
We thank E. Pradel for the gift of strains and plasmids and for critical reading of the manuscript and C. Bollet and A. Davin-Regli for fruitful discussions.
This work was supported by the Université de la Méditerranée.
REFERENCES
- 1.Berisio, R., J. Harms, F. Schluenzen, R. Zavirach, H. A. S. Hansen, P. Fucini, and A. Yonath. 2003. Structural insight into the antibiotic action of telithromycin against resistant mutants. J. Bacteriol. 185:4276-4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bryskier, A. 2000. Ketolides-telithromycin, an example of a new class of antibacterial agents. Clin. Microbiol. Infect. 6:661-669. [DOI] [PubMed] [Google Scholar]
- 3.Chevalier, J., J. Bredin, A. Mahamoud, M. Malléa, J. Barbe, and J.-M. Pagès. 2004. Inhibitor of antibiotic efflux pump in resistant Enterobacter aerogenes and Klebsiella pneumoniae strains. Antimicrob. Agents Chemother. 48:1043-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gayet, S., R. Chollet, G. Molle, J.-M. Pagès, and J. Chevalier. 2003. Modification of outer membrane protein profile and evidence suggesting an active drug pump in Enterobacter aerogenes clinical strains. Antimicrob. Agents Chemother. 47:1555-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldstein, E. J., D. M. Citron, C. V. Merriam, Y. Warren, K. L. Tyrrel, and H. Fernandez. 2003. In vitro activities of telithromycin and 10 oral agents against aerobic and anaerobic pathogens isolated from antral puncture specimens from patients with sinusitis. Antimicrob. Agents Chemother. 47:1963-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagman, K. E., C. E. Lucas, J. T. Balthazar, L. Snyder, M. Nilles, R. C. Judd, and W. M. Shafer. 1997. The MtrD protein of Neisseria gonorrhoeae is a member of the resistance/nodulation/division protein family constituting part of an efflux system. Microbiology 143:2117-2125. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi, N., K. Nishino, and A. Yamaguchi. 2001. Novel macrolide-specific ABC-type efflux transporter in Escherichia coli. J. Bacteriol. 183:5639-5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leclerc, R. 2002. Mechanisms of resistance to macrolides and lincosamides: nature of the resistance elements and their clinical implications. Clin. Infect. Dis. 34:482-492. [DOI] [PubMed] [Google Scholar]
- 9.Levy, S. B. 2002. Active efflux, a common mechanism for biocide and antibiotic resistance. J. Appl. Microbiol. 92(Suppl.):65S-71S. [PubMed] [Google Scholar]
- 10.Lomovskaya, O., M. S. Warren, A. Lee, J. Galazzo, R. Fronko, M. Lee, J. Blais, D. Cho, S. Chamberland, T. Renau, R. Leger, S. Hecker, W. Watkins, K. Hoshino, H. Ishida, and V. J. Lee. 2001. Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: novel agents for combination therapy. Antimicrob. Agents Chemother. 45:105-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lorenz, J. 2003. Telithromycin: the first ketolide antibacterial for the treatment of community-acquired respiratory infections. Int. J. Clin. Pract. 57:519-529. [PubMed] [Google Scholar]
- 12.Malléa, M., A. Mahamoud, J. Chevalier, S. Alibert-Franco, P. Brouant, J. Barbe, and J.-M. Pagès. 2003. Alkylaminoquinolines inhibit bacterial antibiotic efflux pump in multidrug resistant clinical isolates. Biochem. J. 376:801-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malléa, M., J. Chevalier, C. Bornet, A. Eyraud, A. Davin-Regli, C. Bollet, and J.-M. Pagès. 1998. Porin alteration and active efflux: two in vivo drug resistance strategies used by Enterobacter aerogenes. Microbiology 144:3003-3009. [DOI] [PubMed] [Google Scholar]
- 14.Ng, L. K., I. Martin, G. Liu, and L. Bryden. 2002. Mutation in 23S rRNA associated with macrolide resistance in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 46:3020-3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peric, M., B. Bozdogan, M. R. Jacobs, and P. C. Appelbaum. 2003. Effects of an efflux mechanism and ribosomal mutations on macrolide susceptibility of Haemophilus influenzae clinical isolates. Antimicrob. Agents Chemother. 47:1017-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pradel, E., and J.-M. Pagès. 2002. The AcrAB-TolC pump contributes to multidrug resistance in the nosocomial pathogen Enterobacter aerogenes. Antimicrob. Agents Chemother. 46:2640-2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Retsema, J., and W. Fu. 2001. Macrolides: structures and microbial targets. Int. J. Antimicrob. Agents 18:S3-S10. [DOI] [PubMed] [Google Scholar]
- 18.Roberts, M. C., J. Sutcliffe, P. Courvalin, L. B. Jensen, J. Rood, and H. Seppala. 1999. Nomenclature for macrolide and macrolide-lincosamide-streptogramin B resistance determinants. Antimicrob. Agents Chemother. 43:2823-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanchez, L., W. Pan, M. Vinas, and H. Nikaido. 1997. The acrAB homolog of Haemophilus influenzae codes for a functional multidrug efflux pump. J. Bacteriol. 179:6855-6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seral, C., J. M. Michot, H. Chanteux, M. P. Mingeot-Leclercq, P. M. Tulkens, and F. van Bambeke. 2003. Influence of P-glycoprotein inhibitors on accumulation of macrolides in J774 murine macrophages. Antimicrob. Agents Chemother. 47:1047-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veal, W. L., A. Yellen, J. T. Balthazar, W. Pan, B. G. Spratt, and W. M. Shafer. 1998. Loss-of-function mutations in the Mtr efflux system of Neisseria gonorrhoeae. Microbiology 144:621-627. [DOI] [PubMed] [Google Scholar]
- 22.Yu, E. W., G. McDermott, H. I. Zgurskaya, H. Nikaido, and D. E. Koshland, Jr. 2003. Structural basis of multiple drug-binding capacity of the AcrB multidrug efflux pump. Science 300:976-980. [DOI] [PubMed] [Google Scholar]
- 23.Zhanel, G. G., M. Walters, A. Noreddin, L. M. Vercaigne, A. Wierzbowski, J. M. Embil, A. S. Gin, S. Douthwaite, and D. J. Hoban. 2002. The ketolides: a critical review. Drugs 62:1771-1804. [DOI] [PubMed] [Google Scholar]
- 24.Zong, P., and V. D. Shortridge. 2000. The role of efflux in macrolide resistance. Infect. Dis. Res. 3:325-329. [DOI] [PubMed] [Google Scholar]