Abstract
PURPOSE
To assess the impact of radiation treatment time (RTT) in head and neck cancers on overall survival (OS) in the era of chemoradiation.
MATERIALS & METHODS
Patients diagnosed with tongue, hypopharynx, larynx, oropharynx, or tonsil cancer were identified using the National Cancer Database. RTT was defined as date of first radiation treatment to date of last radiation treatment. In the definitive setting, prolonged RTT was defined as >56 days, accelerated RTT was defined as ≤47 days, and standard RTT was defined as 48–56 days. In the post-operative setting, prolonged RTT was defined as >49 days, accelerated RTT was defined as ≤40 days, and standard RTT was defined as 41–49 days. Chi-squared tests were used to identify predictors of RTT. The Kaplan-Meier method was used to compare OS amongst groups. Cox proportional hazards model was used for OS analysis in patients with known comorbidity status.
RESULTS
19,531 patients were included; 12,987 (67%) had a standard RTT, 4,369 (34%) had an accelerated RTT, and 2,165 (11%) had a prolonged RTT. On multivariable analysis, accelerated RTT (HR 0.84 95% CI 0.73–0.97) was associated with an improved OS while prolonged RTT (HR 1.25 95% CI 1.14–1.37) was associated with a worse OS relative to standard RTT. When examining the 9,200 (47%) patients receiving definitive concurrent chemoradiation, prolonged RTT (HR 1.29 95% CI 1.11–1.50) was associated with a worse OS relative to standard RTT while there was no significant association between accelerated RTT and OS (HR 0.76 95% CI 0.57–1.01).
CONCLUSION
Prolonged RTT is associated with worse OS in patients receiving radiotherapy for head and neck cancer, even in the setting of chemoradiation. Expeditious completion of radiation should continue to be a quality metric for the management of head and neck malignancies.
Keywords: Treatment time, Head and Neck Cancer, Squamous Cell, National Cancer Database
INTRODUCTION
Prolonged radiation treatment time (RTT) is associated with poor oncologic outcomes (1–3). This is thought to be referable to accelerated repopulation - the proliferation of surviving tumor clonogens during a standard course of radiotherapy leading to radioresistance (4, 5). Understanding the consequences of prompt completion of fractionated external beam radiation involves two separate but related concepts, the avoidance of unplanned treatment breaks and the elective institution of accelerated treatment schedules.
Most treatment prolongation is unplanned and likely due to acute toxicity; thus the results of prolonged RTT alone are difficult to study. Nonetheless, multiple retrospective studies have demonstrated that it is associated with inferior outcomes in patients treated with radiation alone (6–9). Similarly, prolonged time to complete a head and neck ‘treatment package’ involving an operation and adjuvant therapy is associated with worse outcomes (10–12). In view of the worse outcome with prolonged RTT, it is not surprising that prospective trials demonstrate a significant benefit to accelerated therapy (13–15).
However, despite small series demonstrating the continued importance of RTT in the modern era, most RTT data emphasizing the importance of treatment time predates the widespread adoption of chemoradiation (16). Chemoradiation is currently the standard of care for locally advanced head and neck cancer treated with primary radiation, demonstrating a survival benefit when administered concurrently (17). As a consequence, the application of chemoradiation has increased by 200% from 1998 to 2011 (18). Modern prospective trials investigating acceleration in the setting of concurrent chemoradiation do not demonstrate a benefit to treatment acceleration (19). Whether or not treatment time matters in the era of chemoradiation is of substantial concern, because accelerated radiation with concurrent chemotherapy has proven to be the most toxic mechanism of delivering non-surgical therapy (20). The purpose of this study was to identify the impact of RTT on clinical outcomes in patients with head and neck cancer treated in the era of chemoradiation using a large prospective national database.
MATERIALS & METHODS
Patient Selection
A program of the American College of Surgeons, Commission on Cancer (CoC), and the American Cancer Society, the National Cancer Data Base (NCDB) is a national cancer registry that was established in 1989 and serves as a comprehensive clinical surveillance resource for cancer care in the United States. The NCDB compiles data from more than 1500 commission-accredited cancer programs in the United States and Puerto Rico and captures approximately 70% of all newly diagnosed cancer cases (21).
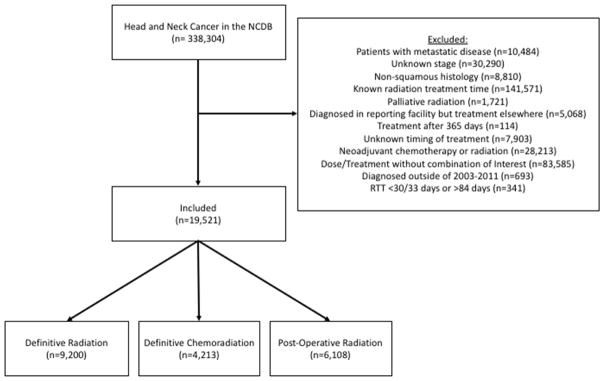
Figure 1 demonstrates the CONSORT diagram for this analysis. Patients with invasive squamous cell carcinoma of the oral tongue, oropharynx, larynx and hypopharynx (codes 8000, 8053, 8070, 8071, 8072, 8076, 8082, 8083, 8084, 8052, 8074, 8023, 8430, 8560) treated with radiation therapy between 2003–2011 were identified. The following patients were excluded: those who had distant metastatic disease or an unknown stage at presentation, those known to have received therapy with palliative intent (specifically coded in the NCDB by treating institution), those who initiated treatment>365 days after the diagnosis date, those receiving induction chemotherapy, and those with incomplete data regarding length of radiation treatment. The NCDB records the prescribed radiation dose/fractionation and the dates of radiation initiation/completion. However it does not report the radiation dose received. In order to eliminate patients who likely did not complete therapy, we excluded patients with an RTT <30 days for post-operative radiotherapy and <33 days for definitive radiotherapy. In order to eliminate patients with excessively long RTT whose outcome was likely confounded by other fundamental issues not captured by the database, we excluded the few patients with a at least a 50% increase over the maximum “standard” RTT (>84 days).
Figure 1.
CONSORT Diagram
Radiation Treatment Time
RTT was defined by the date of first radiation treatment to date of last radiation treatment. For patients undergoing definitive radiation (with or without chemotherapy) the four most common fractionation schedules were included: 70 Gy in 35 fractions (n=10,851), 70.2 Gy in 39 fractions (n=970), 69.96 Gy in 33 fractions (1,404), 72 Gy in 42 fractions (n=188). For patients undergoing post-operative radiation the three most common fractionation schedules were included: 60 Gy in 30 fractions (n=2,575), 66 Gy in 33 fractions (n=3,192), 63 Gy in 35 fractions (n=341). Patients were divided into three groups according to their RTT: prolonged RTT was defined as >56 days for definitive radiation and >49 days for adjuvant radiation, accelerated RTT was defined as <47 days for definitive radiation and <40 days for adjuvant radiation, and standard RTT was defined as 48–56 days for definitive radiation and 41–49 days for adjuvant radiation. When defining our accelerated fractionation schedules, we estimated the minimum time needed to complete a radiotherapy treatment course assuming treatment initiated on a Monday and completed on a Friday. For example the 70 Gy in 35 fraction course would be completed in 47 days and therefore completing radiotherapy in <47 days would be considered accelerated. When identifying a prolonged course we also utilized previously published assessments of a major head and neck fractionation trial investigating both a six and seven week radiation courses (19).
Statistical Analysis
We compared patient and tumor factors versus RTT category (standard, accelerated, prolonged) using Pearson Chi-squared tests. We then used pairs of multivariate logistic regressions (accelerated vs standard, prolonged vs standard) to determine simultaneously which factors were associated with RTT. These models used robust sandwich variance estimates with generalized estimating equations to account for within-hospital correlations (22). We estimated survival functions for RTT groups using the methods of Kaplan and Meier, testing for significance using log-rank tests. We used Cox proportional hazards regressions with robust standard errors to determine the effect of RTT adjusted for patient, tumor, and treatment factors (23). Survival cohorts were limited by year based on available data. To ensure sufficient follow-up time, our survival analysis was composed only of patients diagnosed in 2006 or earlier. In multivariate survival models, we only considered cases diagnosed in years 2003–2006 because this is the only cohort that includes data on Charlson comorbidities; including other years does not allow for the control of comorbidity in the survival model. When estimating the impact of RTT on overall survival in the post-operative radiation cohort, we included time between surgery and radiation initiation as a binary variable (<8 weeks versus ≥8 weeks) secondary to prior analyses highlighting the importance of treatment package time (12). We did not include treatment package time itself due to collinearity with RTT (a component of package time). Median follow-up time was estimated using the Kaplan-Meier potential follow-up method (24).
RESULTS
Patient Demographics
A total of 19,521 patients met the inclusion criteria. The median follow-up was 75.7 months (range 2.1–120.5). A total of 13,413 (69%) patients received definitive radiation while 6,108 (31%) patients received post-operative radiation. A majority of patients (56%) received concurrent chemotherapy. The median RTT was 50 days (range 30–84) for the entire cohort. The median RTT for patients receiving definitive radiation and post-operative radiation were 51 (range 33–84) and 43 (range 30–84), respectively. Further details regarding RTT according to subgroup is detailed in Table 1.
Table 1.
Radiation Treatment Time According to Definitive and Post-Operative Radiotherapy Groups
| Mean | Minimum | Median | Maximum | |
|---|---|---|---|---|
| Entire Cohort | 50.89 | 30 | 50 | 84 |
| Definitive (Accelerated) | 43.16 | 33 | 44 | 46 |
| Definitive (Standard) | 50.87 | 47 | 51 | 56 |
| Definitive (Delayed) | 62.62 | 57 | 60 | 84 |
| Post-Operative (Accelerated) | 40.04 | 30 | 40 | 42 |
| Post-Operative (Standard) | 45.75 | 43 | 46 | 49 |
| Post-Operative (Delayed) | 54.60 | 50 | 52 | 84 |
In the entire cohort, 12,987 (67%) had a standard RTT, 4,369 (22%) had an accelerated RTT, and 2,165 (11%) had a prolonged RTT. Clinical factors associated with a prolonged RTT included female gender (p<0.001), African American race (p<0.001), Charlson/Deyo score 2 (p<0.001), hypopharynx cancer (p<0.001), stage III or stage IV disease (p<0.001), definitive treatment (p<0.001), receipt of chemotherapy (p<0.001), and tumor grade 1/2 disease (p<0.001) (Supplementary Table 1). On multivariable analysis the only clinical factors associated with a prolonged RTT were Charlson/Deyo score of 1 (OR 1.20 95% CI 1.09–1.32 p=0.0002) or 2 (OR 1.38 95% CI 1.17–1.63 p<0.0001), and hypopharynx tumor location (OR 1.16 95% CI 1.01–1.33 p=0.035). Male gender (OR 0.73 95% CI 0.68–0.79 p<0.001), stage I (OR 0.60 95% CI 0.52–0.68 p<0.0001) or II (OR 0.79 95% CI 0.70–0.89 p<0.001) disease, and radiation alone (OR 0.54 95% CI 0.49–0.60 p<0.001) were associated with a decreased likelihood of having a prolonged RTT (Supplementary Table 2).
Radiation Treatment Time
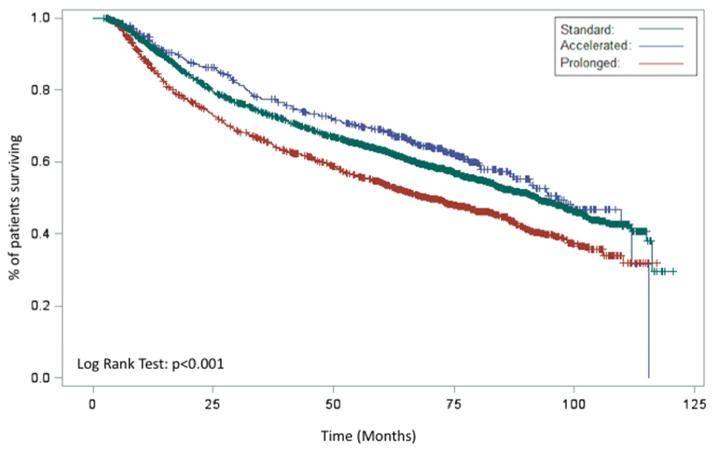
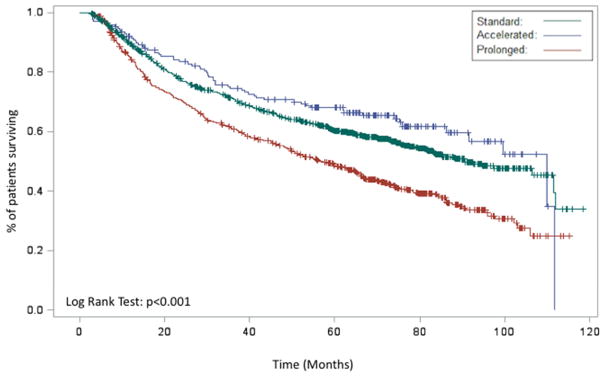
In the entire cohort, Kaplan-Meier analysis demonstrated an improved OS for patients with an accelerated RTT relative to patients with a standard or prolonged RTT (p<0.001, Figure 2A). When analyzed with respect to an operation, both definitive and adjuvant radiation demonstrated similar findings (p<0.001, Figure 2B and p=0.0315, Figure 2C, respectively). On multivariable analysis, accelerated RTT (HR 0.84 95% CI 0.73–0.97 p=0.02) remained associated with an improved OS while prolonged RTT (HR 1.25 95% CI 1.14–1.37 p<0.001) remained associated with a worse OS relative to standard RTT.
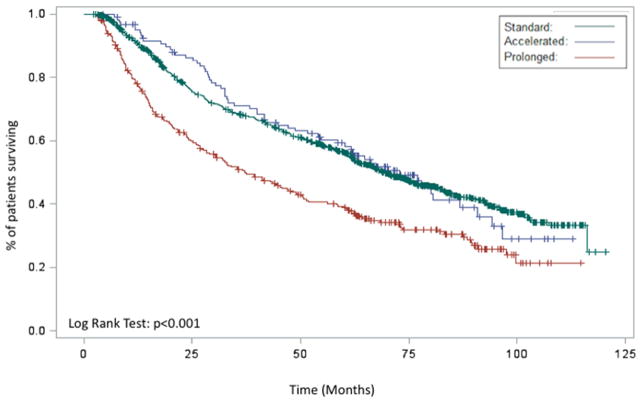
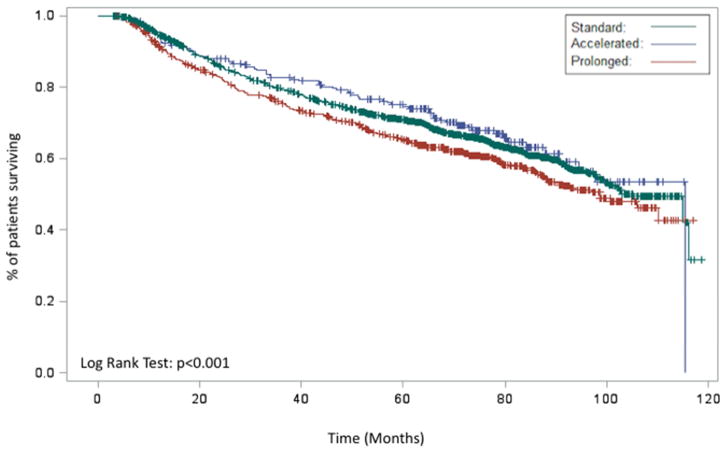
Figure 2.
Kaplan-Meier demonstrating the association between RTT (prolonged, acceleration, standard) and overall survival for entire cohort (A), patients receiving definitive radiation (B), patients receiving post-operative radiation (C), and patients receiving chemoradiation (D)
In the subset of patients receiving definitive radiation, prolonged RTT was associated with a worse OS (HR 1.37 95% CI 1.13–1.66 p=0.0013) while accelerated RTT was no longer associated with an OS benefit (HR 0.86 95% CI 0.67–1.11 p=0.2445). When examining patients receiving post-operative radiation, there was a trend towards improved OS with an accelerated RTT (OR 0.77 95% CI 0.58–1.03 p=0.0824) while prolonged RTT was no longer associated with a worse OS (HR 1.01 95% CI 0.86–1.18 p=0.9151) relative to standard RTT. Other predictors of OS on multivariable analysis included age, tumor location, chemotherapy use, surgery, stage, Charlson-Deyo score, insurance status, facility location, disease site, education status, receipt of surgery, tumor grade, and interval between surgery and radiation (Table 2).
Table 2.
Multivariable of overall survival for definitive radiation, definitive chemoradiation, and post-operative radiation patients
| All Patients (n=19,521) | Definitive Radiation (n=9,200) | Definitive Chemoradiation (n=4,213) | Post-Operative Radiation (n=6,108) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | |||||
| Radiation Treatment Time | ||||||||||||||||
| Standard | Reference | Reference | Reference | Reference | ||||||||||||
| Accelerated | 0.84 | 0.73 | 0.97 | 0.02 | 0.86 | 0.67 | 1.11 | 0.2445 | 0.76 | 0.57 | 1.01 | 0.0556 | 0.77 | 0.58 | 1.03 | 0.0824 |
| Prolonged | 1.25 | 1.14 | 1.37 | <.0001 | 1.37 | 1.13 | 1.66 | 0.0013 | 1.29 | 1.11 | 1.50 | 0.0008 | 1.01 | 0.86 | 1.18 | 0.9151 |
| Age | ||||||||||||||||
| 1.<=30 | Reference | Reference | ||||||||||||||
| 2.31–40 | 0.34 | 0.16 | 0.71 | 0.0043 | Reference | 0.27 | 0.11 | 0.65 | 0.0033 | |||||||
| 3.41–50 | 0.43 | 0.22 | 0.85 | 0.016 | 1.49 | 0.43 | 5.13 | 0.5305 | 0.44 | 0.21 | 0.92 | 0.0301 | ||||
| 4.51–60 | 0.52 | 0.26 | 1.03 | 0.0621 | 1.87 | 0.56 | 6.21 | 0.3089 | 0.53 | 0.26 | 1.12 | 0.0953 | ||||
| 5.61–70 | 0.67 | 0.34 | 1.34 | 0.2584 | 2.58 | 0.77 | 8.67 | 0.1263 | 0.63 | 0.30 | 1.33 | 0.2268 | ||||
| 6.70+ | 1.10 | 0.55 | 2.18 | 0.7952 | 4.27 | 1.28 | 14.29 | 0.0183 | 1.19 | 0.57 | 2.50 | 0.6382 | ||||
| Charlson/Deyo Score | Reference | |||||||||||||||
| 0 | Reference | Reference | Reference | |||||||||||||
| 1 | 1.21 | 1.07 | 1.36 | 0.0019 | 1.13 | 0.92 | 1.37 | 0.2461 | 1.08 | 0.88 | 1.34 | 0.4627 | 1.38 | 1.13 | 1.68 | 0.0016 |
| 2 | 1.83 | 1.51 | 2.21 | <.0001 | 1.51 | 1.09 | 2.10 | 0.0129 | 1.91 | 1.31 | 2.79 | 0.0007 | 2.21 | 1.63 | 2.99 | <.0001 |
| Insurance Status | ||||||||||||||||
| Private | Reference | Reference | Reference | Reference | ||||||||||||
| Not Insured | 1.58 | 1.28 | 1.94 | <.0001 | 1.47 | 0.93 | 2.33 | 0.0995 | 1.96 | 1.46 | 2.63 | <.0001 | 1.32 | 0.90 | 1.92 | 0.1541 |
| Medicaid | 1.98 | 1.68 | 2.32 | <.0001 | 2.15 | 1.51 | 3.04 | <.0001 | 1.95 | 1.53 | 2.49 | <.0001 | 1.69 | 1.21 | 2.35 | 0.0019 |
| Medicare | 1.40 | 1.25 | 1.57 | <.0001 | 1.40 | 1.14 | 1.72 | 0.0012 | 1.60 | 1.33 | 1.93 | <.0001 | 1.30 | 1.06 | 1.61 | 0.0136 |
| Other Government | 2.04 | 1.60 | 2.59 | <.0001 | 2.25 | 1.44 | 3.53 | 0.0004 | 2.31 | 1.59 | 3.36 | <.0001 | 1.34 | 0.83 | 2.18 | 0.2336 |
| Unknown | 1.46 | 1.17 | 1.82 | 0.0009 | 1.88 | 1.14 | 3.12 | 0.0139 | 1.54 | 0.97 | 2.42 | 0.0644 | 1.17 | 0.79 | 1.73 | 0.4305 |
| Facility Location | ||||||||||||||||
| Pacific | Reference | Reference | ||||||||||||||
| New England | 1.17 | 0.93 | 1.48 | 0.1898 | 1.36 | 0.91 | 2.01 | 0.1299 | ||||||||
| Middle Atlantic | 1.27 | 1.05 | 1.54 | 0.0137 | 1.71 | 1.19 | 2.44 | 0.0035 | ||||||||
| South Atlantic | 1.25 | 1.03 | 1.50 | 0.0215 | 1.60 | 1.16 | 2.19 | 0.0039 | ||||||||
| East North Central | 1.17 | 0.97 | 1.41 | 0.1068 | 1.41 | 1.01 | 1.95 | 0.041 | ||||||||
| East South Central | 1.15 | 0.92 | 1.44 | 0.2113 | 1.71 | 1.20 | 2.43 | 0.0028 | ||||||||
| West North Central | 1.22 | 0.99 | 1.51 | 0.0654 | 1.35 | 0.92 | 1.97 | 0.1205 | ||||||||
| West South Central | 1.03 | 0.80 | 1.31 | 0.8326 | 1.47 | 0.91 | 2.36 | 0.1134 | ||||||||
| Mountain | 1.20 | 0.90 | 1.60 | 0.2161 | 1.27 | 0.83 | 1.95 | 0.2756 | ||||||||
| Disease Site | ||||||||||||||||
| Tonsil | Reference | Reference | Reference | Reference | ||||||||||||
| Hypopharynx | 2.17 | 1.84 | 2.57 | <.0001 | 1.60 | 1.10 | 2.33 | 0.0148 | 1.95 | 1.56 | 2.44 | <.0001 | 2.98 | 1.96 | 4.52 | <.0001 |
| Larynx | 1.36 | 1.19 | 1.56 | <.0001 | 1.00 | 0.77 | 1.29 | 0.9907 | 1.28 | 1.04 | 1.57 | 0.0195 | 2.11 | 1.64 | 2.71 | <.0001 |
| Oropharynx | 1.68 | 1.33 | 2.12 | <.0001 | 1.37 | 0.91 | 2.06 | 0.135 | 1.51 | 1.08 | 2.11 | 0.0152 | 2.21 | 1.23 | 3.99 | 0.0083 |
| Tongue | 1.37 | 1.19 | 1.59 | <.0001 | 0.97 | 0.71 | 1.33 | 0.8593 | 0.95 | 0.77 | 1.17 | 0.6476 | 2.92 | 2.30 | 3.70 | <.0001 |
| Stage | ||||||||||||||||
| 4 | Reference | Reference | Reference | Reference | ||||||||||||
| 0 | 0.42 | 0.31 | 0.58 | <.0001 | 0.45 | 0.27 | 0.75 | 0.0025 | 0.87 | 0.18 | 4.12 | 0.8567 | 0.34 | 0.22 | 0.53 | <.0001 |
| 1 | 0.44 | 0.38 | 0.51 | <.0001 | 0.41 | 0.30 | 0.54 | <.0001 | 0.97 | 0.54 | 1.76 | 0.9204 | 0.42 | 0.33 | 0.53 | <.0001 |
| 2 | 0.64 | 0.56 | 0.74 | <.0001 | 0.55 | 0.42 | 0.72 | <.0001 | 0.87 | 0.67 | 1.11 | 0.2635 | 0.59 | 0.46 | 0.76 | <.0001 |
| 3 | 0.83 | 0.74 | 0.93 | 0.0014 | 0.90 | 0.67 | 1.19 | 0.4426 | 0.75 | 0.64 | 0.88 | 0.0004 | 0.75 | 0.58 | 0.97 | 0.0292 |
| % of adults not graduating from high school | ||||||||||||||||
| 29% or more | Reference | Reference | ||||||||||||||
| 20% – 28.9% | 0.87 | 0.67 | 1.13 | 0.2845 | 0.78 | 0.61 | 0.99 | 0.0427 | ||||||||
| 14%–19.9% | 0.71 | 0.52 | 0.97 | 0.0333 | 0.90 | 0.69 | 1.17 | 0.4256 | ||||||||
| Less than 14% | 0.84 | 0.61 | 1.18 | 0.3152 | 0.85 | 0.64 | 1.14 | 0.2729 | ||||||||
| Chemotherapy | ||||||||||||||||
| Yes | Reference | |||||||||||||||
| No | 1.34 | 1.20 | 1.50 | <.0001 | ||||||||||||
| Surgery | ||||||||||||||||
| Yes | Reference | |||||||||||||||
| No | 1.58 | 1.42 | 1.75 | <.0001 | ||||||||||||
| Grade | ||||||||||||||||
| 1/2 | Reference | Reference | Reference | |||||||||||||
| 3/4 | 0.93 | 0.83 | 1.02 | 0.1349 | 0.93 | 0.80 | 1.08 | 0.3157 | 1.01 | 0.85 | 1.21 | 0.8785 | ||||
| Unknown | 0.89 | 0.80 | 0.99 | 0.0352 | 0.81 | 0.68 | 0.97 | 0.0199 | 0.92 | 0.73 | 1.15 | 0.4578 | ||||
| Race | ||||||||||||||||
| White | Reference | Reference | ||||||||||||||
| African American | 0.97 | 0.77 | 1.22 | 0.7752 | 1.23 | 0.98 | 1.55 | 0.0715 | ||||||||
| Asian | 0.43 | 0.15 | 1.25 | 0.1205 | 1.98 | 1.10 | 3.54 | 0.0223 | ||||||||
| Other | 0.45 | 0.20 | 0.99 | 0.0483 | 0.99 | 0.66 | 1.48 | 0.9585 | ||||||||
| Time from Surgery to Radiation | ||||||||||||||||
| <8 weeks | Reference | |||||||||||||||
| ≥8 weeks | 1.21 | 1.01 | 1.44 | 0.0347 | ||||||||||||
Model controlled for radiation treatment time, age, sex, race, Charlson/Deyo score, insurance status, facility location, disease site location, stage, year of diagnosis, income, education, receipt of surgery, receipt of chemotherapy, and grade. Only statistically significant groups demonstrated, grayed out subgroups did not reach statistical significance or were not included in the model.
Chemoradiation Treatment Time
When examining only patients receiving definitive chemoradiation (n=9,200), a total of 6,217 (68%) had a standard RTT, 958 (10%) had an accelerated RTT, and 2,025 (22%) patients had a prolonged RTT. Kaplan-Meier analysis demonstrated a worse OS for patients with a prolonged RTT relative to patients with a standard or accelerated RTT in patients receiving chemoradiation (p<0.001, Figure 2D). On multivariable analysis, prolonged RTT (HR 1.29 95% CI 1.11–1.50 p=0.008) remained associated with a worse OS relative to standard RTT. There was a trend towards an improved OS with accelerated RTT (HR 0.76 95% CI 0.57–1.01 p=0.0556) (Table 2).
Interaction of RTT with Clinical Variables and OS
We examining the survival impact of RTT on clinical covariates associated with mortality on multivariable analysis. There was no interaction between tumor grade (p=0.4501) or disease site (p=0.0979) with RTT. When examining tumor stage, Stage III disease was associated with a worse OS with a prolonged RTT (HR 1.56 95% CI 1.20–2.03) relative to a standard RTT. There was no interaction across any other stage groupings (Table 3).
Table 3.
Interaction between Radiation Treatment Time and Stage
| HR | 95% CI | ||
|---|---|---|---|
| Stage 1 | |||
| Standard | Reference | ||
| Accelerated* | N/A | N/A | N/A |
|
|
|||
| Prolonged | 3.15 | 1.17 | 8.50 |
| Stage 2 | |||
| Standard | Reference | ||
|
|
|||
| Accelerated | 1.37 | 0.39 | 4.81 |
|
|
|||
| Prolonged | 0.93 | 0.55 | 1.57 |
| Stage 3 | |||
| Standard | Reference | ||
|
|
|||
| Accelerated | 0.79 | 0.46 | 1.37 |
|
|
|||
| Prolonged | 1.56 | 1.20 | 2.03 |
| Stage 4 | |||
| Standard | Reference | ||
|
|
|||
| Accelerated | 0.72 | 0.52 | 1.00 |
|
|
|||
| Prolonged | 1.21 | 1.01 | 1.46 |
No deaths in the stage 1/accelerated group
DISCUSSION
Fraction size is chosen to exploit the differential responses of tumors and normal tissues to external radiation. The Radiation Therapy Oncology Group (RTOG) ran multiple trials investigating accelerated fractionation schedules for head and neck cancer, culminating in a large 4-arm trial of radiation alone demonstrating, in part, that delivering 72 Gy in 6 weeks rather than 70 Gy in 7 weeks improved 2 year local-regional control by 8% (13). Although proving that treatment in 6 weeks is superior to 7 is not the same as proving that treatment in 7 weeks is superior to 8, a logical conclusion was that an 8 week treatment of head and neck cancer was no longer considered acceptable (25). By comparison, in the era of chemoradiation, the result of increased RTT is uncertain and unplanned radiation treatment breaks resulting in 8 week (and longer) courses are all too common in daily practice.
Given the complexities of daily attendance to a radiation facility, it is not surprising that many patients experience prolonged RTT. Nonetheless, it is surprising that such a high percentage (22%) met this study’s generous definition of prolonged RTT – an entire week longer than anticipated. Identifying patients at risk for a lengthy RTT may allow for closer monitoring during treatment and promote pre-emptive supportive measures. Female patients and those with higher stage disease, poor performance status, chemotherapy use or a hypopharynx primary were more likely to have an increased RTT.
It is not unexpected that patients predisposed to prolonged RTT are also those known to have increased acute and late toxicity (20, 26). They may benefit from close attention during radiation therapy. Helpful interventions include multiple weekly on-treatment visits, aggressive symptom management (including a low threshold for elective gastrostomy tube placement if unable to maintain weight before or during radiation), and scheduled intravenous fluid administration on days not receiving systemic therapy.
RTT and radiation alone
The role of RTT in the setting of radiation alone is clear – randomized trials support a locoregional control benefit from acceleration. Although the lack of OS improvement in this analysis is consistent with randomized trials, it conflicts with a large meta-analysis that demonstrated a survival benefit for patients receiving altered fractionation (15). The meta-analysis, including nearly twice the number of patients (compared to this monograph) with a HR of 0.92 is similar to the HR of 0.86 in our series. While the OS benefit was not demonstrated in the analysis, this may reflect statistical power to substantiate a difference. Accelerated fractionation should still be considered for all patients receiving definitive radiation therapy.
In contrast to the known benefits of acceleration, which are probably best displayed by locoregional control and not captured by the NCDB, this work demonstrates worse survival among patients treated with radiation alone who sustain an unduly long RTT. Although prolonged RTT has many causes, this survival detriment persists when controlling for comorbidity, suggesting that inadequately treated head and neck cancer is a direct cause of mortality. Thus prolonged RTT must be recognized early by practitioners. In view of this study’s generous definitions (treatment needed to be extended at least a week prior to be classified as ‘prolonged’); mitigation of prolonged RTT encountered during a treatment course would likely require unsustainable acceleration (increased dose per fraction and/or increased fractions in a given week) (27). The best way to address prolonged RTT, as suggested above, is through prevention.
RTT and concurrent chemoradiation
The role of RTT in the setting of chemoradiation is less clear. Randomized trials suggest that the addition of concurrent chemotherapy improves the results of hyperfractionated radiotherapy but acceleration of radiation does not enhance concurrent chemoradiation (19, 28). However, similar to radiation alone, there appears to be no significant survival benefit to acceleration with concurrent chemotherapy. Although the hazard ratio in this analysis suggests a possible benefit (and there are many accelerated fractionation regimens not evaluated in this analysis), the toxicity of concurrent accelerated chemoradiation coupled with the absence of a significant survival benefit even with a large dataset discourages the use of these regimens.
Similar to the setting of radiation alone, prolonged RTT adversely affects survival, even in the setting of concurrent chemoradiation. This is of considerable interest because receiving concurrent chemotherapy is an independent predictor of increased RTT. Prospective analyses of different concurrent regimens suggest that delay is more closely associated with some regimens (29). Although concurrent systemic therapy may limit the benefit of accelerated fractionation, it does not obviate the need to complete therapy in a timely fashion.
RTT and post-operative therapy
RTT is a component of treatment package time — the time needed to complete postoperative radiation after resection of a head and neck cancer. Although historical reports differ on the importance of package time, most prospective investigations stipulate that adjuvant therapy must initiate within 8 weeks of the extirpative operation in order to reduce the package time (12, 30). This analysis demonstrates that prolonged RTT in the adjuvant setting does not worsen survival regardless of whether the adjuvant therapy begins within 8 weeks from the operation. The explanation for this different impact of prolonged RTT on the survival of adjuvant patients (HR 1.01) when compared to definitive radiation (HR 1.37) and definitive chemoradiation (HR 1.29) is likely multifactorial. Although we evaluated delay between operation and radiation start along with RTT, this may somehow be related to issues of package time - unlike in definitive therapy, RTT only represents a component of the total time subject to accelerated repopulation.
Alternatively, this may be related to the very nature of adjuvant therapy. A proportion of patients with either extracapsular spread or positive margins will survive for years after surgery alone (with no post-operative radiation) (31). Demonstrating a worse survival among a group of patients that are not all ‘high risk’ secondary to a 1 week delay in the timing of adjuvant radiotherapy may therefore be difficult. Nonetheless, we continue to advocate for prompt institution of adjuvant therapy and avoidance of prolonged RTT in the adjuvant setting. (32–35).
This work has limitations consistent with all large national database analyses which include coding errors, selection bias, and missing data. The NCDB also only codes for OS as an endpoint and there for there are no data regarding cancer-specific survival or treatment-related toxicity. Furthermore, we were did not include treatment package time within the model for postoperative radiation due to the collinearity of package time with RTT. It is difficult to ascertain the impact of package time on RTT in this analysis.
This analysis demonstrates that RTT is an important prognostic factor for patients with head and neck malignancies. Although the application of concurrent chemotherapy may reduce the benefit of a shorter RTT in patients receiving concurrent chemoradiation, it cannot overcome the decline in tumor control among patients with a prolonged RTT. In patients receiving radiation alone or post-operative radiation, shorter RTT may improve outcomes and should be considered when evaluating the potential for increased toxicity with accelerated schedules.
Supplementary Material
Supplemental Table 1: Patient and treatment characteristics
Supplementary Table 2: Multivariable analysis of predictors of prolonged radiation treatment time (versus standard)
Summary.
Despite the increasing adoption of chemoradiation for patients with locally advanced head and neck cancer, the impact of radiation treatment time (RTT) in patients receiving of chemoradiation is unclear. We utilize the National Cancer Database to identify the impact of RTT on outcomes in patients with head and neck cancer treated in the era of chemoradiation. Our results demonstrate that a prolonged radiation course is associated with an inferior overall survival regardless of chemotherapy use.
Acknowledgments
This publication was supported by grant number P30 CA006927 from the National Cancer Institute, NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. This publication was supported in part by a grant from Varian Medical Systems, Inc. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of Varian Medical Systems, Inc.
Footnotes
Conflicts of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fyles A, Keane TJ, Barton M, Simm J. The effect of treatment duration in the local control of cervix cancer. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 1992;25(4):273–279. doi: 10.1016/0167-8140(92)90247-r. [DOI] [PubMed] [Google Scholar]
- 2.Chen M, Jiang GL, Fu XL, et al. The impact of overall treatment time on outcomes in radiation therapy for non-small cell lung cancer. Lung Cancer. 2000;28(1):11–19. doi: 10.1016/s0169-5002(99)00113-0. [DOI] [PubMed] [Google Scholar]
- 3.Perez CA, Grigsby PW, Castro-Vita H, Lockett MA. Carcinoma of the uterine cervix. I. Impact of prolongation of overall treatment time and timing of brachytherapy on outcome of radiation therapy. International journal of radiation oncology, biology, physics. 1995;32(5):1275–1288. doi: 10.1016/0360-3016(95)00220-S. [DOI] [PubMed] [Google Scholar]
- 4.Kim JJ, Tannock IF. Repopulation of cancer cells during therapy: An important cause of treatment failure. Nature reviews Cancer. 2005;5(7):516–525. doi: 10.1038/nrc1650. [DOI] [PubMed] [Google Scholar]
- 5.Withers HR, Taylor JM, Maciejewski B. The hazard of accelerated tumor clonogen repopulation during radiotherapy. Acta oncologica. 1988;27(2):131–146. doi: 10.3109/02841868809090333. [DOI] [PubMed] [Google Scholar]
- 6.Barton MB, Keane TJ, Gadalla T, Maki E. The effect of treatment time and treatment interruption on tumour control following radical radiotherapy of laryngeal cancer. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 1992;23(3):137–143. doi: 10.1016/0167-8140(92)90323-m. [DOI] [PubMed] [Google Scholar]
- 7.Fowler JF, Lindstrom MJ. Loss of local control with prolongation in radiotherapy. International journal of radiation oncology, biology, physics. 1992;23(2):457–467. doi: 10.1016/0360-3016(92)90768-d. [DOI] [PubMed] [Google Scholar]
- 8.Van den Bogaert W, Van der Leest A, Rijnders A, Delaere P, Thames H, van der Schueren E. Does tumor control decrease by prolonging overall treatment time or interrupting treatment in laryngeal cancer? Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 1995;36(3):177–182. doi: 10.1016/0167-8140(95)01597-a. [DOI] [PubMed] [Google Scholar]
- 9.Withers HR, Peters LJ, Taylor JM, et al. Local control of carcinoma of the tonsil by radiation therapy: An analysis of patterns of fractionation in nine institutions. International journal of radiation oncology, biology, physics. 1995;33(3):549–562. doi: 10.1016/0360-3016(95)00228-Q. [DOI] [PubMed] [Google Scholar]
- 10.Rosenthal DI, Liu L, Lee JH, et al. Importance of the treatment package time in surgery and postoperative radiation therapy for squamous carcinoma of the head and neck. Head & neck. 2002;24(2):115–126. doi: 10.1002/hed.10038. [DOI] [PubMed] [Google Scholar]
- 11.Parsons JT, Mendenhall WM, Stringer SP, Cassisi NJ, Million RR. An analysis of factors influencing the outcome of postoperative irradiation for squamous cell carcinoma of the oral cavity. International journal of radiation oncology, biology, physics. 1997;39(1):137–148. doi: 10.1016/s0360-3016(97)00152-1. [DOI] [PubMed] [Google Scholar]
- 12.Ang KK, Trotti A, Brown BW, et al. Randomized trial addressing risk features and time factors of surgery plus radiotherapy in advanced head-and-neck cancer. International journal of radiation oncology, biology, physics. 2001;51(3):571–578. doi: 10.1016/s0360-3016(01)01690-x. [DOI] [PubMed] [Google Scholar]
- 13.Fu KK, Pajak TF, Trotti A, et al. A radiation therapy oncology group (rtog) phase iii randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: First report of rtog 9003. International journal of radiation oncology, biology, physics. 2000;48(1):7–16. doi: 10.1016/s0360-3016(00)00663-5. [DOI] [PubMed] [Google Scholar]
- 14.Horiot JC, Le Fur R, N’Guyen T, et al. Hyperfractionation versus conventional fractionation in oropharyngeal carcinoma: Final analysis of a randomized trial of the eortc cooperative group of radiotherapy. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 1992;25(4):231–241. doi: 10.1016/0167-8140(92)90242-m. [DOI] [PubMed] [Google Scholar]
- 15.Bourhis J, Overgaard J, Audry H, et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: A meta-analysis. Lancet. 2006;368(9538):843–854. doi: 10.1016/S0140-6736(06)69121-6. [DOI] [PubMed] [Google Scholar]
- 16.Cannon DM, Geye HM, Hartig GK, et al. Increased local failure risk with prolonged radiation treatment time in head and neck cancer treated with concurrent chemotherapy. Head & neck. 2014;36(8):1120–1125. doi: 10.1002/hed.23419. [DOI] [PubMed] [Google Scholar]
- 17.Pignon JP, Bourhis J, Domenge C, Designe L. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: Three meta-analyses of updated individual data. Mach-nc collaborative group. Meta-analysis of chemotherapy on head and neck cancer. Lancet. 2000;355(9208):949–955. [PubMed] [Google Scholar]
- 18.Murphy CT, Galloway TJ, Handorf EA, et al. Increasing time to treatment initiation for head and neck cancer: An analysis of the national cancer database. Cancer. 2015;121(8):1204–1213. doi: 10.1002/cncr.29191. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen-Tan PF, Zhang Q, Ang KK, et al. Randomized phase iii trial to test accelerated versus standard fractionation in combination with concurrent cisplatin for head and neck carcinomas in the radiation therapy oncology group 0129 trial: Long-term report of efficacy and toxicity. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(34):3858–3866. doi: 10.1200/JCO.2014.55.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trotti A, Pajak TF, Gwede CK, et al. Tame: Development of a new method for summarising adverse events of cancer treatment by the radiation therapy oncology group. The lancet oncology. 2007;8(7):613–624. doi: 10.1016/S1470-2045(07)70144-4. [DOI] [PubMed] [Google Scholar]
- 21.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The national cancer data base: A powerful initiative to improve cancer care in the united states. Annals of surgical oncology. 2008;15(3):683–690. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang KY, Zeger SL. Longitudinal data-analysis using generalized linear-models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 23.Lee EW, Wei LJ, Amato D. Cox-type regression analysis for large numbers of small groups of correlated failure time observations. Netherlands: Kluwer Academic; 1992. pp. 237–247. [Google Scholar]
- 24.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17(4):343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 25.Withers HR, Peters LJ. Transmutability of dose and time. Commentary on the first report of rtog 90003 (k. K. Fu et al) International journal of radiation oncology, biology, physics. 2000;48(1):1–2. doi: 10.1016/s0360-3016(00)00644-1. [DOI] [PubMed] [Google Scholar]
- 26.Machtay M, Moughan J, Farach A, et al. Hypopharyngeal dose is associated with severe late toxicity in locally advanced head-and-neck cancer: An rtog analysis. International journal of radiation oncology, biology, physics. 2012;84(4):983–989. doi: 10.1016/j.ijrobp.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bese NS, Hendry J, Jeremic B. Effects of prolongation of overall treatment time due to unplanned interruptions during radiotherapy of different tumor sites and practical methods for compensation. International journal of radiation oncology, biology, physics. 2007;68(3):654–661. doi: 10.1016/j.ijrobp.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 28.Brizel DM, Albers ME, Fisher SR, et al. Hyperfractionated irradiation with or without concurrent chemotherapy for locally advanced head and neck cancer. The New England journal of medicine. 1998;338(25):1798–1804. doi: 10.1056/NEJM199806183382503. [DOI] [PubMed] [Google Scholar]
- 29.Magrini SM, Buglione M, Corvo R, et al. Cetuximab and radiotherapy versus cisplatin and radiotherapy for locally advanced head and neck cancer: A randomized phase ii trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34(5):427–435. doi: 10.1200/JCO.2015.63.1671. [DOI] [PubMed] [Google Scholar]
- 30.Schiff PB, Harrison LB, Strong EW, et al. Impact of the time interval between surgery and postoperative radiation therapy on locoregional control in advanced head and neck cancer. Journal of surgical oncology. 1990;43(4):203–208. doi: 10.1002/jso.2930430403. [DOI] [PubMed] [Google Scholar]
- 31.Huang DT, Johnson CR, Schmidt-Ullrich R, Grimes M. Postoperative radiotherapy in head and neck carcinoma with extracapsular lymph node extension and/or positive resection margins: A comparative study. International journal of radiation oncology, biology, physics. 1992;23(4):737–742. doi: 10.1016/0360-3016(92)90646-y. [DOI] [PubMed] [Google Scholar]
- 32.Beadle BMGAS, Morrison WH, Rosenthal DI, Gunn GB, Frank SJ, Hessel AC, Ang KK. Outcomes and risk factors in patients treated with surgery and postoperative radiation therapy alone: A subset update of a randomized trial. ASTRO 53rd Annual Meeting.2011. [Google Scholar]
- 33.Calais G, Alfonsi M, Bardet E, et al. Randomized trial of radiation therapy versus concomitant chemotherapy and radiation therapy for advanced-stage oropharynx carcinoma. Journal of the National Cancer Institute. 1999;91(24):2081–2086. doi: 10.1093/jnci/91.24.2081. [DOI] [PubMed] [Google Scholar]
- 34.Adelstein DJ, Li Y, Adams GL, et al. An intergroup phase iii comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21(1):92–98. doi: 10.1200/JCO.2003.01.008. [DOI] [PubMed] [Google Scholar]
- 35.Pignon JP, le Maitre A, Maillard E, Bourhis J, Group M-NC. Meta-analysis of chemotherapy in head and neck cancer (mach-nc): An update on 93 randomised trials and 17,346 patients. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2009;92(1):4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Patient and treatment characteristics
Supplementary Table 2: Multivariable analysis of predictors of prolonged radiation treatment time (versus standard)