Abstract
Objectives
In a pilot randomized clinical trial of active stellate ganglion blockade (SGB) versus sham control, SGB significantly reduced the frequency of reported moderate to severe vasomotor symptoms (VMS) and the frequency of physiologic VMS measured using ambulatory skin conductance monitors. Here we examine secondary effects of SGB on verbal learning and memory.
Study Design
In a randomized, sham-controlled study, 36 women met eligibility criteria for cognitive assessments, of whom 17 were randomized to receive fluoroscopy-guided SGB and 19 to sham control.
Main Outcome Measures
At baseline and three months post-treatment, women completed tests of verbal learning and memory (primary outcome) and other cognitive measures and also wore an ambulatory monitor for 24 hours to measure physiologic VMS and VMS reported in real time.
Results
Verbal learning improved following active SGB (p<0.05) but not sham treatment; however, the interaction between group and time was not significant (p values 0.13-0.20). Two secondary cognitive measures improved only in the sham group. Improvements in physiologic VMS correlated significantly with improvements in verbal learning (r=0.51, p<0.05).
Conclusions
SGB might confer benefits to memory in relation to the magnitude of improvement in physiologic VMS. Broadly these findings suggest a possible link between physiologic VMS and memory problems in midlife women.
Keywords: Menopause, Vasomotor Symptoms, Memory, Stellate Ganglion Blockade, Hot Flash
1.0 INTRODUCTION
The stellate ganglion is a bilateral neural structure located in the C6-T2 region of the anterior cervical spine, and can be safely blocked via the image-guided injection of local anesthetic at the C6 level. As part of the sympathetic nervous system, the stellate ganglia act through noradrenergic pathways to modulate blood flow, sweating, piloerection, and visceral homeostasis. Four uncontrolled open-label studies showed that SGB reduced vasomotor symptoms (hot flashes and night sweats; VMS)[1-4]. Our pilot randomized, sham-controlled clinical trial compared active SGB with bupivacaine to a sham procedure involving subcutaneous saline injection on VMS in 40 women over 6 months of follow-up [5]. Compared to the sham-control group, the SGB group showed a significantly greater decrease in the frequency of moderate-to-severe patient-reported VMS, the intensity of VMS, and the frequency of physiologic VMS measured with ambulatory skin conductance monitors. These pilot findings suggest that SGB might be an effective, non-hormonal treatment for VMS, but further study is warranted[6]. Here we examine the effects of SGB on secondary memory outcomes from that pilot “parent” study.
VMS are associated with forgetfulness [7], a common menopausal symptom reported by 40% of midlife women[8]. Complaints of forgetfulness correlate with performance on neuropsychological tests of verbal memory, which measure learning and recall of words, stories and other verbal material[9]. Verbal memory worsens during the menopausal transition and after surgical menopause [10-12]. Whether VMS relate to verbal memory depends on whether VMS are self-reported or recorded with ambulatory skin conductance monitors. Among midlife women with moderate-to-severe VMS, physiologic VMS, but not patient-reported VMS, related to poorer verbal memory [13]. Similarly, magnetic resonance imaging (MRI) studies have revealed an association between physiologic VMS and adverse structural and functional brain outcomes that were not found with patient-reported VMS [14, 15]. On structural MRI scans, physiologic VMS were associated with white matter hyperintensities[14], ischemic brain abnormalities which increase risk for cognitive decline, stroke, and dementia [16]. On functional MRI scans, physiologic VMS related to alterations in the network of brain regions active during wakeful rest [15]. Thus, physiologic VMS appear more sensitive to memory and brain changes than do patient reported outcomes. This may be expected because physiologic VMS are not subject to wakefulness, attention, stress, emotional state, and other factors that influence perception and reporting of VMS [17]. Further, physiologic VMS appear to be measuring a more objective marker of VMS, since randomized clinical trials show a large placebo effect in reported VMS but no placebo effect for physiologic VMS [17, 18].
It is unknown whether treatments like SGB that reduce VMS, especially physiologic VMS, confer benefits to memory and whether any benefit to memory might relate to reductions in physiologic VMS. To address this knowledge gap, we used data from our randomized, sham-controlled trial of SGB for the treatment of VMS to examine the effect of SGB on verbal learning and memory. We hypothesized that: a) SGB treatment would improve verbal learning and memory when compared to sham control; and b) the magnitude of reduction in physiologic, but not reported VMS would correlate with the magnitude of improvement in verbal learning and memory. Based on a previous cross-sectional study [13], we predicted that physiologic VMS would relate specifically to verbal memory and not to other cognitive domains including attention, visual memory, visuospatial abilities, verbal fluency, working memory, and executive function.
2.0 MATERIALS AND METHODS
2.1 Participants
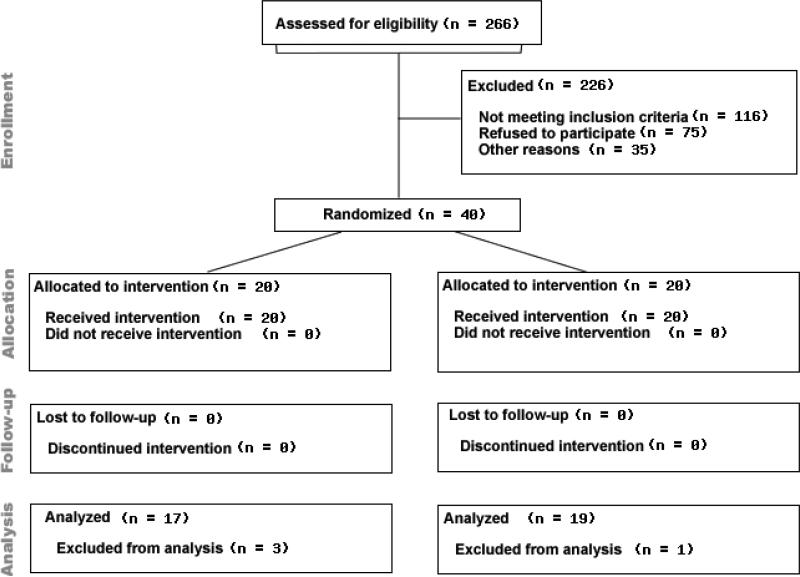
Women were eligible for this study if they were 30-70 years-old, experienced natural or surgical menopause, and reported moderate to very severe VMS (i.e., ≥ 25 VMS per week) in diaries ≥ 2 weeks before treatment randomization. Exclusionary criteria included: American Society of Anesthesiologists physical status score > 2 (indicating severe systemic disease and functional impairment); contraindications to SGB (anatomic abnormalities of the anterior cervical spine; cardiac/pulmonary compromise; acute illness/infection; coagulopathy/bleeding disorder; allergic reactions/contraindications to local anesthetic or contrast dye); current use of hormone therapy, SSRIs or other VMS treatment; conditions that affect cognition (e.g., stroke, severe brain injury); history of psychosis, depression, alcohol or substance abuse; and conditions that invalidate cognitive procedures (e.g., inability to write, speak, or read in English). The Institutional Review Boards at Northwestern University and the University of Illinois at Chicago approved this study. Participants underwent an informed consent process before completing any study procedure and were compensated for time and effort. Four of the 40 women in the parent study [5] were ineligible for the cognitive evaluation; one had a history of multiple sclerosis, two had a previous brain aneurysm, and one had a history of coma following a motor vehicle accident (See Figure 1). The trial study was registered on www.clinicaltrials.gov (NCT00992914).
Figure 1.
Participant Flow Diagram.
2.2 Randomization and Masking
A computer-generated 1:1 block randomization scheme was used to assign participants to receive either a SGB with bupivacaine or sham injection with saline. Randomization was performed by the injectionist immediately before the injection procedure by opening an opaque envelope to reveal the participant number and group assignment printed on an index card. Participants and all other study personnel were blinded to group assignment. Only the injectionist and statistician were unblinded after study completion. One board certified anesthesiologist (DRW) with 15 years of injection experience performed all injections.
2.3 Procedures
For active SGB, a right-sided SGB was performed. Using fluoroscopic guidance, 0.5% bupivacaine (5 mL) was injected into the anterolateral portion of the C6 vertebra[5]. For sham control, the same positioning, monitoring, sterile preparation and technique were used, except the needle was placed in the superficial tissues overlying the C6 tubercle. Participants were then monitored in a reclining position for at least 30 minutes to assess potential adverse effects. Presence of a Horner's sign (miosis, ptosis, anhydrosis) was recorded and validated successful SGB.
2.4 Outcome Measures
The primary cognitive outcomes were verbal learning and memory, assessed only at baseline and 3-months post-treatment to minimize carryover effects in a small sample. Cognitive and VMS outcomes were compared between baseline and 3-months post-treatment.
2.4.1 Reported VMS
Participants recorded the frequency and severity of daily VMS in a diary for at least two weeks before the injection and for six months thereafter. Each hot flash was rated as “mild”, “moderate”, “severe” or “very severe”[18]. Hot flash intensity was calculated as follows: Intensity = Frequency*Severity = [(frequency of mild*1) + (frequency of moderate*2) + (frequency of severe*3) + (frequency of very severe*4)]. Frequency of night sweats was recorded daily via self-report the following morning. Baseline VMS frequency was estimated as the daily count totals on diaries averaged over the two screening weeks. VMS frequency at Month 3 was estimated as the daily count totals averaged over the previous 30 days.
2.4.2 Physiologic VMS
Participants were fitted with the Biolog Model 3991 x/2-HFI (UFI, Morro Bay, CA), an ambulatory sternal skin conductance monitor with two skin conductance electrodes connected to the sternum by adhesive electrode pads (UFI, 1081-HFD). They wore the monitor for 24 hours following the baseline and 3-month visit. The monitor was placed inside a small pouch that was slung over the shoulder. Physiologic (i.e., > 2.0 μmho increase in 30 sec) and reported (button press) VMS were recorded with the monitor according to standard procedures. Participants were instructed to push two red buttons simultaneously on the monitor when they experienced a hot flash. These events were time stamped on the continuous skin conductance data to record the time of each subjective hot flash. Participants also kept a diary of the time, severity, and intensity of each hot flash or night sweat. Raw physiologic VMS data were analyzed by a combination of automated computer software and two trained data coders according to standard procedures. Data were independently double-scored and double-entered into the database by coders blinded to treatment assignment. Discrepancies between the two coders were resolved before analysis. Subclassification of VMS during sleeping versus waking hours was based on the time participants reported going to bed and waking up while being monitored.
2.4.3 Other Outcomes
Participants completed the Center for Epidemiological Studies – Depression Scale (CES¬D), a 20-item self-report measure of depressive symptoms (cut-off > 16)[19]. Sleep quality was assessed with the Modified Pittsburgh Sleep Quality Index, with higher scores indicating greater reported sleep disturbance [20]. These questionnaires were completed at the baseline and 3-month visits.
2.5 Cognitive Measures
Participants completed a neuropsychological test battery at baseline and three months after treatment. Testing sessions lasted approximately 1 hour and were led by a certified test administrator who was blind to treatment. Study visits took place at Northwestern University, Chicago, IL. Parallel versions of the California Verbal Learning Test, Logical Memory Subtest of Wechsler Memory Scale, and phonemic fluency were used to limit carryover effects.
2.5.1 Primary outcome: Verbal memory
California Verbal Learning Test (CVLT) [21]
The CVLT assesses verbal learning and memory, with both short- and long-delay recall trials. A list of 16 words from four semantic categories was read aloud on five consecutive trials. Participants were asked to recall as many words as possible after each trial (verbal learning; max score = 80). Next, a 16-word “interference” list was read once, and participants were asked to recall the words. Participants were then instructed to recall the original word list (short-delay recall; max score = 16) and again 20-minutes later (long-delay recall; max score = 16).
Logical Memory Subtest of Wechsler Memory Scale-Revised [22]
This verbal memory test assesses immediate and delayed recall of a short story. Participants were read a brief story and asked to recall as many elements as possible immediately after the reading (immediate recall) and again 30 minutes later (delayed recall). Scores range from 0-25 for both immediate and delayed recall.
2.5.2 Secondary outcome measures
Secondary outcome measures included: 1) Benton Visual Retention Test: short-term figural memory[23]; 2) Digit Span (Forward and Backward)[24]: attention and working memory; 3) Brief Test of Attention[25]: sustained attention; 4) Verbal Fluency[26]: letter and category fluency; and 5) Finding A's: visuoperceptual speed and attention.
2.6 Statistical Analyses
Baseline characteristics were compared between treatment groups using t-tests for continuous variables and chi-square test for categorical variables. For our primary hypothesis, a series of mixed-effects regressions (random intercept only) were conducted to examine the change in cognitive performance over time as a function of Treatment Group. Independent predictors included Treatment Group (active SGB vs. sham control), a dummy variable for month 3 (vs. baseline), and their interaction. The same analyses were conducted on the secondary cognitive outcome measures. A power analysis based on a repeated measures design with one between-subjects factor (Treatment Group) and one within-subjects factor (Time) revealed that the smallest detectable effect size was 0.48. For our second hypothesis, Pearson correlations were conducted to examine associations between changes in cognitive performance and changes in VMS; partial correlations were conducted to examine whether these associations remained significant after controlling for changes in sleep and mood. SAS statistical software version 9.2 (SAS Institute Inc., Cary, NC, USA) was used. Significance was set at p<0.05.
3.0 RESULTS
Table 1 shows demographic and clinical characteristics for the 36 participants. Participants ranged in age from 45-58 years (mean = 52), and 50% were African-American. There were no group differences in sociodemographic or clinical variables, and no baseline differences in cognitive outcomes or the frequency of physiologic or reported VMS. The mean subjective detection of physiologic VMS was 55%. As in the parent trial[5], the frequency of physiologic VMS was significantly reduced from baseline to the 3-month assessment in the SGB group (p=0.03) but not in the sham-control group (p=0.31), for a significant group by time interaction (p=0.02). There was no significant difference between treatment groups in reported VMS from baseline to 3-months post-injection (p=0.69 for the Group × Time interaction), due to a 34% placebo effect in the sham group.
Table 1.
Demographic and Clinical Characteristics by Treatment Group at Baseline.
| Treatment Group |
|||
|---|---|---|---|
| SGB (n=17) M (SD) |
Sham-control (n=19) M (SD) |
p-value | |
| Demographics | |||
| Age (years) | 51.47 (2.60) | 53.26 (4.07) | 0.13 |
| Years of Education | 15.29 (1.66) | 14.43 (1.78) | 0.21 |
| Body Mass Index | 28.71 (5.01) | 28.68 (5.74) | 0.99 |
| Race, n (%) | 0.58 | ||
| White | 6 (35) | 10 (53) | |
| Black | 10 (59) | 8 (42) | |
| Hispanic | 1 (6) | 1 (5) | |
| Natural menopause, n (%) | 15 (88) | 12 (63) | 0.09 |
| Postmenopausal status, <5 years since LMP (%) | 11 (65) | 12 (63) | 0.92 |
| Menopausal Symptoms | |||
| CES-D | 11.88 (7.01) | 12.11 (9.06) | 0.94 |
| Pittsburgh Sleep Quality Index | |||
| Latency | 1.24 (1.03) | 1.68 (1.06) | 0.21 |
| Quality | 1.76 (0.76) | 1.84 (0.76) | 0.76 |
| Disturbances | 2.00 (0.50) | 1.84 (0.60) | 0.40 |
| Green Climacteric Scale | |||
| Psychological | 8.35 (4.14) | 8.74 (5.02) | 0.81 |
| Vasomotor | 4.06 (1.25) | 4.21 (1.18) | 0.71 |
| Somatic | 3.94 (3.51) | 4.11 (3.49) | 0.89 |
| Utian Quality of Life (UQOL) | 80.53 (8.32) | 82.27 (11.34) | 0.60 |
| Vasomotor Symptoms (Daily Count)‡ | |||
| Objective (via monitor) | |||
| Total | 15.13 (12.84) | 10.01 (10.22) | 0.26 |
| Awake | 12.47 (11.00) | 7.08 (7.23) | 0.15 |
| Asleep | 2.67 (2.16) | 2.92 (4.27) | 0.84 |
| Reported (via monitor) | |||
| Total | 16.93 (7.53) | 17.25 (9.32) | 0.92 |
| Awake | 13.33 (8.04) | 13.71 (6.72) | 0.90 |
| Asleep | 3.60 (2.29) | 3.54 (3.95) | 0.96 |
Note. LMP=last menstrual period. SGB= Stellate Ganglion Blockade. CES-D = Center for Epidemiological Studies - Depression Scale.
Valid objective data was available on 28/36 women (15 SGB; 13 sham-control).
Table 2 shows changes in verbal memory from baseline to three months post-treatment in the SGB and sham-control groups. As predicted, verbal learning on the CVLT significantly improved in the active SGB group post-treatment (B=4.76, SE=1.95, p<0.05). The SGB group also showed a trend towards improved delayed verbal memory on the CVLT (B=0.88, SE=0.49, p=0.08). Conversely, the sham-control group did not show improvements on these primary outcomes (p's 0.52-0.69). However, the interactions between treatment group and time were not significant (p's 0.13-0.20). Table 3 shows the change in secondary cognitive outcomes from baseline to three months post-treatment. The sham-control group showed significant improvements in visuospatial performance on the Card Rotations Test (B=13.47, SE=4.03, p<0.01), and the SGB group showed a trend for improvement on that measure (B=7.71, SE=3.94, p=0.06). The sham-control group also showed a significant improvement in psychomotor speed on the Finding A's test (B=2.99, SE=1.44, p<0.05), whereas the active SGB group showed no change in that outcome (B=0.47, SE=1.44, p=0.74). The interactions between treatment group and time were not significant for Card Rotations (p=0.22) or Finding A's (p=0.31). Neither group showed a change in any other secondary outcome measure from baseline to three months post-treatment.
Table 2.
Estimated means (SE) and estimated change scores over time for the memory outcomes in active SGB and sham-control groups.
| Group |
Differential Mean change between Groups | ||||||
|---|---|---|---|---|---|---|---|
| SGB (n=17) | Placebo (n=19) | ||||||
| Baseline | Post | Change | Baseline | Post | Change | ||
| Test, mean (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) |
| CVLT | |||||||
| Total across 5 trials | 51.41 (2.54) | 56.18 (2.70) | 4.76 (1.95)* | 51.89 (2.55) | 53.14 (2.61) | 1.24 (1.94) | 3.52 (2.74) |
| Short-delay free recall | 11.12 (0.82) | 11.24 (0.82) | 0.12 (0.69) | 10.79 (0.77) | 11.19 (0.80) | 0.40 (0.69) | −0.28 (0.97) |
| Long-delay free recall | 11.12 (0.72) | 12.00 (0.72) | 0.88 (0.49)T | 11.37 (0.68) | 11.17 (0.69) | −0.19 (0.48) | 1.08 (0.69) |
| Logical Memory Test | |||||||
| Immediate total score | 12.35 (0.95) | 11.82 (0.95) | −0.53 (0.85) | 12.95 (0.89) | 12.71 (0.93) | −0.24 (0.84) | −0.29 (1.20) |
| Delayed total score | 10.35 (0.99) | 9.65 (0.99) | −0.71 (0.87) | 11.37 (0.95) | 12.27 (0.98) | 0.89 (0.86) | −1.60 (1.23) |
Note.
**p<0.01
p<0.05
p=0.08.
CVLT=California Verbal Learning Test. Results are based on mixed effects regression models (random intercept only).
Table 3.
Estimated means (SE) and change scores over time for the secondary cognitive outcomes in active SGB and sham-control groups.
| Group |
Differential Mean change between Groups | ||||||
|---|---|---|---|---|---|---|---|
| SGB (n=17) | Placebo (n=19) | ||||||
| Baseline | Post | Change | Baseline | Post | Change | ||
| Test, mean (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) |
| BVRT | 6.29 (0.58) | 6.53 (0.58) | 0.24 (0.35) | 5.95 (0.55) | 5.98 (0.56) | 0.03 (0.35) | 0.20 (0.50) |
| Card Rotation Test | 55.24 (7.42) | 62.94 (7.42) | 7.71 (3.94)T | 66.63 (7.01) | 80.10 (7.19) | 13.47 (4.03)** | −5.76 (5.64) |
| Verbal Fluency | |||||||
| Letter | 29.53 (2.06) | 29.59 (2.06) | 0.06 (1.48) | 29.74 (1.95) | 30.20 (1.99) | 0.47 (1.47) | −0.41 (2.09) |
| Semantic | 48.47 (2.38) | 46.93 (2.43) | −1.54 (2.26) | 48.95 (2.25) | 49.77 (2.34) | 0.82 (2.18) | −2.36 (3.14) |
| Finding A's | 29.65 (1.98) | 30.12 (1.98) | 0.47 (1.44) | 30.78 (1.93) | 33.77 (1.95) | 2.99 (1.44)* | −2.52 (2.03) |
| Digit Span Test | |||||||
| Forward | 8.06 (0.60) | 8.18 (0.60) | 0.12 (0.49) | 8.74 (0.57) | 8.79 (0.59) | 0.05 (0.48) | 0.07 (0.69) |
| Backward | 6.06 (0.49) | 6.65 (0.49) | 0.59 (0.44) | 6.47 (0.46) | 6.43 (0.48) | −0.05 (0.43) | 0.64 (0.61) |
| BTA | 15.53 (0.77) | 16.00 (0.77) | 0.47 (0.65) | 15.50 (0.75) | 15.77 (0.76) | 0.27 (0.64) | 0.20 (0.92) |
Note.
p<0.01
p<0.05
p=0.06.
BVRT = Benton Visual Retention Test; BTA = Brief Test of Attention. Results are based on mixed effects regression models (random intercept only).
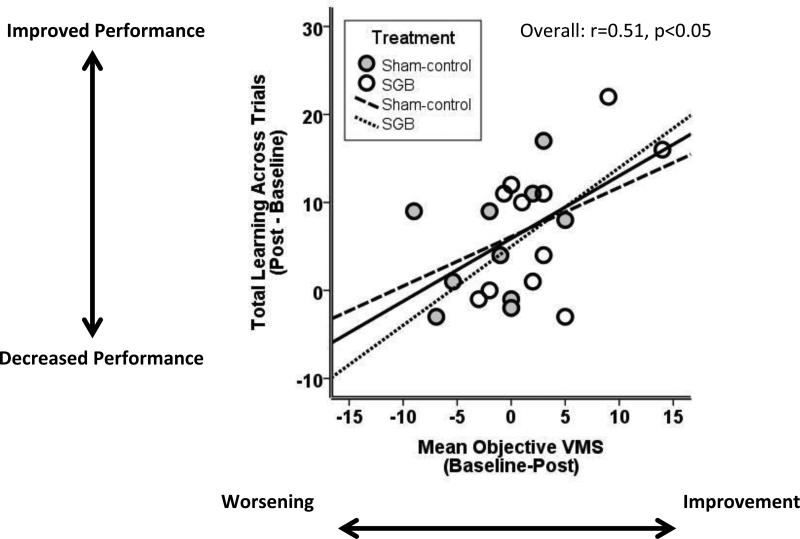
As predicted, decreases in total daily physiologic VMS from baseline to 3-months follow-up significantly correlated with improvements in verbal memory over that same period (r=0.51, p<0.05; Figure 2) even after adjustment for mood and sleep (r=0.54, p=0.02). However, changes in reported VMS did not correlate with changes in memory (r=0.05, p=0.85).
Figure 2.
Improvements in verbal learning were significantly correlated with improvements in physiologic vasomotor symptoms following intervention with stellate ganglion blockade or sham.
4.0 DISCUSSION
This study addressed whether SGB as a treatment for VMS also confers benefits to verbal memory. As hypothesized, treatment with active SGB led to significant improvements in verbal learning and near-significant (p = .08) improvements in delayed verbal recall, whereas sham control did not. The interaction between treatment group and time did not reach statistical significance, though the effect size was 0.33, suggesting limited statistical power. Second, as hypothesized[13], the magnitude of reduction in physiologic VMS correlated significantly with the magnitude of improvement in memory. These findings suggest that the extent to which memory improves following SGB may relate to, and potentially depend upon, the extent to which physiologic VMS improve following treatment. Larger trials are needed to rule out low power as a factor underlying the lack of a main effect of SGB on memory and to test the reproducibility of the correlation between improvements in memory and VMS.
Participants reported only 55% of physiologic VMS. In our cross-sectional study, women reported 60% of physiologic VMS, and physiologic VMS were similarly associated with worse verbal memory[13]. Our present findings provide stronger support, albeit tentative given small numbers, of a possible causal relationship between reductions in physiologic VMS and improvements in memory. Changes in memory were unrelated to changes in reported VMS, consistent with previous findings that memory[9] and neuroimaging measures [14, 15] related to physiologic VMS but not reported VMS. Further investigation in larger studies is needed to determine the potential clinical utility of physiologic VMS monitoring to identify women at risk for memory declines.
The factors accounting for the correlation between improvements in physiologic VMS and memory are unknown. Although declines in estrogen elicit both VMS[27] and memory dysfunction[28], SGB is not known to alter estradiol. Improved sleep or mood might account for the correlation, but controlling for reported sleep and mood did not alter the magnitude of the correlation. Additionally, in the parent trial, SGB did not significantly improve subjective sleep, and although depressive symptoms improved more with SGB than with sham treatment, this difference (p < 0.10) was not statistically significant [5]. Generally, sleep complaints and depressive symptoms relate to reported VMS but not physiologic VMS[29-31]. Given the discrepancy between self-reported sleep outcomes and objective sleep measures [32, 33], larger studies are needed to examine the potential role of improved sleep (including objective sleep parameters) and mood as mediators of the relationship between VMS and memory.
The mechanisms underlying VMS and SGB are not fully elucidated, so the biological mechanisms linking physiologic VMS and memory are also unknown. In a case report, SGB improved post-traumatic stress disorder and memory [34]. Cortisol levels increase following a hot flash[35], and women with higher overnight levels of cortisol report more severe VMS compared to women with low cortisol [36]. Cortisol has been linked to decreases in memory, particularly in women[37, 38]. Alternatively, SGB may alter the sympathetic nervous system or brain derived neurotrophic factor[39, 40]. The duration of VMS effect in the parent trial lasted six months, far beyond the duration of sympatholysis and bupivacaine half-life. Mechanistic studies are needed to test these potential mediators.
Broadly, our findings raise the question of whether increases in physiologic VMS contribute to declines in verbal memory during the menopausal transition [36]. As predicted based on our cross-sectional study [13], verbal memory was the only cognitive function shown to relate to physiologic VMS. Verbal memory decreases from the premenopausal to postmenopausal stage, even after adjusting for age and other factors[10-12]. Declines in estradiol likely contribute to decreases in verbal memory during the menopausal transition; estrogen therapy reverses the declines in verbal memory that occur after hysterectomy and bilateral oophorectomy[41] and also reverses declines that occur with suppression of estradiol and progesterone with leuprolide acetate [42]. However, the role of physiological VMS in these changes is unknown. Although a randomized clinical trial suggested no benefit of HT on memory in naturally postmenopausal women [43], that trial was not conducted in women with moderate-to-severe VMS, and it is unknown if improvements in memory following HT might be observed in relation to the magnitude of reduction in physiologic VMS.
The study has notable limitations. First, the sample size is small and the follow-up was only three months. A mediation analysis was not possible. Second, further investigation is needed to discern whether the lack of improvement in the SGB group in processing speed is a chance finding or a potential adverse cognitive effect of SGB. Third, we did not measure estradiol. Participants were postmenopausal, but changes in estradiol might have contributed to changes in memory. Fourth, participants were highly symptomatic, reporting on average 17 daily VMS. Results may not generalize to less symptomatic women.
5.0 CONCLUSION
This pilot trial provides tentative new evidence that improvements in physiologic VMS following SGB are significantly related to improvements in verbal memory. SGB may confer benefits to memory.
Highlights.
This randomized, sham-controlled trial suggests that improvements in vasomotor symptoms following stellate ganglion blockade are significantly related to improvements in verbal memory.
Stellate ganglion blockade may confer secondary benefits to memory.
Broadly the findings suggest a possible link between physiologic vasomotor symptoms and memory problems in midlife women.
Acknowledgments
Funding
Funding was provided by the Department of Obstetrics and Gynecology, Northwestern University. Dr. Rubin's effort was supported by grant number K12HD055892 from the National Institute of Child Health and Human Development and the National Institutes of Health Office of Research on Women's Health.
Abbreviations
- SGB
Stellate Ganglion Blockade
- CES-D
Center for Epidemiological Studies – Depression Scale
- CVLT
California Verbal Learning Test
- VMS
vasomotor symptoms
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
PMM contributed to study conception and design, study administration, technical support, acquisition of data, data analysis, interpretation of data, drafting of the manuscript, and final review of the manuscript.
LHR contributed to study conception and design, data analysis (as lead statistician), interpretation of data, drafting of the manuscript, and final review of the manuscript.
AS contributed to study administration, technical support, acquisition of data, interpretation of data and final review of the manuscript.
LD contributed to study administration, technical support, acquisition of data, interpretation of data and final review of the manuscript.
LPS contributed to study conception and design and final review of the manuscript.
SB contributed to study conception and design, study administration, technical support, and final review of the manuscript.
DRW contributed to study conception and design, study administration, data analysis, interpretation of data, drafting of the manuscript, and final review of the manuscript.
ClinicalTrials.gov Identifier: NCT00992914
Conflict of interest
PMM has received consulting honoraria from Abbott and Noven.
LHR has no conflict of interest to declare.
AS has no conflict of interest to declare.
LD has no conflict of interest to declare.
LPS has received consulting fees from Bayer, Merck, Roche, Allergan, BioPharmX.
SB has no conflict of interest to declare.
DRW has no conflict of interest to declare.
Ethical approval
Northwestern University Office for the Protection of Research Subjects issued approval for IRB #STU00006644.
University of Illinois at Chicago Office for the Protection of Research Subjects issued approval for IRB #2009-0204.
Provenance and peer review
This article has undergone peer review.
REFERENCES
- 1.Haest K, Kumar A, Van Calster B, Leunen K, Smeets A, Amant F, et al. Stellate ganglion block for the management of hot flashes and sleep disturbances in breast cancer survivors: an uncontrolled experimental study with 24 weeks of follow-up. Ann Oncol. 2012;23:1449–54. doi: 10.1093/annonc/mdr478. [DOI] [PubMed] [Google Scholar]
- 2.Pachman DR, Barton D, Carns PE, Novotny PJ, Wolf S, Linquist B, et al. Pilot evaluation of a stellate ganglion block for the treatment of hot flashes. Support Care Cancer. 2011;19:941–7. doi: 10.1007/s00520-010-0907-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lipov EG, Joshi JR, Sanders S, Wilcox K, Lipov S, Xie H, et al. Effects of stellate-ganglion block on hot flushes and night awakenings in survivors of breast cancer: a pilot study. Lancet Oncol. 2008;9:523–32. doi: 10.1016/S1470-2045(08)70131-1. [DOI] [PubMed] [Google Scholar]
- 4.van Gastel P, Kallewaard JW, van der Zanden M, de Boer H. Stellate-ganglion block as a treatment for severe postmenopausal flushing. Climacteric. 2013;16:41–7. doi: 10.3109/13697137.2012.709889. [DOI] [PubMed] [Google Scholar]
- 5.Walega DR, Rubin LH, Banuvar S, Shulman LP, Maki PM. Effects of stellate ganglion block on vasomotor symptoms: findings from a randomized controlled clinical trial in postmenopausal women. Menopause. 2014;21:807–14. doi: 10.1097/GME.0000000000000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause. 2015 doi: 10.1097/GME.0000000000000546. [DOI] [PubMed] [Google Scholar]
- 7.Woods NF, Smith-Dijulio K, Percival DB, Tao EY, Taylor HJ, Mitchell ES. Symptoms during the menopausal transition and early postmenopause and their relation to endocrine levels over time: observations from the Seattle Midlife Women's Health Study. J Womens Health (Larchmt) 2007;16:667–77. doi: 10.1089/jwh.2006.0138. [DOI] [PubMed] [Google Scholar]
- 8.Gold EB, Sternfeld B, Kelsey JL, Brown C, Mouton C, Reame N, et al. Relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40-55 years of age. American Journal of Epidemiology. 2000;152:463–73. doi: 10.1093/aje/152.5.463. [DOI] [PubMed] [Google Scholar]
- 9.Drogos LL, Rubin LH, Geller SE, Banuvar S, Shulman LP, Maki PM. Objective cognitive performance is related to subjective memory complaints in midlife women with moderate to severe vasomotor symptoms. Menopause. 2013;20:1236–42. doi: 10.1097/GME.0b013e318291f5a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epperson CN, Sammel MD, Freeman EW. Menopause effects on verbal memory: findings from a longitudinal community cohort. J Clin Endocrinol Metab. 2013;98:3829–38. doi: 10.1210/jc.2013-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber MT, Rubin LH, Maki PM. Cognition in perimenopause: the effect of transition stage. Menopause. 2013;20:511–7. doi: 10.1097/GME.0b013e31827655e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greendale GA, Huang MH, Wight RG, Seeman T, Luetters C, Avis NE, et al. Effects of the menopause transition and hormone use on cognitive performance in midlife women. Neurology. 2009;72:1850–7. doi: 10.1212/WNL.0b013e3181a71193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maki PM, Drogos LL, Rubin LH, Banuvar S, Shulman LP, Geller SE. Objective hot flashes are negatively related to verbal memory performance in midlife women. Menopause. 2008;15:848–56. doi: 10.1097/gme.0b013e31816d815e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thurston RC, Aizenstein HJ, Derby CA, Sejdic E, Maki PM. Menopausal hot flashes and white matter hyperintensities. Menopause. 2015 doi: 10.1097/GME.0000000000000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thurston RC, Maki PM, Derby CA, Sejdic E, Aizenstein HJ. Menopausal hot flashes and the default mode network. Fertil Steril. 2015;103:1572–8 e1. doi: 10.1016/j.fertnstert.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thurston RC, Blumenthal JA, Babyak MA, Sherwood A. Emotional antecedents of hot flashes during daily life. Psychosomatic medicine. 2005;67:137–46. doi: 10.1097/01.psy.0000149255.04806.07. [DOI] [PubMed] [Google Scholar]
- 18.Sloan JA, Loprinzi CL, Novotny PJ, Barton DL, Lavasseur BI, Windschitl H. Methodologic lessons learned from hot flash studies. J Clin Oncol. 2001;19:4280–90. doi: 10.1200/JCO.2001.19.23.4280. [DOI] [PubMed] [Google Scholar]
- 19.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 20.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 21.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test - Research Edition. The Psychological Corporation; New York: 1987. [Google Scholar]
- 22.Wechsler D. Manual for the Wechsler Memory Scale- Revised. Psychological Corporation; San Antonio, TX: 1987. [Google Scholar]
- 23.Benton AL. The Revised Visual Retention Test: clinical and experimental applications (3rd edition) Psychological Corporation; New York: 1963. [Google Scholar]
- 24.Wechsler D. Wechsler Adult Intelligence Scale - Revised. Psychological Corporation; New York: 1981. [Google Scholar]
- 25.Schretlen D, Bobholz JH, Brandt J. Development and psychometric properties of the Brief Test of Attention. Clinical Neuropsychologist. 1996;10:80–9. [Google Scholar]
- 26.Benton AL. Differential behavioral effects in frontal lobe disease. Neuropsychologia. 1968;6:53–60. [Google Scholar]
- 27.Hunter MS, Gentry-Maharaj A, Ryan A, Burnell M, Lanceley A, Fraser L, et al. Prevalence, frequency and problem rating of hot flushes persist in older postmenopausal women: impact of age, body mass index, hysterectomy, hormone therapy use, lifestyle and mood in a cross-sectional cohort study of 10,418 British women aged 54-65. BJOG. 2012;119:40–50. doi: 10.1111/j.1471-0528.2011.03166.x. [DOI] [PubMed] [Google Scholar]
- 28.Sherwin BB. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology. 1988;13:345–57. doi: 10.1016/0306-4530(88)90060-1. [DOI] [PubMed] [Google Scholar]
- 29.Thurston RC, Santoro N, Matthews KA. Are vasomotor symptoms associated with sleep characteristics among symptomatic midlife women? Comparisons of self-report and objective measures. Menopause. 2012;19:742–8. doi: 10.1097/gme.0b013e3182422973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thurston RC, Blumenthal JA, Babyak MA, Sherwood A. Association between hot flashes, sleep complaints, and psychological functioning among healthy menopausal women. Int J Behav Med. 2006;13:163–72. doi: 10.1207/s15327558ijbm1302_8. [DOI] [PubMed] [Google Scholar]
- 31.Carpenter JS, Elam JL, Ridner SH, Carney PH, Cherry GJ, Cucullu HL. Sleep, fatigue, and depressive symptoms in breast cancer survivors and matched healthy women experiencing hot flashes. Oncol Nurs Forum. 2004;31:591–5598. doi: 10.1188/04.onf.591-598. [DOI] [PubMed] [Google Scholar]
- 32.Regestein QR, Friebely J, Shifren JL, Scharf MB, Wiita B, Carver J, et al. Self-reported sleep in postmenopausal women. Menopause. 2004;11:198–207. doi: 10.1097/01.gme.0000097741.18446.3e. [DOI] [PubMed] [Google Scholar]
- 33.Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19:838–45. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lipov EG, Navaie M, Brown PR, Hickey AH, Stedje-Larsen ET, McLay RN. Stellate ganglion block improves refractory post-traumatic stress disorder and associated memory dysfunction: a case report and systematic literature review. Mil Med. 2013;178:e260–4. doi: 10.7205/MILMED-D-12-00290. [DOI] [PubMed] [Google Scholar]
- 35.Meldrum DR, Defazio JD, Erlik Y, Lu JK, Wolfsen AF, Carlson HE, et al. Pituitary hormones during the menopausal hot flash. Obstet Gynecol. 1984;64:752–6. [PubMed] [Google Scholar]
- 36.Woods NF, Carr MC, Tao EY, Taylor HJ, Mitchell ES. Increased urinary cortisol levels during the menopausal transition. Menopause. 2006;13:212–21. doi: 10.1097/01.gme.0000198490.57242.2e. [DOI] [PubMed] [Google Scholar]
- 37.Seeman TE, McEwen BS, Singer BH, Albert MS, Rowe JW. Increase in urinary cortisol excretion and memory declines: MacArthur studies of successful aging. Journal of Clinical Endocrinology & Metabolism. 1997;82:2458–65. doi: 10.1210/jcem.82.8.4173. [DOI] [PubMed] [Google Scholar]
- 38.Greendale GA, Kritz-Silverstein D, Seeman T, Barrett-Connor E. Higher basal cortisol predicts verbal memory loss in postmenopausal women: Rancho Bernardo Study. J Am Geriatr Soc. 2000;48:1655–8. doi: 10.1111/j.1532-5415.2000.tb03878.x. [DOI] [PubMed] [Google Scholar]
- 39.Lipov EG, Joshi JR, Sanders S, Slavin KV. A unifying theory linking the prolonged efficacy of the stellate ganglion block for the treatment of chronic regional pain syndrome (CRPS), hot flashes, and posttraumatic stress disorder (PTSD). Medical hypotheses. 2009;72:657–61. doi: 10.1016/j.mehy.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 40.Lipov EG, Lipov S, Joshi JR, Santucci VD, Slavin KV, Beck Vigue SG. Stellate ganglion block may relieve hot flashes by interrupting the sympathetic nervous system. Medical hypotheses. 2007;69:758–63. doi: 10.1016/j.mehy.2007.01.082. [DOI] [PubMed] [Google Scholar]
- 41.Phillips SM, Sherwin BB. Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology. 1992;17:485–95. doi: 10.1016/0306-4530(92)90007-t. [DOI] [PubMed] [Google Scholar]
- 42.Sherwin BB, Tulandi T. “Add-back” estrogen reverses cognitive deficits induced by a gonadotropin-releasing hormone agonist in women with leiomyomata uteri. Journal of Clinical Endocrinology and Metabolism. 1996;81:2545–9. doi: 10.1210/jcem.81.7.8675575. [DOI] [PubMed] [Google Scholar]
- 43.Gleason CE, Dowling NM, Wharton W, Manson JE, Miller VM, Atwood CS, et al. Effects of Hormone Therapy on Cognition and Mood in Recently Postmenopausal Women: Findings from the Randomized, Controlled KEEPS-Cognitive and Affective Study. PLoS medicine. 2015;12:e1001833. doi: 10.1371/journal.pmed.1001833. [DOI] [PMC free article] [PubMed] [Google Scholar]