Abstract
Azomethine linked pyrrole bishetarylazoles containing benzimidazole/pyrazolone/1,3,4-oxadiazole were synthesized in satisfactory yields. Their structures were confirmed by IR, 1H-NMR, 13C-NMR and elemental analysis. Evaluation for the cytotoxic activities In vitro against a panel of breast cancer cell lines (MDA-AB-231, BT-474 and Ishikawa cells) revealed that the pyrrole-benzimidazole hybrids are more potent than the pyrazolone and 1,3,4-oxadiazole hybrids in all cell lines. Compound (9) displayed promising cytotoxicity against BT-474 cell line with IC50 values, 7.7 µM.
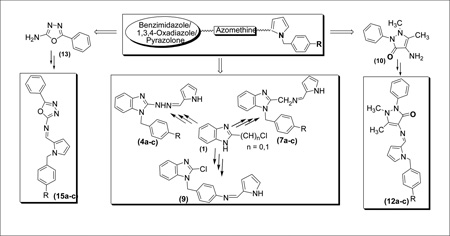
Graphical Abstract
1. Introduction
Hetarylazoles form by far the largest class of heterocyclic compounds and are of immense importance biologically and industrially. Owing to their versatile chemotherapeutic activities, safety profiles and high therapeutic indices a significant amount of research effort has been directed towards combining hetarylazoles to investigate additative biological activities.[1–9] Benzimidazoles, their aryl and alkyl substituted derivatives have evoked considerable attention as members of hetarylazoles with antifungal, antioxidant, antihypertensive, cardiotonic, antithrombotic, antiulcer, antitubercular, antitumor activity against several tumor cell lines etc.[10–12]
On the other hand, five membered 1,3,4-oxadiazole heterocyclics are associated with a variety of pharmacological actions and serve as intermediates for the development of bioactive molecules. Molecular modeling and pharmacokinetic studies have also demonstrated that incorporating the 1,3,4-oxadiazole moiety in drug-like molecules changes polarity, flexibility as well as metabolic profiles and the ability to engage in hydrogen bonding interaction with receptors. Hence, 1,3,4-oxadiazoles have been widely employed as isoesteric substituents for esters and amides in a number of biological targets [13–18]. The pyrazolone derivative, 4-aminoantipyrine, is a strong inhibitor of cyclooxygenase isoenzymes, platelet thromboxane synthesis, and prostanoids synthesis. The biological activity of the 4-aminoantipyrine has been attributed to its scavenging activity against reactive oxygen and nitrogen species (ROS and RNS), as well as to the inhibition of neutrophil’s oxidative burst [19–26].
A recent survey of novel small-molecule therapeutics has revealed that the majority of the drugs result from an analog-based approach and that their market share represents two-thirds of all drug sales. Hence, combination of two pharmacophores into a single molecule or molecular hybridization represents an important part of the efforts to overcome drug resistance in anticancer and antimicrobial agents search. The reported individual/chemical/pharmacological properties of benzimidazole, 1,3,4-oxadiazoleand pyrazolone functionalities compelled us to design and synthesize hetarylazole molecule bearing strategic hybrid combinations of the above moieties, and investigate the anticancer activities of these novel agents.[27–31]. Herein we report the synthesis and preliminary anticancer evaluation of bishetarylazole hybrids bearing pyrrole moieties and benzimidazole/4-aminoantipyrine/1,3,4-oxadiazole nucleus.
Results and Discussion
1.1 Chemistry
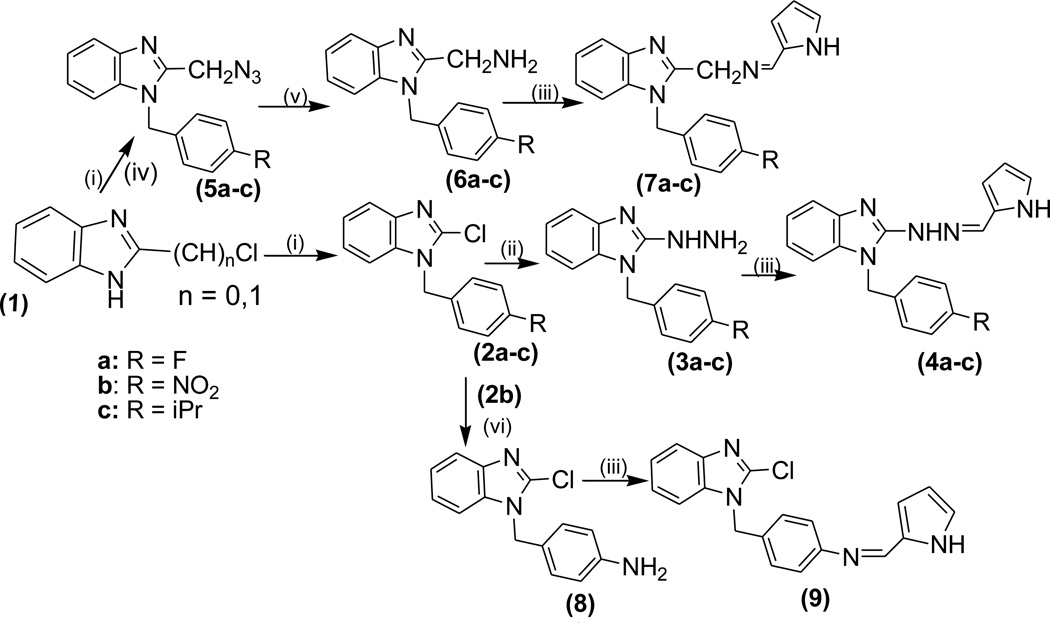
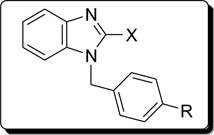
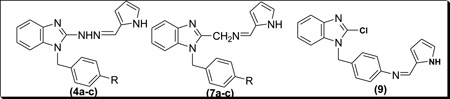
The synthetic strategies adopted for the synthesis of the intermediates and target compounds are depicted in schemes 1, 2 and 3. In Scheme 1, 1-(4-substituted benzyl)-2-chlorobenzimidazoles, 2a-c were prepared from the commercially available 2-chlorobenzimidazole under basic condition. Treatment of 2 with hydrazine hydrate resulted in the benzimidazol-2-hydrazine derivatives 3a-c. The physical properties of compounds 2&3 are summarized in Table 1. The reaction of compound 3 with 2-pyrrole carboxaldehyde under acidic condition afforded the targeted bishetaryls of benzimidazole and pyrrole (4a-c) in good to excellent yields. The physical and analytical data of compounds 4a-c are outlined in Table-2. Similarly, 2-cyanobenzimidazole obtained from 2, were reduced to afford benzimidazol methanamine intermediates 6a-c. Condensation of 6 with pyrrole-2-carboxaldehyde resulted in 7a-c in good yields. Compound 9, was prepared in two steps from 2b, that is reduction of nitro group followed by condensation with an aldehyde. The 1 NMR spectra of hydrazines (4) indicates multiplet aromatic protons at 6.9–7.4 ppm and the signals of the pyrrolyl ring protons at 6.0, 6.3 and 6.7 ppm. The azomethine and methylene protons appear at 7.4 and 5.2 ppm, respectively. The 13C-NMR shows the azomethine carbon signal at 152 ppm. In the IR spectrum of 4, the absorption band for stretching vibrations of the C=N group of the azomethine fragment is observed at 1640 cm−1.
Scheme 1.
Reagents and Conditions: (i) 4-R-PhCH2Cl, K2CO3, Acetone, reflux, 8h (ii) N2H4. H2O 160°C, 5h (iii) ArCHO, EtOH, 60°C, 2h (iv) NaN3, DMSO, rt-50°C, 4h (v) NH4Cl, EtOH/H2O, Zn, reflux, 4h (vi) 10% Pd/C, H2, EtOH
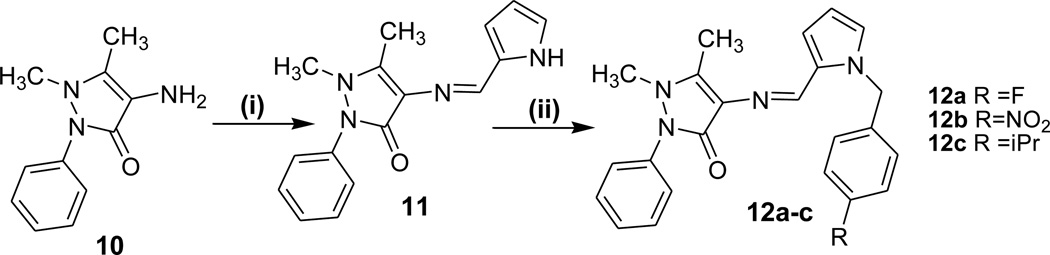
Scheme 2.
Reagents and Conditions: (i) 2-pyrrole carboxaldehyde, EtOH, AcOH (iii) NaH/DMF, RC6H4CH2X
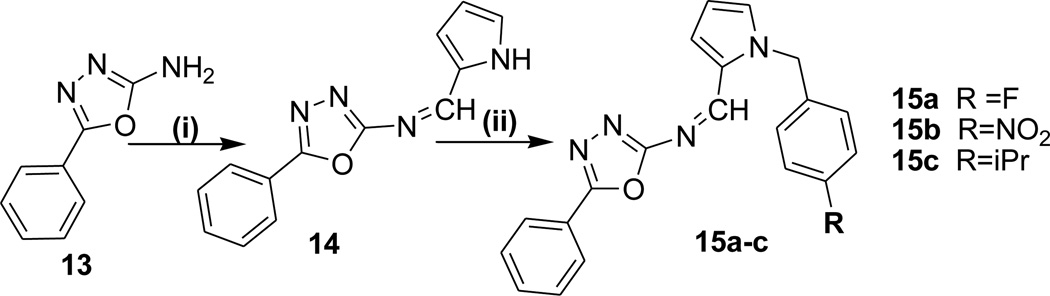
Scheme 3.
Reagents and Conditions: (i) 2-pyrrole carboxaldehyde, EtOH, AcOH (ii) NaH/DMF, RC6H4CH2X
Table 1.
Physical Properties of N-benzyl-2-chloroBenzimidazoles and 2-mehanaminebenzimidazoles
Table 2.
Physical and Analytical Data of pyrrolyl-benzimidazole Hybrids
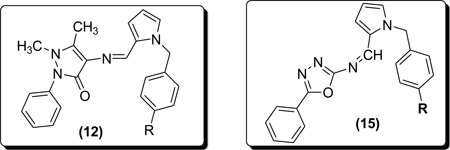
In Scheme-2, 4-aminoantipyrine (10) was condensed with pyrrole-caboxaldehyde and afforded the expected Schiff bases in excellent yields. In the 1H-NMR spectrum of the Schiff base of 4-aminoantipyrine (11), the signal for azomethine proton (-CH=N-) appears as a singlet at 9.2 – 10.5 ppm. The pyrrole (NH) proton appears as a singlet at 11.0–11.8 ppm. The multiplet signals obtained in the δ 7.0–8.0 ppm range are due to the aromatic protons. The signal for pyrazolone ring carbon attached methyl protons (-CH3) appear as a singlet at δ 2.42 ppm while pyrazolone ring nitrogen attached methyl protons (=N-CH3) appear as a singlet at δ3.06 ppm. In the 13C-NMR spectrum, the azomethine carbon signal has appeared at 152 ppm. The pyrazolone ring carbon attached methyl carbon (-CH3) and pyrazolone ring nitrogen attached methyl carbon (=N-CH3) peaks have been observed in the expected range at 8 – 32 ppm. The aromatic carbon signals are seen at 106–157 ppm range depending on their electronic environment. The IR spectrum of the Schiff base displays a sharp band at 1600–1630 cm-1 which can be assigned to -C=N stretching frequency. Further, the Schiff base exhibits a band at 1655 cm-1 due to -C=O.
In Scheme-3, Condensation of 2-amino-5-phenyl 1,3,4-oxadiazole (13) with 2-pyrrole carbaldehyde generated the Schiff base derivatives (14) in good yields. The three protons of pyrrole ring in targeted compounds appeared as multiplets between δ 6.27 – 7.49. The 1H NMR spectra also supported the proposed structure as there was no signal corresponding to NH2 proton.
1.2 Cytotoxcity
All of the newly synthesized compounds were evaluated for their anticancer effects using breast cancer cell lines, (MDA-MB-231), Ishikwa cells and BT-474 cells. Each compound stored at 20 mM was diluted from 100 µM to 10 µM by five-fold serial dilutions. Cells were treated with each compound for 48 h, followed by measuring cell growth rates by sulforhodamine B (SRB) based spectrophotometry. The IC50 concentration for each compound was calculated with reference to a control sample, which represents the concentration that results in a 50% decrease in cell growth after 48 h incubation in the presence of the test compound and the values are presented in Table 4. The data for pacletaxel was included as a reference.
Table 4.
Cytotoxicity activity on breast cancer cell lines.
| MDA-AB-231 | Ishikwa | BT-474 | |
|---|---|---|---|
| IC50 (µM) | IC50 (µM) | IC50 (µM) | |
| 4a | 84.1 | 77.2 | >500 |
| 4b | 72.2 | 67.7 | 53 |
| 4c | 82 | 55 | >500 |
| 7a | 76.22 | 88.2 | >500 |
| 7b | 64.1 | >500 | >500 |
| 7c | 37.8 | 65.2 | 78.41 |
| 9 | 23.26 | 9,07 | 7.7 |
| 12a | 66.7 | >500 | >500 |
| 12b | 49.8 | >500 | >500 |
| 12c | 53.26 | 89.07 | 77.7 |
| 15a | 84.2 | 78.5 | 68.3 |
| 15b | >500 | 66.9 | 88.1 |
| 15c | >500 | >500 | >500 |
| Pacletaxel | 0.003 | 0.003 | 0.005 |
The resultant data showed that all the synthesized compounds exhibited moderate activity against MDA-MB-231 cell lines except 15b and 15c. Compound 15c was inactive in all cell lines. Compounds 4b, 7c, 9, 12c and 15a were active against all the three cell lines. Compound 9 being the most active and compound 15c the least from the series. The pyrrole-benzimidazole series exhibited moderate activity compared to the pyrazolone and 1,3,4-oxadiazole series.
3. Experimental
3.1 Synthesis
Reagents and solvents were purchased from Sigma-Aldrich Chemical Company Inc., and used as received. The melting points (mp) were determined using Mel-Temp apparatus and were uncorrected. The infrared spectra were obtained using Perkin-Elmer 1430 FT spectrometer and are reported in cm-1. 1H-NMR and 13C-NMR spectra were recorded on Bruker-300 MHz spectrometer. Chemical shifts (in ppm) are reported relative to TMS as internal standard for solutions in DMSO-d6 and CDCl3. Column chromatography was performed using silica gel (200–425 mesh). Analytical thin layer chromatography was performed on 250 µm. layer flexible plates. Spots were visualized under UV light. Elemental analyses for C, H, and N were within (0.4% of the calculated values.
Synthesis of 1-(4-substituted benzyl)-2-chlorobenzimidazoles (2a–c)
2-Chloro-1H-benzoimidazole (20 mmol) was dissolved in dry DMF (15 mL) at 0 °C, to the solution was added NaH (22.7 mmol), and the mixture was stirred for 1 h at 0 °C, then halide (21.6 mmol) was added. The mixture was stirred overnight at room temperature and was poured into water (50 mL) and stirred for 1 h, filtrated, washed with water and dried to afford 2a-c. Yields, recrystallization solvents, and melting points of the products are reported in Table 1.
2-Chloro-1-(4-fluorobenzyl) benzimidazole (2a): White solid, IR (KBr): 1450, 1360, 720. 1H NMR (CDCl3):, δ 5.13 (s,2H, CH2), 7.10-7.49 (m, 8H, Ar-H).
2-Chloro-1-(4-nitrobenzyl)benzimidazole (2b): White solid, IR (KBr): 1450, 1360, 720. 1H NMR (CDCl3):, δ5.44 (s,2H, CH2), 7.10-7.49 (m, 8H, Ar-H).
2-chloro-1-(4-isopropylbenzyl)benzimidazole (2c): White solid, IR (KBr): 1450, 1360, 720.1H NMR (CDCl3): δ 1.08 (d, 6H, -CH(CH3)2), 3.12 (m, 1H, CH(CH3)2), 5.09 (s, 2 H), 7.18 (d, J = 6.5 Hz, 2H, Ar-H), 7.23–7.34 (m, 6H, Ar-H), 7.72 (d, J = 7.0 Hz, 1H, Ar-H).
Synthesis of 2-benzimidazole hydrazine (3a–c)
N-benzyl-2-chlorobenzimidazole (2a–c) (1mmol) was heated at 160 °C in a Pyrex capped tube with 0.1 mL of hydrazine hydrate for 5 h. After the mixture was cooled, a white solid separated which was collected and recrystallized. Yields, recrystallization solvents, and melting points of the products are reported in Table 1. Spectral data for 3c, which is representative of the title compounds, are listed below.
1-(4-isopropylbenzyl)-2-benzimidazole hydrazine (3c): IR (KBr):3320, 3225, 1550, 720. 1H NMR, (DMSO-d6,): δ 1.08 (d, 6H, -CH (CH3)2, 6.91-7.30 (m, 4H, Ar-H).
Synthesis of the hydrazine Schiff base (4a–c)
A mixture of pyrrole-2-carboxaldehyde (10 mmol) and 3a - c (10 mmol) in methanol (10 ml) containing 3 drops of glacial acetic acid was heated to reflux for 1.5 h. The resulting Schiff base precipitated on cooling. The precipitate was filtered off and recrystallized from absolute ethanol.
2-(2-pyrolylmethylene)-1-(4-fluorobenzyl)-benzimidazol-2-hydrazine (4a): White solid; 72% yield; m.p. 162–164°C; 1H NMR (CDCl3, δ, ppm): 5.58 (s, 2H, Ph–CH2), 7.05-7.15 (m, 3H, CH-pyrrole),6.97–7.82 (m, 8H, Ar-H), 10.77 (s, 1H, CH=N). Analysis for: 19H16FN5. Found, %: C 68.62; H 4.86; N 21.88. Calc, %: C 68.46; H 4.84; N 21.01.
2-(2-pyrolylmethylene)-1-(4-nitrobenzyl)-benzimidazol-2-hydrazine (4b): yellow solid, 65% yield; m.p. 155°–158°C; IR (KBr):1660 (C=O), 1614 (C=N). 1H NMR (CDCl3, δ, ppm): 5.85 (s, 2H, Ph–CH2), 6.65-7.35 (m, 3H, CH-pyrrole), 7.30 –7.90 (m,8H, Ar-H), 10.85 (s, 1H, –CH=N). Analysis for: C19H16N6O2, Found, %: C 63.43; H 4.12; N 23.64. Calc, %: C 63.32; H 4.48; N 23.32.
2-(2-pyrolylmethylene)-1-(4-isopropylbenzyl)-benzimidazol-2-hydrazine(4c): White powder, 64% yield; m.p. 186°–188°C; IR (KBr): 1643 (C=O), 1586 (C=N). 1H NMR (CDCl3, δ, ppm): 1.08 (d, 6H, -CH(CH3)2), 3.12 (m, 1H, CH(CH3)2), 5.58 (s, 2H, Ph–CH2), 6.81-7.57 (m, 3H, CH-pyrrole), 6.98–7.20 (m, 8H, Ar-H), 7.0 (1H, NH), 10.41 (s, 1H, –N=CH). Analysis for: C22H23N5. Found, %: C 73.62; H 6.16; N 19.87. Calc, %: C 73.92; H 6.49; N 19.59.
General procedures for the synthesis of compounds 5a–c
1-(4-fluorobenzyl)-2-chloromethylbenzimidazole (10 mmol) was added to 11 mmol NaN3 in DMSO solution. The mixture was heated at 50°C for 4h. The reaction was quenched with water (10 mL), extracted with chloroform, washed with water and brine and the organic layer was dried over MgSO4, and evaporated under reduced pressure and purified by column chromatography to afford pure azide.
1-(4-Fluorobenzyl)-2-azidomethyl-1H-benzoimidazole (5a): Yield: (67%), mp 139–141 °C. 1H NMR (CDCl3): δ 4.55 (s, 2H, CH2), 7.33-7.87 (m, 8H).
General procedure for the synthesis of compounds 6a–c
To the solution of azide (15 mmole) and ammonium chloride (35 mmol) in ethyl alcohol (20 mL) and water (7 mL), zinc powder (20 mmol) was added and refluxed for 4h. The mixture was diluted with ethylacetate and aqueous ammonia (5 mL) was added. The mixture was filtered and washed with brine and dried over anhydrous Sodium sulphate. The solvent was removed under reduced pressure and the residue was purified by flash chromatography.
1-(4-Fluorobenzyl)-2-benzimidazol methanamine (6a): recrystallized from EtOAc to give 1.78 g (75% yield) of cream crystals; m.p. 269°–271°C; 1H NMR (CDCl3, δ, ppm):3.90 (s, 2H, -CH2–NH2), 5.18 (s, 2H, Ph–CH2), 6.97–7.82 (m, 8H, Ar-H).
1-(4-nitrobenzyl)-2-benzimidazol methanamine (6b): Light yellow solid; 75% yield; m.p. 123–125°C; IR (KBr):1660 (C=O), 1614 (C=N). 1H NMR (CDCl3, δ, ppm): 3.94 (s, 2H, -CH2–NH2), 5.30 (s, 2H, Ph–CH2), 7.01–7.82 (m, 8H, Ar-H).
1-(4-isopropylbenzyl)-2-benzimidazol methanamine (6c): White solid; 75% yield; m.p. 132–134°C; IR (KBr): 1643 (C=O), 1586 (C=N). 1H NMR (CDCl3, δ, ppm): 1.08 (d, 6H, -CH(CH3)2), 3.83 (s, 3H CH3), 5.58 (s, 2H, Ph–CH2), 6.98–7.20 (m, 8H, Ar-H).
General procedure for the synthesis of compounds 7a–c
2-pyrrole carboxaldehyde (10 mmol) and few drops of glacial acetic acid were added to a solution of 4-amino-1,5-dimethyl-2-phenylpyrazol-3-one (10 mmol) in anhydrous ethanol (20 mL) at room temperature. The reaction mixture was refluxed for 2 h and then cooled to room temperature. The solid formed was filtered and washed with ether gave the desired Schiff base.
N-(2-pyrolylmethylene)-1-(4-fluorobenzyl)-benzimidazol-2-methanamine(7a): White powder, 56% yield; m.p. 143°–145°C; 1H NMR (CDCl3): δ 3.90 (s, 2H, -CH2–NH2), 5.18 (s, 2H, Ph–CH2), 6.97–7.82 (m, 8H, Ar-H). Analysis for: C20H17FN4. Found, %: C 72.52; H 5.18; N 16.80. Calc, %: C 72.27; H 5.16; N16.86.
N-(2-pyrolylmethylene)-1-(4-nitrobenzyl)-benzimidazol-2-methanamine(7b): yellow solid; 76% yield; m.p. 269°–271°C; IR (KBr):1660 (C=O), 1614 (C=N). 1H NMR (CDCl3): δ 3.94 (s, 2H, -CH2–NH2), 5.30 (s, 2H, Ph–CH2), 7.01–7.82 (m, 8H, Ar-H). Analysis for: C20H17N5O2, Found, %: C 66.66; H 4.38; N 19.32. Calc, %: C 66.84; H 4.77; N 19.49.
N-(2-pyrolylmethylene)-1-(4-isopropylbenzyl)-benzimidazol-2-methanamine(7c): Brownish solid; 67% yield; m.p. 169°–171°C; IR (KBr): 1643 (C=O), 1586 (C=N). 1H NMR (CDCl3): δ 1.08 (d, 6H, -CH(CH3)2), 3.83 (s, 3H CH3), 5.58 (s, 2H, Ph–CH2), 6.98–7.20 (m, 8H, Ar-H). Analysis for: C23H24N4. Found, %: C 77.96; H 6.89; N 15.22. Calc, %: C 77.50; H 6.79; N 15.72.
Preparation of Compound (8)
To a suspension of (2b, 5 mmol) and 10% Pd-C (0.25g) in methanol (2–5 mL) , 90% formic acid (2.5 mL) was added. The resulting solution was stirred at room temperature for 4 h, the mixture was filtered through celite and washed with methanol. The filtrate evaporated under reduced pressure, suspended in water and neutralized with ammonia. The resulting solid was extracted with ether and dried over anhydrous Na2SO4. The organic layer on evaporation afforded 8 (76%) as yellow solid, IR (KBr): 1450, 1360, 720. 1H NMR (CDCl3): δ5.44 (s, 2H, CH2), 7.10-7.49 (m, 8H, Ar-H).
N-(2-pyrolylmethylene)-4-(2-chlorobenzimidazolyl)methyl benznamine (9)
A mixture of pyrrole-2-carboxaldehyde (10 mmol) and 8 (10 mmol) in methanol (10 ml) containing 3 drops of glacial acetic acid was heated to reflux for 1.5 h. The Schiff base (9) was precipitated on cooling to 5°C. The solid was filtered off and recrystallized from EtOAc to give white solid (56% ) yield; m.p. 145°–147°C; 1H NMR (CDCl3, δ, ppm): 5.08 (s, 2H, Ph–CH2), 6.81-7.57 (m, 3H, CH-pyrrole), 7.12–7.28 (m, 8H, Ar-H). Analysis for: C19H15ClN4. Found, %: C 68.66; H 4.34; N 16.87. Calc, %: C 68.16; H 4.52; N 16.73.
Synthesis of 4-(2-pyrrolyl methylene amino)-1,2-dihydro-1,5-dimethyl-2-phenylpyrazol-3-one (11)
2-pyrrole carboxaldehyde (10 mmol) and few drops of glacial acetic acid were added to a solution of 4-amino-1,5-dimethyl-2-phenylpyrazol-3-one (10) (10 mmol) in anhydrous ethanol (20 mL) at room temperature. The reaction mixture was refluxed for 2 h and then cooled to room temperature. The solid formed was filtered and washed with ether gave the desired Schiff base (11): Yellow solid (82.7%), mp 194–195°C; IR (KBr, cm-1):1665 (C=O), 1615 (C=N). 1H-NMR (CDCl3): δ 2.41 (s, 3H, =C–CH3), 3.09 (s, 3H, N-CH3), 7.05-7.15 (m, 3H, CH-pyrrole), 7.26-7.49 (m, 5H, Ar-H), 10.75 (s, 1H, CH=N), 11.35 (s, 1H, NH).
Synthesis of N-Substituted-benzyl-pyrroleaminopyrine Schiff Bases (12a–c)
NaH (60% in mineral oil, 11.8 mmol) was added to a solution of 11 (10 mmol) in DMF at 0°C. The mixture was stirred at 0°C for 30 min. and 4-substituted benzyl chloride (10 mmol) was added. The mixture was stirred at room temperature for 8 h, acidified with saturated aqueous solution of NH4Cl and extracted twice with EtOAc. The organic layers were combined, washed with brine, dried over anhydrous MgSO4, and concentrated in vacuo. The residue was purified by silica gel column chromatography.
4-((1-(4-Fluorobenzyl)-1H-pyrrol-2-yl)methylene-amino)-1,2-dihydro-1,5-dimethyl-2-phenyl-pyrazol-3-one (12a): yellow solid (89%), mp 165–168°C; IR (KBr): 1655 (C=O), 1605 (C=N). 1H NMR (CDCl3): δ 2.47 (s, 3H, =C–CH3), 3.18 (s, 3H, N–CH3), 5.58 (s, 2H, Ph–CH2), 7.05-7.15 (m, 3H, CH-pyrrole),6.97–7.82 (m, 9H, Ar-H), 10.77 (s, 1H, CH=N). 13C NMR (CDCl3, δ, ppm): 124.19, 129.08, 126.70, 149.20,131.17, 134.85, 160.88 (C=O), 156.76 (CH=N), 131.71 (C(py)), 110.41 (C(py)), 108.08 (C(py)), 122.57 (C(py)), 134.8, 130.5, 128.6, 131.2, 10.09 (C-CH3), 35.85 (CH3-N), 50.42 (Ph-CH2-N). Analysis for: C23H21FN4O, Found, %: C 71.52; H 5.18; N 14.80. Calculated, %: C 71.12; H 5.45; N 14.42.
4-((1-(4-Nitrobenzyl)-1H-pyrrol-2-yl)-methylene-amino)-1,2-dihydro-1,5-dimethyl-2-phenyl-pyrazol-3-one (12b): orange solid (68%), mp 155–158°C; IR (KBr):1660 (C=O), 1614 (C=N). 1H NMR (CDCl3): δ 2.45 (s, 3H, =C–CH3), 3.18 (s, 3H, N–CH3), 5.85 (s, 2H, Ph–CH2), 6.65-7.35 (m, 3H, CH-pyrrole),7.30–7.90 (m,9H, Ar-H), 10.85 (s, 1H, –CH=N). 13C NMR (CDCl3, δ, ppm): 124.19, 129.08, 126.70, 149.20,131.17, 134.85, 160.88 (C=O), 156.76 (CH=N), 131.71 (C(py)), 110.41 (C(py)), 108.08 (C(py)), 122.57 (C(py)), 142.78, 130.0, 121.6, 145.2, 10.09 (C-CH3), 35.85 (CH3-N), 50.42 (Ph-CH2-N). Analysis for: C23H21N5O3, Found, %: C 66.43; H 5.12; N 16.64. Calc, %: C 66.49; H 5.09; N 16.86.
4-((1-(4-isopropylbenzyl)-1H-pyrrol-2-yl)methylene-amino)-1,2-dihydro-1,5-dimethyl-2-phenyl-pyrazol-3-one (12c): yellow solid (89%), mp 165–168°C; IR (KBr): 1655 (C=O), 1605 (C=N). 1H NMR (CDCl3): δ 2.47 (s, 3H, =C–CH3), 3.18 (s, 3H, N–CH3), 5.58 (s, 2H, Ph–CH2), 7.05-7.15 (m, 3H, CH-pyrrole),6.97–7.82 (m, 9H, Ar-H), 10.77 (s, 1H, CH=N). 13C NMR (CDCl3, δ, ppm): 124.19, 129.08, 126.70, 149.20,131.17, 134.85, 160.88 (C=O), 156.76 (CH=N), 131.71 (C(py)), 110.41 (C(py)), 108.08 (C(py)), 122.57 (C(py)), 134.8, 130.5, 128.6, 131.2, 10.09 (C-CH3), 35.85 (CH3-N), 50.42 (Ph-CH2-N). Analysis for: C26H28N4O. Found, %: C 75.62; H 6.16; N 13.87. Calc, %: C 75.70; H 6.84; N 13.58.
Synthesis of 2-pyrrolylmethylene-5-phenyl-1,3,4-oxadiazol-2-amine (14)
2-pyrrole-carbox- aldehyde(10 mmol) and few drops of glacial acetic acid were added to a solution of 13 (10 mmol) in anhydrous ethanol (20 mL) at room temperature. The reaction mixture was refluxed for 2 h and then cooled to room temperature. The solid formed was filtered and washed with ether to provide the desired Schiff base.(14): White solid, yield ( 74%), m.p. 203–205°C 1H NMR (DMSO,) δ:7.68–7.45 (m, 5H, Ar-H), 8.73 (s, 1 H, CH=N) ppm. IR (KBr): 1713, 1618, 1582, 1391, 1293, 1245, 1023, 694 cm-1.
Synthesis of N-Substituted benzyl pyrrole-1,3,4-oxadiazole Schiff Bases (15a–c): General procedure
To an ice-cooled solution of 14 (10 mmol) in DMF was added NaH (60% in mineral oil, 11.8 mmol) and the mixture was stirred at 0°C for 30 min. To the mixture were added 4-substituted benzyl chlorides (10 mmol) at 0°C and stirred at room temperature for 8 h. The mixture was acidified with saturated aqueous solution of NH4Cl and extracted twice with EtOAc. The organic layers were combined, washed with brine, dried over anhydrous MgSO4, and concentrated under vacuum. The residue was purified by silica gel column chromatography.
N-((1-(4-Fluorobenzyl)-1H-pyrrol-2-yl)methylene)-5-phenyl-1,3,4-oxadiazol-2-amine (15a): yellow solid (89%), mp 165–168°C; 1H NMR (CDCl3): δ 5.58 (s, 2H, Ph–CH2), 7.05-7.15 (m, 3H, CH-pyrrole),6.97–7.82 (m, 9H, Ar-H), 10.77 (s, 1H, CH=N). Analysis for: C20H15FN4O, Found, %: C 69.36; H 4.08; N 16.52. Calc, %: C 69.35; H 4.37; N 16.18.
N-((1-(4-Nitrobenzyl)-1H-pyrrol-2-yl)methylene)-5-phenyl-1,3,4-oxadiazol-2-amine (15b): orange solid (68%), mp 155–158°C; 1H NMR (CDCl3): δ 5.85 (s, 2H, Ph–CH2), 6.65-7.35 (m, 3H, CH-pyr),7.30–7.90 (m, 9H, Ar-H), 10.85 (s, 1H, –CH=N). Analysis for: C20H15N5O3, Found, %: C 64.12; H 4.36; N 18.43. Calc, %: C 64.34; H 4.05; N18.76.
N-((1-(4-isopropylbenzyl)-1H-pyrrol-2-yl)methylene)-5-phenyl-1,3,4-oxadiazol-2-amine (15c): yellow crystal (91%); mp 168–169°C; 1H NMR (CDCl3): δ 3.83 (s, 3H CH3), 6.81-7.57 (m, 9H, Ar-H), 10.41 (s, 1H, –N=CH). Analysis for: C23H22N4O, Found, %: C 74.38; H 5.41; N 15.06. Calc, %: C 74.57; H 5.99; N15.12.
4. Conclusions
Bis-hetarylazoles of benzimidazole, 1,3,4-oxadiazole and pyrazolone bearing pyrrole moieties were synthesized and assessed for cytotoxicity in vitro against breast cancer cell lines. The benzimidazole series displayed the optimal profiles with IC50 in µM range. The most promising compound 9 with an IC50 = 7.7µM is an attractive candidate for further assessment. This observation could be attributed to the synergetic effect that may result from combining the N-benzylpyrrolyl core with the typical benzimidazole core.
Table 3.
Physical and Analytical Data of Compounds 12a-c and 15a-c
Acknowledgment
The authors are grateful to the National Institute of Health, the National Institute of General Medical Sciences, the MBRS Program (GM 08111) and the Research Center at Minority Institutions Grant (RCMI) RR 03020. Also, much gratitude goes to the FAMU School of Graduate Studies, Faculty Research Award Program.
References
- 1.Kumar V, Kaue K, Gupta GK, Sharma AK. Eur. J. Med. Chem. 2013;69:735–753. doi: 10.1016/j.ejmech.2013.08.053. [DOI] [PubMed] [Google Scholar]
- 2.Spasov A, L Yozhitsa I, Bugaeva LI, Amisimova VA. Pharm. Chem. J. 1999;33(5):232. [Google Scholar]
- 3.Gaba Monika, Singh Sarbjot, Mohan Chander. Eur. J. Med. Chem. 2014;76(9):494. doi: 10.1016/j.ejmech.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 4.Khokra SI, Choudhary D. Asian J. Biochem. Pharm. Res. 2011;3(1):476. [Google Scholar]
- 5.Kharb R, ShaharYar M, Sharma PC. Mini Rev Med Chem. 2011;11(1):84. doi: 10.2174/138955711793564051. [DOI] [PubMed] [Google Scholar]
- 6.Keri Rangappa S, Patil Mahadeo R, Patil Siddappa A, SrinivasaBudagumpi Eur. J. Med. Chem. 2015;89(7):207. doi: 10.1016/j.ejmech.2014.10.059. [DOI] [PubMed] [Google Scholar]
- 7.Khalilullah H, Ahsan MJ, Hedaitullah M, Khan S, Ahmed B. Mini Rev. Med. Chem. 2012;12(8):789. doi: 10.2174/138955712801264800. [DOI] [PubMed] [Google Scholar]
- 8.Zarighi A, Hajimahdi Z. Expert. Opin. Ther. Pat. 2013;23(9):1209. doi: 10.1517/13543776.2013.797409. [DOI] [PubMed] [Google Scholar]
- 9.Kharb R, Yar MS, Sharma PC. Curr Med Chem. 2011;18(21):3265. doi: 10.2174/092986711796391615. [DOI] [PubMed] [Google Scholar]
- 10.YogitaBansal, Om Silakari Bio. Org. Med. Chem. 2012;20:6208. doi: 10.1016/j.bmc.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Ansari KF, Lal C. Eur. J. Med. Chem. 2009;44(10):4028. doi: 10.1016/j.ejmech.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 12.Krishnanjaneyulu IS, Saravanan G, Vamsi J, Supriya P, Bhavana JU, Sunil Kumar MV. J Adv Pharm Technol Res. 2014;5(1):21. doi: 10.4103/2231-4040.126983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pace A, Pierro P. Org. Biomol. Chem. 2009;7:4337. doi: 10.1039/b908937c. [DOI] [PubMed] [Google Scholar]
- 14.De Oliveira CS, Lira BF, Barbosa-Filho JM, Lorenzo JG, deAthavde-Filho PF. Molecules. 2012;17(9):10192. doi: 10.3390/molecules170910192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun J, Makawana JA, Zhu HL. Mini Rev Med Chem. 2013;13(12):1725. doi: 10.2174/13895575113139990071. [DOI] [PubMed] [Google Scholar]
- 16.Basoglu S, Yolal M, Demirci S, Demirbas N, Bektas H, Karaoglu SA. Acta Pol. Pharm. 2013;70(2):229. [PubMed] [Google Scholar]
- 17.Chawla R, Arora A, Parameswaran MK, Chan P, Sharma D, Michael S, Ravi TK. Acta Pol Pharm. 2010;67(3):247. [PubMed] [Google Scholar]
- 18.Tanwar Saha R, Mareella A, Alam MM, Akhter M. Mini Rev Med. Chem. 2013;13:1027. doi: 10.2174/1389557511313070007. [DOI] [PubMed] [Google Scholar]
- 19.Bondock S, Rabie R, Etman HA, Fadda A. Eur. J. Med. Chem. 2008;43:2122. doi: 10.1016/j.ejmech.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Marella A, Ali MR, Alam MT, Saha R, Tanwar O, Akhter M, Shaquiquzzaman M, Alam MM. Mini Rev Med Chem. 2013;13(6):921. doi: 10.2174/1389557511313060012. [DOI] [PubMed] [Google Scholar]
- 21.Summit S, Balasubramanian N, Vasudevan M, Rakesh KM, Abu Baker AM. Med Chem Res. 2012;21:3863. [Google Scholar]
- 22.Ghorab MM, El-Gazzar MG, Alsaid MS. Int J Mol Sci. 2014 May 2;15(5):7539. doi: 10.3390/ijms15057539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar V, Kaur K, Gupta GK, Shama AK. Eur. J. Med. Chem. 2013;69:735. doi: 10.1016/j.ejmech.2013.08.053. [DOI] [PubMed] [Google Scholar]
- 24.Khalil NA, Ahmed EM, Mohamed KO, Nissan YM, Abo-BakrZaitone S. Bioorg Med Chem. 2014;22(7):2080. doi: 10.1016/j.bmc.2014.02.042. [DOI] [PubMed] [Google Scholar]
- 25.Pedro M, Santos MP, Alexandra M, Antunes M, Noronha J, Fernandes E, Abel J, Veira SC. Eur J Med Chem. 2010;45(6):2258. doi: 10.1016/j.ejmech.2010.01.071. [DOI] [PubMed] [Google Scholar]
- 26.Metwally MA, Gouda MA, Ammar N, Harmal A, Khalil M. Eur J Med Chem. 2012;56:254. doi: 10.1016/j.ejmech.2012.08.034. [DOI] [PubMed] [Google Scholar]
- 27.Gopishetty B, Zhang S, Kharkar PS, Antonio T, Reith M, Dutta AK. Bioorg. Med. Chem. 2013;21(11):3164–3174. doi: 10.1016/j.bmc.2013.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dutta AK, Venkataraman SK, Fei XS, Kolhatkar R, Zhang S, Reith ME. Bioorg. Med. Chem. 2004;12(16):4361–4373. doi: 10.1016/j.bmc.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 29.Vijesh AM, Isloor AM, Shetty P, Sundershan S, Fun HK. Eur. J. Med. Chem. 2013;62:410. doi: 10.1016/j.ejmech.2012.12.057. [DOI] [PubMed] [Google Scholar]
- 30.Xu Yu-Ling, Lin Hong-Yan, Cao Run-Jie, Ming Ze-Zhong, Yang Wen-Chao, Yang Guang-Fu. Bioorg. Med. Chem. 2014;22(19):5194. doi: 10.1016/j.bmc.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Wang X-Q, Liu LX, Li Y, Sun CJ, Chen W, LI L, Zhang HB, Yang XD. Eur. J. Med. Chem. 2013;62:111. doi: 10.1016/j.ejmech.2012.12.040. [DOI] [PubMed] [Google Scholar]