Abstract
The objective of this study was to determine the steady-state plasma and intrapulmonary pharmacokinetic parameters of orally administered cethromycin in healthy volunteers. The study design included administering 150 or 300 mg of cethromycin once daily to 25 or 35 healthy adult subjects, respectively, for a total of five doses. Standardized and timed bronchoalveolar lavage (BAL) was performed after the last dose. Blood was obtained for drug assay prior to the first and last dose, at multiple time points following the last dose, and at the time of BAL. Cethromycin was measured in plasma, BAL, and alveolar cell (AC) by using a combined high-performance liquid chromatography-mass spectrometric technique. Plasma, epithelial lining fluid (ELF), and AC pharmacokinetics were derived by noncompartmental methods. Cmax/90% minimum inhibitory concentration (MIC90) ratios, area under the concentration-time curve (AUC)/MIC90 ratios, intrapulmonary drug exposure ratios, and percent time above MIC90 during the dosing interval (%T > MIC90) were calculated for recently reported respiratory pathogens. The kinetics were nonlinear, i.e., not proportional to dose. In the 150-mg-dose group, the Cmax (mean ± standard deviations), AUC0-24, and half-life for plasma were 0.181 ± 0.084 μg/ml, 0.902 ± 0.469 μg · h/ml, and 4.85 ± 1.10 h, respectively; for ELF the values were 0.9 ± 0.2 μg/ml, 11.4 μg · h/ml, and 6.43 h, respectively; for AC the values were 12.7 ± 6.4 μg/ml, 160.8 μg · h/ml, and 10.0 h, respectively. In the 300-mg-dose group, the Cmax (mean ± standard deviations), AUC0-24, and half-life for plasma were 0.500 ± 0.168 μg/ml, 3.067 ± 1.205 μg · h/ml, and 4.94 ± 0.66 h, respectively; for ELF the values were 2.7 ± 2.0 μg/ml, 24.15 μg · h/ml, and 5.26 h, respectively; for AC the values were 55.4 ± 38.7 μg/ml, 636.2 μg · h/ml, and 11.6 h, respectively. We concluded that the Cmax/MIC90 ratios, AUC/MIC90 ratios, %T > MIC90 values, and extended plasma and intrapulmonary half-lives provide a pharmacokinetic rationale for once-daily administration and are favorable for the treatment of cethromycin-susceptible pulmonary infections.
Cethromycin is an investigational ketolide antibiotic that is active against common respiratory pathogens, such as Streptococcus pneumoniae, Mycoplasma pneumoniae, Chlamydia pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, group A streptococci, and Streptococcus viridans (14, 15, 17, 18, 29). Cethromycin is also active, in vitro and in a murine model, against Mycobacterium avium infection (10), and related macrolides are active against other mycobacteria (12, 21). Doses of cethromycin ranging from 100 to 1,200 mg have been administered to humans in phase I clinical studies. The plasma elimination half-life (t1/2) ranges from 3.6 to 6.7 h (R. S. Pradhan, L. E. Gustavson, D. D. Londo, Y. Shang, J. Zhang, and M. Paris, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 2138, 2000). The maximum concentration of drug in serum (Cmax), area under the concentration-time curve from 0 to 24 h (AUC0-24), and plasma clearance in healthy subjects receiving an orally administered once-daily dose of 300 mg have been reported to be approximately 0.9 μg/ml, 5.9 μg · h/ml, and 63 liters/h, respectively; in human plasma, the protein binding ranges from approximately 86.7 to 95.6% over a drug concentration range of 0.1 to 30.0 μg/ml (Abbott Laboratories) (data not shown). Cethromycin is presently being studied for the treatment of nosocomial and community-acquired pneumonia, but its in vivo penetration into pulmonary alveolar cells (AC) and pulmonary epithelial lining fluid (ELF) in humans has not been reported.
Techniques for the in vivo measurement of the concentration of antibiotics in ELF and AC have been reported previously (4-9). The purpose of this investigation was to determine the steady-state plasma and intrapulmonary pharmacokinetic and pharmacodynamic parameters of two dosage regimens of orally administered cethromycin in healthy volunteers.
MATERIALS AND METHODS
Study design and subjects.
This study was a prospective, nonblinded study of the plasma and intrapulmonary concentrations of cethromycin at steady state. All subjects gave written informed consent and were required to be between 21 and 55 years of age and have a body mass index of 18 to 29 (1). The evaluation included a medical history; physical examination; baseline laboratory testing, including complete blood count with differential, platelet count, blood urea nitrogen, serum creatinine, aspartate aminotransferase, alanine aminotransferase, gamma glutaryl transferase, calcium, inorganic phosphates, sodium, potassium chloride, 5-nucleotidase, leucineaminopeptidase, reticulocyte count, serum human chorionic gonadotropin (if female), alkaline phosphatase, total bilirubin, glucose, total protein, albumin, and uric acid; urinalysis with urine drug and alcohol screen; and electrocardiogram. The evaluation was repeated prior to administration of cethromycin and, except for the urine drug and alcohol screen, following bronchoscopy. Subjects were excluded included those who had a history of clinically significant disease; clinically significant abnormal findings at the screening physical examination (including laboratory tests); intolerance to cethromycin, macrolides, or lidocaine; positive drug screen; history of smoking within the previous 6 months. Other subjects excluded were those who were required to take chronic medications other than birth control pills and hormone replacement therapy and those receiving any investigational drug within 30 days prior to the study. Twenty-five subjects in the 150-mg-dose group were assigned to one of five groups of five subjects each according to the time of bronchoscopy: 2, 4, 8, 12, and 24 h following the last dose. Thirty-five subjects in the 300-mg-dose group were assigned to one of seven groups of five subjects each according to the time of bronchoscopy: 2, 4, 6, 8, 12, 24, and 48 h following the last dose. The 2- and 4-h time periods were chosen to approximate the peak (Cmax) intrapulmonary concentration of cethromycin; the 8-h time was chosen as an approximate midpoint between the Cmax and minimum concentration of drug in serum (Cmin) (24 h) in a once-daily dosing regimen. The 48-h time point (300-mg-dose group) was selected to examine the possibility of a long intrapulmonary t1/2.
Cethromycin was administered orally in a dose of 150 or 300 mg, once daily, for a total of five doses. The first and last doses of study medication were administered under direct supervision in the General Clinical Research Center (GCRC) at the University of California, San Francisco (UCSF). Subjects were observed for 30 min after the first dose for adverse effects. Subsequent doses were taken according to oral and written instructions and were documented in a written diary by the subjects.
Bronchoscopy and BAL.
Standardized bronchoscopy, bronchoalveolar lavage (BAL), and clinical monitoring (7, 8, 9) were performed at the GCRC at 2, 4, 8, 12, and 24 h (150-mg-dose group) or at 2, 4, 6, 8, 12, 24, and 48 h (300-mg-dose group) after the administration of the last dose. Systemic sedation was not used.
A fiber optic bronchoscope (Pentax FB-18BS) was inserted in the right middle lobe. Four 50-ml aliquots of normal saline were instilled, and each was immediately aspirated into a trap. The average duration of the bronchoscopy was 4 min. The specimens were kept on ice until they were frozen. The first aspirate was discarded. The second, third, and fourth aspirates were pooled (pooled BAL). The volume of the pooled BAL was measured and recorded. Measured aliquots of the pooled BAL were sent to the clinical laboratory for cell count and differential. A known volume of the pooled BAL was immediately spun at 400 × g for 5 min in a refrigerated centrifuge. The supernatant and the cells were separated and frozen at −70°C until assay. A small aliquot of the supernatant was frozen separately for urea assay.
Blood samples.
Blood was obtained for cethromycin assay just prior to administration of the last dose, at 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 5.0, 6.0, 8.0, 10.0, 12.0, 16.0, 20.0, 24.0, and 48.0 h (48 h in the 300-mg-dose group only) following the last dose and at the time of bronchoscopy for each of these subjects.
Specimen handling.
Blood samples were kept on ice until centrifugation. The plasma was separated and then frozen at −70°C until assay. Immediately prior to analysis, the BAL cell pellets were resuspended in 3.0 ml of deionized water and were sonicated for 2 min on ice.
Cethromycin assay.
Quantitation of cethromycin in plasma, BAL, and AC was conducted by using a specific and sensitive high-performance liquid chromatographic-tandem mass spectrometry method (22). This method measures total cethromycin (protein bound and unbound). Briefly, the mobile phase consists of 50% acetonitrile, 0.05% acetic acid, and 5 mM ammonium acetate; the column used is a C8 reverse-phase column. The retention times for cethromycin and the internal standard were approximately 2.0 and 2.7 min, respectively, with a total run time of 3.5 min. Detection was carried out with electrospray mass spectrometry in a multiple-reaction monitor mode. Preparation of samples requires a solvent extraction step. After addition of an internal standard, 0.5 ml of plasma was extracted with 3 ml of methyl t-butyl ether, evaporated, and resuspended in 50% acetonitrile. BAL and AC suspensions were extracted in a similar manner, but prior to the extraction step the pH was adjusted by adding 50 μl of a 0.1 M NaOH solution. Plasma and BAL and AC standard curves were linear from 1.0 to 1,000 ng/ml and from 0.2 to 200 ng/ml, respectively.
The mean (± standard deviation [SD]) coefficients of variation and ranges of the assay for intraday and interday determination together for plasma, BAL supernatants, and AC suspensions were 6.65 ± 3.16 (range, 2.51 to 10.5%), 6.64 ± 2.43 (range, 2.74 to 8.92%), and 7.14 ± 2.70 (range, 2.08 to 9.58%), respectively. The accuracy ranges for all determinations in plasma, BAL supernatants, and AC suspensions were −8.89 to 12.0%, −5.67 to 11.6%, and −0.229 to 11.9%, respectively.
Quantitation of volume of ELF and concentration of antibiotics in ELF and AC.
The amount of ELF recovered was calculated by the urea dilution method as described by Rennard et al. (23) and as reported in other previous pulmonary pharmacokinetic studies (4-9). The concentration of urea in serum was analyzed by the clinical laboratory at UCSF using a coupled urease-glutamate dehydrogenase enzymatic method, modified by Boehringer Mannheim Corporation (Indianapolis, Ind.) (24). Measurements were made at a fixed time interval, permitting automated analysis with a BM 747 Analyzer (Boehringer Mannheim). Urea was measured in BAL supernatant utilizing a modified enzymatic assay (Infinity BUN kit UV-63; Sigma, St Louis, Mo.) as previously reported (4-9). The assay is linear (R2 = 0.99) for concentrations of urea in BAL from 0.047 to 0.750 mg/dl. Controls were included with every run and, if not within 10% of the known value, the standard curve, controls, and specimen assays were repeated.
The volume of ELF in BAL fluid, the concentration of antibiotic in the ELF, and the concentration of antibiotic in AC were derived using methods and calculations that have been previously published (4-9).
The volume of AC collected in the pellet suspension was determined from the cell count in the BAL fluid. Because of cell loss during centrifugation, the actual number of cells recovered may be lower than the number counted, and the antibiotic concentration may be approximately 20% greater than we calculated (26). Differential cell counting was performed after spinning the specimen in a cytocentrifuge. The volume of AC in the pellet suspension was determined by using a mean macrophage cell volume of 2.42 μl/106 cells (2).
Statistical, pharmacokinetic, and pharmacodynamic analyses.
Descriptive statistics, graphic representations, database management, and kinetic analysis were performed with Kinetica 2000 (version 4.1.1; InnaPhase Corporation, Philadelphia, Pa.), SPSS (version 11.0.1; SPSS, Inc., Chicago, Ill.), and PROPHET (version 6.0; AbTech). Because the interpatient variability of plasma, ELF, and AC cethromycin concentrations at each of the selected time periods was not known prior to the study, we used sample sizes (five in each group) based upon prior experience with rifapentine and linezolid and a similar study design (7, 9). The log trapezoidal rule was used to compute the AUC0-24 for the mean concentration-time data in plasma, ELF, and AC after the fifth dose. The plasma, intracellular, and ELF concentration-time data declined monoexponentially; the means of the observed concentrations at each BAL time, from 6 to 24 h (150-mg-dose group) or 6 to 48 h (300-mg-dose group) were used to calculate kel, the elimination rate constant. Fitting was performed by using a weighting function (1/Y2), where 1/Y was the reciprocal of the observed concentration. The plasma, ELF, and AC t1/2s were calculated by using the relationship t1/2 = 0.693/kel. The intrapulmonary drug exposure ratio was defined as the AUC0-24 for ELF or AC divided by the AUC0-24 for plasma.
Analysis of variance was used to compare the concentrations in plasma, AC, and ELF and to compare ELF recovery and AC recovery at each of the different time periods. Prior to performing the analysis of variance, the data sets were tested for normality (Wilk-Shapiro) and equality of variances (Levene's test). Parametric and nonparametric analyses were performed with the Newman-Keuls or Friedman test, respectively (28). Linear regression was performed by using the method of least-squares estimation. A P value of <0.05 was regarded as significant.
For the pharmacodynamic calculations, the concentrations of cethromycin required to inhibit 90% (MIC90) of the following respiratory pathogens were obtained from recently reported literature (see Tables 1 to 6): M. pneumoniae (103 strains; MIC90 = 0.001 μg/ml) (25); S. pneumoniae (6,691 isolates; MIC90 = 0.0008 μg/ml; 9.6% intermediately resistant and 9.9% resistant to macrolides) (29); C. pneumoniae (13 isolates; MIC90 = 0.0.15 μg/ml) (15); 2,314 isolates; M. catarrhalis (MIC90 = 0.12 μg/ml) (30); and H. influenzae (25 strains; MIC90 = 4.0 μg/ml) (14). Standard pharmacokinetic/pharmacodynamic indices, as suggested by Mouton et al. (19), were used to calculate the Cmax/MIC90 and AUC/MIC90 ratios and the percent time above MIC (%T > MIC).
TABLE 1.
Recovery of cells and ELF from BAL in healthy adult subjects in the 150-mg/day-dose group (n = 25)
| Value | Result at time point (h)b:
|
||||
|---|---|---|---|---|---|
| 2 | 4a | 8a | 12a | 24a | |
| Mean (cells/liter)a | 1.2 × 108 | 1.0 × 108 | 0.9 × 108 | 1.2 × 108 | 1.0 × 108 |
| SD (cells/liter) | 0.5 × 108 | 0.3 × 108 | 0.3 × 108 | 0.4 × 108 | 0.4 × 108 |
| PMNs (%) | 0.2 ± 0.4 | 1.8 ± 1.1 | 1.4 ± 2.2 | 1.4 ± 1.1 | 3.0 ± 2.5 |
| Lymphocytes (%) | 1.8 ± 1.9 | 10.2 ± 13.0 | 11.0 ± 12.3 | 8.0 ± 2.4 | 9.8 ± 4.8 |
| Mono/macro (%) | 91.6 ± 6.8 | 84.6 ± 13.1 | 82.4 ± 10.2 | 81.8 ± 14.5 | 82.6 ± 6.1 |
| Eosinophils (%) | 0.0 ± 0.0 | 0.6 ± 0.9 | 0.2 ± 0.4 | 0.4 ± 0.5 | 1.0 ± 1.0 |
| Degen cells (%) | 5.0 ± 5.4 | 2.8 ± 4.4 | 4.6 ± 5.0 | 8.4 ± 16.6 | 3.6 ± 5.3 |
| ELF volume (ml) | 0.7 ± 0.2 | 0.4 ± 0.2 | 0.5 ± 0.4 | 0.5 ± 0.2 | 0.4 ± 0.1 |
No significant differences among the groups for cell recovery, differential cell count, or volume of ELF (P > 0.05). Comparison testing was not applied to zero values.
All data given as mean±SD. PMN, polymorphonuclear leukocyte. Mono/macro, monocytes and macrophages. Degen, degenerated.
TABLE 6.
Plasma pharmacodynamic parameters
| Organism (MIC90) |
Cmax/MIC90 ratio for dose group:
|
AUC/MIC90 ratio for dose group:
|
%T > MIC90 for dose group:
|
|||
|---|---|---|---|---|---|---|
| 150 mg | 300 mg | 150 mg | 300 mg | 150 mg | 300 mg | |
| M. pneumoniaea (≤0.001 μg/ml) | 184 | 504 | 906 | 3,064 | 100 | 100 |
| S. pneumoniae (0.008 μg/ml) | 23 | 63 | 113 | 383 | 78 | 100 |
| C. pneumoniae (0.015 μg/ml) | 12 | 33 | 60 | 204 | 59 | 100 |
| M. catarrhalis (0.12 μg/ml) | 2 | 4 | 8 | 25 | 10 | 39 |
| H. influenzae (4 μg/ml) | 0.05 | 0.13 | 0.23 | 0.77 | 0 | 0 |
MIC90 of 0.001 was used for the pharmacodynamic calculations.
RESULTS
Sixty subjects were enrolled in the study. Seventy subjects were given consent, 10 were excluded after enrollment and were replaced with 10 additional subjects, 4 withdrew consent, 3 withdrew due to illness, 2 did no meet eligibility criteria, and 1 had an abnormal electrocardiogram.
In the 150-mg group, the mean age of the 25 subjects was 31.7 ± 7.3 years; 15 were men and 10 were women; 17 were Caucasian, 4 were Asian, 2 were African-American, and 2 were Hispanic; the mean (± SD) weight and serum creatinine values were 67.6 ± 11.6 kg and 0.9 ± 0.2 mg/dl, respectively; the remaining screening laboratory tests were within normal limits.
In the 300-mg group, the age (mean ± SD) of the 35 subjects was 29.5 ± 6.7 years; 20 were men and 15 were women; 25 were Caucasian, 5 were Asian, 3 were African-American, 1 was Hispanic, and 1 was other; the mean (± SD) weight and serum creatinine values were 69.1 ± 11.2 kg and 0.9 ± 0.2 mg/dl, respectively; the remaining screening laboratory tests were within normal limits. None of the differences in age, weight, or serum creatinine between the 300- and 150-mg groups were significant (P > 0.05).
For both dose groups, there were no serious adverse events and the subjects returned to their normal duties following the bronchoscopy and BAL. Six subjects experienced transient chest discomfort or elevated temperature. Self-limited lightheadedness occurred in 23 subjects. Transient rales and/or diminished breath sounds were present in 42 subjects following the procedure. This is an expected finding following instillation of fluid for the purpose of BAL. On repeat laboratory testing, eight subjects had elevated liver function tests, two had elevated serum creatinine, five had decreased hemoglobin, and one had an elevated white blood cell count.
AC recovery was not significantly different among the five time groups in either dose group (P > 0.05) (Tables 1 and 2). For both dose groups, the majority of the cells (mean ± SD) at all time periods were in the monocyte/macrophage class and ranged from 81.8% ± 14.5% to 91.6% ± 6.8% in the 150-mg-dose group and from 69.2% ± 23.3% to 86.0% ± 10.7% in the 300-mg-dose group. AC recovery was not correlated with concentrations of cethromycin in AC for either the 150-mg-dose group (R2 = 0.007; P = 0.68) or the 300-mg-dose group (R2 = 0.008; P = 0.62). The volume (mean ± SD) of ELF recovered from the 150-mg-dose group was not significantly different among the time groups (P > 0.05) (Tables 1 and 2). The volume (mean ± SD) of ELF recovered from the 300-mg-dose group was not significantly different among the time groups (P > 0.05) (Table 2). ELF recovery was not correlated with concentrations of cethromycin in ELF for either the 150-mg-dose group (R2 = 0.02; P = 0.45) or the 300-mg-dose group (R2 = 0.06; P = 0.17).
TABLE 2.
Recovery of cells and ELF from BAL in healthy adult subjects in the 300-mg-day-dose group (n = 35)
| Value | Result at time point (h)a:
|
||||||
|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 8 | 12 | 24 | 48 | |
| Mean (cells/liter) | 1.2 × 108 | 1.4 × 108 | 1.0 × 108 | 1.0 × 108 | 0.7 × 108 | 1.1 × 108 | 0.9 × 108 |
| SD (cells/liter) | 0.6 × 108 | 1.2 × 108 | 0.5 × 108 | 0.4 × 108 | 0.4 × 108 | 0.4 × 108 | 0.4 × 108 |
| PMNs (%) | 1.0 ± 1.2 | 3.6 ± 3.8 | 1.4 ± 0.5 | 1.6 ± 0.9 | 1.6 ± 2.1 | 1.2 ± 0.4 | 1.0 ± 0.0 |
| Lymphocytes (%) | 10.0 ± 10.9 | 12.4 ± 11.8 | 10.8 ± 7.9 | 16.4 ± 11.0 | 9.2 ± 7.6 | 8.4 ± 5.9 | 10.0 ± 8.2 |
| Mono/macro (%) | 69.2 ± 23.3 | 73.0 ± 13.6 | 84.2 ± 7.7 | 74.4 ± 8.3 | 74.0 ± 16.7 | 86.0 ± 10.7 | 85.2 ± 9.8 |
| Eosinophils (%) | 0.4 ± 0.9 | 0.0 ± 0.0 | 0.8 ± 1.1 | 0.2 ± 0.4 | 2.4 ± 4.3 | 0.2 ± 0.4 | 1.6 ± 3.0 |
| Degen cells (%) | 19.4 ± 28.0 | 11.0 ± 9.2 | 2.8 ± 4.1 | 7.4 ± 6.8 | 12.4 ± 15.6 | 4.2 ± 9.4 | 2.2 ± 3.2 |
| ELF volume (ml) | 0.6 ± 0.2 | 0.5 ± 0.1 | 0.9 ± 0.6 | 0.6 ± 0.5 | 0.7 ± 0.1 | 0.5 ± 0.2 | 0.6 ± 0.2 |
No significant differences among the groups for cell recovery differential cell count, or volume of ELF (P > 0.05). Comparison testing was not applied to zero values. All data are given as means±SD. Mono/macro, monocytes and macrophages. Degen, degenerated.
Plasma.
Comparison of the plasma concentrations determined at the time of bronchoscopy indicated that drug absorption among subjects within each time and dose group was equivalent (P > 0.05 for all comparisons) (Tables 3 and 4). The plasma Cmax, Tmax values for the 150-mg-dose group (n = 25) and 300-mg-dose group (n = 35) were 0.181 ± 0.084 μg/ml, 2.01 ± 1.30 h, and 0.500 ± 0.168 μg/ml, 2.09 ± 0.03 h (Table 5, Fig. 1 and 2). The plasma t1/2 and AUC for the 150- and 300-mg-dose groups were 4.85 ± 1.10 h, 0.902 ± 0.469 μg · h/ml and 4.94 ± 0.66 h, 3.067 ± 1.205 μg · h/ml, respectively (Table 5). Cethromycin exhibited nonlinear plasma pharmacokinetics as previously reported (R. S. Pradhan, L. E. Gustavson, D. D. Londo, Y. Shang, J. Zhang, and M. Paris, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 2138, 2000). Doubling of the dose resulted in an approximate threefold increase in the Cmax and AUC during the dosing interval. The plasma t1/2s for the 150- and 300-mg-dose groups were not affected by dose and were not significantly different (P > 0.05). There was no correlation between the weights of the subjects and the trough concentrations of cethromycin at 24 h following the fourth dose for the 150-mg-dose (R2 = 0.02; P = 0.54) or the 300-mg-dose (R2 = 0.03; P = 0.31) groups. Thus, although these doses were not weight corrected, the plasma concentrations were not affected by the size of the subjects within the limits of the criteria used for enrollment.
TABLE 3.
Cethromycin concentrations in plasma, ELF, and AC at the time of bronchoscopy in the 150-mg-dose group (n = 25)a
| BAL time (h) | Plasma Cmax (μg/ml)b | Plasma concn (μg/ml)c | AC concn (μg/ml)c | ELF concn (μg/ml)c |
|---|---|---|---|---|
| 2 | 0.15 ± 0.08 | 0.12 ± 0.08 | 8.1 ± 4.9 | 0.9 ± 1.0 |
| 4 | 0.19 ± 0.09 | 0.09 ± 0.06 | 9.5 ± 2.5 | 0.9 ± 0.2 |
| 8 | 0.18 ± 0.08 | 0.04 ± 0.02 | 12.7 ± 6.4 | 0.8 ± 0.5 |
| 12 | 0.17 ± 0.08 | 0.02 ± 0.01 | 5.7 ± 4.5 | 0.3 ± 0.3 |
| 24 | 0.20 ± 0.11 | 0.01 ± 0.01 | 2.9 ± 2.4 | 0.1 ± 0.1 |
Data are given as means±SD.
There were no significant differences among the plasma Cmaxs (P > 0.05).
AC concentrations were significantly higher than ELF concentrations at 2, 4, and 8 h (P < 0.05) but not at 12 (P = 0.06) and at 24 h (P = 0.06); ELF concentrations were significantly higher than plasma concentrations at 4 (P = 0.000) and 8 h (P = 0.029) but not at 2 (P > 0.120), 12 (P = 0.059), or 24 h (P = 0.151).
TABLE 4.
Cethromycin concentrations in plasma, ELF, and AC at the time of bronchoscopy in the 300-mg-dose group (n = 35)a
| BAL time (h) | Plasma Cmax (μg/ml)b | Plasma concn (μg/ml)c | AC concn (μg/ml)c | ELF concn (μg/ml)c |
|---|---|---|---|---|
| 2 | 0.39 ± 0.09 | 0.25 ± 0.15 | 22.6 ± 12.4 | 2.5 ± 1.6 |
| 4 | 0.52 ± 0.23 | 0.38 ± 0.21 | 48.5 ± 33.0 | 2.7 ± 2.0 |
| 6 | 0.47 ± 0.13 | 0.18 ± 0.07 | 55.4 ± 38.7 | 1.6 ± 0.8 |
| 8 | 0.43 ± 0.17 | 0.09 ± 0.03 | 22.6 ± 8.9 | 0.9 ± 0.8 |
| 12 | 0.61 ± 0.21 | 0.10 ± 0.06 | 33.6 ± 17.8 | 0.8 ± 0.4 |
| 24 | 0.52 ± 0.17 | 0.01 ± 0.01 | 6.7 ± 3.4 | 0.1 ± 0.1 |
| 48 | 0.56 ± 0.14 | 0.00 ± 0.0 | 3.7 ± 4.9 | 0.0 ± 0.0 |
Data are given as means±SD.
There were no significant differences among the plasma Cmaxs (P > 0.05).
AC concentrations were significantly higher than ELF or plasma concentrations at 2, 4, 8, 12, 24, and 48 h (P < 0.05). ELF concentrations were significantly higher than plasma concentrations at 2, 6, 12, and 24 h (P < 0.05) but not at 4 h (P = 0.057), 8 h (P = 0.074), or 48 h (P = 0.69).
TABLE 5.
Plasma pharmacokinetic parameters in the 150- and 300-mg-dose groups
| Dose group | Parameter
|
||||||
|---|---|---|---|---|---|---|---|
| Cmax (μg/ml) | Tmax (h) | Cmin (μg/ml) | AUC0-24 (μg · hr/ml) | t1/2 (h) | Vz (liters)a | Vss (liters)b | |
| 150 mg | 0.181 ± 0.084 | 2.01 ± 1.30 | 0.004 ± 0.004 | 0.902 ± 0.469 | 4.85 ± 1.10 | 1,433 ± 843 | 1,453 ± 997 |
| 300 mg | 0.500 ± 0.168 | 2.09 ± 0.03 | 0.014 ± 0.008 | 3.067 ± 1.205 | 4.94 ± 0.66 | 761 ± 293 | 769 ± 272 |
Vz, volume of distribution at the terminal elimination phase.
Vss, volume of distribution at steady state.
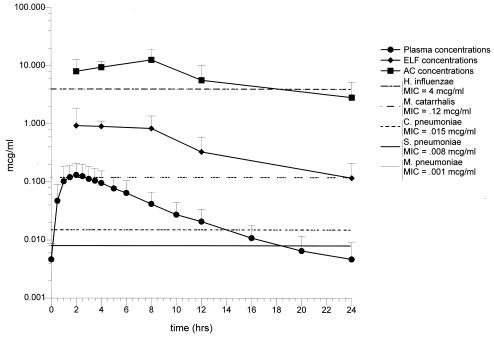
FIG. 1.
One-hundred fifty-milligram group. Mean concentrations of cethromycin in plasma, ELF, and AC at the time of bronchoscopy. Standard deviations and ranges for each time period are given in Table 3. The MIC90s for S. pneumoniae, M. pneumoniae, C. pneumoniae, H. influenzae, and M. catarrhalis are included for comparison.
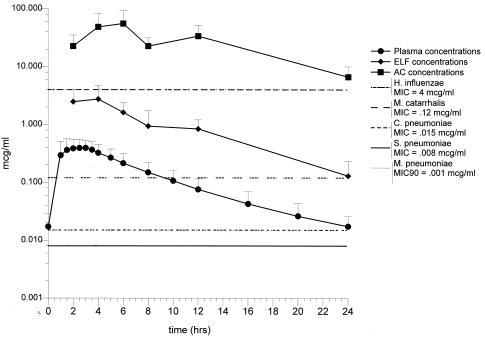
FIG. 2.
Three-hundred-milligram group. Mean concentrations of cethromycin in plasma, ELF, and AC at the time of bronchoscopy. Standard deviations and ranges for each time period are given in Table 4. The MIC90s for S. pneumoniae, M. pneumoniae, C. pneumoniae, H. influenzae, and M. catarrhalis are included for comparison.
The plasma Cmax/MIC90, AUC/MIC90 ratios and %T > MIC90 for S. pneumoniae, M. pneumoniae, C. pneumoniae, H. influenzae, and M. catarrhalis are summarized in Table 6.
ELF.
For the 24-h dosing interval, the ELF concentrations (mean ± SD) determined at the time of bronchoscopy ranged from 0.9 ± 1.0 μg/ml at 2 h (Cmax, Tmax) to 0.1 ± 0.1 μg/ml at 24 h (Cmin, Tmin) for the 150-mg-dose group and from 2.7 ± 2.0 μg/ml at 4 h (Cmax, Tmax) to 0.1 ± 0.1 μg/ml at 24 h (Cmin, Tmin) for the 300-mg-dose group (Tables 3 and 4). The t1/2 and AUC0-24 in ELF were 6.43 h and 11.4 μg · h/ml in the 150-mg-dose group and 5.26 h and 24.15 μg · h/ml in the 300-mg-dose group, respectively (Table 7). The calculated intrapulmonary drug exposure ratio for ELF was 12.6 and 7.9 for the 150- and 300-mg doses, respectively. The ELF pharmacokinetics were also nonlinear. Doubling of the dose resulted in an approximately threefold increase in the Cmax. The effect of dose was less (approximately 2.1-fold increase) on AUC. The ELF t1/2s for the 150- and 300-mg-dose groups were not affected by dose and were not significantly different (P > 0.05).
TABLE 7.
ELF and AC pharmacokinetic parameters in the 150- and 300-mg-dose groups
| Dose group | Parameter
|
||||
|---|---|---|---|---|---|
| Cmax (μg/ml) | Tmax (h) | Cmin (μg/ml) | AUC0-24 (μg · h · ml) | t1/2 (h) | |
| 150 mg | |||||
| ELF | 0.94 | 2.0 | 0.118 | 11.4 | 6.43 |
| AC | 12.7 | 8.0 | 2.88 | 160.8 | 10.0 |
| 300 mg | |||||
| ELF | 2.75 | 4.0 | 0.13 | 24.15 | 5.26 |
| AC | 55.4 | 6.0 | 6.66 | 636.2 | 11.6 |
For the 150-mg group, the mean cethromycin concentrations at each time point in ELF were above the MIC90s for M. catarrhalis, S. pneumoniae, M. pneumoniae, and C. pneumoniae for the entire 24-h dosing interval, i.e., %T > MIC was 100% (Fig. 1). None of the mean drug concentrations were above the MIC90 value for H. influenzae, i.e., the %T > MIC was 0. For the 300-mg group, the mean cethromycin concentrations at each time point in ELF were above the MIC90s for M. catarrhalis, S. pneumoniae, M. pneumoniae, and C. pneumoniae for the entire 24-h dosing interval, i.e., %T > MIC was 100% (Fig. 2). None of the mean drug concentrations in ELF were above the MIC90 value for H. influenzae, i.e., the %T > MIC was 0. The ELF Cmax/MIC90, AUC/MIC90 ratios and %T > MIC90 for S. pneumoniae, M. pneumoniae, C. pneumoniae, H. influenzae, and M. catarrhalis are summarized in Table 8.
TABLE 8.
ELF pharmacodynamic parameters
| Organism (MIC90) |
Cmax/MIC90 ratio for dose group:
|
AUC/MIC90 ratio for dose group:
|
%T > MIC90 for dose group:
|
|||
|---|---|---|---|---|---|---|
| 150 mg | 300 mg | 150 mg | 300 mg | 150 mg | 300 mg | |
| M. pneumoniaea (≤0.001 μg/ml) | 936 | 2,752 | 11,400 | 25,792 | 100 | 100 |
| S. pneumoniae (0.008 μg/ml) | 117 | 344 | 1,425 | 3,224 | 100 | 100 |
| C. pneumoniae (0.015 μg/ml) | 62 | 183 | 760 | 1,800 | 100 | 100 |
| M. catarrhalis (0.12 μg/ml) | 8 | 23 | 95 | 215 | 100 | 100 |
| H. influenzae (4 μg/ml) | 0.24 | 0.69 | 2.9 | 6 | 0 | 0 |
MIC90 of 0.001 was used for the pharmacodynamic calculations.
AC.
For the 24-h dosing interval, the mean (± SD) AC concentrations determined at the time of bronchoscopy ranged from 12.7 ± 6.4 μg/ml at 8 h (Cmax, Tmax) to 2.9 ± 2.4 μg/ml at 24 h (Cmin, Tmin) for the 150-mg-dose group and from 55.4 ± 38.7 μg/ml at 6 h (Cmax, Tmax) to 6.7 ± 3.4 μg/ml at 24 h (Cmin, Tmin) for the 300-mg-dose group (Table 3 and 4). The T1/2 and AUC0-24 in AC were 10.0 h and 160.8 μg · h/ml and 11.6 h and 636.2 μg · h/ml in the 150- and 300-mg-dose groups, respectively. The calculated intrapulmonary drug exposure ratios for AC were 178 and 705 for the 150- and 300-mg-dose groups, respectively. The effect of the nonlinear pharmacokinetics was greater in AC than in plasma or ELF. Doubling of the dose resulted in an approximately fourfold increase in the Cmax and AUC. The AC t1/2s for the 150- and 300-mg-dose groups were not affected by dose and were not significantly different (P > 0.05).
For the 150- and 300-mg groups, the mean cethromycin concentrations at each time point in AC were above the MIC90s of the drug for M. catarrhalis, S. pneumoniae, and C. pneumoniae, i.e., the %T > MIC was 100%. The pattern was similar for H. influenzae with the exception of the mean AC concentration at 24 h in the 150-mg group (Fig. 1 and 2). The AC Cmax/MIC90, AUC/MIC90 ratios and %T > MIC90 for S. pneumoniae, M. pneumoniae, C. pneumoniae, H. influenzae, and M. catarrhalis are summarized in Table 9.
TABLE 9.
AC pharmcodynamic parameters
| Organism (MIC90) | Cmax/MIC90 ratio for dose group:
|
AUC/MIC90 ratio for dose group:
|
%T > MIC90 for dose group:
|
|||
|---|---|---|---|---|---|---|
| 150 mg | 300 mg | 150 mg | 300 mg | 150 mg | 300 mg | |
| M. pneumoniaea (≤0.001 μg/ml) | 12,704 | 55,400 | 160,848 | 760,400 | 100 | 100 |
| S. pneumoniae (0.008 μg/ml) | 1,588 | 6,925 | 20,106 | 95,050 | 100 | 100 |
| C. pneumoniae (0.015 μg/ml) | 847 | 3,693 | 10,723 | 50,693 | 100 | 100 |
| M. catarrhalis (0.12 μg/ml) | 106 | 462 | 1,340 | 6,083 | 100 | 100 |
| H. influenzae (4 μg/ml) | 3.2 | 14 | 40 | 159 | 75 | 100 |
MIC90 of 0.001 was used for the pharmacodynamic calculations.
DISCUSSION
The nonlinearity of the plasma pharmacokinetics of cethromycin has previously been reported for animal models (16, 27) and for a single-dose study on healthy human subjects (R. S. Pradhan, L. E. Gustavson, D. D. Londo, Y. Shang, J. Zhang, and M. Paris, 40th ICAAC, abstr. 2138, 2000). This study confirms the nonlinearity of cethromycin kinetics and extends the observation to multiple-dose administration and to the intrapulmonary drug concentrations. Dose-related efficacy data for the treatment of community-acquired pneumonia in humans have not been reported. We would expect that the plasma and intrapulmonary drug concentrations and ratios observed in this study would be considerably greater with higher daily-dose administration. The T1/2s, Cmax values, and AUCs we observed in plasma were comparable to those previously reported (R. S. Pradhan, L. E. Gustavson, D. D. Londo, Y. Shang, J. Zhang, and M. Paris, 40th ICAAC, abstr. 2138, 2000).
The MIC90s used for the pharmacokinetic/pharmacodynamic calculations in this study were those reported in recent literature. Thus, the calculated pharmacokinetic/pharmacodynamic indices are clinically relevant and are those that are likely to be achieved in the present treatment of community-acquired respiratory infection; however, these indices apply only for those organisms whose MIC90s fall within the concentrations used in this study.
In a murine pneumococcal pneumonia model, the serum AUC/MIC ratio and the Cmax/MIC ratio were best correlated with reduction in pulmonary bacterial counts (16). Cethromycin exhibited bactericidal activity, irrespective of macrolide susceptibility. In this model, an AUC/MIC ratio of >50 or a Cmax/MIC ratio of 1 was associated with bacteriostatic effects and a twofold or greater ratio of maximized survival. In a rabbit model of pneumococcal pneumonia, in which telithromycin was studied, pulmonary bacterial clearance was correlated with time (in serum) above minimal bacterial concentrations of >33% of the dosing interval; bacteriologic failure was correlated with time (in serum) above minimal bacterial concentrations of <25% of the dosing interval (20). The importance of concentration-dependent activity of cethromycin has also been shown in a murine thigh infection model (D. R. Andes and W. A. Craig, 42nd ICAAC, abstr. 2139, 2002). These authors demonstrated that the most important correlate of efficacy was the AUC/MIC ratio (r2 = 0.88), followed by the Cmax/MIC ratio (r2 = 0.78) and the %T > MIC value (r2 = 0.64).
In this study, the high intrapulmonary Cmax/MIC and AUC/MIC ratios, high intrapulmonary drug exposure values, and prolonged %T > MIC values support a once-daily dosing regimen for the treatment of respiratory infection due to susceptible pathogens. Based on these data, it is likely that a greater daily dose than was used in this study would yield AC drug concentrations that also exceed the MIC90 for H. influenzae (see Fig. 2). Further investigation is warranted in this area. We did not directly measure intracellular antimicrobial activity in AC, and the presence of the drug does not confirm antimicrobial activity. However, intracellular cethromycin has been reported to accumulate and to retain its antimicrobial activity within polymorphonuclear cells (13). A larger dose would also yield Cmax ELF concentrations that would likely exceed the MIC90 for H. influenzae; however, the determination of the duration above MIC90 and other pharmacodynamic parameters requires further investigation. Six individuals were included at each bronchoscopy time period. The pharmacokinetic/pharmacodynamic calculations are based upon the mean data, and because of interpatient variability our conclusions may not apply to all individuals in the group.
At all time periods, plasma cethromycin concentrations were less than those observed in AC and ELF, suggesting that cethromycin was concentrated in the pulmonary compartments. The physiological basis for this differential penetration into the lungs is unknown. The high concentrations in AC suggest that this drug may also be clinically useful in the treatment of respiratory infection that is due to susceptible intracellular pathogens, such as Legionella spp. (11) or Listeria spp. (3). The demonstration of efficacy would require controlled clinical trials.
This study was not designed to measure the effect of protein binding on cethromycin concentrations or the calculated pharmacodynamics. Our assay measured total (free and protein-bound) antibiotic concentrations in plasma, ELF, and AC. The fraction of free drug in these compartments was not determined. Because the protein binding is concentration dependent and the total drug concentrations varied widely between the compartments and with time, it is likely that free drug concentrations and the pharmacodynamic ratios were less than those that we have reported and that this effect would be greatest in serum. Further investigation is warranted to determine the effect of protein binding on the pharmacodynamics of cethromycin in these compartments.
In summary, our data indicate that cethromycin in a once-daily dosing regimen is likely to be effective for the treatment of respiratory infection due to susceptible pathogens.
Acknowledgments
This work was carried out with funds provided by Abbott Pharmaceuticals and with funds provided by NIH grant no. MO1RR00079 (GCRC) at the UCSF.
We thank Qiulei Ren for assay development and analysis and Rebecca Helgeson for manuscript preparation.
REFERENCES
- 1.Anonymous. 2000. Dietary guidelines for Americans, 2000. U.S. Department of Agriculture and U.S. Department of Human Health Services, Washington, D.C.
- 2.Baldwin, D. R., R. Wise, J. M. Andrews, J. P. Ashby, and D. Honeybourne. 1990. Azithromycin concentrations at the sites of pulmonary infection. Eur. Respir. J. 3:886-890. [PubMed] [Google Scholar]
- 3.Conejo, M. C., L. Martinez-Martinez, A. Pascual, A. I. Suarez, and E. J. Perea. 2003. Activities of ABT-773 against Listeria monocytogenes and coryneform bacteria of clinical interest. Antimicrob. Agents Chemother. 47:1403-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conte, J. E., Jr., J. Golden, S. Duncan, E. McKenna, E. Lin, and E. Zurlinden. 1996. Single-dose intrapulmonary pharmacokinetics of azithromycin, clarithromycin, ciprofloxacin, and cefuroxime in volunteer subjects. Antimicrob. Agents Chemother. 40:1617-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conte, J. E., Jr., J. A. Golden, S. Duncan, E. McKenna, and E. Zurlinden. 1995. Intrapulmonary pharmacokinetics of clarithromycin and of erythromycin. Antimicrob. Agents Chemother. 39:334-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conte, J. E., Jr., J. A. Golden, J. Kipps, E. T. Lin, and E. Zurlinden. 2001. Effects of AIDS and gender on steady-state plasma and intrapulmonary ethambutol concentrations. Antimicrob. Agents Chemother. 45:2891-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conte, J. E., Jr., J. A. Golden, J. Kipps, and E. Zurlinden. 2002. Intrapulmonary pharmacokinetics of linezolid. Antimicrob. Agents Chemother. 46:1475-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conte, J. E., Jr., J. A. Golden, M. McQuitty, J. Kipps, S. Duncan, E. McKenna, and E. Zurlinden. 2002. Effects of gender, AIDS, and acetylator status on intrapulmonary concentrations of isoniazid. Antimicrob. Agents Chemother. 46:2358-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conte, J. E., Jr., J. A. Golden, M. McQuitty, J. Kipps, E. T. Lin, and E. Zurlinden. 2000. Single-dose intrapulmonary pharmacokinetics of rifapentine in normal subjects. Antimicrob. Agents Chemother. 44:985-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cynamon, M. H., J. L. Carter, and C. M. Shoen. 2000. Activity of ABT-773 against Mycobacterium avium complex in the beige mouse model. Antimicrob. Agents Chemother. 44:2895-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edelstein, P. H., F. Higa, and M. A. Edelstein. 2001. In vitro activity of ABT-773 against Legionella pneumophila, its pharmacokinetics in guinea pigs, and its use to treat guinea pigs with L. pneumophila pneumonia. Antimicrob. Agents Chemother. 45:2685-2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez-Roblas, R., J. Esteban, F. Cabria, J. C. Lopez, M. S. Jimenez, and F. Soriano. 2000. In vitro susceptibilities of rapidly growing mycobacteria to telithromycin (HMR 3647) and seven other antimicrobials. Antimicrob. Agents Chemother. 44:181-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia, I., A. Pascual, S. Ballesta, C. del Castillo, and E. J. Perea. 2003. Accumulation and activity of cethromycin (ABT-773) within human polymorphonuclear leucocytes. J. Antimicrob. Chemother. 52:24-28. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein, E. J., G. Conrads, D. M. Citron, C. V. Merriam, Y. Warren, and K. Tyrrell. 2001. In vitro activities of ABT-773, a new ketolide, against aerobic and anaerobic pathogens isolated from antral sinus puncture specimens from patients with sinusitis. Antimicrob. Agents Chemother. 45:2363-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammerschlag, M. R., T. Reznik, P. M. Roblin, J. Ramirez, J. Summersgill, and S. Bukofzer. 2003. Microbiological efficacy of ABT-773 (cethromycin) for the treatment of community-acquired pneumonia due to Chlamydia pneumoniae. J. Antimicrob. Chemother. 51:1025-1028. [DOI] [PubMed] [Google Scholar]
- 16.Kim, M. K., W. Zhou, P. R. Tessier, D. Xuan, M. Ye, C. H. Nightingale, and D. P. Nicolau. 2002. Bactericidal effect and pharmacodynamics of cethromycin (ABT-773) in a murine pneumococcal pneumonia model. Antimicrob. Agents Chemother. 46:3185-3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Low, D. E., J. de Azavedo, K. Weiss, T. Mazzulli, M. Kuhn, D. Church, K. Forward, G. Zhanel, A. Simor, and A. McGeer. 2002. Antimicrobial resistance among clinical isolates of Streptococcus pneumoniae in Canada during 2000. Antimicrob. Agents Chemother. 46:1295-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mason, E. O., Jr., L. B. Lamberth, E. R. Wald, J. S. Bradley, W. J. Barson, and S. L. Kaplan. 2003. In vitro activities of cethromycin (ABT-773), a new ketolide, against Streptococcus pneumoniae strains that are not susceptible to penicillin or macrolides. Antimicrob. Agents Chemother. 47:166-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mouton, J. W., M. N. Dudley, O. Cars, H. Derendorf, and G. L. Drusano. 2002. Standardization of pharmacokinetic/pharmacodynamic (PK/PD) terminology for anti-infective drugs. Int. J. Antimicrob. Agents 19:355-358. [DOI] [PubMed] [Google Scholar]
- 20.Piroth, L., N. Desbiolles, V. Mateo-Ponce, L. Martin, C. Lequeu, P. E. Charles, H. Portier, and P. Chavanet. 2001. HMR 3647 human-like treatment of experimental pneumonia due to penicillin-resistant and erythromycin-resistant Streptococcus pneumoniae. J. Antimicrob. Chemother. 47:33-42. [DOI] [PubMed] [Google Scholar]
- 21.Rastogi, N., K. S. Goh, M. Berchel, and A. Bryskier. 2000. In vitro activities of the ketolides telithromycin (HMR 3647) and HMR 3004 compared to those of clarithromycin against slowly growing mycobacteria at pHs 6.8 and 7.4. Antimicrob. Agents Chemother. 44:2848-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren, Q., J. E. Conte, Jr., E. Zurlinden, and E. T. Lin. 2003. A high performance liquid chromatographic-tandem mass spectrometric method for the determination of cethromycin (ABT-773) in human plasma, bronchoalveolar lavage fluid, and alveolar cells. J. Chromatogr. Sci. 41:494-499. [DOI] [PubMed] [Google Scholar]
- 23.Rennard, S. I., G. Basset, D. Lecossier, K. M. O'Donnell, P. Pinkston, P. G. Martin, and R. G. Crystal. 1986. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J. Appl. Physiol. 60:532-538. [DOI] [PubMed] [Google Scholar]
- 24.Talke, H. S., and G. E. Enzymatische. 1965. harnstoffbestimmung im blut und serum im optischem test nach warburg. Klin. Wochschr. 43:174. [DOI] [PubMed] [Google Scholar]
- 25.Waites, K. B., D. M. Crabb, and L. B. Duffy. 2003. In vitro activities of ABT-773 and other antimicrobials against human mycoplasmas. Antimicrob. Agents Chemother. 47:39-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willcox, M., A. Kervitsky, L. C. Watters, and T. E. King, Jr. 1988. Quantification of cells recovered by bronchoalveolar lavage. Comparison of cytocentrifuge preparations with the filter method. Am. Rev. Respir. Dis. 138:74-80. [DOI] [PubMed] [Google Scholar]
- 27.Xuan, D., M. Ye, M. Kim, C. H. Nightingale, and D. P. Nicolau. 2002. Pharmacokinetics of ABT-773, a new semi-synthetic ketolide in neutropenic lung-infected mice: a population approach. J. Pharm. Pharmacol. 54:71-75. [DOI] [PubMed] [Google Scholar]
- 28.Zar, J. H. 1984. Multisample hypotheses: the analysis of variance. Multiple comparisons, p. 162-205. In J. H. Zar (ed.), Biostatistical analysis. Prentice-Hall, Englewood Cliffs, N.J.
- 29.Zhanel, G. G., L. Palatnick, K. A. Nichol, T. Bellyou, D. E. Low, and D. J. Hoban. 2003. Antimicrobial resistance in respiratory tract Streptococcus pneumoniae isolates: results of the Canadian Respiratory Organism Susceptibility Study, 1997 to 2002. Antimicrob. Agents Chemother. 47:1867-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhanel, G. G., L. Palatnick, K. A. Nichol, D. E. Low, and D. J. Hoban. 2003. Antimicrobial resistance in Haemophilus influenzae and Moraxella catarrhalis respiratory tract isolates: results of the Canadian respiratory organism susceptibility study, 1997 to 2002. Antimicrob. Agents Chemother. 47:1875-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]