Abstract
Enzymes such as lactoperoxidase and glucose oxidase (GOx) are used as antimicrobial agents in oral care products. Their low specificities and substantiveness can be reduced by covalent coupling of antimicrobial molecules to antibodies. Variable domains (VHH) derived from llama heavy-chain antibodies are particularly suited for such an approach. The antibodies are composed solely of heavy-chain dimers; therefore, production of active fusion proteins by using molecular biology-based techniques is less complicated than production by use of conventional antibodies. In this study, a fusion protein consisting of VHH and GOx was constructed and expressed by Saccharomyces cerevisiae. A llama was immunized with Streptococcus mutans strain HG982. Subsequently, B lymphocytes were isolated and cDNA fragments encoding the VHH fragments were obtained by reverse transcription-PCR. After construction of a VHH library in Escherichia coli and screening of the library against mutans group streptococci and Streptococcus sanguinis strains, we found two VHH fragments with high specificities for S. mutans strains. A GOx gene was linked to the two VHH genes and cloned into S. cerevisiae yeasts. The yeasts expressed and secreted the recombinant proteins into the growth medium. The test of binding of fusion proteins to oral bacteria through their VHH fragments showed that S. mutans had been specifically targeted by GOx-S120, one of the fusion protein constructs. A low concentration of the fusion protein was also able to selectively kill S. mutans within 20 min in the presence of lactoperoxidase and potassium iodide. These findings demonstrate that the fusion protein GOx-VHH is potentially valuable in the selective killing of target bacteria such as S. mutans.
The presence of specific microorganisms in dental plaque, such as Streptococcus mutans and Streptococcus sobrinus, has been highly correlated with the development of dental decay (8, 25). Several different attempts have been made to eliminate potential oral pathogens. One approach involves the enhancement of the peroxidase system, part of the innate salivary defense system, by applying a mixture of glucose oxidase (GOx), lactoperoxidase (LPO), and iodide (I−) or thiocyanate (SCN−). GOx catalyzes the oxidation of β-d-glucose to glucono-δ-lactone, concomitantly producing hydrogen peroxide. Subsequently, in the presence of hydrogen peroxide, LPO delivers the antimicrobial agents by oxidation of iodide or thiocyanate. The bactericidal effect of this system has been shown in various in vitro studies that have tested oral bacteria. It has been shown that gram-positive bacteria, such as S. mutans and Streptococcus rattus, are more resistant than gram-negative bacteria, such as Actinobacillus actinomycetemcomitans, Fusobacterium nucleatum, and Porphyromonas gingivalis. Moreover, the antimicrobial effect of the peroxidase system is dependent on the amount of hydrogen peroxide and an oxidizable substrate, such as a halide (I− or Cl−) or a pseudohalide (SCN−) (17, 18). Furthermore, many in vivo studies in which commercial products have been tested have not confirmed the antimicrobial activity of the peroxide system either in saliva (22) or in plaque (29), nor have they confirmed the activities of the system against plaque development and gingivitis (1, 10, 31). Therefore, it seems necessary to target the antimicrobials to the pathogenic bacteria not only to avoid the killing of noncariogenic bacteria but also to increase the local antimicrobial concentration.
One of the potential solutions for targeting of the antimicrobials is construction of a fusion protein composed of an antimicrobial compound coupled to an antibody. Since the GOx gene from Aspergillus niger has been successfully cloned in the yeast Saccharomyces cerevisiae and the active protein has been expressed and secreted into the growth medium, the enzyme is a good candidate for the antimicrobial moiety of the hybrid molecule (11). In the proposed system, we have chosen the antigen-binding fragments of heavy-chain immunoglobulin G as the specifically binding fragments. Unlike the conventional immunoglobulins, these antibodies, which have been found only in the family Camelidae, are devoid of the light chains. The N-terminal variable domain of the heavy-chain antibodies, referred to as VHH (13), has an overall sequence and structure homologous to the variable domain (VH) of the heavy chain of a classical human antibody (5). Although the VHH domain comprises only one single-immunoglobulin domain with three antigen-binding loops, the specificity and affinity of heavy-chain antibodies are comparable to or higher than those of conventional antibodies (2, 12, 35). Furthermore, the variable-region fragments can easily be produced in large quantities by fermentation by S. cerevisiae (12).
In the present study we examined combinations of the specific antibodies and enzyme-induced killing. The gene of the single-chain antibodies of a llama immunized with S. mutans was coupled to the GOx gene of A. niger and cloned into the yeast S. cerevisiae. After expression and secretion of the fusion protein into the medium, the binding specificity of the protein was investigated, as was the ability of the protein to kill S. mutans.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Escherichia coli JM109 was used for DNA manipulations, and S. cerevisiae was used for expression of the proteins. For the binding specificity assay, the following oral bacteria were tested: A. actinomycetemcomitans HG683 (NCTC 9710); Actinomyces naeslundii HG401 (ATCC 12104); Bacteroides gracilis HG853 (our own clinical isolate); Campylobacter concisus HG348 (a gift from A. C. Tanner, The Forsyth Institute, Boston, Mass.); Lactobacillus acidophilus HG1112 (ATCC 4356); P. gingivalis HG184 (our own clinical isolate); Prevotella melaninogenica HG616 (our own clinical isolate); Streptococcus anginosus HG1481 (SK52); Streptococcus gordonii HG222 (our own clinical isolate); S. mutans HG723 (our own clinical isolate), HG724 (SE11; a gift from W. H. van Palenstein Helderman, World Health Organization Collaborating Centre, Nijmegen, The Netherlands), HG735 (OM7175; a gift from A. L. Coykendall, University of Connecticut Health Center, Farmington), HG748 (NCTC 10449), and HG982 (our own clinical isolate); Streptococcus oralis HG1477 (SK23); Streptococcus rattus HG693 (BHT); Streptococcus salivarius HG475 (our own clinical isolate); Streptococcus sanguinis HG1470 (SK1) and HG1472 (SK150); and S. sobrinus HG200 (our own clinical isolate) and HG718 (50B4; a gift from W. H. van Palenstein Helderman). The SK strains were obtained as a gift from M. Kilian, University of Aarhus, Aarhus, Denmark.
Strain JM109 was grown in 2× TY (16 g of Bacto tryptone, 10 g of yeast extract, and 5 g of NaCl per liter [pH 7]) medium supplemented with 1% glucose and 100 μg of ampicillin per ml. Oral bacteria were cultivated anaerobically in Todd-Hewitt broth at 37°C without agitation. For expression of recombinant proteins, S. cerevisiae was grown in selective minimal medium, which comprised 0.7% yeast nitrogen base without amino acids (291940; Becton Dickinson) and 2% glucose supplemented with 0.02 mg of histidine per ml, for 48 h at 30°C. Subsequently, the cultures were washed in phosphate-buffered saline (PBS) and diluted 10 times in YPGal medium, which comprised 1% yeast extract, 2% Bacto Peptone, and 5% galactose. After 48 h of growth, the cells were pelleted and the culture supernatants were split, placed into 1-ml vials, and stored at −20°C and used for the subsequent experiments.
Library construction and screening.
Antibody fragments were obtained essentially as described previously (12, 35). Briefly, a young adult male llama (Lama glama) was immunized with a mixture of disrupted and intact S. mutans HG982 cells. After isolation of RNA from lymphocytes and purification of the RNA, the first cDNA was synthesized. DNA fragments encoding VHH fragments and part of the long and short hinge regions were amplified by PCR with specific primers (35). Subsequently, DNA fragments with lengths of between 300 and 450 bp were purified and ligated into the E. coli phagemid vector pUR4536 or the episomal S. cerevisiae expression vector pUR4548. pUR4536 is derived from pHEN (16) and contains the lacIq gene and unique restriction sites to allow cloning of the llama VHH genes. pUR4548 is derived from pSY1 (14). The BstEII site in the leu2 gene was removed from this plasmid by PCR, and the cloning sites between the SUC2 signal sequence and the terminator were replaced in order to facilitate the cloning of the VHH gene fragments. In order to facilitate the detection of protein expression, the nucleotide sequence encoding the myc tag (EQKLISEEDLN) was introduced before the terminator by a subsequent PCR.
On transformation, individual E. coli JM109 colonies were transferred to 96-well microtiter plates containing 150 μl of 2× TY medium supplemented with 1% glucose and 100 mg of ampicillin per liter. After growth at 37°C overnight, the plates were duplicated in 2× TY medium supplemented with 100 mg of ampicillin per liter and 0.1 mM isopropyl-β-d-thiogalactopyranoside. After another overnight incubation, the cells were pelleted and the supernatant was used in an enzyme-linked immunosorbent assay (ELISA).
Individual S. cerevisiae colonies were transferred to test tubes containing selective minimal medium and were grown for 48 h at 30°C. Subsequently, the cultures were diluted 10 times in YPGal medium. After 24 and 48 h of growth, the cells were pelleted and the culture supernatant was analyzed by an ELISA. Supernatants from two clones (clones S36 and S120) were reactive against S. mutans HG982.
Construction of recombinant GOx specific for S. mutans.
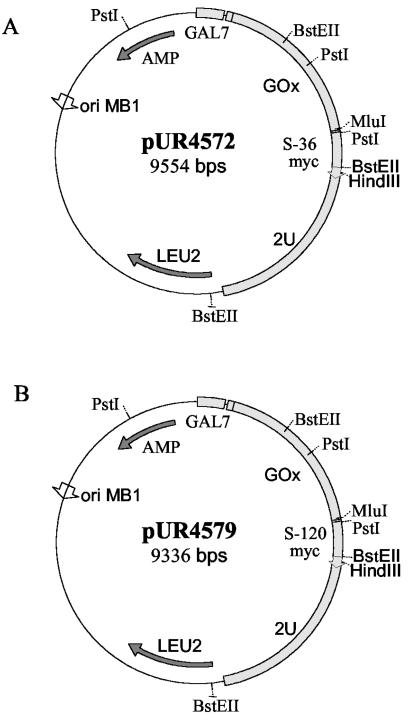
Episomal S. cerevisiae expression vectors were derived from pUR2741 (9). For the production of GOx, the GOx gene from A. niger (11) was modified. A BspHI site was introduced at the 5′ end of the gene; this allowed a fusion between the GOx gene and the GAL7 promoter. At the 3′ end, a MluI site was introduced just before the stop codon and a HindIII site was introduced just downstream of the stop codon. The modified GOx gene was cloned into the pSY1 derivative, resulting in pUR4504. For the production of GOx-VHH fusion proteins, the VHH genes preceded by a linker sequence (which encodes a GSSGGSS linker peptide) were introduced into pUR4504, downstream of the GOx gene. In this way pUR4572 (S36) and pUR4579 (S120) were obtained. The plasmids maps are presented in Fig. 1. Expression of GOx or the GOx-VHH fusion protein was performed essentially as described above.
FIG. 1.
Schematic representation of GOx-S36 (A) and GOx-S120 (B) expression vectors. Abbreviations: GAL7, promoter of the α-d-galactose-phosphate uridyl transferase; GOx, glucose oxidase gene; S-120 and S-36, genes encoding variable domains of llama heavy-chain antibodies; 2U, 2 μm yeast replication sequence; LEU2, LEU2 gene selection marker; ORI MB1, bacterial origin of replication; AMP, β-lactamase gene.
GOx assay.
GOx activity was determined with yeast culture supernatants containing GOx or GOx-VHH. One hundred microliters of a staining mixture was added to 100 μl of twofold serial dilutions of the yeast supernatants. The staining mixture was composed of 125 mM phosphate-citrate buffer (pH 4.8), 2 μg of horseradish peroxidase (P-8375; Sigma) per ml, 150 mM glucose, and 0.25 mM 3,3′,5,5′-tetramethylbenzidine (TMB; T-2885; Sigma). The reaction was stopped after 20 min by the addition of 50 μl of 1 M H2SO4. The absorbances of the TMB reaction products were determined at 450 nm.
Determination of c-myc tagged proteins.
The presence and the amount of VHH and GOx-S120 in yeast supernatants were determined by detection of the c-myc tag. The assay was a modification of the method of de Soet et al. (7). Briefly, a culture of S. mutans HG982 was prepared by inoculating Todd-Hewitt (TH) broth. After 16 h of anaerobic growth at 37°C without agitation, the culture was washed once in PBS and was resuspended to an optical density at 600 nm (OD600) of 0.7 in coating buffer containing 18 mM Na2CO3 and 32 mM NaHCO3. The bacterial suspension was added to each well of a microtiter plate (F 655001; Greiner) and incubated at 4°C for 16 h. After blocking of the wells with 4% skim milk (L31; Oxoid) for 30 min at room temperature, the plate was incubated with a serial dilution of yeast culture supernatants containing S120 or GOx-S120 in 2% skim milk for 2 h at room temperature. Monoclonal antibodies against c-myc (3800-1; Clontech) were added after a washing step and were incubated for 2 h at room temperature. Subsequently, the monoclonal antibodies were detected with secondary antibodies conjugated to horseradish peroxidase. The substrate solution contained 125 mM phosphate-citrate buffer (pH 4.8), 0.25 mM TMB, and 0.06% H2O2. The reaction was stopped after 20 min by adding 50 μl of 1 M H2SO4. The absorbances of the TMB reaction products were determined at 450 nm.
Binding specificity.
The specificities of the recombinant antibodies coupled to GOx were determined by a method similar to the one used for determination of c-myc-tagged proteins, but instead of using anti c-myc antibodies, the activity of GOx was measured as described above for the GOx assay.
Bactericidal test.
Bacterial cultures were grown anaerobically in 10 ml of TH broth at 37°C overnight. After the bacteria were washed with PBS, they were resuspended in PBS until the OD600 reached 1. One-half milliliter of the bacterial suspension was mixed with 5 μl of 10% Tween 80 and 0.5 ml of a dilution of the yeast culture supernatant in PBS. The mixtures of bacteria and growth media were incubated for 1 h at room temperature with continuous shaking. After the cells were harvested, the pellet was washed once with PBS and resuspended in 0.5 ml of PBS and then 0.5 ml of a killing mixture, which was made up of 10% glucose, 2 mM KI, and 200 μg of LPO (batch 4300123; DMV International) per ml in PBS, was added. After 20 min of incubation at room temperature, the samples were serially diluted in PBS and plated onto blood agar. The plates were incubated anaerobically at 37°C for 2 days, and the colonies were counted. Survival was calculated as a percentage of the growth of each strain treated with PBS instead of the yeast supernatant.
In order to determine the influence of the S120 and GOx moieties on bacterial survival in comparison to the antimicrobial activity of fusion protein GOx-S120, bactericidal tests were performed with the different dilutions of yeast growth media but with equimolar concentrations of S120 and active GOx. The yeast growth supernatant dilutions were chosen on the basis of determination of the GOx activity and the presence of the c-myc tag.
RESULTS
Expression and binding specificities of the fusion proteins.
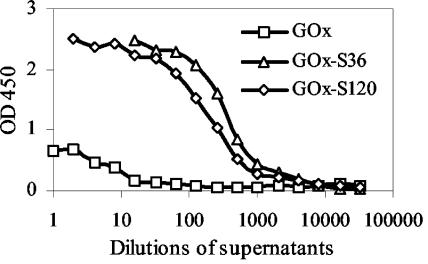
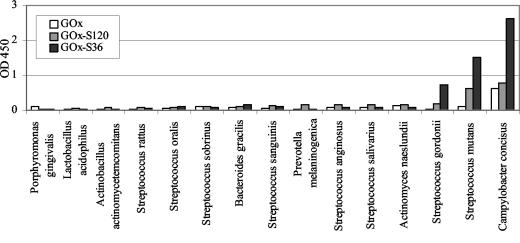
The screening of the E. coli and S. cerevisiae libraries resulted in the selection of two clones which expressed different llama VHH fragments able to bind to S. mutans and which are referred to as clones S120 and S36, respectively. Subsequently, a modified GOx gene was cloned into the episomal S. cerevisiae expression vector, followed by introduction of the S120 or S36 gene. The presence of the GAL7 promoter and the SUC2 signal sequence in the S. cerevisiae vectors enabled expression of recombinant proteins in the presence of galactose and secretion into the yeast growth medium. The levels of expression of recombinant proteins GOx-S36, GOx-S120, and GOx were checked by determining GOx activity by the GOx assay. We used a commercially available GOx enzyme (G-6891; Sigma) as a standard for that assay, in which the GOx activity was designated by the manufacturer. Yeasts carrying fusion protein vectors were able to secrete comparable amounts of GOx-S36 and GOx-S120, and the GOx activities were 4 and 2.4 U/ml, respectively. The activity of the enzyme in the yeast culture supernatant, in which S. cerevisiae carried the GOx gene only, was almost 20 times higher and was equal to 39 U/ml. This was probably due to the fact that this molecule is smaller and therefore easier to express and secrete than the larger fusion proteins. The binding specificities of the recombinant proteins were tested with S. mutans HG982, and the results are shown in Fig. 2. On the basis of the results it was concluded that GOx-S120 and GOx-S36 are specific for S. mutans, while GOx is not able to bind specifically to the bacterial cells. Figure 3 shows the binding capacities of the fusion proteins and GOx to various oral bacterial species. Among those bacteria, S. mutans and C. concisus were able to bind both of the fusions proteins. However, C. concisus also interacted with GOx alone, indicating that GOx-S36 and GOx-S120 might attach to these bacteria by its GOx moiety. In addition, S. gordonii bound GOx-S36 at a higher ratio than the other bacterial species tested.
FIG. 2.
Binding of GOx, GOx-S36, and GOx-S120 to S. mutans HG982 determined by the detection of GOx activities after incubation of bacterium-coated microtiter plates with serial dilutions of yeast growth medium containing GOx, GOx-S36, or GOx-S120.
FIG. 3.
Binding of GOx, GOx-S36, and GOx-S120 to various oral bacterial species determined by the detection of GOx activities after incubation of yeast growth medium diluted 64 times in bacterium coated microtiter plates. The bars for S. sobrinus, S. sanguinis, and S. mutans represent the averages for two S. sobrinus strains, two S. sanguinis strains, and five S. mutans strains. The other bars represent the results for one strain of each species.
Bactericidal activities of immunotoxins.
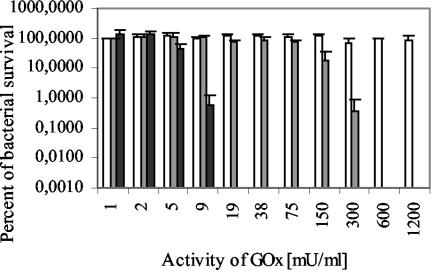
The antimicrobial activity of one of the new constructed fusion proteins was measured by detecting the minimal bactericidal concentration (MBC), defined as the lowest concentration of an antimicrobial agent causing at least a 99.9% reduction in the number of microorganisms, evaluated as the number of CFU (33). Since GOx-S120 was more specific for S. mutans than the other fusion protein, we chose to use GOx-S120 to examine its killing effects against three different bacterial strains: S. mutans HG982, S. gordonii HG222, and S. sanguinis HG1472 (Fig. 4). S. sanguinis was not affected by GOx-S120, whereas the MBC for S. mutans was 32 times lower than that for S. gordonii and was equal to the 19 mU of GOx activity per ml in the yeast supernatant containing GOx-S120.
FIG. 4.
Survival of S. mutans HG982 (black bars), S. gordonii HG222 (grey bars), and S. sanguinis HG1472 (white bars) after 1 h of incubation with yeast growth medium containing GOx-S120, followed by incubation with a killing mixture containing 10% glucose, 2 mM KI, and 200 μg of LPO per ml.
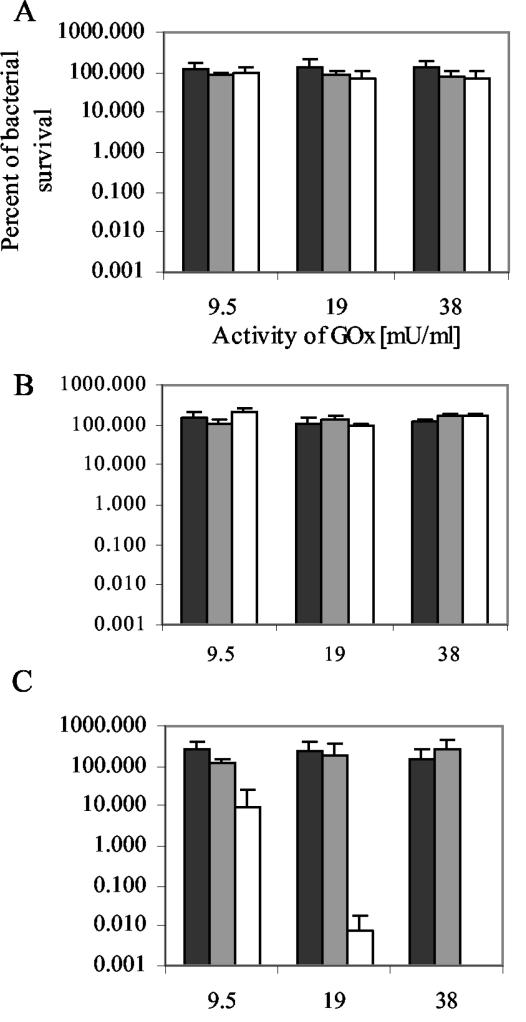
Since antibodies might reveal antimicrobial activity and GOx alone at a low concentration could influence the growth of the bacteria, we studied the effect of the yeast culture medium containing S120 or GOx alone on bacterial survival. On the basis of the estimate of the GOx activity in the yeast culture medium containing GOx alone, we selected the dilutions of the medium possessing GOx activity equal to the MBC of GOx-S120 for S. mutans. The three bacteria tested were not affected by yeast growth medium containing GOx alone with activity equal to 9.5, 19, or 38 mU/ml (Fig. 5). To estimate the bactericidal activity of S120, we chose the dilutions of the S120-containing medium which had the same amount of the c-myc tag as the dilution of the growth medium containing GOx-S120 with GOx activity of 19 mU/ml. The results showed that at about the MBC of GOx-S120, equimolar concentrations of S120 did not affect the survival of the bacteria (Fig. 5).
FIG. 5.
Survival of S. gordonii HG222 (A), S. sanguinis HG1472 (B), and S. mutans HG982 (C) after incubation with yeast supernatants containing equimolar concentrations of GOx-S120 (white bars), GOx (grey bars), and S120 (black bars), followed by incubation with the killing mixture.
DISCUSSION
Several studies have indicated that inhibition of early colonization by S. mutans inhibits the dental caries process at a later age. Dental caries can therefore be prevented by reducing the load of cariogenic bacteria. Kohler et al. (20) proved that the passage of S. mutans colonization from mother to child can be inhibited by improving oral hygiene, reducing sugar consumption, and applying chlorhexidine gel. In general, a reduction in the levels of sugar intake is thought to have the best results (36). It is doubtful the chlorhexidine has an effect in an environment in which sugar is used abundantly. A recent study has shown that chlorhexidine is unable to reduce mutans group streptococci significantly in situations with a high frequency of sucrose consumption (6, 34). It therefore seems to be important to use an agent that, on the one hand, will decrease the amount of sugar in saliva and that, on the other hand, will be involved in killing the pathogenic bacteria or at least inhibiting their growth. GOx may be a good candidate not only because it is an enzyme that catalyzes the oxidation of glucose but also because it is an H2O2 -generating system (19) and may therefore support the peroxidase system, one of the innate salivary defense systems. Moreover, because the iodide was shown to be a much more potent antimicrobial agent against periodontal and cariogenic pathogens than other halides and pseudohalides (17, 18), the combination of GOx with LPO and iodide is beneficial. Although various in vitro experiments have shown the effectiveness of the peroxidase system against pathogens, in vivo studies do not generally support these findings (22, 37). This problem may be solved by increasing the iodide level in saliva because its concentration in general is very low (13.8 ± 8.5 μM) (4), as has been suggested by Ihalin et al. (18). The concentration of hydrogen peroxide also influences the sensitivity of bacteria to the peroxidase system. Therefore, a local increase in the H2O2 level may result in selective killing of the target microorganism and protect nonpathogenic bacteria from the bactericidal effects of the peroxidase system.
In the present study a hybrid molecule consisting of GOx and the variable domain of a llama heavy-chain antibody (VHH) was constructed. Although significant progress has been made with the production of monoclonal and recombinant immunoglobulins against S. mutans (21, 26, 27), the creation of the active fusion proteins consisting of these antibodies and an antimicrobial agent is uncertain. The antibodies are based on conventional immunoglobulins; thus, the recognition of antigen requires proper folding of variable domains of light and heavy chains (12). To avoid potential difficulties, we applied the recently developed single-chain antibody technology. In the early 1990s, the novel class of immunoglobulin G antibodies was discovered in the family Camelidae (camels and llamas) (13). Because these antibodies lack light chains, their binding domains consist only of the variable domains of the heavy chains. An additional advantage is the possibility of the production of active VHH in E. coli (2) and S. cerevisiae (12). Not only can both of these organisms be easily modified genetically, but S. cerevisiae is also generally regarded as safe. Moreover, the combined findings from immunization of the llama (L. glama) with S. mutans and the determination of two heavy-chain fractions (the long hinge and the short hinge) with activities against S. mutans (35) facilitated the acquisition of DNA fragments encoding VHH, which were subsequently cloned into E. coli and S. cerevisiae. Screening of libraries enabled the detection of two clones expressing VHH against S. mutans. The subsequent joining of VHH genes to the GOx gene of A. niger (11) resulted in the formation of two hybrid molecules, GOx-S120 and GOx-S36, which were expressed by baker's yeast and secreted into the growth medium. Usually, the C terminus of single-chain Fv fragments is connected to large foreign protein domains, since joining to the N terminus resulted in the loss of binding activity (28, 32). However, using a flexible linker, we were able to isolate active fusion proteins in which the C terminus of GOx was coupled to the N terminus of VHH. Moreover, the binding specificity assay showed that both moieties maintained their biological activities.
As we were particularly interested in the delivery of GOx to S. mutans, we tested the specificities of the fusion proteins. Most of the oral bacteria examined did not interact with any of the recombinant molecules. Only S. mutans and C. concisus were bound by GOx-S120, as well as by GOx-S36. The recognition of the S. mutans antigens by VHH was not surprising, since the llamas were immunized with these bacteria. However, the binding of C. concisus by hybrid molecules was probably due to an interaction of the GOx moiety, since GOx alone was able to bind to these bacteria. A similar phenomenon of nonspecific binding of an enzyme to bacteria was previously reported (24) for galactose oxidase and S. sanguinis. Additionally, GOx-S36 recognized S. gordonii. Since the antigens for S120 and S36 are still not known, it is difficult to assess which bacterial epitopes are responsible for this cross-reaction. However, it has been shown that cross-reactions between S. mutans and S. gordonii occur with monoclonal antibodies (23, 38), rabbit anti-SR antibodies, and sera from patients with rheumatoid arthritis (30). Therefore, we suspect that the resemblance of the antigens on the surfaces of both bacteria can be responsible for the cross-reaction in the case of GOx-S36. Since the S120 moiety was not responsible for the binding to the bacteria tested, GOx-S120 is considered more specific than GOx-S36.
Bactericidal test data showed that the killing effect of GOx-S120 is dependent on the binding of the hybrid molecule to the bacteria. S. sanguinis was not affected by GOx-LPO-I−, since this species was not able to bind GOx-S120 at all. The amount of GOx-S120 required to coat S. gordonii and to finally kill the bacteria was much higher than the amount required for S. mutans. This suggests that the affinity of GOx-S120 to S. mutans is higher than that to S. gordonii. Because some antibodies have antimicrobial activities (3) and GOx alone might also contribute to bacterial death, we tested VHH and GOx separately with the MBC of GOx-S120. Neither of the molecules affected the growth of S. mutans, S. sanguinis, or S. gordonii.
So far, only a few target systems against oral bacteria have been investigated. Hill et al. (15) proposed one of them, in which GOx was encapsulated with horseradish peroxidase in liposomes. In order to target S. gordonii biofilms, Hill et al. (15) chose the optimum polyhydroxy lipid level. Although the reactive liposomes had antimicrobial effects on monoculture biofilms, it is difficult to assess whether more complex and diverse biofilms are targets for the liposomes. As another delivery system, the fusion protein composed of galactose oxidase (GAO) joined with the glucan binding domain (GBD) was suggested (24). As in our system, the bacteria were more susceptible to the hybrid molecule than to the antimicrobial agent alone. Nevertheless, all the bacteria tested were affected by GAO-GBD, and S. mutans seemed to be more resistant than S. gordonii and S. sanguinis. The application of the VHH fragment instead of the less specific GBD made it possible to kill the target bacteria by increasing the local concentration of antimicrobials: consequently, this may protect other microorganisms in the oral cavity against the activities of broad-spectrum antimicrobial agents. Of course, although we should not ignore the nonspecific binding of the hybrid molecules, the application of relatively low concentrations of the fusion protein may solve the problem.
It can be concluded that the active fusion protein composed of an antimicrobial moiety, such as GOx, and a target moiety, such as anti-S. mutans VHH antibody fragments, can be expressed and secreted into the medium by S. cerevisiae. Furthermore, the hybrid molecule specifically binds to the target bacteria and leads to a local increase in antimicrobial activity. In addition, such a construct needs to be tested with mixed biofilm cultures and in an animal model system to assess its effectiveness under more complex conditions.
Acknowledgments
We thank Arie van Nieuw Amerongen for advice and review of the manuscript, and we thank Enno Veerman (Department of Oral Biochemistry, ACTA, Amsterdam, The Netherlands) for helpful discussions.
REFERENCES
- 1.Afseth, J., and G. Rolla. 1983. Clinical experiments with a toothpaste containing amyloglucosidase and glucose oxidase. Caries Res. 17:472-475. [DOI] [PubMed] [Google Scholar]
- 2.Arbabi, G. M., A. Desmyter, L. Wyns, R. Hamers, and S. Muyldermans. 1997. Selection and identification of single domain antibody fragments from camel heavy-chain antibodies. FEBS Lett. 414:521-526. [DOI] [PubMed] [Google Scholar]
- 3.Conti, S., W. Magliani, S. Arseni, R. Frazzi, A. Salati, L. Ravanetti, and L. Polonelli. 2002. Inhibition by yeast killer toxin-like antibodies of oral streptococci adhesion to tooth surfaces in an ex vivo model. Mol. Med. 8:313-317. [PMC free article] [PubMed] [Google Scholar]
- 4.Courtois, P., A. Vanden Abbeele, N. Amrani, and M. Pourtois. 1995. Streptococcus sanguinis survival rates in the presence of lactoperoxidase-produced OSCN and OI. Med. Sci. Res. 23:195-197. [Google Scholar]
- 5.Desmyter, A., K. Decanniere, S. Muyldermans, and L. Wyns. 2001. Antigen specificity and high affinity binding provided by one single loop of a camel single-domain antibody. J. Biol. Chem. 276:26285-26290. [DOI] [PubMed] [Google Scholar]
- 6.de Soet, J. J., R. J. Gruythuysen, J. A. Bosch, and W. E. Van Amerongen. 2002. The effect of 6-monthly application of 40% chlorhexidine varnish on the microflora and dental caries incidence in a population of children in Surinam. Caries Res. 36:449-455. [DOI] [PubMed] [Google Scholar]
- 7.de Soet, J. J., P. J. van Dalen, B. J. Appelmelk, and J. de Graaff. 1987. Identification of Streptococcus sobrinus with monoclonal antibodies. J. Clin. Microbiol. 25:2285-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Soet, J. J., T. M. J. van Steenbergen, and J. de Graaff. 1992. Streptococcus sobrinus: taxonomy, virulence and pathogenicity. Alpe Adria Microbiol. 3:127-145. [Google Scholar]
- 9.Driedonks, R. A., H. Y. Toschka, J. W. van Almkerk, I. M. Schaffers, and J. M. Verbakel. 1995. Expression and secretion of antifreeze peptides in the yeast Saccharomyces cerevisiae. Yeast 11:849-864. [DOI] [PubMed] [Google Scholar]
- 10.Etemadzadeh, H., J. Ainamo, and H. Murtomaa. 1985. Plaque growth-inhibiting effects of an abrasive fluoride-chlorhexidine toothpaste and a fluoride toothpaste containing oxidative enzymes. J. Clin. Periodontol. 12:607-616. [DOI] [PubMed] [Google Scholar]
- 11.Frederick, K. R., J. Tung, R. S. Emerick, F. R. Masiarz, S. H. Chamberlain, A. Vasavada, S. Rosenberg, S. Chakraborty, L. M. Schopfer, L. M. Schopter, and V. Massey. 1990. Glucose oxidase from Aspergillus niger. Cloning, gene sequence, secretion from Saccharomyces cerevisiae and kinetic analysis of a yeast-derived enzyme. J. Biol. Chem. 265:3793-3802. [PubMed] [Google Scholar]
- 12.Frenken, L. G., R. H. van der Linden, P. W. Hermans, J. W. Bos, R. C. Ruuls, B. de Geus, and C. T. Verrips. 2000. Isolation of antigen specific llama VHH antibody fragments and their high level secretion by Saccharomyces cerevisiae. J. Biotechnol. 78:11-21. [DOI] [PubMed] [Google Scholar]
- 13.Hamers-Casterman, C., T. Atarhouch, S. Muyldermans, G. Robinson, C. Hamers, E. B. Songa, N. Bendahman, and R. Hamers. 1993. Naturally occurring antibodies devoid of light chains. Nature 363:446-448. [DOI] [PubMed] [Google Scholar]
- 14.Harmsen, M. M., A. C. Langedijk, E. van Tuinen, R. H. Geerse, H. A. Raue, and J. Maat. 1993. Effect of a pmr 1 disruption and different signal sequences on the intracellular processing and secretion of Cyamopsis tetragonoloba alpha-galactosidase by Saccharomyces cerevisiae. Gene 125:115-123. [DOI] [PubMed] [Google Scholar]
- 15.Hill, K. J., M. Kaszuba, J. E. Creeth, and M. N. Jones. 1997. Reactive liposomes encapsulating a glucose oxidase-peroxidase system with antibacterial activity. Biochim. Biophys. Acta 1326:37-46. [DOI] [PubMed] [Google Scholar]
- 16.Hoogenboom, H. R., J. C. Raus, and G. Volckaert. 1990. Cloning and expression of a chimeric antibody directed against the human transferrin receptor. J. Immunol. 144:3211-3217. [PubMed] [Google Scholar]
- 17.Ihalin, R., V. Loimaranta, M. Lenander-Lumikari, and J. Tenovuo. 1998. The effects of different (pseudo)halide substrates on peroxidase-mediated killing of Actinobacillus actinomycetemcomitans. J. Periodontal Res. 33:421-427. [DOI] [PubMed] [Google Scholar]
- 18.Ihalin, R., V. Loimaranta, M. Lenander-Lumikari, and J. Tenovuo. 2001. The sensitivity of Porphyromonas gingivalis and Fusobacterium nucleatum to different (pseudo)halide-peroxidase combinations compared with mutans streptococci. J. Med. Microbiol. 50:42-48. [DOI] [PubMed] [Google Scholar]
- 19.Klebanoff, S. J. 1982. The iron-H2O2-iodide cytotoxic system. J. Exp. Med. 156:1262-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohler, B., I. Andreen, and B. Jonsson. 1988. The earlier the colonization by mutans streptococci, the higher the caries prevalence at 4 years of age. Oral Microbiol. Immunol. 3:14-17. [DOI] [PubMed] [Google Scholar]
- 21.Kruger, C., Y. Hu, Q. Pan, H. Marcotte, A. Hultberg, D. Delwar, P. J. van Dalen, P. H. Pouwels, R. J. Leer, C. G. Kelly, C. van Dollenweerd, J. K. Ma, and L. Hammarstrom. 2002. In situ delivery of passive immunity by lactobacilli producing single-chain antibodies. Nat. Biotechnol. 20:702-706. [DOI] [PubMed] [Google Scholar]
- 22.Lenander-Lumikari, M., J. Tenovuo, and H. Mikola. 1993. Effects of a lactoperoxidase system-containing toothpaste on levels of hypothiocyanite and bacteria in saliva. Caries Res. 27:285-291. [DOI] [PubMed] [Google Scholar]
- 23.Ligtenberg, A. J., E. Walgreen-Weterings, E. C. Veerman, J. J. de Soet, J. de Graaff, and A. V. van Nieuw Amerongen. 1992. Influence of saliva on aggregation and adherence of Streptococcus gordonii HG 222. Infect. Immun. 60:3878-3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lis, M., and H. K. Kuramitsu. 1997. Galactose oxidase-glucan binding domain fusion proteins as targeting inhibitors of dental plaque bacteria. Antimicrob. Agents Chemother. 41:999-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loesche, W. J. 1986. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 50:353-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma, J. K., A. Hiatt, M. Hein, N. D. Vine, F. Wang, P. Stabila, C. van Dolleweerd, K. Mostov, and T. Lehner. 1995. Generation and assembly of secretory antibodies in plants. Science 268:716-719. [DOI] [PubMed] [Google Scholar]
- 27.Ma, J. K., T. Lehner, P. Stabila, C. I. Fux, and A. Hiatt. 1994. Assembly of monoclonal antibodies with IgG1 and IgA heavy chain domains in transgenic tobacco plants. Eur. J. Immunol. 24:131-138. [DOI] [PubMed] [Google Scholar]
- 28.Martsev, S. P., A. A. Chumanevich, A. P. Vlasov, A. P. Dubnovitsky, Y. I. Tsybovsky, S. M. Deyev, A. Cozzi, P. Arosio, and Z. I. Kravchuk. 2000. Antiferritin single-chain Fv fragment is a functional protein with properties of a partially structured state: comparison with the completely folded V(L) domain. Biochemistry 39:8047-8057. [DOI] [PubMed] [Google Scholar]
- 29.Modesto, A., K. C. Lima, and M. de Uzeda. 2000. Effects of three different infant dentifrices on biofilms and oral microorganisms. J. Clin. Pediatr. Dent. 24:237-243. [PubMed] [Google Scholar]
- 30.Moisset, A., N. Schatz, Y. Lepoivre, S. Amadio, D. Wachsmann, M. Scholler, and J. P. Klein. 1994. Conservation of salivary glycoprotein-interacting and human immunoglobulin G-cross-reactive domains of antigen I/II in oral streptococci. Infect. Immun. 62:184-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moran, J., M. Addy, and R. Newcombe. 1989. Comparison of the effect of toothpastes containing enzymes or antimicrobial compounds with a conventional fluoride toothpaste on the development of plaque and gingivitis. J. Clin. Periodontol. 16:295-299. [DOI] [PubMed] [Google Scholar]
- 32.Thompson, J., S. Stavrou, M. Weetall, J. M. Hexham, M. E. Digan, Z. Wang, J. H. Woo, Y. Yu, A. Mathias, Y. Y. Liu, S. Ma, I. Gordienko, P. Lake, and D. M. Neville, Jr. 2001. Improved binding of a bivalent single-chain immunotoxin results in increased efficacy for in vivo T-cell depletion. Protein Eng 14:1035-1041. [DOI] [PubMed] [Google Scholar]
- 33.Tossi, A., M. Scocchi, M. Zanetti, R. Gennaro, P. Storici, and D. Romeo. 1997. An approach combining rapid cDNA amplification and chemical synthesis for the identification of novel, cathelicidin-derived, antimicrobial peptides. Methods Mol. Biol. 78:133-150. [DOI] [PubMed] [Google Scholar]
- 34.van der Hoeven, J. S., and M. J. Schaeken. 1995. Streptococci and actinomyces inhibit regrowth of Streptococcus mutans on gnotobiotic rat molar teeth after chlorhexidine varnish treatment. Caries Res. 29:159-162. [DOI] [PubMed] [Google Scholar]
- 35.van der Linden, R. H., B. de Geus, W. Stok, W. Bos, D. van Wassenaar, T. Verrips, and L. Frenken. 2000. Induction of immune responses and molecular cloning of the heavy chain antibody repertoire of Lama glama. J. Immunol. Methods 240:185-195. [DOI] [PubMed] [Google Scholar]
- 36.van Palenstein Helderman, W. H., M. I. Matee, J. S. van der Hoeven, and F. H. Mikx. 1996. Cariogenicity depends more on diet than the prevailing mutans streptococcal species. J. Dent. Res. 75:535-545. [DOI] [PubMed] [Google Scholar]
- 37.van Steenberghe, D., E. E. Van den, R. Jacobs, and M. Quirynen. 1994. Effect of a lactoperoxidase containing toothpaste in radiation-induced xerostomia. Int. Dent. J. 44:133-138. [PubMed] [Google Scholar]
- 38.Wyatt, J. E., M. D. Willcox, R. R. Russell, and P. S. Handley. 1988. Fibrillar strains of Streptococcus sanguis biotype I carry a surface protein which cross-reacts with antigen B from Streptococcus mutans Ingbritt. Oral Microbiol. Immunol. 3:162-168. [DOI] [PubMed] [Google Scholar]