Abstract
Bacterial keratitis is a serious infectious ocular disease requiring prompt treatment to prevent frequent and severe visual disabilities. Standard treatment of bacterial keratitis includes topical administration of concentrated antibiotic solutions repeated at frequent intervals in order to reach sufficiently high drug levels in the corneal tissue to inhibit bacterial growth. However, this regimen has been associated with toxicity to the corneal epithelium and requires patient hospitalization. In the present study, a mucoadhesive polymer extracted from tamarind seeds was used for ocular delivery of 0.3% rufloxacin in the treatment of experimental Pseudomonas aeruginosa and Staphylococcus aureus keratitis in rabbits. The polysaccharide significantly increased the intra-aqueous penetration of rufloxacin in both infected and uninfected eyes. Rufloxacin delivered by the polysaccharide reduced P. aeruginosa and S. aureus in the cornea at a higher rate than that obtained by rufloxacin alone. In particular, use of the polysaccharide allowed a substantial reduction of S. aureus in the cornea to be achieved even when the time interval between drug administrations was extended. These results suggest that the tamarind seed polysaccharide prolongs the precorneal residence times of antibiotics and enhances drug accumulation in the cornea, probably by reducing the washout of topically administered drugs. The tamarind seed polysaccharide appears to be a promising candidate as a vehicle for the topical treatment of bacterial keratitis.
The eye is a unique organ that is virtually impermeable to most environmental agents. Continuous tear flow, aided by the blink reflex, mechanically washes substances from the ocular surface and prevents the accumulation of microorganisms. In addition, lysozyme, lactoferrin, secretory immunoglobulins, and defensins are present at high levels in tears and can specifically reduce bacterial colonization of the ocular surface (16, 23).
Since most pathogens cannot penetrate the intact corneal layer, corneal infections derive essentially from a failure of the protective mechanisms that maintain ocular surface integrity. Defects in the tear film, chemical or foreign body trauma, allergic hypersensitivity reactions, and overuse of contact lenses, as well as complications after laser in situ keratomileusis, can result in injury to the ocular surface and predispose the cornea to infection (14, 21).
Because of its high incidence and potential complications, bacterial keratitis is one of the most threatening ocular infections. Pseudomonas aeruginosa and Staphylococcus aureus frequently cause severe keratitis that may lead to progressive destruction of the corneal epithelium and stroma (2, 3). Infectious keratitis due to these organisms often causes corneal scarring, corneal perforation, and blindness if aggressive and appropriate therapy is not promptly initiated (7, 18).
Successful therapy of bacterial keratitis must be able to rapidly attain high drug concentrations at the site of infection. Since the cornea is not vascularized, it is not readily permeated by systemically administered drugs, which are therefore generally not used for the treatment of keratitis (7). On the other hand, topical treatment may fail to achieve therapeutically active drug levels in the cornea, as continuous tear flow reduces the bioavailability of topically applied antibiotics and the corneal epithelium acts as a barrier against drug penetration. For this reason, standard treatment of severe bacterial keratitis requires administration at frequent intervals (every 15 to 60 min for 48 to 72 h) of eyedrops often containing fortified (more concentrated than commercially available solutions) solutions of fluoroquinolones or multiple antibiotics, usually a cephalosporin and an aminoglycoside (7, 13, 28, 29). However, this regimen not only is disruptive to the patient and usually necessitates hospitalization, but it has also been associated with in vitro toxicity to the corneal epithelium (9, 22). Efforts are now directed to testing new antimicrobials that better permeate the cornea and to developing systems capable of prolonging the contact time between antibiotics and the corneal tissue, thereby potentially enhancing intracorneal delivery of ophthalmic medicaments.
A mucoadhesive polymer extracted from tamarind seeds (xyloglucan, or tamarind seed polysaccharide [TSP]) has been described as a viscosity enhancer showing mucomimetic, mucoadhesive, and bioadhesive activities (M. F. Saettone, S. Burgalassi, E. Boldrini, P. Bianchini, and G. Luciani, 1997, international patent application PCT/IT97/00026). Several features make TSP an attractive candidate as a vehicle for ophthalmic medicaments, since it (i) is completely devoid of ocular toxicity (4; Saettone et al., patent application); (ii) has recently been put on the market (TSP; Farmigea S.p.A., Pisa, Italy) as a tear fluid substitute because of its activity in preventing alterations of the corneal surface known as keratoconjunctivitis sicca (4); (iii) increases the corneal-wound healing rate (5); (vi) reduces the in vitro toxicity exerted by timolol, methiolate, and fluoroquinolones on human conjunctival cells (27); and (v) significantly increases the corneal accumulation and intraocular penetration of gentamicin and ofloxacin when administered topically to healthy rabbits (15).
In this study, we used TSP for ocular delivery of rufloxacin, a broad-spectrum monofluorinated quinolone that has never been tested for its effectiveness in the topical treatment of bacterial keratitis. Rufloxacin is administered orally and displays a long half-life in plasma (28 to 30 h), consistently high bactericidal concentrations at the site of infection, and good penetration into infected tissues and cerebrospinal fluid (24, 31). To evaluate the intraocular penetration and therapeutic benefit of rufloxacin, whether delivered by TSP or not, an experimental model of P. aeruginosa and S. aureus keratitis in rabbits was developed; the drug efficacy was then compared with that of ofloxacin, which is commonly used for the topical therapy of this ocular pathology (13, 26, 29).
MATERIALS AND METHODS
Strains used and preparation of inocula.
P. aeruginosa (n = 10) and S. aureus (n = 10) clinical isolates, recovered from patients with ocular infections, were identified using the Vitek2 system (BioMeriéux, Paris, France). The strains were used to calculate the MICs of rufloxacin and ofloxacin, which were compared to those obtained with P. aeruginosa ATCC 27853 and S. aureus ATCC 29213. The last two strains were used to induce experimental bacterial keratitis in rabbits. To this end, inocula for each organism were prepared by propagating cultures in tryptic soy broth (Difco Laboratories, Detroit, Mich.) at 37°C for 5 h. Bacteria were suspended in phosphate-buffered saline (PBS; pH 7.4) to a concentration of ∼1.5 × 104 CFU/ml. Retrospective quantitation of inocula was done by plating triplicate serial 10-fold dilutions on tryptic soy agar (Difco Laboratories). Bacillus subtilis ATCC 6633 was used as the indicator organism for determining rufloxacin and ofloxacin concentrations in biological samples by an agar diffusion assay (15). B. subtilis spores were prepared in 0.1 M PBS, pH 8.0, and stored at 4°C as sterile suspensions.
Drug formulations.
Sterile water solutions of rufloxacin (Mediolanum Farmaceutici, Milan, Italy) or ofloxacin (Sigma, St. Louis, Mo.) contained mannitol (50 mg/ml; Sigma), benzalkonium chloride (0.05 mg/ml; A.C.E.F., Milan, Italy), and rufloxacin or ofloxacin (3 mg/ml). The viscous preparation of rufloxacin (TSP-rufloxacin) was obtained by adding 10 mg of TSP (Farmigea S.p.A.)/ml to the rufloxacin-water solution. A 10-mg/ml TSP solution containing the excipients (50 mg of mannitol/ml and 0.05 mg of benzalkonium chloride/ml) was used as a control.
Experimental keratitis and therapeutic regimen.
Male New Zealand rabbits (body weight, 2.3 to 2.7 kg), free from preexisting corneal defects, were anesthetized by intramuscular injection of 35 mg of ketamine-HCl per kg of body weight and by topical application in each eye of 0.4% oxibuprocaine HCl (Novesina; Mipharm, Milan, Italy). Bacterial suspensions (20 μl) were injected intrastromally in both eyes via a 30-gauge needle attached to a 1.0-ml syringe. Three animals were injected with 20 μl of sterile PBS in one eye (surgical control), while the contralateral eye was left undisturbed (absolute control). Eye disease was evaluated by slit lamp biomicroscopy, and inflammation was scored as described by Johnson et al. (19). Briefly, conjunctival injection, conjunctival chemosis, iritis, fibrin in the anterior chamber, hypopyon, stromal infiltrate, and stromal edema were scored on a scale from 0 (absent) to 4 (severe). For each eye, individual parameter scores were summed to give a total slit lamp examination (SLE) score ranging from 0 (normal eye) to 28 (maximally inflamed eye). Infected animals were assigned randomly to the treatment groups or left untreated (five rabbits in each group). At 16 h postinfection, rabbits included in the treatment groups were subjected to instillation of 50-μl drops of rufloxacin, TSP-rufloxacin, ofloxacin, or TSP solution in the lower conjunctival sacs of both eyes. Animals infected with P. aeruginosa received one drop in the eye every 30 min for 6 h (treatment A). Three different treatment schedules were followed for rabbits infected with S. aureus: administration of one drop in the eye every 30 min for 6 h (treatment A), every 90 min for 6 h (treatment B), or every 90 min for 3 h (treatment C). To evaluate antibiotic penetration in the aqueous humors of uninfected eyes, groups of five healthy rabbits were subjected to instillation of rufloxacin, TSP-rufloxacin, or ofloxacin in both eyes following treatment A. At fixed time intervals after the last administration (1 h after treatment A; 2 h after treatment B or C), the infected and uninfected animals were killed by injecting an overdose of ethyl urethane into the marginal ear vein. Aqueous humor was recovered from the anterior chamber by paracentesis. The eyes were rapidly enucleated, trimmed of all adventitial tissue, and rinsed with PBS. The corneas were dissected away, weighed, suspended in PBS (25 mg of tissue/ml), and homogenized with 1.0-mm-diameter glass beads in a Mini-BeadBeater (Biospec Products, Bartesville, Okla.) at 5,000 rpm for 30 s. The study was approved by the Ethics Committee of Pisa University Hospital.
Microbiological determinations.
The MICs of rufloxacin, TSP-rufloxacin, and ofloxacin against P. aeruginosa and S. aureus strains were determined by the microdiluton technique (25) with microtiter plates. In vitro susceptibility testing was performed five times for each strain, and modal MICs for each strain were calculated. Bacterial strains were also assayed for susceptibility to the TSP solution.
To determine rufloxacin and ofloxacin levels in aqueous humor samples, B. subtilis spores were incorporated into molten Antibiotic Medium No. 2 (Difco), and the assay was performed as described previously (15). Calibration curves were constructed by adding from 0.1 to 100 μg of rufloxacin or ofloxacin/ml to pooled aqueous humor samples taken from untreated healthy rabbits. The assays were shown to be linear in the range of 1.6 to 15 μg/ml for rufloxacin and 0.2 to 10 μg/ml for ofloxacin. Each determination was performed in triplicate.
Bacterial CFU were quantified in corneal homogenates by plating triplicate serial 10-fold dilutions on tryptic soy agar and incubating them at 37°C for 18 h.
Statistical analysis.
All values were expressed as the mean ± standard error of the mean. Statistical analysis was performed using the two-tailed Student t test. A P value of ≤0.05 was considered significant.
RESULTS
Antibacterial activity in vitro.
Ofloxacin and rufloxacin were found to be similarly active against the S. aureus strains analyzed, showing a MIC range of 0.125 to 4 and 0.5 to 4 μg/ml, respectively. In contrast, ofloxacin was more active than rufloxacin against P. aeruginosa in vitro, with respective MIC ranges of 1 to 4 and 4 to 32 μg/ml. Modal MICs were 2 μg of rufloxacin or ofloxacin per ml for S. aureus ATCC 29213 and 8 μg of rufloxacin and 2 μg of ofloxacin per ml for P. aeruginosa ATCC 27853. Identical MICs of rufloxacin and TSP-rufloxacin were obtained against all microorganisms tested, indicating that the drug maintains its activity in vitro when made viscous with TSP. The TSP solution exerted no inhibitory activity against any of the organisms tested.
Bacterial keratitis in rabbits.
Experimental infections were initiated by injection of the following inocula (log10): P. aeruginosa, 2.51 ± 0.06; S. aureus, 2.49 ± 0.04. Sham-injected and uninjected control eyes did not develop appreciable signs of inflammation. S. aureus-infected eyes showed moderate to severe chemosis and iritis, mild conjunctival injection, and trace amounts of fibrin in the anterior chamber (SLE score, 11.32 ± 0.60) 16 h postinfection. At a later stage (23 h postinfection), the eyes showed moderate conjunctival injection, mild stromal edema, severe chemosis and iritis, and moderate to severe fibrin accumulation in the anterior chamber (SLE score, 15.07 ± 0.85). Experimental P. aeruginosa keratitis appeared to evolve more slowly than that induced by S. aureus. Essentially, mild to moderate chemosis and iritis and trace to moderate conjunctival injection and stromal edema were observed at 16 (SLE score, 7.43 ± 0.24) and 23 h (SLE score, 9.15 ± 0.97) postinfection.
Ocular penetration of antibiotics.
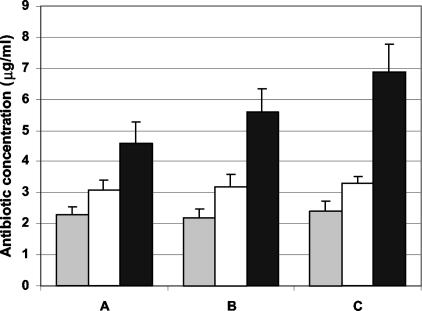
Antibiotic concentrations were measured in the aqueous humors taken from both uninfected and infected eyes following treatment A (Fig. 1). When administered alone, rufloxacin tended to permeate the cornea better than ofloxacin; the average rufloxacin concentration in the aqueous humor was significantly higher than that of ofloxacin in uninfected (3.14 ± 0.32 versus 2.31 ± 0.26 μg/ml), P. aeruginosa-infected (3.25 ± 0.40 versus 2.24 ± 0.26 μg/ml), and S. aureus-infected (3.33 ± 0.21 versus 2.68 ± 0.33 μg/ml) eyes (P < 0.05). Administration of TSP-rufloxacin gave rise to intraocular drug levels significantly higher than those obtained with rufloxacin alone, as shown by values recorded in uninfected (4.62 ± 0.68 μg/ml; P < 0.01), P. aeruginosa-infected (5.63 ± 0.73 μg/ml; P < 0.01), and S. aureus-infected (6.91 ± 0.89 μg/ml; P < 0.001) eyes. In addition, it was considered of some relevance that, while comparable rufloxacin or ofloxacin levels were observed in the aqueous humors of infected and uninfected eyes treated with the drugs alone, a significantly higher concentration of rufloxacin was found in the aqueous humors of infected versus uninfected eyes when rufloxacin was administered in association with TSP (uninfected versus P. aeruginosa-infected eyes, P < 0.05; uninfected versus S. aureus-infected eyes, P < 0.01).
FIG. 1.
Antibiotic concentrations in aqueous humor samples following topical administration of 50 μl of ofloxacin (shaded bars), rufloxacin (open bars), and TSP-rufloxacin (solid bars) every 30 min for 6 h in uninfected (A), P. aeruginosa-infected (B), and S. aureus-infected (C) rabbit eyes. The error bars indicate standard errors of the mean.
Therapeutic treatment of P. aeruginosa keratitis.
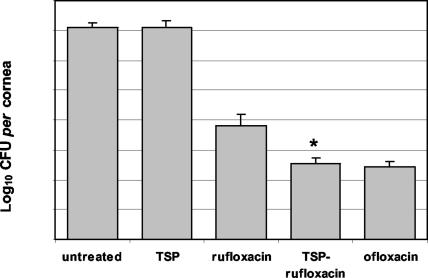
Based upon its in vitro activity, rufloxacin is regarded as an inappropriate antibiotic in the treatment of infections by some frequently encountered species of Enterobacteriaceae and P. aeruginosa (30). In our study, the MIC of rufloxacin (8 μg/ml) was considerably higher than that of ofloxacin (2 μg/ml) for P. aeruginosa ATCC 27853. The poor rufloxacin activity against P. aeruginosa was exploited to evaluate whether TSP would improve its therapeutic efficacy in the experimental model of P. aeruginosa keratitis. Animals infected with P. aeruginosa were subjected to ocular instillation of rufloxacin, TSP-rufloxacin, ofloxacin, or TSP solution following treatment A, and their corneas were removed 23 h after infection. At this time, the SLE scores for the eyes treated with rufloxacin (3.28 ± 0.66), TSP-rufloxacin (2.91 ± 0.48), or ofloxacin (3.11 ± 0.75) were statistically lower than the scores for the P. aeruginosa-infected eyes that did not receive any treatment (P < 0.05) or that were treated with the TSP solution (8.72 ± 0.84) (P < 0.05). The untreated eyes harbored an average level of 1.3 × 107 CFU of P. aeruginosa in the cornea. A similar bacterial burden was recorded in corneas taken from animals treated with the TSP solution (1.5 × 107 ± 0.8 × 107 CFU/cornea) (Fig. 2), indicating that the polysaccharide exerted no antibacterial activity in vivo. All the antibiotic-treated eyes exhibited a significant decrease in the corneal P. aeruginosa burden compared to the untreated eyes (Fig. 2). Administration of rufloxacin, TSP-rufloxacin, and ofloxacin produced a significant reduction in corneal bacteria compared to that in untreated eyes (rufloxacin versus control, P < 0.01; TSP-rufloxacin and ofloxacin versus control, P < 0.001). TSP-rufloxacin was significantly more active than rufloxacin in controlling bacterial proliferation in the cornea (3.4 × 102 ± 2.2 × 102 versus 6.3 × 103 ± 9.4 × 103 CFU/cornea; P < 0.05). In contrast, no relevant difference was detected between treatments with TSP-rufloxacin and ofloxacin (3.4 × 102 ± 2.2 × 102 versus 2.7 × 102 ± 1.4 × 102 CFU/cornea; P > 0.05). Therefore, administration of rufloxacin in association with TSP allows an intracorneal drug concentration to be achieved that is sufficiently high to inhibit P. aeruginosa proliferation as effectively as ofloxacin, even though the MIC of rufloxacin against P. aeruginosa ATCC 27853 is four times higher than that of ofloxacin.
FIG. 2.
Quantification of P. aeruginosa in the cornea following administration to infected eyes of 50 μl of TSP solution, rufloxacin, TSP-rufloxacin, and ofloxacin every 30 min for 6 h. Untreated control eyes did not receive any treatment. The error bars indicate standard errors of the mean. *, P < 0.05.
Therapeutic treatment of S. aureus keratitis.
The comparable in vitro activities of rufloxacin and ofloxacin against S. aureus and the high frequency and severity of corneal infections due to the organism (2, 7) prompted us to examine whether TSP could improve the therapeutic efficacy of rufloxacin in an experimental model of S. aureus keratitis. At 16 h postinfection, S. aureus-infected eyes were subjected to instillation of rufloxacin, TSP-rufloxacin, ofloxacin, or TSP solution according to the treatment schedules A, B, and C. The animals were sacrificed, and their corneas were excised at 21 (treatment C), 23 (treatment A), and 24 h (treatment B). At these times, no difference between the SLE scores for untreated and TSP-treated eyes (mean values ranging from 13.5 to 15.7) was recorded. In contrast, at the end of treatment A, there was a dramatic improvement in the clinical features of animals treated with rufloxacin (SLE score, 4.03 ± 0.43), TSP-rufloxacin (SLE score, 3.26 ± 0.24), or ofloxacin (SLE score, 4.51 ± 0.30) compared to untreated eyes (P < 0.01). Good improvement was also obtained following treatment B with rufloxacin (SLE score, 6.97 ± 0.60), TSP-rufloxacin (SLE score, 5.44 ± 0.24), and ofloxacin (SLE score, 7.52 ± 0.49) (P < 0.05). However, at the end of treatment C, only eyes treated with TSP-rufloxacin (SLE score, 7.42 ± 0.63) showed a significant reduction in the clinical signs of infection (P < 0.05).
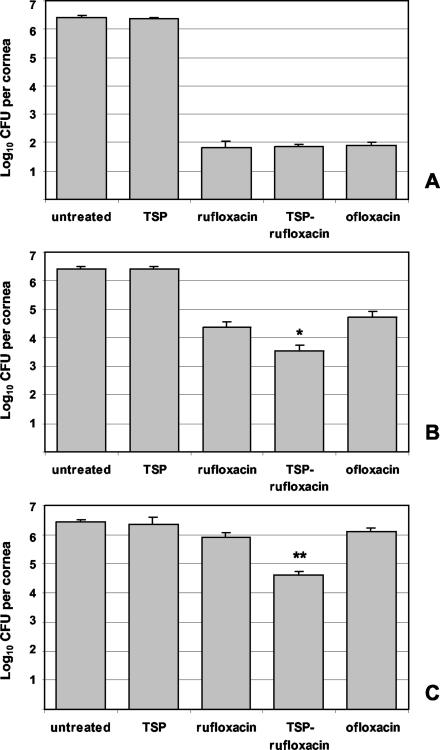
Untreated and TSP-treated animals harbored comparable average levels of S. aureus in the cornea at 21, 23, and 24 h postinfection (values ranging from 2.3 × 106 to 3.1 × 106 CFU/cornea). Following treatment A or B, administration of rufloxacin, TSP-rufloxacin, and ofloxacin produced significant reductions in corneal bacteria compared to untreated eyes (treatment A, P < 0.001; treatment B, P < 0.01) (Fig. 3A and B). However, while no difference among the therapeutic efficacies of the three compounds was observed following treatment A, administration of TSP-rufloxacin produced a significant reduction in corneal bacteria (3.5 × 103 ± 2.0 × 103 CFU/cornea) compared to both rufloxacin-treated (2.3 × 104 ± 1.3 × 104 CFU/cornea) and ofloxacin-treated (5.2 × 104 ± 3.1 × 104 CFU/cornea) eyes following treatment B (P < 0.05) (Fig. 3B). Even more evident was the difference in efficacy of TSP-rufloxacin compared with rufloxacin and ofloxacin after the end of treatment C (Fig. 3C). Indeed, while eyes treated with rufloxacin (8.1 × 105 ± 3.5 × 105 CFU/cornea) and ofloxacin (1.3 × 106 ± 0.4 × 106 CFU/cornea) displayed no appreciable reduction in corneal bacteria compared to untreated eyes, a significant reduction was obtained following administration of TSP-rufloxacin (4.1 × 104 ± 1.5 × 104 CFU/cornea) (P < 0.01). These results indicate that all the antibiotic formulations can reach high drug concentrations at the site of infection and efficiently control bacterial growth when administered at very frequent time intervals and for a prolonged period of time (i.e., treatment A, every 30 min for 6 h). In contrast, with longer time intervals between treatments (i.e., treatment B, every 90 min for 6 h) and less prolonged treatments (i.e., treatment C, every 90 min for 3 h), the use of TSP allows a more efficacious antibacterial effect to be obtained, possibly improving the precorneal residence time of the antibiotic and enhancing drug accumulation in the cornea.
FIG. 3.
Quantification of S. aureus in the cornea following administration to infected eyes of 50 μl of TSP solution, rufloxacin, TSP-rufloxacin, and ofloxacin every 30 min for 6 h (A), every 90 min for 6 h (B), and every 90 min for 3 h (C). Untreated control eyes did not receive any treatment. The error bars indicate standard errors of the mean. *, P < 0.05; **, P < 0.01.
DISCUSSION
Successful treatment of bacterial keratitis requires multiple administrations and/or fortified solutions of antibacterial agents to maintain drug concentrations in the corneal tissue high enough and for a sufficient period of time to have a useful antibacterial effect. For this reason, the interest in both new antibiotics, able to reach high concentrations in the cornea, and ocular drug delivery systems has grown over recent years.
Fluoroquinolones (particularly ciprofloxacin and ofloxacin) have become widely used as antibacterial agents in the treatment of ocular infections, with topical, intravitreal, and systemic routes of administration being used. Therapeutic concentrations are achieved in the cornea after topical administration, so that fluoroquinolones have largely replaced combination therapy for treatment of bacterial keratitis (26, 29). Compared to ciprofloxacin, however, ofloxacin reaches higher concentrations in the cornea and aqueous humor and is considered the primary choice for treatment of this ocular infection (1, 12). In the present study, assessment of the intraocular penetration of rufloxacin following topical administration of a 0.3% solution demonstrated that the drug reaches significantly higher levels in the aqueous humor than ofloxacin in both infected and uninfected eyes. This finding suggests that rufloxacin can be at least as efficacious as ofloxacin in the topical therapy of ocular infections caused by bacteria that are similarly susceptible to the two drugs.
Several systems have been developed for ocular delivery of antimicrobials (6, 8, 10, 11). Among these, bioadhesive polymers, usually consisting of macromolecular hydrocolloids with numerous hydrophilic functional groups, are known to adhere to the precorneal mucin layer via noncovalent bonds. Consequently, they rely on mucoadhesion as the primary mechanism for prolonging the residence of ocular dosage forms in the precorneal area (10). TSP is a high-molecular-weight, nonionic, neutral, branched polysaccharide consisting of a cellulose-like backbone that carries xylose and galactoxylose substituents (Saettone et al., patent application), chemical residues similar to those of mucin MUC-1 and episialin (17). Being similar to mucins, TSP is able to bind to the cell surface and intensify the contact between drugs and the adsorbing biological membrane (5). As previously reported for ocular delivery of ofloxacin and gentamicin (15), this study demonstrates that TSP enhances transcorneal disposition and intra-aqueous penetration of rufloxacin in healthy rabbits when administered topically in a drop regimen. The increased aqueous concentration of rufloxacin due to TSP could be exploited for future treatment of intraocular microbial infections. It is worth pointing out, in this context, that the antibiotic levels we obtained in uninfected eyes approached the MIC at which 90% of isolates are inhibited (MIC90) for most gram-negative bacteria and were greater than the MIC90s of Staphylococcus epidermidis and S. aureus (30), the most common organisms in postoperative endophthalmitis. Another relevant finding was that administration of TSP-rufloxacin to eyes intracorneally infected with P. aeruginosa or S. aureus produced significantly higher aqueous drug concentrations than those obtained in uninfected eyes subjected to the same treatment, although the integrity of the corneal epithelial surface appeared to be conserved. This result may be explained by the fact that bacterial components, such as lipopolysaccharide and lipoteichoic acid, activate mucin production by epithelial cells (20) and can therefore increase the availability of substrates capable of adhering to TSP. A greater amount of mucins may have led to prolonged residence of TSP-rufloxacin on the corneal tissue, allowing increased penetration of rufloxacin into the aqueous humor.
The animal keratitis model developed in this study was reproducible in terms of the time course of pathological ocular changes and bacterial growth in vivo. Rufloxacin and ofloxacin significantly decreased P. aeruginosa- or S. aureus-induced ocular inflammation, indicating that neither of the drugs was toxic to rabbit eyes. The two drugs appeared to be similarly efficacious in the treatment of S. aureus keratitis, with bacterial growth in the cornea controlled at comparable levels. In contrast, as expected from the higher MIC of rufloxacin against the P. aeruginosa strain used for the experimental infection, rufloxacin was less active than ofloxacin in therapeutic studies of P. aeruginosa keratitis.
TSP-delivered rufloxacin reduced P. aeruginosa and S. aureus in the cornea at a higher rate than that achieved by rufloxacin alone. Use of TSP rendered the 0.3% rufloxacin solution as effective as ofloxacin in reducing P. aeruginosa in the cornea and more active than rufloxacin and ofloxacin in control of S. aureus keratitis when the time interval between two consecutive drug administrations was extended. These results indicate that rufloxacin bioavailability in the cornea is increased by TSP which, as a viscosity enhancer (Saettone et al., patent application), can prolong preocular retention time compared to the reference solution. It may therefore be assumed that even scantily active antibiotics, when delivered by the polysaccharide, can reach adequate intracorneal concentrations and be effective in controlling bacterial replication in the cornea.
In conclusion, we suggest that TSP can be considered a promising vehicle for topical ocular administration of antibiotics. Its application could possibly replace the use of fortified solutions of antimicrobials and reduce the necessity for repeated drug administration at frequent intervals, thereby potentially lowering corneal toxicity and increasing patient compliance.
Acknowledgments
This work was supported by University of Pisa grants (2001 to 2003) and by Farmigea S.p.A., Pisa, Italy.
REFERENCES
- 1.Akkan, A. G., I. Mutlu, S. Ozyazgan, A. Gok, U. Yigit, Z. Ozuner, V. Senses, and H. Pekel. 1997. Penetration of topically applied ciprofloxacin, norfloxacin and ofloxacin into the aqueous humor of the uninflamed human eye. J. Chemother. 9:257-262. [DOI] [PubMed] [Google Scholar]
- 2.Alexandrakis, G., E. C. Alfonso, and D. Miller. 2000. Shifting trends in bacterial keratitis in south Florida and emerging resistance to fluoroquinolones. Ophthalmology 107:1497-1502. [DOI] [PubMed] [Google Scholar]
- 3.Bourcier, T., F. Thomas, V. Borderie, C. Chaumeil, and L. Laroche. 2003. Bacterial keratitis: predisposing factors, clinical and microbiological review of 300 cases. Br. J. Ophthalmol. 87:234-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burgalassi, S., L. Panichi, P. Chetoni, M. F. Saettone, and E. Boldrini. 1999. Development of simple dry eye model in the albino rabbit and evaluation of some tear substitutes. Ophthalmic Res. 31:229-235. [DOI] [PubMed] [Google Scholar]
- 5.Burgalassi, S., L. Raimondi, R. Pirisino, G. Banchelli, E. Boldrini, and M. F. Saettone. 2000. Effect of xyloglucan (tamarind seed polysaccharide) on conjunctiva cell adhesion to laminin and on corneal epithelium wound healing. Eur. J. Ophthalmol. 10:71-76. [DOI] [PubMed] [Google Scholar]
- 6.Callegan, M. C., J. A. Hobden, R. J. O'Callaghan, and J. M. Hill. 1995. Ocular drug delivery: a comparison of transcorneal iontophoresis to corneal collagen shields. Int. J. Pharm. 123:173-179. [Google Scholar]
- 7.Callegan, M. C., R. J. O'Callaghan, and J. M. Hill. 1994. Pharmacokinetic considerations in the treatment of bacterial keratitis. Clin. Pharmacokinet. 27:129-149. [DOI] [PubMed] [Google Scholar]
- 8.Cavalli, R., M. R. Gasco, P. Chetoni, S. Burgalassi, and M. F. Saettone. 2002. Solid lipid nanoparticles (SLN) as ocular delivery system for tobramycin. Int. J. Pharm. 238:241-245. [DOI] [PubMed] [Google Scholar]
- 9.Cutarelli, P. E., J. H. Lass, H. M. Lazarus, S. C. Putman, and M. R. Jacobs. 1991. Topical fluoroquinolones: antimicrobial activity and in vitro corneal epithelial toxicity. Curr. Eye Res. 10:557-563. [DOI] [PubMed] [Google Scholar]
- 10.Davies, N. M., S. J. Farr, J. Hadgraft, and I. W. Kellaway. 1991. Evaluation of mucoadhesive polymers in ocular drug delivery. I. Viscous solutions. Pharm. Res. 8:1039-1043. [DOI] [PubMed] [Google Scholar]
- 11.Di Colo, G., S. Burgalassi, M. P. Fiaschi, Y. Zambito, and M. F. Saettone. 2001. Gel-forming erodible inserts for ocular controlled delivery of ofloxacin. Int. J. Pharm. 215:101-111. [DOI] [PubMed] [Google Scholar]
- 12.Donnenfeld, E. D., A. Schrier, H. D. Perry, T. Aulicino, M. E. Gombert, and R. Snyder. 1994. Penetration of topically applied ciprofloxacin, norfloxacin, and ofloxacin into the aqueous humor. Ophthalmology 101:902-905. [DOI] [PubMed] [Google Scholar]
- 13.Gangopadhyay, N., M. Daniell, L. Weih, and H. R. Taylor. 2000. Fluoroquinolone and fortified antibiotics for treating bacterial corneal ulcers. Br. J. Ophthalmol. 84:378-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garg, P., A. K. Bansal, S. Sharma, and G. K. Vemuganti. 2001. Bilateral infectious keratitis after laser in situ keratomileusis: a case report and review of the literature. Ophthalmology 108:121-125. [DOI] [PubMed] [Google Scholar]
- 15.Ghelardi, E., A. Tavanti, F. Celandroni, A. Lupetti, C. Blandizzi, E. Boldrini, M. Campa, and S. Senesi. 2000. Effect of a novel mucoadhesive polysaccharide obtained from tamarind seeds on the intraocular penetration of gentamicin and ofloxacin in rabbits. J. Antimicrob. Chemother. 46:831-834. [DOI] [PubMed] [Google Scholar]
- 16.Haynes, R. J., P. J. Tighe, and H. S. Dua. 1999. Antimicrobial defensin peptides of the human ocular surface. Br. J. Ophthalmol. 83:737-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hilkens, J., M. J. Ligtenberg, H. L. Vos, and S. V. Litvinov. 1992. Cell membrane-associated mucins and their adhesion modulating property. Trends Biochem. Sci. 17:359-363. [DOI] [PubMed] [Google Scholar]
- 18.Holland, S. P., J. S. Pulido, T. K. Shires, and J. W. Costerton. 1993. Pseudomonas aeruginosa ocular infections, p. 160-176. In R. B. Fick (ed.), Pseudomonas aeruginosa: the opportunist. CRC Press Inc., Boca Raton, Fla.
- 19.Johnson, M. K., J. A. Hobden, M. Hagenah, R. J. O'Callaghan, J. M. Hill, and S. Chen. 1990. The role of pneumolysin in ocular infections with Streptococcus pneumoniae. Curr. Eye Res. 9:1107-1114. [DOI] [PubMed] [Google Scholar]
- 20.Lemjabbar, H., and C. Basbaum. 2002. Platelet-activating factor receptor and ADAM10 mediate responses to Staphylococcus aureus in epithelial cells. Nat. Med. 8:41-46. [DOI] [PubMed] [Google Scholar]
- 21.Liesegang, T. J. 1988. Bacterial and fungal keratitis, p. 217-270. In H. E. Kaufman, B. A. Barron, M. B. McDonald, and S. R. Waltman (ed.), The cornea. Churchill Livingstone, New York, N.Y.
- 22.Mallari, P. L., D. J. McCarty, M. Daniell, and H. Taylor. 2001. Increased incidence of corneal perforation after topical fluoroquinolone treatment for microbial keratitis. Am. J. Ophthalmol. 131:131-133. [DOI] [PubMed] [Google Scholar]
- 23.McClellan, K. A. 1997. Mucosal defense of the outer eye. Surv. Ophthalmol. 42:233-246. [DOI] [PubMed] [Google Scholar]
- 24.Moretti, M. V., S. Pauluzzi, and M. Cesana. 2000. Penetration of rufloxacin into the cerebrospinal fluid in patients with inflamed and uninflamed meninges. Antimicrob. Agents Chemother. 44:73-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osato, M. S., H. G. Jensen, M. D. Trousdale, J. A. Bosso, L. R. Borrmann, J. Frank, and P. Akers. 1989. The comparative in vitro activity of ofloxacin and selected ophthalmic antimicrobial agents against ocular bacterial isolates. Am. J. Ophthalmol. 108:380-386. [DOI] [PubMed] [Google Scholar]
- 26.Prajna, N. V., C. Geaorge, S. Selvaraj, K. L. Lu, P. J. McDonnell, and M. Srinivasan. 2001. Bacteriologic and clinical efficacy of ofloxacin 0.3% versus ciprofloxacin 0.3% ophthalmic solutions in the treatment of patients with culture-positive bacterial keratitis. Cornea 20:175-178. [DOI] [PubMed] [Google Scholar]
- 27.Raimondi, L., G. Sgromo, R. Banchelli, I. Corti, R. Pirisino, and E. Boldrini. 2000. A new viscosity enhancer for ophthalmic preparations devoid of toxicity for human conjunctiva cells. J. Toxicol. Cut. Ocular Toxicol. 19:31-42. [Google Scholar]
- 28.Schaefer, F., O. Bruttin, L. Zografos, and Y. Guex-Crosier. 2001. Bacterial keratitis: a prospective clinical and microbiological study. Br. J. Ophthalmol. 85:842-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith, A., P. M. Pennefather, S. B. Kaye, and C. A. Hart. 2001. Fluoroquinolones: place in ocular therapy. Drugs 61:747-761. [DOI] [PubMed] [Google Scholar]
- 30.Wise, R., J. M. Andrews, R. Matthews, and M. Wolstenholme. 1992. The in-vitro activity of two new quinolones: rufloxacin and MF 961. J. Antimicrob. Chemother. 29:649-660. [DOI] [PubMed] [Google Scholar]
- 31.Wise, R., J. Johnson, N. O'Sullivan, J. M. Andrews, and B. P. Imbimbo. 1991. Pharmacokinetics and tissue penetration of rufloxacin, a long acting quinolone antimicrobial agent. J. Antimicrob. Chemother. 28:905-909. [DOI] [PubMed] [Google Scholar]