Abstract
Background
Risk scores for patients who are at high risk for major bleeding complications during treatment with vitamin K antagonists (VKAs) do not perform that well. BLEEDS was initiated to search for new biomarkers that predict bleeding in these patients.
Objectives
To describe the outline and objectives of BLEEDS and to examine whether the study population is generalizable to other VKA treated populations.
Methods
A cohort was created consisting of all patients starting VKA treatment at three Dutch anticoagulation clinics between January-2012 and July-2014. We stored leftover plasma and DNA following analysis of the INR.
Results
Of 16,706 eligible patients, 16,570 (99%) were included in BLEEDS and plasma was stored from 13,779 patients (83%). Patients had a mean age of 70 years (SD 14), 8713 were male (53%). The most common VKA indications were atrial fibrillation (10,876 patients, 66%) and venous thrombosis (3920 patients, 24%). 326 Major bleeds occurred during 17,613 years of follow-up (incidence rate 1.85/100 person years, 95%CI 1.66–2.06). The risk for major bleeding was highest in the initial three months of VKA treatment and increased when the international normalized ratio increased. These results and characteristics are in concordance with results from other VKA treated populations.
Conclusion
BLEEDS is generalizable to other VKA treated populations and will permit innovative and unbiased research of biomarkers that may predict major bleeding during VKA treatment.
Introduction
Vitamin K antagonists (VKAs) are used to treat and prevent thromboembolic events [1]. Monitoring of VKA treatment is required because VKAs have a narrow therapeutic window and the dosage depends on inter-individual, but also intra-individual factors [1]. In the Netherlands, patients on VKA treatment are monitored by specialized anticoagulation clinics [2]. The clinics are regionally organized and all patients who live in a certain area are monitored by the same clinic [2]. At these clinics, the international normalized ratios (INRs) are measured on a regular basis, after which a specialized physician determines the VKA dosage and the time interval between INR measurements [2].
Despite this monitoring system, the most common side effects of VKAs remain bleeding complications [1]. Bleeding complications are, depending on the severity, categorized as minor or major bleeding complications. Minor bleedings, such as skin bruises or nosebleeds, occur annually in 6–10% of patients on VKAs and major bleedings, including (fatal) intra-organ bleeds, occur in 1–3% of VKA treated patients per year [2–4]. Risk factors for major bleeding events have been identified and subsequent bleeding risk scores have been developed [5–10]. However, these risk scores do not accurately predict major bleeding (range of C statistics: 0.59–0.69) [11]. Additional biomarkers and genetic variants potentially yield a better accuracy of predicting major bleeding, but information on such predictors is scarce. The goal of the Biomarkers in the Leiden Etiology and Epidemiology of bleeding in vitamin K antagonists Drug users Study (BLEEDS) is to identify novel biomarkers and genetic variants that predict patients at risk for major bleeding events during treatment with VKAs. Here, we delineate the outline of the study. In addition, we provide an overview on classical risk factors for major bleeding to ensure that our population is generalizable to other VKA treated populations.
Methods
Study design
BLEEDS is a population based cohort study with longitudinal follow-up in 16,570 patients who started VKA treatment and were recruited from three anticoagulation clinics in the Netherlands.
Study population
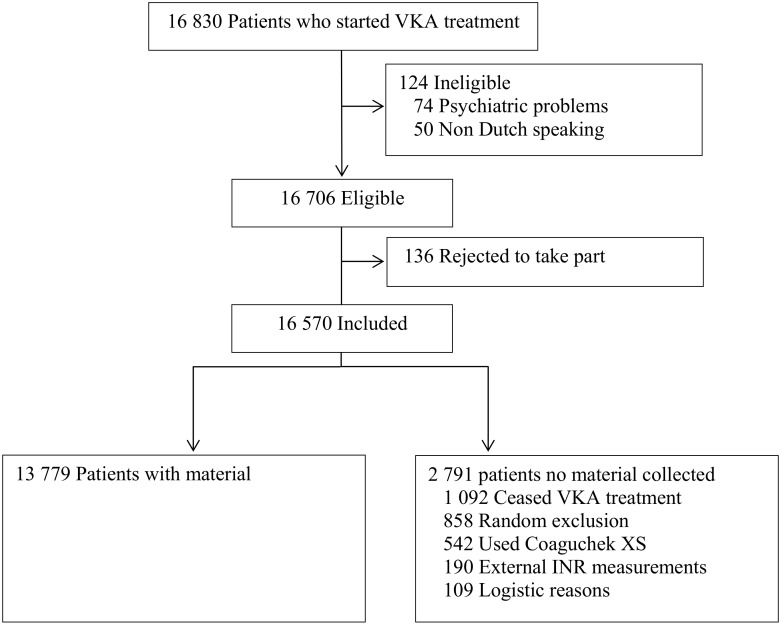
Consecutive patients aged 18 years or older who started VKA treatment at one of the three participating anticoagulation clinics in the Netherlands (Leiden, The Hague and Hoofddorp) between January 2012 and July 2014 were eligible (Fig 1). These regional anticoagulation clinics monitor the VKA therapy of those patients living in well-defined geographical areas surrounding Leiden, The Hague and Hoofddorp. Patients were included if the planned treatment duration was at least six weeks, and patients who did not speak Dutch (n = 50) or experienced psychiatric problems (n = 74) were excluded.
Fig 1. Flow chart of number of individuals included.
Considering an alpha value of 5%, statistical power of 80%, exposure prevalence of 10%, a relative risk of 1.8, an incidence rate of bleeding of 1.8 per year 100 patient years and a mean follow-up of one year, we estimated the necessary sample size at approximately 16,500 patients. All eligible patients received information regarding the study and were included if they did not decline to take part (i.e. an opt-out procedure was followed). This is in accordance with the Dutch law as long as the patient does not have to perform any additional actions for the study and if the privacy of the patients is guaranteed. As more extensively described in the Material collection, left-over plasma was used for the study and patient numbers were encoded to guarantee privacy of the patients. Therefore, the BLEEDS was approved by the medical ethical committee of the Leiden University Medical Center in which an ‘opt-out procedure’ was followed. The included study population consisted of 16,706 eligible patients, of whom 136 opted out (< 1%), resulting in 16,570 included patients.
Baseline examination and surveillance
When enlisted by the anticoagulation clinic, several patient characteristics were registered, including date of birth, sex, co-medication, indication for VKA treatment, planned duration of VKA treatment and INR target range. To monitor the INR, appointments are made with a frequency of at least every six weeks. The time interval between these measurements depends on the stability of the INR. In case of an unstable INR, the INR will be reassessed more frequently. In case of a stable INR, INR measurements will be performed after a maximum period of six weeks.
To measure the INR, venous blood is drawn into vacuum tubes containing 0.1-volume 0.109 mol/L trisodium citrate as anticoagulant. Blood was centrifuged (10 minutes at 2800 G) within 4 hours of collection, upon which the INR was measured. Another second, less frequently performed method to measure the INR is by using a point-of-care device (CoaguChek XS). At each appointment, a standardized short questionnaire is taken (and electronically stored) by a nurse in order to document changes in co-medication, onset of co-morbidities, the occurrence of bleeding events, or scheduled invasive procedures (e.g. planned surgery or dental extractions).
Data collection
Patient characteristics were extracted from the computerized patient records of the anticoagulation clinics. Baseline characteristics included sex, age, indication for VKA treatment, type of VKA, INR target range, and co-medications. The study population included 16 185 patients, and because some patients stopped VKA treatment and started again, these patients represented a total of 16,570 treatment periods. There were 8713 male patients (53%) and the mean age was 70 years (standard deviation [SD] 14; Table 1). The most common indications for VKA treatment were atrial fibrillation (10,876 treatment periods, 66%) and venous thrombosis (3920 treatment periods, 24%). Phenprocoumon was used during 12,083 (73%) periods, and approximately half of the patients used antihypertensive medication (8354 patients, 50%) or glucose lowering drugs (8 013 patients, 48%).
Table 1. Baseline characteristics.
| General characteristics | |
| Patients | 16,185 |
| Treatment periods | 16,570 |
| Men | 8713 (53) |
| Age | 70 (14) |
| INR target range | |
| 2.5–3.5 | 15,509 (93) |
| 3.0–4.0 | 1061 (7) |
| Treatment indication | |
| Atrial fibrillation | 10,876 (66) |
| Venous thrombosis | 3920 (24) |
| Mechanical heart valves | 435 (3) |
| Ischemic heart disease | 519 (3) |
| Vascular disease | 412 (3) |
| Postoperative | 125 (1) |
| Other | 348 (2) |
| Vitamin K antagonist | |
| Phenprocoumon | 12,068 (73) |
| Acenocoumarol | 4481 (27) |
| Warfarin | 18 (0) |
| Fluindione | 3 (0) |
| Co-Medication | |
| Anti-platelet drugs | 2705 (16) |
| NSAIDs | 1004 (6) |
| Glucose lowering drugs | 8013 (48) |
| Anti-hypertensive drugs | 8354 (50) |
| Cholesterol lowering drugs | 6288 (38) |
| Digoxin | 1767 (11) |
| Anti-cancer drugs | 339 (2) |
| Opioids | 1306 (8) |
| Methotrexate | 155 (1) |
Material collection
For his study, we used patient’s blood and plasma samples that were leftover following INR analyses. The sample collection started three weeks after initiations of VKA therapy and, if applicable, two weeks after termination of low-molecular-weight-heparin (LMWH) treatment. To guarantee the privacy of the patients, technicians who were not involved in the study recoded patient numbers to study numbers. After recoding, patient specific characteristics were concealed and samples were labelled according to study number. The ‘key’ linking patient to study numbers is maintained by a data manager who is not involved in the study. Per patient, a minimum volume of 2.0 ml plasma was collected, which resulted from blood samples of two to three subsequent visits to the anticoagulation clinic. Plasma samples were initially stored at -20°C up to one week. The remaining white blood cells, also encoded with the corresponding study number, were stored for up to one week at 2–8°C, after which DNA was isolated [12]. Plasma and DNA were both long-term stored at -80°C.
Plasma and DNA was collected from 13,779 patients (83%). Material collection failed for 2791 patients because they ceased VKA treatment early (1092 patients), were randomly excluded due to a high workload at the anticoagulation clinic (858 patients), the INR was established by the point-of-care device CoaguChek XS (542 patients), the INR measurement was performed externally (190 patients), and due to logistic complications (109 patients).
Follow-up and outcome
The follow-up lasted from starting VKA therapy to either the termination of VKA treatment, migration to an area not covered by the three anticoagulation clinics, death, the occurrence of a major bleeding event, or end of the study (31st December 2014), whichever occurred first, resulting in a total follow-up of 17 613 years and a mean follow-up time of 13 months. Patients were followed according to the routine procedures of the anticoagulation clinic. During the appointments, major bleeding events were identified through short interviews that were part of the standard procedures of the anticoagulation clinic. If patients mentioned any bleeding event or hospitalization related to a bleeding event, information was obtained from the hospital, general practitioner or patient to classify the bleeding event as minor or major. Major bleeding events were defined according to the guidelines of the Federation of Dutch Anticoagulation clinics (FNT) by trained anticoagulation clinic physicians, who were not involved in the current study. Bleeding events were classified as major if these were fatal, lead to a blood transfusion or hospital admission, were an intracranial bleeding, objectively diagnosed joint bleed, or a bleeding event in a critical organ [13]. In total, 326 major bleeding events were identified during 17,613 years of follow-up.
Results and Discussion
BLEEDS provides a large set of patient information and material that will be used to discover new information on risk factors for major bleeding events during VKA treatment. The large number of major bleeding events (n = 326) provides a unique opportunity to perform subgroup analyses and study relatively rare risk factors.
Previously, three studies have been performed in which plasma and DNA were collected to discover new risk factors for major bleeding during VKA treatment [14–16]. However, in all of these studies, only a subgroup of the total VKA-treated patients was included. The first study excluded patients who died or became mentally disabled by the bleeding event and included only 22% of all patients who experienced a major bleeding complication in the final analyses [16]. This makes the results susceptible to survivor bias [17]. The second study’s inclusion criterion was a time in therapeutic range (TTR) of 100% [15], while the third study included only 75% of the warfarin-treated patients. Furthermore, the patients were followed up to 5.5 years [14], which may have diluted the results. By including only subsets of patients on VKA therapy, results can be biased and cannot be extrapolated to all VKA-treated patients [16, 17]. In BLEEDS, follow-up was short which creates the possibility to study risk factors that predict the short term risk for major bleeding. In addition, we have included 99% of the eligible patients treated with VKAs, thereby providing a strong case that our population represents real world patients.
Further support for this stems from the observation that the incidence rate of the major bleeding events (1.85 per 100 patient-years, 95% CI 1.66–2.06; see Table 2) compares well with major bleeding rates of other population-based studies [2, 18, 19]. The major bleeding complications observed in our study also concur with previous findings, given that intracranial bleedings were the most common fatal major bleeding complications (37, 54% of all fatal major bleeding events), while the non-fatal bleedings mostly resulted from digestive (106, 41%) and intracranial (40, 15%; see Table 3) bleedings [2, 18, 19].
Table 2. Incidence rates of bleeding events stratified by clinical characteristics.
| No. ofevents | Patient time (years) | Events/100 patient-years(95% CI) |
|
|---|---|---|---|
| Total | 326 | 17,613 | 1.85 (1.66–2.06) |
| Sex | |||
| Male | 184 | 9224 | 1.99 (1.72–2.30) |
| Female | 142 | 8387 | 1.69 (1.43–1.99) |
| INR target range | |||
| 2.5–3.5 | 306 | 16,454 | 1.86 (1.66–2.08) |
| 3.0–4.0 | 20 | 1157 | 1.73 (1.09–2.62) |
| Vitamin K antagonist | |||
| Phenprocoumon | 262 | 13,278 | 1.97 (1.75–2.22) |
| Acenocoumarol | 64 | 4333 | 1.48 (1.15–1.87) |
| Indication | |||
| Atrial fibrillation | 241 | 13,162 | 1.83 (1.61–2.07) |
| Venous thrombosis | 53 | 2702 | 1.96 (1.48–2.55) |
| Mechanical heart valves | 4 | 351 | 1.14 (0.36–2.75) |
| Ischemic hearts disease | 7 | 555 | 1.26 (0.55–2.50) |
| Vascular | 9 | 433 | 2.08 (1.01–3.81) |
| Postoperative | 3 | 105 | 2.86 (0.72–7.78) |
| Other | 9 | 384 | 2.34 (1.14–4.30) |
Table 3. Number of bleeding events stratified by area.
| Fatal | Non-fatal | |
|---|---|---|
| Total | 68 | 260 |
| Gastrointestinal | 12 (18) | 106 (41) |
| Intracranial | 37 (54) | 40 (15) |
| Muscle | 0 | 13 (5) |
| Joint | 0 | 17 (7) |
| Epistaxis | 0 | 16 (6) |
| Respiratory | 3 (4) | 9 (3) |
| Urinary tract | 1 (1) | 16 (6) |
| Ocular | 0 | 1 (0) |
| Skin | 0 | 8 (3) |
| Circulatory | 9 (13) | 6 (2) |
| Retroperitoneal | 0 | 1 (0) |
| Other | 6 (9) | 27 (10) |
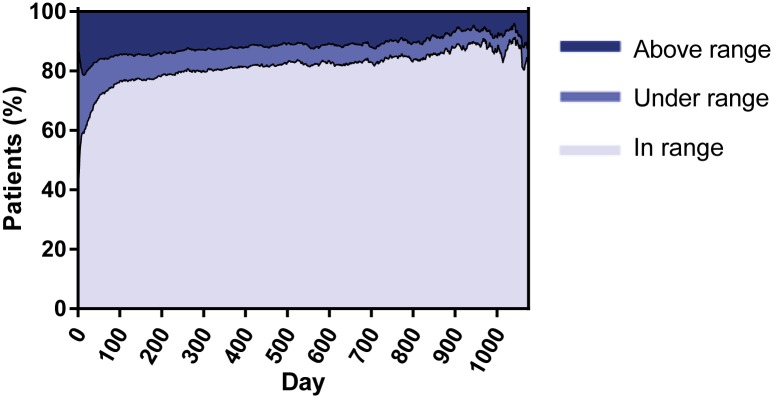
To further analyze if BLEEDS agrees with other VKA treated populations in terms of predictors for bleeding, we decided to calculate the TTR by linear interpolation as described by Rosendaal et al. [20] Previous studies have shown that high TTRs are associated with lower bleeding rates as compared with low TTRs. The lowest TTR was observed shortly after the initiation of VKA treatment, which increased to approximately 80% and stabilized until the end of follow-up (Fig 2), which in agreement with previous findings. This pattern is as expected based on other population based studies, although it should be mentioned that a TTR of 80% is within the upper range of normal for Western European anticoagulation clinics that on average achieve a TTR of 70% [21].
Fig 2. Time in therapeutic range per day after starting VKA treatment.
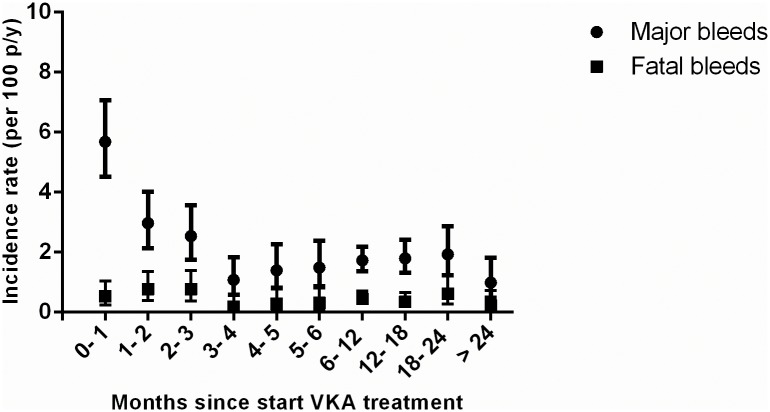
Further TTR assessment revealed that a low TTR was dose-dependently associated with increased bleeding rates (see Table 4) [20, 22, 23]. Additional analyses confirmed that, similar to other population based cohorts, in BLEEDS the bleeding rates i) increased with age (Fig 3) [2, 10, 18, 24–26], ii) were highest shortly after initiation of VKA treatment and became stable after four months of VKA treatment (Fig 4) [18, 25, 27, 28], iii) and increased after a high INR (Fig 5) [18, 26]. The number of fatal bleeding events was stable from start of therapy, but increased with age and INR (Figs 3 to 5). Results of the subgroup analyses (atrial fibrillation patients, venous thrombosis patients, and patients with an INR target range between 2.5 and 3.5) showed similar results as compared with the main analyses (see S1–S4 Figs, S1 and S2 Tables).
Table 4. Association of TTR with bleeding events.
| No. of events | Patient years | Events/100 patient-years (95% CI) |
|
|---|---|---|---|
| Time in range | |||
| < 35% | 63 | 448 | 13.02 (10.09–16.54) |
| ≥ 35% and < 50% | 33 | 1 145 | 2.88 (2.02–4.00) |
| ≥ 50% and < 60% | 35 | 1 538 | 2.28 (1.61–3.13) |
| ≥ 60% and < 70% | 48 | 2 212 | 2.17 (1.62–2.85) |
| ≥ 70% and < 80% | 40 | 2 821 | 1.42 (1.03–1.91) |
| ≥ 80% and < 90% | 37 | 3 059 | 1.21 (0.86–1.65) |
| ≥ 90% | 62 | 5 361 | 1.16 (0.89–1.47) |
Fig 3. Incidence rates of bleeding events stratified by age.
Fig 4. Incidence rates of bleeding events stratified by time since start of VKA treatment.
Fig 5. Incidence rates of bleeding events stratified by INR.
Two characteristics of the study deserve a further comment. Material (plasma and DNA) of all patients was collected from the third week after initiating VKA therapy and, if applicable, two weeks after termination of LMWH therapy. As such, we did not collect material of 32 patients who experienced a major bleeding complication before blood could be collected of which 10 patients died and 22 ceased VKA treatment. As a possible consequence, risk estimates may dilute because the patients with the strongest risk factors ‘dropped out’ of the study, which may disfavor the study of patients with the strongest and thus shortest term risk factors. Second, the low INR target range is 2.5–3.5 in the Netherlands as decided by the Federation of Dutch Anticoagulation Clinics, while it is 2.0–3.0 in other countries. This may result in slightly higher major bleeding rates in these patients, but may also favor the detection of risk factors for major bleeding complications.
In this study, 99% of the eligible patients were included which results in a unique, unselected study population. The number of major bleeding events is relatively high, which creates the possibility to perform subgroup analyses and study whether protein levels are dose dependently associated with major bleeding events. In addition, the availability of stored biological specimens (citrated plasma and DNA) will allow us to uncover new risk factors for major bleeding complications during VKA treatment, with the goal of discovering new predictors for VKA-treated patients at high risk for major bleeding events. We would like to emphasize that results of BLEEDS with respect to classical risk factors for major bleeding are similar to other cohorts of patients who received VKAs for all long term indications, which indicates that this population is generalizable to other populations. In summary, the BLEEDS will permit innovative and unbiased research of multiple exposures for major bleeding events and will assist in the prevention of major bleeding events in patients treated with VKAs.
Supporting Information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
The authors would like to thank all technicians, nurses and physicians at the anticoagulation clinics for their devotion and work on BLEEDS. We would also like to thank M.M. Burgers, M.L.C. Noorlander and R. de Winter for their excellent work.
Data Availability
Data from this study are available in Figshare (URL: https://figshare.com/articles/Objectives_and_design_of_BLEEDS_a_cohort_study_to_identify_new_risk_factors_and_predictors_for_major_bleeding_during_treatment_with_vitamin_K_antagonists/4246745; DOI: https://dx.doi.org/10.6084/m9.figshare.4246745.v1).
Funding Statement
Hartstichting (2011 T12) https://www.hartstichting.nl/, Center for Translational Molecular Medicine (01 C-201) http://www.ctmm.nl/nl, and Hartstichting (99.165) https://www.hartstichting.nl/.
References
- 1.Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):160S–98S. 10.1378/chest.08-0670 [DOI] [PubMed] [Google Scholar]
- 2.van der Meer FJ, Rosendaal FR, Vandenbroucke JP, Briet E. Bleeding complications in oral anticoagulant therapy. An analysis of risk factors. Arch Intern Med. 1993;153(13):1557–62. [DOI] [PubMed] [Google Scholar]
- 3.Linkins LA. Bleeding risks associated with vitamin K antagonists. Blood Rev. 2013;27(3):111–8. 10.1016/j.blre.2013.02.004 [DOI] [PubMed] [Google Scholar]
- 4.Schulman S, Beyth RJ, Kearon C, Levine MN. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):257S–98S. 10.1378/chest.08-0674 [DOI] [PubMed] [Google Scholar]
- 5.Beyth RJ, Quinn LM, Landefeld CS. Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin. Am J Med. 1998;105(2):91–9. [DOI] [PubMed] [Google Scholar]
- 6.Fang MC, Go AS, Chang Y, Borowsky LH, Pomernacki NK, Udaltsova N, et al. A new risk scheme to predict warfarin-associated hemorrhage: The ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol. 2011;58(4):395–401. 10.1016/j.jacc.2011.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gage BF, Yan Y, Milligan PE, Waterman AD, Culverhouse R, Rich MW, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J. 2006;151(3):713–9. 10.1016/j.ahj.2005.04.017 [DOI] [PubMed] [Google Scholar]
- 8.Kuijer PM, Hutten BA, Prins MH, Buller HR. Prediction of the risk of bleeding during anticoagulant treatment for venous thromboembolism. Arch Intern Med. 1999;159(5):457–60. [DOI] [PubMed] [Google Scholar]
- 9.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263–72. 10.1378/chest.09-1584 [DOI] [PubMed] [Google Scholar]
- 10.Shireman TI, Howard PA, Kresowik TF, Ellerbeck EF. Combined anticoagulant-antiplatelet use and major bleeding events in elderly atrial fibrillation patients. Stroke. 2004;35(10):2362–7. 10.1161/01.STR.0000141933.75462.c2 [DOI] [PubMed] [Google Scholar]
- 11.Zhu W, He W, Guo L, Wang X, Hong K. The HAS-BLED Score for Predicting Major Bleeding Risk in Anticoagulated Patients With Atrial Fibrillation: A Systematic Review and Meta-analysis. Clin Cardiol. 2015;38(9):555–61. 10.1002/clc.22435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16(3):1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trombosediensten FvN. FNT- NORMEN. 2015 2015. Report No.
- 14.Lind M, Boman K, Johansson L, Nilsson TK, Ohlin AK, Birgander LS, et al. Thrombomodulin as a marker for bleeding complications during warfarin treatment. Arch Intern Med. 2009;169(13):1210–5. 10.1001/archinternmed.2009.170 [DOI] [PubMed] [Google Scholar]
- 15.Roldan V, Marin F, Muina B, Torregrosa JM, Hernandez-Romero D, Valdes M, et al. Plasma von Willebrand factor levels are an independent risk factor for adverse events including mortality and major bleeding in anticoagulated atrial fibrillation patients. J Am Coll Cardiol. 2011;57(25):2496–504. 10.1016/j.jacc.2010.12.033 [DOI] [PubMed] [Google Scholar]
- 16.van der Heijden JF, Rekke B, Hutten BA, van der Meer FJ, Remkes MG, Vermeulen M, et al. Non-fatal major bleeding during treatment with vitamin K antagonists: influence of soluble thrombomodulin and mutations in the propeptide of coagulation factor IX. J Thromb Haemost. 2004;2(7):1104–9. 10.1111/j.1538-7836.2004.00768.x [DOI] [PubMed] [Google Scholar]
- 17.van Rein N, Cannegieter SC, Rosendaal FR, Reitsma PH, Lijfering WM. Suspected survivor bias in case-control studies: stratify on survival time and use a negative control. J Clin Epidemiol. 2014;67(2):232–5. 10.1016/j.jclinepi.2013.05.011 [DOI] [PubMed] [Google Scholar]
- 18.Palareti G, Leali N, Coccheri S, Poggi M, Manotti C, D'Angelo A, et al. Bleeding complications of oral anticoagulant treatment: an inception-cohort, prospective collaborative study (ISCOAT). Italian Study on Complications of Oral Anticoagulant Therapy. Lancet. 1996;348(9025):423–8. [DOI] [PubMed] [Google Scholar]
- 19.Go AS, Hylek EM, Chang Y, Phillips KA, Henault LE, Capra AM, et al. Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? JAMA. 2003;290(20):2685–92. 10.1001/jama.290.20.2685 [DOI] [PubMed] [Google Scholar]
- 20.Rosendaal FR, Cannegieter SC, van der Meer FJ, Briet E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69(3):236–9. [PubMed] [Google Scholar]
- 21.Singer DE, Hellkamp AS, Piccini JP, Mahaffey KW, Lokhnygina Y, Pan G, et al. Impact of global geographic region on time in therapeutic range on warfarin anticoagulant therapy: data from the ROCKET AF clinical trial. J Am Heart Assoc. 2013;2(1):e000067 10.1161/JAHA.112.000067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Connolly SJ, Pogue J, Eikelboom J, Flaker G, Commerford P, Franzosi MG, et al. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation. 2008;118(20):2029–37. 10.1161/CIRCULATIONAHA.107.750000 [DOI] [PubMed] [Google Scholar]
- 23.White HD, Gruber M, Feyzi J, Kaatz S, Tse HF, Husted S, et al. Comparison of outcomes among patients randomized to warfarin therapy according to anticoagulant control: results from SPORTIF III and V. Arch Intern Med. 2007;167(3):239–45. 10.1001/archinte.167.3.239 [DOI] [PubMed] [Google Scholar]
- 24.Fang MC, Chang Y, Hylek EM, Rosand J, Greenberg SM, Go AS, et al. Advanced age, anticoagulation intensity, and risk for intracranial hemorrhage among patients taking warfarin for atrial fibrillation. Ann Intern Med. 2004;141(10):745–52. [DOI] [PubMed] [Google Scholar]
- 25.Landefeld CS, Goldman L. Major bleeding in outpatients treated with warfarin: incidence and prediction by factors known at the start of outpatient therapy. Am J Med. 1989;87(2):144–52. [DOI] [PubMed] [Google Scholar]
- 26.Cannegieter SC, Rosendaal FR, Wintzen AR, van der Meer FJ, Vandenbroucke JP, Briet E. Optimal oral anticoagulant therapy in patients with mechanical heart valves. N Engl J Med. 1995;333(1):11–7. 10.1056/NEJM199507063330103 [DOI] [PubMed] [Google Scholar]
- 27.Fihn SD, McDonell M, Martin D, Henikoff J, Vermes D, Kent D, et al. Risk factors for complications of chronic anticoagulation. A multicenter study. Warfarin Optimized Outpatient Follow-up Study Group. Ann Intern Med. 1993;118(7):511–20. [DOI] [PubMed] [Google Scholar]
- 28.Petitti DB, Strom BL, Melmon KL. Duration of warfarin anticoagulant therapy and the probabilities of recurrent thromboembolism and hemorrhage. Am J Med. 1986;81(2):255–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
Data from this study are available in Figshare (URL: https://figshare.com/articles/Objectives_and_design_of_BLEEDS_a_cohort_study_to_identify_new_risk_factors_and_predictors_for_major_bleeding_during_treatment_with_vitamin_K_antagonists/4246745; DOI: https://dx.doi.org/10.6084/m9.figshare.4246745.v1).