Abstract
Posaconazole is a potent, broad-spectrum triazole antifungal agent currently in clinical development for the treatment of refractory invasive fungal infections. Eight healthy male subjects received a single 399-mg (81.7 μCi) oral dose of [14C]posaconazole after consuming a high-fat breakfast. Urine, feces, and blood samples were collected for up to 336 h postdose and assayed for total radioactivity; plasma and urine samples were also assayed for parent drug. Posaconazole was orally bioavailable, with a median maximum posaconazole concentration in plasma achieved by 10 h postdose. Thereafter, posaconazole was slowly eliminated, with a mean half-life of 20 h. The greatest peak in the radioactivity profile of pooled plasma extracts was due to posaconazole, with smaller peaks due to a monoglucuronide, a diglucuronide, and a smaller fragment of the molecule. The mean total amount of radioactivity recovered was 91.1%; the cumulative excretion of radioactivity in feces and in urine was 76.9 and 14.0% of the dose, respectively. Most of the fecal radioactivity was associated with posaconazole, which accounted for 66.3% of the administered dose; however, urine contained only trace amounts of unchanged posaconazole. The radioactivity profile of pooled urine extracts included two monoglucuronide conjugates and a diglucuronide conjugate of posaconazole. These observations suggest that oxidative (phase 1) metabolism by cytochrome P450 isoforms represents only a minor route of elimination for posaconazole, and therefore cytochrome P450-mediated drug interactions should have a limited potential to impact posaconazole pharmacokinetics.
Posaconazole (SCH 56592) is a potent, extended-spectrum triazole antifungal agent currently in late-phase clinical development for the treatment and prophylaxis of invasive fungal infections due to a wide range of common, rare, and emerging molds and yeasts. Posaconazole has demonstrated in vitro activity against several commonly encountered pathogens, including Candida, Aspergillus, Cryptococcus, and Coccidioides species (1, 7, 12, 13). In addition, its activity against several emerging pathogens, such as the filamentous fungus Scedosporium (3, 10), has been explored clinically. In an immunocompromised patient, posaconazole has been used to effectively treat Scedosporium-induced brain abscesses that were refractory to itraconazole and amphotericin B therapy (11).
The anticipated clinical dosage regimen of posaconazole oral suspension for the treatment of refractory invasive fungal infections is 400 mg twice daily. The purpose of the present study was to determine the absorption, metabolism, and excretion of a single 400-mg dose of [14C]posaconazole oral suspension in human subjects.
MATERIALS AND METHODS
Radiolabeled posaconazole and dosage forms.
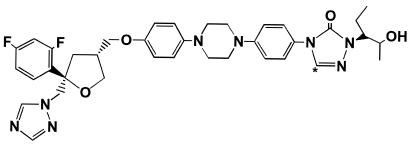
[14C]posaconazole (Fig. 1) was synthesized and formulated by the radiochemistry group at the Schering-Plough Research Institute (Kenilworth, N.J.). Its specific activity was 0.205 μCi/mg, and its radiochemical purity was 98.7%. The radiolabeled drug was formulated as a 40-mg/ml suspension with the same excipients as the commercial suspension. Each unit dose bottle contained 10 ml of the suspension. The mean doses and amounts of radioactivity administered were 399 mg (range, 397 to 401 mg) and 81.7 μCi (range, 81.4 to 82.1 μCi), respectively.
FIG. 1.
Structure of [14C]posaconazole. The asterisk indicates the position of the 14C label.
Subjects and dose administration.
Eight healthy white male subjects between the ages of 22 and 43 years (mean, 28 years), whose body mass index was between 22.6 and 25 (mean, 23.6) and who met the eligibility criteria, participated in the study. Subjects were in good health based on their medical history, a physical examination, a 12-lead electrocardiogram, and clinical laboratory test results. After an overnight fast, the subjects ingested radiolabeled posaconazole ∼5 min after consumption of a high-fat breakfast. The bottles containing the suspension were rinsed four times with 50 ml of noncarbonated water, and the contents were administered to the subjects. All subjects were confined to the study site until at least 168 h postdose or until 90% of the administered radioactive dose was excreted and recovered (total duration of up to 14 days postdose).
The present study was reviewed and approved by the Covance Clinical Research Unit Institutional Review Board. Informed consent was obtained from each volunteer before study initiation. The study was conducted in accordance with Good Clinical Practice and the Declaration of Helsinki.
Sample collection.
Blood samples (10 ml each) were collected into chilled, heparinized tubes at 0, 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 16, 24, 36, 48, 72, 96, 120, 144, 168, 192, 216, 240, 264, 288, 312, and 336 h postdose. An additional 20 ml of blood was collected at 5, 12, and 24 h postdose for metabolite profiling. One sample of blood per time point was placed into a polypropylene cryotube and frozen to −20°C until analyzed for total radioactivity. The remaining blood was centrifuged, and the plasma was frozen to −20°C until analyzed for total radioactivity and posaconazole concentrations. Plasma samples collected for metabolite profiling were stored at −70°C. Blood and plasma samples were assayed daily for total radioactivity.
The urine samples were collected before [14C]posaconazole administration and then in block intervals of 0 to 12, 12 to 24, 24 to 36, and 36 to 48 h and in 24-h blocks thereafter until 336 h postdose. Samples were refrigerated until the end of the block collection periods, when the total weight and volume of each urine block were recorded. The amount of total radioactivity in each urine sample was determined on a daily basis, and the samples were stored at −20°C until assayed. To completely solubilize posaconazole, a volume of methanol equal to 40% of the weight of the urine sample was added. After a thorough mixing, samples were removed for the determination of total radioactivity concentrations, posaconazole concentrations, and metabolite profiling.
All bowel movements and fecal wipes were collected before [14C]posaconazole administration and up to 336 h postdose. The fecal samples and wipes were refrigerated until assayed for total radioactivity on a daily basis. After homogenization and sampling, the remainder of the samples were stored at −20°C until extracted for metabolite profiling.
Measurement of radioactivity.
All samples were assayed for total radioactivity by liquid scintillation spectroscopy (LSS; model no. 1900TR or 2300TR liquid scintillation counter; Packard Instrument Co., Meriden, Conn.). Quench corrections were made by the external standard method. Samples with less than three times the background disintegrations per minute were recorded as 0. Liquid scintillation fluid (Ultima Gold; Packard Instrument Co.) was added directly to the plasma and urine samples before analysis by LSS. Blood samples were combusted by using a Packard 307 sample oxidizer; the resulting 14CO2 was trapped in Carbo-Sorb (Packard Instrument Co.) and quantitated by LSS. Feces and the fecal wipes were homogenized with a sufficient volume of water/methanol (1:1 [vol/vol]) by using a probe-type homogenizer and combusted to generate 14CO2. The ranges of lower limits of detection for radioactivity in plasma, blood, urine, fecal homogenates, and fecal wipes were 246 to 354, 428 to 740, 94 to 134, 492 to 822, and 97 to 132 ng equivalents (equiv)/g, respectively.
Posaconazole plasma and urine assays.
Liquid chromatography with tandem mass spectrometric (LC-MS/MS) detection was used to determine the concentrations of posaconazole in human plasma and urine. Sample preparation involved the addition of ammonium hydroxide and the internal standard to 0.1 ml of plasma, or a mixture of urine and methanol, followed by solid-phase extraction with a 96-well plate format on a Quadra 96 model 320 automated liquid-handling system (TOMTEC, Hamden, Conn.). The samples were chromatographed by using a C18 column and a mobile phase consisting of methanol and 5 mM ammonium acetate (85:15 [vol/vol]). Detection was performed on a PE Sciex API 3000 tandem mass spectrometer equipped with TurboIon spray interface (Applied Biosystems/MDS Sciex, Concord, Ontario, Canada) and was operated in a positive-ion, multiple-reaction monitoring mode. The precursor → product transition pair was mass-to-charge ratio (m/z) 701.3 → 683.3 for posaconazole and m/z 687.2 → 669.3 for the internal standard. The method was validated at posaconazole concentration ranges of 1.00 to 4,000 ng/ml in plasma and 35 to 1,400 ng/ml in urine.
Pharmacokinetic analyses.
Individual posaconazole and radioactivity concentration in plasma-time data above the lower limit of quantitation (LLOQ) for the respective assays were used for the pharmacokinetic analyses by using model-independent methods (6). The maximum concentration in plasma (Cmax) and the time to maximum concentration (Tmax) were the observed values. The terminal rate constant of posaconazole, k, was calculated as the negative gradient of the log-linear terminal portion of the concentration in plasma-time curve by using linear regression. The terminal-phase half-life (t1/2) was calculated as ln(2)/k. The area under the plasma concentration-time curve from time zero to the time of the last quantifiable sample (AUCtf) was calculated by using the linear trapezoidal method. The AUC was extrapolated to infinity (AUCI) by using the equation: AUCI = AUCtf + Ctfest/k, where Ctfest is the estimated concentration at tf determined from the linear regression. The apparent total body clearance (CL/F) of posaconazole was calculated as the dose/AUCI. The renal clearance (CLR) was calculated as the total amount of posaconazole excreted into urine (Ae) divided by the AUCI. The apparent volume of distribution (V/F) was calculated by using the following equation: V/F = dose/AUCI/k.
Metabolite profiling and identification. (i) Plasma.
For each time point (5, 12, and 24 h), equal volumes were pooled from individual subjects' plasma collections. After the pooled samples were analyzed by LSS, they were extracted in duplicate by using tC18 solid-phase extraction (SPE) cartridges (Waters, Milford, Mass.). The cartridges were washed with water under vacuum, and the drug-derived material bound to the cartridges was eluted with methanol; the methanol eluants were then concentrated under vacuum by using a Speed-Vac concentrator (Savant Instruments, Inc., Farmingdale, N.Y.). After reconstitution in water-methanol-dimethyl sulfoxide (50:45:5 [vol/vol/vol]), duplicate samples were analyzed by LSS to determine the extraction recovery. The recovery of radioactivity in the methanol extracts from the pooled plasma samples was between 94.7 and 124%.
(ii) Urine.
Approximately 5% of the volume was pooled from each subject's 0- to 120-h interval urine collection sample. Then, 10% of each individual's pooled urine was combined to generate a 0- to 120-h pooled urine sample for all eight subjects. Samples from the pooled urine were extracted in a manner similar to that used for the pooled plasma. The recovery of extracted radioactivity in the methanol eluant was between 84.7 and 101%.
(iii) Feces.
Approximately 5% of the volume was pooled from each subject's fecal collections from 0 to 120 h postdose. Then, 5% of each individual's pooled fecal homogenate was combined to generate a 0- to 120-h pooled fecal sample for all eight subjects. An aliquot was extracted twice with approximately two times the volume of methanol by using sonication and vortex mixing. After centrifugation, the supernatants were combined and analyzed by LSS. Methanol was concentrated under vacuum and reconstituted in water. The sample was applied to an Extrelut column (EM Sciences, Gibbstown, N.J.) and eluted with isopropanol-ethyl acetate-dichloromethane (20:60:20 [vol/vol/vol]). The elution solvent was evaporated by using a flash evaporator. The recovery rate of radioactivity in the initial methanol extract was 98.5%, whereas 98.4% of the radioactivity applied to the Extrelut column was recovered.
(iv) HPLC and radiometric instrumentation.
Extracted samples were profiled for metabolites by using a high-performance liquid chromatography (HPLC) assay coupled with UV, mass spectrometric, and radiometric flow detection. HPLC equipment consisted of an HP 1100 pump (model G1312A), an injector (model G1313A) (Hewlett-Packard, Palo Alto, Calif.), an ABI Analytical Spectroflow 757 UV detector (Kratos Division, Chestnut Ridge, N.Y.), a Zorbax Rx-C8 column (250 by 4.6 mm, 5 μm-particle size; Agilent Technologies, Palo Alto, Calif.), and an HP 1100 column switcher (Hewlett-Packard model G1316A). A separate pump (SSI model 222C; Scientific Systems, Inc., State College, Pa.) was used to inject solvent into the column eluant before it was introduced into the mass spectrometer. The initial mobile phase was 90% water-10% methanol, and the flow rate was 1.0 ml/min. After 5 min, the percentage of methanol was increased in a linear manner such that the mobile phase constituted 100% methanol at 51 min after injection. After the column was maintained at 100% methanol for another 4 min, it was reequilibrated to the initial mobile-phase proportions, and ∼30% of the column eluant was diverted to the mass spectrometer. Before it was introduced into the mass spectrometer, 0.05% formic acid (0.05 ml/min) was added by using the separate pump to increase ionization of the metabolites. The other 70% of the HPLC eluant was mixed with Ultima Flow M scintillation fluid (3 ml/min; Packard Instrument Co.) and introduced into a Radiomatic detector model A525A (Packard Instrument Co.) equipped with a 0.5-ml TRLSC flow cell. The software for the flowthrough radiometric detector was Flo-One version 3.65 (Packard Instrument Co.). To determine the percentage of the total dose in each radioactive metabolite from the pooled urine and fecal extracts, the percentage of the radioactivity that eluted in each peak was multiplied by the percentage of administered radioactivity excreted in the sample from 0 to 120 h postdose.
(v) MS and MS/MS instrumentation.
Full-scan analyses were performed by using a Quattro mass spectrometer (Fison, Inc., Danvers, Mass.), while the MS/MS analysis was performed with a Quattro II with a Z-Spray source (Micromass, Withenshawe, United Kingdom). The MS software was MassLynx version 3.4 (Micromass). For the full-scan analyses, the source temperature was 150°C, the cone voltage was 65 V, and the analyses were performed in the positive ionization mode. Nitrogen was used as both the ESI nebulizer gas (20 liters/h) and the bath gas (350 liters/h). For the MS/MS analyses, the source and desolvation temperatures were 130 and 300°C, respectively, and the instrument was used in the positive ionization mode. The cone voltage was 40 V, and the collision energy was 38 eV. Nitrogen was used as both the ESI nebulizer gas (20 liters/h) and the bath gas (340 liters/h), and argon was used as the collision gas.
RESULTS
Posaconazole and drug-derived radioactivity pharmacokinetic parameters.
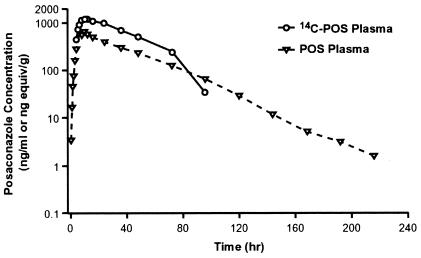
Posaconazole was orally bioavailable after oral administration; the mean maximum concentration in plasma of 654 ng/ml was achieved at a median time of ∼10 h postdose (Table 1 and Fig. 2). Thereafter, posaconazole concentrations declined slowly with a mean t1/2 of ∼20 h and an apparent total body clearance of 16.3 liters/h. Posaconazole had a large apparent volume of distribution (465 liters). The mean time of the final (tf) concentrations in plasma for posaconazole and drug-derived radioactivity were 183 and 66 h, respectively. Based on the ratio of the AUC0-24 h values of posaconazole and the total drug-derived radioactivity, the plasma contained metabolites in addition to the parent drug (Table 1). The mean pharmacokinetic parameters were consistent with the results from a previous single-dose study in healthy volunteers (4). The renal clearance of posaconazole was only 0.0114 ml/min (0.684 ml/h), which was negligible compared to the mean total body clearance of 16.3 liters/h. A mean value of only 0.06 μg of posaconazole was excreted as unchanged drug in the urine during the same collection period.
TABLE 1.
Mean pharmacokinetic parameters of posaconazole and drug-derived radioactivity
| Parameter | Posaconazole
|
Radioactivity
|
||
|---|---|---|---|---|
| Unit | Mean value (CV) in plasma | Unit | Mean value (CV) in plasma | |
| Cmax | ng/ml | 654 (19) | ng equiv/g | 1,260 (18) |
| Tmax [median (range)] | h | 9.25 (26) [10 (6-12)] | h | 11.0 (22) [10 (8-16)] |
| t1/2 | h | 20 (19) | NAb | NA |
| AUC0-24 h | ng·h/ml | 10,300 (22) | ng equiv·h/g | 21,300 (21) |
| AUCtf | ng·h/ml | 26,000 (26) | ng equiv·h/g | 48,100 (31) |
| AUCI | ng·h/ml | 26,100 (26) | NA | NA |
| tf | h | 183 (14) | h | 66 (26) |
| CL/F | Liter/h | 16.3 (30) | NA | NA |
| V/F | Liter | 465 (31) | NA | NA |
| CLR | ml/min | 0.0114 (76)a | NA | NA |
n = 3; remaining subjects had <3 urine concentrations above the LLOQ (25 ng/ml).
NA, not applicable.
FIG. 2.
Mean posaconazole- and drug-derived radioactivity concentration in plasma-time profiles after a single oral dose of 399 mg of [14C]posaconazole given to eight healthy male subjects.
The concentration of drug-derived radioactivity in most whole blood samples was generally less than the LLOQ (428 to 740 ng equiv/g). However, in samples with measurable concentrations, the concentration of radioactivity in whole blood was less than that in plasma at the same time point. This indicates that there was minimal to no accumulation of drug-derived radioactivity in the red blood cells.
Recovery of radioactivity in urine and feces.
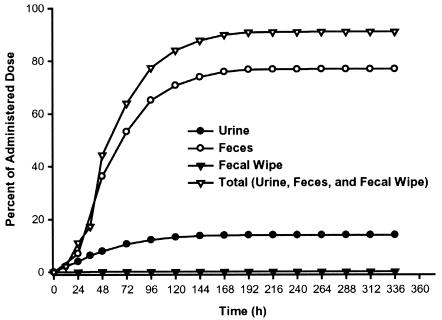
The mean total recovery of the administered radioactivity across all subjects was 91.1% (coefficient of variation [CV] = 9%) (Fig. 3), of which the feces contained 76.9% (CV = 12%) of the radioactive dose and the urine contained 14.0% (CV = 11%). Only 0.15% of the dose was detected on the fecal wipes. In one subject, the recovery of radioactivity in the feces was low (55%), even though all of the excreta were collected. For the last 3 days of collection, the level of radioactivity in the excreta of this subject was less than the lower limit of detection. It is interesting that the urinary excretion of [14C]posaconazole in this subject was similar to that in each of the other seven subjects (17%).
FIG. 3.
Mean cumulative percentage of radioactive dose in feces, urine, and fecal wipes after a single oral dose of 399 mg of [14C]posaconazole given to eight healthy male subjects.
Metabolite profiling. (i) Plasma.
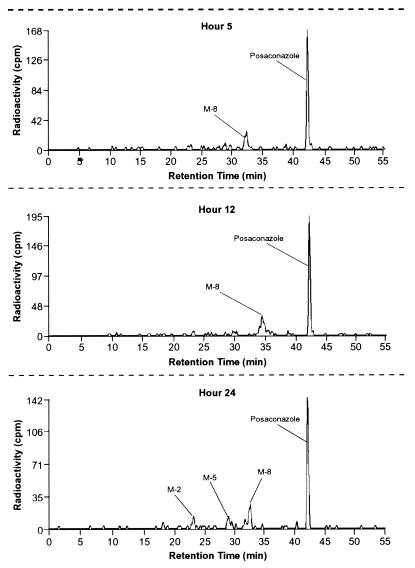
Posaconazole accounted for most of the profiled radioactivity in the pooled plasma extracts at 5, 12, and 24 h (Table 2). A glucuronide conjugate of posaconazole, designated M8, accounted for 17.5 to 27.6% of the profiled radioactivity in the pooled plasma. In addition, the 24-h pooled plasma extract contained a diglucuronide conjugate of posaconazole (M5) and a smaller fragment of the compound, designated M2 (Table 2 and Fig. 4). Other minor metabolites were detected by MS but were below the level of radiometric detection.
TABLE 2.
Distribution of radioactivity in pooled human plasma extracts after a single oral dose of 399 mg of [14C]posaconazole to healthy human subjects
| Peak | Rt (min) | m/z | Δa | % of profiled plasma radioactivity at:
|
||
|---|---|---|---|---|---|---|
| 5 h | 12 h | 24 h | ||||
| M2 | 23.2 | 263 | −438 | BITb | BIT | 7.88 |
| M5 | 29.0 | 1053 | +352 | BIT | ND | 8.97 |
| M8 | 32.6 | 877 | +176 | 21.1 | 27.6 | 17.5 |
| M7 | 33.2 | 424 | −277 | NDc | BIT | BIT |
| M9 | 38.8 | 877 | +176 | BIT | BIT | BIT |
| M12 | 39.7 | 717 | +16 | BIT | BIT | BIT |
| M13 | 40.2 | 717 | +16 | BIT | BIT | BIT |
| M14 | 40.9 | 717 | +16 | BIT | BIT | BIT |
| M15 | 41.6 | 717 | +16 | BIT | BIT | BIT |
| Posaconazole | 42.1 | 701 | 0 | 78.9 | 72.4 | 65.7 |
That is, the difference between the m/z value of the metabolite and that of posaconazole.
BIT, below the integration threshold of the 14C detector but detected by MS.
ND, not detected by 14C or the MS detector.
FIG. 4.
Radioactivity profile of pooled plasma extracts at 5, 12, and 24 h after a single oral dose of 399 mg of [14C]posaconazole given to eight healthy male subjects.
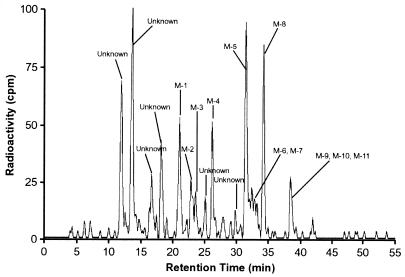
(ii) Urine.
The pooled urine extract contained only trace amounts of unchanged posaconazole (Table 3 and Fig. 5). This confirms the result of the pharmacokinetic analysis, which indicated that renal elimination was a minor excretion pathway and that only negligible amounts of posaconazole were excreted unchanged in the urine. Other metabolites in the urine extracts were composed of glucuronide conjugates of posaconazole and oxidative metabolites. None of the urinary metabolites accounted for >2% of the administered dose. Together, the two monoglucuronide conjugates, M8 and M9, and the diglucuronide conjugate, M5, accounted for <4% of the administered dose.
TABLE 3.
Distribution of radioactivity in pooled human urine extracts after a single oral dose of 399 mg of [14C]posaconazole to healthy human subjects
| Peak | Rt (min) | m/z | Δa | % of profiled radioactivity | % of dose (0-120 h)b |
|---|---|---|---|---|---|
| Unknown | 12.0 | NDe | NAf | 9.38 | 1.24 |
| Unknown | 12.6 | ND | NA | 1.38 | 0.18 |
| Unknown | 13.7 | ND | NA | 15.5 | 2.04 |
| Unknown | 16.7 | ND | NA | 5.19 | 0.69 |
| Unknown | 18.2 | ND | NA | 6.37 | 0.84 |
| M1 | 21.1 | 600 | −101 | 8.20 | 1.08 |
| M2 | 22.9 | 263 | −438 | 4.57 | 0.60 |
| M3 | 23.6 | 600 | −101 | 2.63 | 0.35 |
| Unknown | 25.1 | ND | NA | 2.18 | 0.29 |
| M4 | 26.2 | 305 | −396 | 6.61 | 0.87 |
| Unknown | 29.8 | ND | NA | 1.38 | 0.18 |
| M5 | 31.5 | 1053 | +352 | 15.0 | 1.97 |
| M6c | 32.4 | 332 | −369 | 6.75 | 0.89 |
| M7 | 32.9 | 424 | −277 | ||
| M8 | 34.3 | 877 | +176 | 10.7 | 1.41 |
| M9d | 38.5 | 877 | +176 | 4.29 | 0.57 |
| M10 | 719 | +18 | |||
| M11 | 715 | +14 | |||
| M12 | 39.6 | 717 | +16 | BITg | 0.00 |
| M13 | 40.1 | 717 | +16 | BIT | 0.00 |
| M14 | 40.9 | 717 | +16 | BIT | 0.00 |
| Posaconazole | 42.1 | 701 | 0 | BIT | 0.00 |
That is, the difference between the m/z of the metabolite and that of posaconazole.
The percentage of the dose is the percentage of chromatographic peak integration multiplied by the fraction of the dose excreted in urine. The radioactivity excreted in subjects 1 to 8 (0 to 120 h) was 13.2% of the dose.
Metabolites M6 and M7 were not resolved from each other by the radiometric detector.
Metabolites M9, M10, and M11 were not resolved from each other by the radiometric detector.
ND, not detected. No drug-related ion was detected by MS.
NA, not applicable.
BIT, below the integration threshold of the 14C detector but detected by MS.
FIG. 5.
Radioactivity profile of a pooled (0 to 120 h) urine extract after a single oral dose of 399 mg of [14C]posaconazole given to eight healthy male subjects.
(iii) Feces.
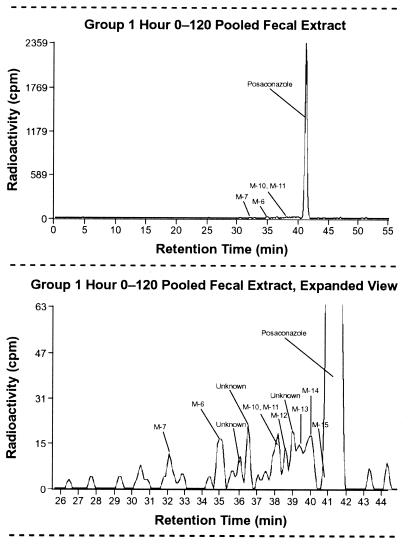
Posaconazole was the major drug-related component detected in the pooled fecal extracts (∼94% of profiled radioactivity), accounting for 66.3% of the dose (Table 4 and Fig. 6). The rest of the radioactivity was divided among a number of minor metabolites, which eluted shortly before the parent compound.
TABLE 4.
Distribution of radioactivity in pooled human fecal extracts after a single oral dose of 399 mg of [14C]posaconazole to healthy human subjects
| Peak | Rt (min) | m/z | Δa | % of profiled radioactivity | % of dose (0-120 h)b |
|---|---|---|---|---|---|
| M7 | 32.1 | 424 | −277 | 0.41 | 0.29 |
| M6 | 34.9 | 332 | −369 | 0.78 | 0.55 |
| Unknown | 36.1 | NDd | NAe | 0.54 | 0.38 |
| Unknown | 36.5 | ND | NA | 0.55 | 0.39 |
| M10c | 38.2 | 719 | +18 | 0.81 | 0.57 |
| M11 | 38.2 | 715 | +14 | ||
| M12 | 38.6 | 717 | +16 | 0.38 | 0.27 |
| Unknown | 39.0 | ND | NA | 0.61 | 0.43 |
| M13 | 39.4 | 717 | +16 | 0.54 | 0.38 |
| M14 | 40.0 | 717 | +16 | 0.96 | 0.68 |
| M15 | 40.9 | 717 | +16 | BITf | 0.00 |
| Posaconazole | 41.3 | 701 | 0 | 93.8 | 66.3 |
| Unknown | 47.0 | ND | NA | 0.32 | 0.23 |
| Unknown | 51.2 | ND | NA | 0.32 | 0.23 |
That is, the difference between the m/z of the metabolite and that of posaconazole.
The percentage of the dose is the percentage of chromatographic peak integration multiplied by the fraction of the dose excreted in feces. The radioactivity excreted in subjects 1 to 8 (0 to 120 h) was 70.7% of the dose.
Metabolites M10 and M11 were not resolved from each other by the radiometric detector.
ND, not detected. No drug-related ion was detected by MS.
NA, not applicable.
BIT, below the integration threshold of the 14C detector but detected by MS.
FIG. 6.
Radioactivity profile of a pooled (0 to 120 h) fecal extract (top) and expanded view (bottom) after a single oral dose of 399 mg of [14C]posaconazole given to eight healthy male subjects.
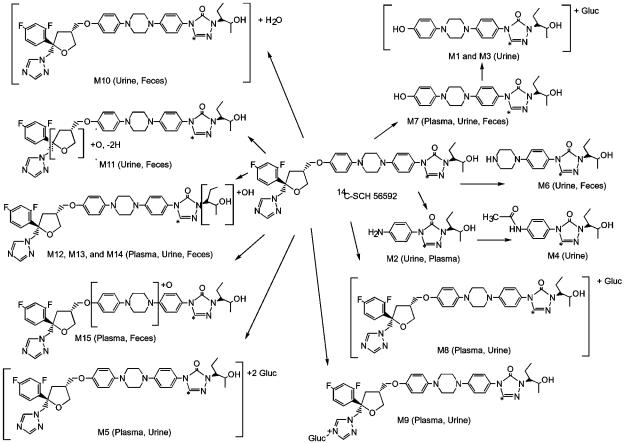
Metabolite structure elucidation.
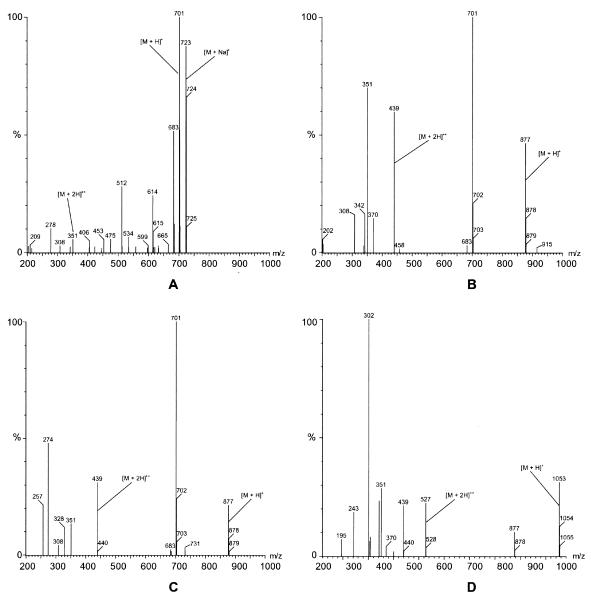
Structures of all identified metabolites are provided in Fig. 7. Posaconazole was detected by LC-MS in plasma (Fig. 8A), urine, and feces. Metabolite M8 was a glucuronide conjugate of posaconazole (Fig. 8B). It was a major circulating metabolite in plasma, constituting up to 28% of the plasma radioactivity at 12 h postdose (Table 2). Approximately 1.4% of the dose was also excreted as this metabolite in the urine. M8 exhibited an m/z of 877, which is 176 atomic mass units (amu) higher than that of posaconazole (Table 3 and Fig. 8). The full-scan mass spectrum gave a diprotonated molecular ion [M + 2H]2+ at m/z 439 and an ion at m/z 701, indicating loss of a glucuronic acid moiety from the protonated molecular ion. Because of the low amount of M8 in urine, the position of the sugar could not be determined.
FIG. 7.
Proposed metabolic pathway of [14C]posaconazole in healthy male subjects.
FIG. 8.
Representative mass spectra of posaconazole and glucuronide conjugates. (A) Posaconazole in pooled plasma collected 24 h postdose; (B) metabolite M8 in 0- to 120-h pooled urine; (C) metabolite M9 in 0- to 120-h pooled urine; (D) metabolite M5 in 0- to 120-h pooled urine from subject 2.
The M9 metabolite (m/z of 877; Fig. 8C) was identified as a glucuronide conjugate of posaconazole and was a minor metabolite in both plasma and urine. Based on the nuclear magnetic resonance spectra, it was determined that the sugar (glucuronide) is attached at the 4" position of the triazole ring. The percentage of the dose in urine associated with M9 could not be accurately determined because of its coelution with metabolites M10 and M11. However, M9 has been generated in vitro with human liver microsomes (5).
The M5 metabolite (m/z of 1,053, 352 amu higher than that of posaconazole; Table 3 and Fig. 8D) made a minor contribution to the radioactivity profile in human plasma (9% at 24 h postdose) and urine (≤2.0% of the dose). Analysis of mass spectral data indicated that M5 was a diglucuronide conjugate of posaconazole; the positions of the conjugation could not be specified.
M12, M13, and M14 were minor metabolites detected in plasma, urine, and fecal extracts. These metabolites accounted for 0.27, 0.38, and 0.68% of the dose in feces, respectively. They were below the limit of detection by radiochromatography in both plasma and urine. All three had a molecular ion with an m/z value of 717, which is 16 amu higher than that of posaconazole. All three metabolites are proposed to have a hydroxyl group on the 2-pentanol side chain.
Approximately 2% of the posaconazole was metabolized to hydroxylated or oxygenated metabolites (M10 to M15). Although M10 coeluted with M11 in urine, the metabolites could be distinguished by their respective LC-MS/MS spectra. As in urine, M10 was a minor metabolite observed in fecal extracts, with a protonated molecular ion 18 amu higher than that of posaconazole. M10 is proposed to be a water-addition product of posaconazole; the position of the addition could not be determined because of the low amount excreted. Both M11 and M15 were minor metabolites with protonated molecular ions with m/z values of 715 and 717, respectively. Based on its LC-MS/MS spectrum, it was postulated that M11 was formed by the addition of oxygen to the furan ring with the subsequent loss of two hydrogen atoms. Based on its LC-MS/MS spectrum, M15 was formed by the addition of oxygen to either the piperazine or the phenyl ring in the center of the molecule. Results from previous metabolism studies in preclinical species suggest that this metabolite is formed by oxidation of one of the piperazine carbons (unpublished data).
The minor metabolite M7 had a protonated molecular ion of m/z 424 and was observed in plasma, urine, and fecal extracts. Full-scan and LC-MS/MS spectra showed prominent ions at m/z values of 406 and 338, indicating the loss of water and the terminal 2-pentanol side chain, respectively, from the protonated molecular ion. Two other prominent ions in the LC-MS/MS spectrum were at m/z values of 203 and 136. Based on the fragmentation, the metabolite was formed by oxidation of the carbon between the furan and phenolic rings and subsequent cleavage of the molecule. M7 was further conjugated to M1 (1.08% of the dose in urine) and M3 (0.35% of the dose in urine). Both metabolites had protonated molecular ions with an m/z value of 600 and a prominent ion at m/z of 424, which indicates loss of the glucuronic acid moiety. The positions of the sugar groups could not be determined.
The M6 metabolite was found in fecal (0.55% of the dose) and urine (0.89% of the dose) extracts. It had a protonated molecular ion m/z value of 332 and prominent ions at m/z of 314 and 246 in the LC-MS/MS spectrum, indicating the loss of water and the 2-pentanol moiety, respectively. M6 was also formed nonenzymatically when posaconazole was incubated at 37°C with boiled human liver microsomes for 2 h (5). The amount of M6 formation due to either enzymatic oxidation or degradation could not be determined.
The M2 metabolite accounted for ≤7.9% of the profiled plasma radioactivity. In urine, M2 accounted for 0.6% of the total dose. In addition to a protonated molecular ion with an m/z value of 263, it showed a prominent ion at m/z 177 in its LC-MS/MS spectrum, indicating loss of the terminal 2-pentanol moiety. The M2 metabolite was then acetylated to the M4 metabolite (m/z, 305), which was detected only in urine.
Safety.
No serious adverse events, clinically significant abnormalities, changes in routine clinical laboratory test results (complete blood count, serum chemistry profiles, and urinalysis), or clinically significant changes in the electrocardiogram evaluations compared with baseline values were reported during the present study.
DISCUSSION
After administration of a single oral dose to healthy male subjects, posaconazole was orally bioavailable with a median Tmax of ∼10 h postdose. Posaconazole was slowly eliminated with a terminal elimination half-life of 20 h and was still detectable in plasma at 183 h postdose. The long half-life and the large volume of distribution (465 L) indicate a prolonged and extensive distribution of the drug into the peripheral tissues.
Most (91%) of the administered radiocarbon was recovered from the eight subjects by 336 h postdose. Elimination occurred primarily through fecal excretion (77%) and to a lesser extent through urinary excretion (14%). Most (66.3%) of the excreted drug in the feces was the parent compound. In addition, unlike other triazole antifungals, administering posaconazole oral suspension resulted in plasma drug concentrations that exceeded those of its metabolites over a 24-h period and thus enabled the active drug to predominate and to have prolonged microbiologic exposure. This is distinct from other triazole antifungals such as voriconazole, which is metabolized to inactive metabolites when absorbed upon passage through the hepatic portal system, before presentation to the systemic circulation. The inactive N-oxide metabolite of voriconazole accounts for 72% of the circulating plasma radioactivity after an oral or intravenous (i.v.) dose of this triazole (9). Conversely, the AUC of hydroxyitraconazole, the active metabolite of itraconazole, is ca. 2.5 times greater than that of the parent drug after a single oral dose (2).
Previous studies in bile duct-cannulated rats demonstrated that significant amounts (14 to 65%) of [14C]posaconazole were excreted into feces after an i.v. dose of the radiolabeled compound (P. A. Krieter, J. Achanfuo-Yeboah, M. Shea, K. Thor, M. Cayen, and J. Patrick, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1199, 1999). Thus, it is unlikely that all of the fecal posaconazole recovered was purely unabsorbed drug. Given that both ketoconazole and itraconazole are substrates for intestinal P glycoprotein (15, 17), it is likely that posaconazole is also a substrate for this transporter and therefore effluxes directly from the systemic circulation into the lumen of the gut. In some clinical studies, multiple peaks in the concentration in plasma-time profile have been observed, indicating that the drug effluxed by the MDR1 transporter is reabsorbed into the systemic circulation, resulting in greater systemic bioavailability (R. Courtney, unpublished data).
Renal excretion is a minor elimination pathway for posaconazole (CLR = 0.0114 ml/min); the small amount of posaconazole excreted in the urine is expected for a drug that is both lipophilic and highly protein bound (97 to 98.6%) to albumin. For some drugs, a low CLR also implies a slow rate of metabolic degradation because CLR can also be expressed as the volume of plasma from which a drug is metabolized per unit time. This assertion is confirmed for posaconazole, as a high proportion of profiled radioactivity in plasma (up to 79%) and then in the feces (94%) is due to parent compound.
Posaconazole metabolites excreted in urine and feces accounted for a small amount (17%) of the total radioactive dose. Most metabolites were glucuronide conjugates (M1, M3, M5, M9, and M8) rather than oxidative products (M10 to M15). Hence, the limited metabolism of posaconazole is mediated predominantly through phase 2 biotransformations via UDP-glucuronosyltransferase (UGT) enzyme pathways. Screening of 10 cDNA-expressed recombinant human UGT enzymes showed that UGT1A4 exhibited catalytic activity with respect to the formation of one of the glucuronide conjugates of posaconazole (5). These data suggest that posaconazole is not oxidized by the cytochrome P450 (CYP) pathway to the same extent as voriconazole and itraconazole (2, 9). Therefore, the metabolism of posaconazole is less likely to be altered by coadministered agents that modify the activity of CYP enzymes. Posaconazole pharmacokinetics are still affected, to a limited extent, by drugs that modify UGT enzymes and/or transporters (e.g., rifabutin).
Although posaconazole is not significantly metabolized by phase 1 enzymes, a recent in vivo study in healthy volunteers demonstrated that posaconazole inhibits hepatic CYP3A4 but no other CYP isoforms (18). Hence, posaconazole metabolism differs from that of voriconazole, which is both a substrate and an inhibitor of CYP3A4 as well as of CYP2C9 and CYP2C19 (8, 9). Because voriconazole saturates the CYP enzymes responsible for its clearance, this agent exhibits nonlinear pharmacokinetics (14), whereas posaconazole pharmacokinetics are dose proportional at up to 800 mg/day (4).
Posaconazole oral suspension, once absorbed, maintains predictable plasma concentrations of parent compound regardless of variations in the activity of the CYP enzymes between subjects. Combined with the prolonged elimination half-life, this results in a concentration in plasma-time profile that has limited fluctuations around its mean. In contrast, interindividual variability of oral and i.v. voriconazole pharmacokinetics is high, in part because of differences in CYP2C19 and CYP2C9 expression. These enzymes, which play a major role in voriconazole metabolism (8), exhibit genetic polymorphism that divides the population into poor (<5% in white and 12 to 20% in Asian populations) and extensive metabolizer phenotypes (16). Further, posaconazole concentrations in plasma should not be affected to any clinically relevant extent by other medications that inhibit or induce CYP enzymes. Although posaconazole is unlikely to be associated with major CYP-mediated drug interactions, the pharmacokinetics of coadministered medications that are metabolized via CYP3A4 will be affected. Overall, the lower drug interaction propensity of posaconazole is an important consideration given that many patients who require systemic antifungal treatment receive comedications that inhibit the CYP elimination pathways (e.g., antidepressants).
In summary, these data indicate that posaconazole is not significantly metabolized, with most of the single dose excreted unchanged in the feces. Only ∼15% of the total [14C]posaconazole dose was metabolized, primarily to multiple glucuronide conjugates. Renal elimination was a minor elimination pathway for posaconazole. The largest single radioactive component in plasma was the microbiologically active parent posaconazole, which was widely distributed to the tissues and was slowly eliminated. This prolonged exposure to the active drug was safe and well tolerated in the healthy volunteers.
Acknowledgments
We thank P. McNamara and S. Saluja (SPRI Chemical Research) for the synthesis and preparation of [14C]posaconazole; Swapan Chowdhury for comments on metabolite identification; Brian Acker (Covance Clinical Research Unit), Claudine Oelke, Amy Brinkman, Dan Albaugh, Al Fick, and Sara Dierdorff (Covance Laboratories) for the clinical conduct of the study and the radioanalysis of the samples; and Mark Laughlin and Angela Sansone (SPRI Early Clinical Research and Experimental Medicine) for applying their clinical expertise throughout.
REFERENCES
- 1.Barchiesi, F., A. M. Schimizzi, F. Caselli, D. Giannini, V. Camiletti, B. Fileni, A. Giacometti, L. F. Di Francesco, and G. Scalise. 2001. Activity of the new antifungal triazole, posaconazole, against Cryptococcus neoformans. J. Antimicrob. Chemother. 48:769-773. [DOI] [PubMed] [Google Scholar]
- 2.Barone, J. A., B. L. Moskovitz, J. Guarnieri, A. E. Hassell, J. L. Colaizzi, R. H. Bierman, and L. Jessen. 1998. Enhanced bioavailability of itraconazole in hydroxypropyl-β-cyclodextrin solution versus capsules in healthy volunteers. Antimicrob. Agents Chemother. 42:1862-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carrillo, A. J., and J. Guarro. 2001. In vitro activities of four novel triazoles against Scedosporium spp. Antimicrob. Agents Chemother. 45:2151-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Courtney, R., S. Pai, M. Laughlin, J. Lim, and V. Batra. 2003. Pharmacokinetics, safety, and tolerability of oral posaconazole administered in single and multiple doses in healthy adults. Antimicrob. Agents Chemother. 47:2788-2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghosal, A., N. Hapangama, Y. Yuan, J. Achanfuo-Yeboah, R. M. Iannucci, S. Chowdhury, K. B. Alton, J. E. Patrick, and S. Zbaida. 2004. Identification of human UDP-glucuronosyltransferase enzyme(s) responsible for the glucuronidation of posaconazole (Noxafil). Drug Metab. Dispos. 32:267-271. [DOI] [PubMed] [Google Scholar]
- 6.Gibaldi, M., and D. Perrier. 1982. Pharmacokinetics. Marcel Dekker, New York, N.Y.
- 7.Gonzalez, G. M., R. Tijerina, L. K. Najvar, R. Bocanegra, M. Rinaldi, D. Loebenberg, and J. R. Graybill. 2002. In vitro and in vivo activities of posaconazole against Coccidioides immitis. Antimicrob. Agents Chemother. 46:1352-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hyland, R., B. C. Jones, and D. A. Smith. 2003. Identification of the cytochrome P450 enzymes involved in the N-oxidation of voriconazole. Drug Metab. Dispos. 31:540-547. [DOI] [PubMed] [Google Scholar]
- 9.Jeu, L., F. J. Piacenti, A. G. Lyakhovetskiy, and H. B. Fung. 2003. Voriconazole. Clin. Ther. 25:1321-1381. [DOI] [PubMed] [Google Scholar]
- 10.Meletiadis, J., J. F. Meis, J. W. Mouton, J. L. Rodriquez-Tudela, J. P. Donnelly, and P. E. Verweij. 2002. In vitro activities of new and conventional antifungal agents against clinical Scedosporium isolates. Antimicrob. Agents Chemother. 46:62-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mellinghoff, I. K., D. J. Winston, G. Mukwaya, and G. J. Schiller. 2002. Treatment of Scedosporium apiospermum brain abscesses with posaconazole. Clin. Infect. Dis. 34:1648-1650. [DOI] [PubMed] [Google Scholar]
- 12.Pfaller, M. A., S. A. Messer, R. J. Hollis, and R. N. Jones. 2001. In vitro activities of posaconazole (SCH 56592) compared to those of itraconazole and fluconazole against 3,685 clinical isolates of Candida spp. and Cryptococcus neoformans. Antimicrob. Agents Chemother. 45:2862-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfaller, M. A., S. A. Messer, R. J. Hollis, R. N. Jones, and the SENTRY Participants Group. 2002. Antifungal activities of posaconazole, ravuconazole, and voriconazole compared to those of itraconazole and amphotericin B against 239 clinical isolates of Aspergillus spp. and other filamentous fungi: report from SENTRY Antimicrobial Surveillance Program, 2000. Antimicrob. Agents Chemother. 46:1032-1037. [DOI] [PMC free article] [PubMed]
- 14.Purkins, L., N. Wood, P. Ghahramani, K. Greenhalgh, M. J. Allen, and D. Kleinermans. 2002. Pharmacokinetics and safety of voriconazole following intravenous- to oral-dose escalation regimens. Antimicrob. Agents Chemother. 46:2546-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thiebaut, F., T. Tsuruo, H. Hamada, M. M. Gottesman, I. Pastan, and M. C. Willingham. 1987. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc. Natl. Acad. Sci. USA 84:7735-7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Venkatakrishnan, K., L. L. Von Moltke, and D. J. Greenblatt. 2000. Effects of the antifungal agents on oxidative drug metabolism: clinical relevance. Clin. Pharmacokinet. 38:111-180. [DOI] [PubMed] [Google Scholar]
- 17.Wang, E. J., K. Lew, C. N. Casciano, R. P. Clement, and W. W. Johnson. 2002. Interaction of common azole antifungals with P glycoprotein. Antimicrob. Agents Chemother. 46:160-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wexler, D., R. Courtney, W. Richards, C. Banfield, J. Lim, and M. Laughlin. 2004. Effect of posaconazole on cytochrome P450 enzymes: a randomized, open-label, two-way crossover study. Eur. J. Pharm. Sci. 21:645-653. [DOI] [PubMed] [Google Scholar]