Abstract
The features of chronic airway diseases, including chronic bronchitis, cystic fibrosis, bronchiectasis, and diffuse panbronchiolitis, include chronic bacterial infection and airway obstruction by mucus. Pseudomonas aeruginosa is one of the most common pathogens in chronic lung infection, and quorum-sensing systems contribute to the pathogenesis of this disease. The quorum-sensing signal molecule [N-(3-oxododecanoyl) homoserine lactone (3O-C12-HSL)] not only regulates bacterial virulence but also is associated with the immune response. In this study, we investigated whether 3O-C12-HSL could stimulate the production of a major mucin core protein, MUC5AC. The effect of a macrolide on MUC5AC production was also studied. 3O-C12-HSL induced NCI-H292 cells to express MUC5AC at both the mRNA and the protein levels in time- and dose-dependent manners. A 15-membered macrolide, azithromycin, inhibited MUC5AC production that was activated by 3O-C12-HSL. 3O-C12-HSL induced extracellular signal-regulated kinase (ERK) 1/2 and I-κB phosphorylation in cells, and this induction was suppressed by azithromycin. 3O-C12-HSL-induced MUC5AC production was blocked by the ERK pathway inhibitor PD98059. Our findings suggest that the P. aeruginosa autoinducer 3O-C12-HSL contributes to excessive mucin production in chronic bacterial infection. Azithromycin seems to reduce this mucin production by interfering with intracellular signal transduction.
Mucus secretion is useful for host protection of mucosal surfaces against pathogens and irritants. However, in chronic airway diseases, mucus hyperproduction is an important hallmark of pathogenesis because excessive mucus secretion causes airway obstruction and impairment of gas exchange. Thus, preventing mucus overproduction is beneficial in chronic airway diseases. The major macromolecular component of mucus is mucin protein. Of the 14 currently identified human MUC genes, the MUC5AC gene encodes the major core protein of mucin secreted from the airway surface epithelium (3, 8, 10).
Among many factors that contribute to mucin hypersecretion, bacterial infection is one of the most important in chronic airway infection, such as diffuse panbronchiolitis (DPB) and cystic fibrosis (CF). Pseudomonas aeruginosa is a major pathogen causing chronic respiratory infection in DPB and CF. Previous studies showed that P. aeruginosa airway infection stimulates mucin production (6, 13, 17, 35). These organisms use cell-to-cell communication, called a quorum-sensing system, to regulate the expression of their virulence factors (4). This communication relies on the production of small, diffusible signal molecules called acyl homoserine lactones. These molecules activate transcriptional regulators and induce the transcription of many genes. There are at least two quorum-sensing systems, Las and Rhl (7, 20). Their signal molecules are N-(3-oxododecanoyl) homoserine lactone (3O-C12-HSL) (22) and N-butyryl homoserine lactone (23), respectively. Recently, several studies suggested that 3O-C12-HSL is a signal transfer molecule and also has immunomodulatory activity (5, 27-29, 33). 3O-C12-HSL has been shown to be a potent activator of transcription factor NF-κB (27). In nonactivated cells, NF-κB is kept in the cytoplasm by the inhibitory protein I-κB. In stimulated cells, I-κB is phosphorylated and degraded by proteolysis, allowing NF-κB to enter the nucleus and activate the transcription of multiple genes. Because NF-κB activation is considered to be important in mucin production, we expected that 3O-C12-HSL may be a potent activator for mucin production.
Macrolide antibiotics have been shown to be effective for the treatment of chronic airway diseases, especially DPB (16). Because macrolides have no bactericidal activities against P. aeruginosa, their effectiveness in chronic airway diseases has been considered to depend on other actions.
In this study, we investigated whether 3O-C12-HSL-induced mucin secretion from airway epithelial cells activates airway epithelial cells to produce the mucin core protein MUC5AC.
MATERIALS AND METHODS
Materials.
3O-C12-HSL was kindly provided by B. H. Iglewski (School of Medicine and Dentistry, University of Rochester, Rochester, N.Y.). PD98059, an extracellular signal-regulated kinase (ERK) pathway inhibitor, was obtained from Promega (Madison, Wis.). Azithromycin was obtained from Pfizer (Tokyo, Japan). RPMI 1640 medium, penicillin-streptomycin, fetal bovine serum, and a reverse transcription (RT)-PCR kit were obtained from Invitrogen (Carlsbad, Calif.). A mouse MUC5AC monoclonal antibody (clone 45M1) was obtained from Neo Markers (Fremont, Calif.). Goat anti-mouse and goat anti-rabbit horseradish peroxidase (HRP)-conjugated secondary antibodies were obtained from Bio-Rad (Hercules, Calif.). Anti-ERK1/2, anti-phospho-ERK1/2, and anti-phospho-I-κB antibodies were obtained from Cell Signaling Technology (Beverly, Mass.).
Cell culture.
The NCI-H292 epithelial cell line was obtained from the American Type Culture Collection (Manassas, Va.). The cells were cultured in RPMI 1640 medium with 10% fetal bovine serum, 100 U of penicillin/ml, and 100 μg of streptomycin/ml. The cells were grown at 37°C with 5% CO2 in fully humidified air and were subcultured twice weekly. The cells were seeded in a 12-well plate at 5 × 105 cells/well. When confluent, the cells were incubated in RPMI 1640 medium containing 0.5% fetal bovine serum for 24 h. The cells then were rinsed with serum-free RPMI 1640 medium and exposed to 1, 10, or 100 μM 3O-C12-HSL for 4, 8, 12, or 24 h. For inhibition studies, cells were pretreated with 10 or 100 μg of azithromycin/ml and 50 μM PD98059 for 30 min before exposure to 3O-C12-HSL. For controls, the cells were incubated with medium alone.
RT-PCR.
The NCI-H292 cells were cultured, harvested, and subsequently washed three times with phosphate-buffered saline (PBS) containing 2% bovine serum albumin. RNA was extracted with Isogen (Nippon Gene, Toyama, Japan), and RT-PCR was performed to determine the mRNA level. Oligonucleotide primers for PCR were designed according to the published sequence for human MUC5AC (sense, ATC ACC GAA GGC TGC TTC TGT C; antisense, GTT GAT GCT GCA CAC TGT CCA G) (15). PCR products were separated by electrophoresis through a 1% agarose gel containing ethidium bromide, and the signal intensity was analyzed with NIH Image software. β-Actin controls were used to standardize the quantification of RNA samples. The β-actin bands for each condition were measured, and MUC5AC/β-actin ratios were compared.
ELISA.
The NCI-H292 cells were plated in a 12-well plate, and MUC5AC protein was measured by an enzyme-linked immunosorbent assay (ELISA) (31). The cells then were washed with cold PBS, exposed to trypsin, and formed into pellets at 700 × g at 4°C. The pellets were resuspended in lysis buffer (20 mM Tris-HCl [pH 8.0], 133 mM NaCl, 1% NP-40, 10% glycerol). The preparation then was cleared by centrifugation, and the supernatant was saved as a whole-cell lysate. Protein concentrations in the lysates were measured, and equal amounts of total protein were incubated at 40°C in a 96-well plate until dry. The plates then were washed three times with PBS, blocked with 2% bovine serum albumin for 1 h at room temperature, washed again three times with PBS, and incubated with MUC5AC antibody diluted in PBS containing 0.05% Tween 20 for 1 h. The wells were washed three times with PBS, HRP-conjugated anti-goat immunoglobulin G was dispensed into each well and, after 4 h, the plates were washed three times with PBS. Color was developed with 3,3′,5,5′-tetramethylbenzidine-peroxidase solution, and the reaction was stopped with 2 N H2SO4. The absorbance was read at 450 nm.
Western blot analysis.
Proteins were separated by reducing sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Amersham Pharmacia Biotech, Piscataway, N.J.) in 20% methanol-25 mM Tris-HCl-0.2 M glycine. Nonspecific binding was blocked by incubating the membranes with 5% skim milk in Tris-buffered saline-0.1% Tween 20 for 1 h at room temperature. Immunoreactive proteins were detected by incubating the membranes with rabbit anti-human ERK1/2, anti-phospho-ERK1/2, or anti-phospho-I-κB antibodies (each at 1:1,000) overnight at 4°C. Between each step, the membranes were washed three times for 5 min each time with Tris-buffered saline-0.1% Tween 20. Subsequently, the membranes were incubated for 1 h with anti-rabbit immunoglobulin G conjugated to HRP (1:10,000), rewashed, and developed with enhanced chemiluminescence reagents (Amersham Pharmacia Biotech).
Statistical analysis.
All data were expressed as the mean and standard error of the mean (SEM). Differences were examined for statistical significance by using one-way analysis of variance for comparisons involving more than two groups and Student's t test for comparisons between two groups. A P value of less than 0.05 denoted the presence of a statistically significant difference.
RESULTS
3O-C12-HSL up-regulates MUC5AC gene and protein expression.
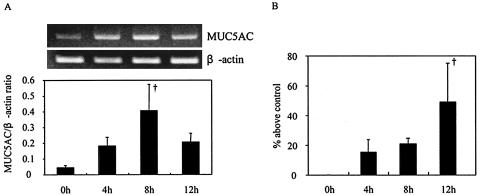
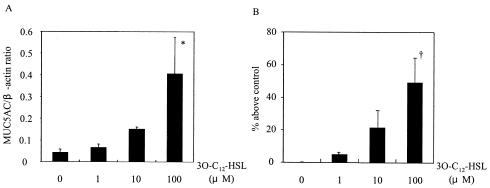
To determine whether 3O-C12-HSL can induce mucin production in NCI-H292 cells, we evaluated MUC5AC expression at both the mRNA and the protein levels after the addition of 3O-C12-HSL to the cells. We found that 3O-C12-HSL activated the cells to express MUC5AC. The level of mRNA expression (Fig. 1A) and the protein level (Fig. 1B) were maximal at 8 and 12 h after the addition of 3O-C12-HSL, respectively. The up-regulation of MUC5AC by the addition of 3O-C12-HSL occurred in a dose-dependent manner, and the level of mRNA expression (Fig. 2A) and the protein level (Fig. 2B) were maximal at 100 μM 3O-C12-HSL.
FIG. 1.
Time-dependent effect of 3O-C12-HSL on MUC5AC synthesis. NCI-H292 cells were stimulated with 100 μM 3O-C12-HSL. (A) Total RNA was isolated, and the MUC5AC mRNA level was analyzed by RT-PCR. (B) MUC5AC protein was measured by an ELISA. Data are expressed as the mean and SEM for three experiments. A dagger indicates a P value of <0.01 for a comparison with culture medium alone.
FIG. 2.
Dose-dependent effect of 3O-C12-HSL on MUC5AC synthesis. Cells were stimulated with 1, 10, or 100 μM 3O-C12-HSL. (A) The mRNA level of MUC5AC expression at 8 h after the addition of 3O-C12-HSL was analyzed by RT-PCR. (B) MUC5AC protein was measured by an ELISA at 12 h after the addition of 3O-C12-HSL. Data are expressed as the mean and SEM for three experiments. An asterisk and a dagger indicate P values of <0.05 and <0.01, respectively, for comparisons with culture medium alone.
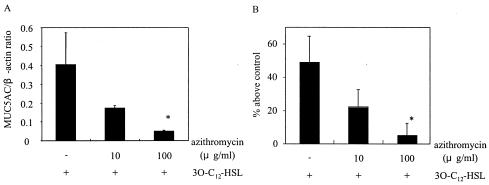
Azithromycin inhibits MUC5AC production by 3O-C12-HSL-activated cells.
Certain macrolide antibiotics are considered to reduce mucus hypersecretion. We evaluated the effect of azithromycin on MUC5AC expression induced by 3O-C12-HSL. The cells were pretreated with azithromycin at 10 or 100 μg/ml for 30 min before the addition of 3O-C12-HSL. Azithromycin at 10 μg/ml reduced MUC5AC expression by more than 50% and azithromycin at 100 μg/ml reduced MUC5AC expression by more than 90% at both the mRNA (Fig. 3A) and the protein (Fig. 3B) levels. Azithromycin had no inhibitory effect on the basal expression of MUC5AC (data not shown).
FIG. 3.
Effect of azithromycin on MUC5AC production in cells activated by 3O-C12-HSL. Cells were pretreated with 10 or 100 μg of azithromycin/ml for 30 min before exposure to 3O-C12-HSL. (A) MUC5AC mRNA expression at 8 h after the addition of 3O-C12-HSL was determined by RT-PCR. (B) MUC5AC protein was measured by an ELISA at 12 h after the addition of 3O-C12-HSL. Data are expressed as the mean and SEM for three experiments. An asterisk indicates a P value of <0.05 for a comparison with culture medium alone.
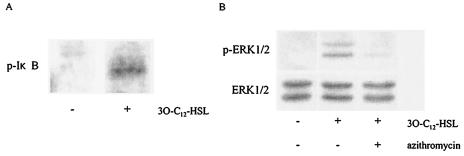
Increased I-κB and ERK1/2 phosphorylation in 3O-C12-HSL-activated cells.
To study the involvement of NF-κB activation in MUC5AC production induced by 3O-C12-HSL stimulation of NCI-H292 cells, we treated the cells with 3O-C12-HSL for 10 min and evaluated I-κB phosphorylation by Western blotting. As shown in Fig. 4A, I-κB phosphorylation was stimulated in 3O-C12-HSL-treated cells but not in untreated cells. To better understand the activation of NF-κB and the subsequent production of MUC5AC, we examined a potential role for mitogen-activated protein kinases. When the cells were stimulated with 3O-C12-HSL, the phosphorylated forms of ERK1/2 were induced. Next, we examined the effect of azithromycin on ERK1/2 phosphorylation. Pretreatment with azithromycin exerted an inhibitory effect on the phosphorylation of ERK1/2 (Fig. 4B).
FIG. 4.
Phosphorylation of I-κB and ERK1/2 induced by 3O-C12-HSL. Cells were treated with 3O-C12-HSL for 10 min and evaluated by Western blotting. (A) I-κB phosphorylation was induced in 3O-C12-HSL-treated cells but not in untreated cells. (B) ERK1/2 phosphorylation was induced in 3O-C12-HSL-treated cells. This phosphorylation was blocked by the addition of azithromycin. Data are representative of three separate experiments. p, phospho.
PD98059, an ERK1/2 inhibitor, blocks 3O-C12-HSL-induced MUC5AC production.
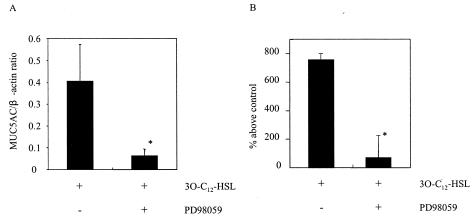
To confirm the involvement of mitogen-activated protein kinases in MUC5AC production, we examined the effect of the ERK pathway inhibitor PD98059 on MUC5AC production induced by 3O-C12-HSL. PD98059 (50 μM) blocked the synthesis of MUC5AC from 3O-C12-HSL-treated cells. This result was observed at both the mRNA (Fig. 5A; treated after 8 h and analyzed by RT-PCR) and the protein (Fig. 5B; treated after 12 h and analyzed by ELISA) levels. PD98059 had no inhibitory effect on the basal expression of MUC5AC (data not shown).
FIG. 5.
Effect of PD98059 on MUC5AC production in cells activated by 3O-C12-HSL. Cells were pretreated with 50 μM PD98059 for 30 min prior to 3O-C12-HSL stimulation. (A) MUC5AC mRNA expression was determined by RT-PCR. (B) MUC5AC protein was measured by an ELISA. Data are expressed as the mean and SEM for three experiments. An asterisk indicates a P value of <0.05 for a comparison with culture medium alone.
DISCUSSION
Chronic airway diseases, such as diffuse DPB and CF, are characterized by inflammation of the airways and mucus hypersecretion (11, 12). MUC5AC is the major core protein of mucin secreted from the airway surface epithelium. MUC5AC expression is up-regulated by various factors, such as inflammatory mediators (30) and cytokines (2, 19, 25, 34, 36).
Clinically, P. aeruginosa infection in the lungs is often accompanied by mucus overproduction. The supernatant of P. aeruginosa contains an activity that up-regulates transcription of the mucin genes (17). Lipopolysaccharide has already been confirmed to activate MUC5AC in the supernatant (6, 35).
In CF patients, P. aeruginosa has been found to grow to high concentrations (108 CFU/ml of sputum) and to live in microcolonies or biofilms (26). 3O-C12-HSL in the biofilm of P. aeruginosa was detected at ∼600 μM in an in vitro model (1). It was also reported that 3O-C12-HSL could be detected in the sputum of CF patients colonized with P. aeruginosa (26). In recent years, it has been revealed that 3O-C12-HSL acts not only as a regulator of virulence factor genes but also as a potent activator of the immune response in eukaryotic cells (5, 27, 29, 33). In this study, we showed that 3O-C12-HSL induced MUC5AC expression in airway epithelial cells (Fig. 1). These data suggest that a quorum-sensing system may play an important role in mucus hypersecretion.
Long-term treatment with macrolide antibiotics is considered to be effective in DPB (16) and CF (24). Although the reason why macrolides are effective in such diseases remains unclear, one possible mechanism is their inhibitory effect on mucus. Some studies reported that erythromycin reduced mucus production in vitro (9, 32). Kaneko et al. recently reported that clarithromycin could directly inhibit MUC5AC expression in a murine model of chronic respiratory P. aeruginosa infection (13). In the present study, we found that 10 and 100 μg of azithromycin/ml inhibited MUC5AC production induced by 3O-C12-HSL. The concentration of azithromycin was reported to reach more than 3 and 400 μg/ml in epithelial lining fluid and in alveolar macrophages after oral administration, respectively (21). Therefore, 10 and 100 μg of azithromycin/ml may be attainable concentrations in the lungs. Because DPB patients are usually treated with macrolide antibiotics at concentrations below the MICs, it has been considered that macrolides may affect the host defense mechanism. Our data support this hypothesis from the aspect of inhibitory efficacy against mucus hypersecretion. We also examined the effect of N-butyryl homoserine lactone on MUC5AC production, but hyperexpression of MUC5AC was not detected (data not shown).
Among a variety of transcriptional regulators, NF-κB has been shown to play an important role in mucin production (18). 3O-C12-HSL is reported to be a potent activator of NF-κB (27). The activation of NF-κB is controlled by an inhibitory subunit, I-κB, which retains NF-κB in the cytoplasm. Phosphorylation of I-κB leads to the nuclear translocation of NF-κB, which activates the expression of target genes in the nucleus. We showed that phosphorylation of I-κB was induced in 3O-C12-HSL-treated cells, suggesting that NF-κB was activated by the addition of 3O-C12-HSL. To determine which signal pathway is involved upstream of I-κB phosphorylation, we examined the ERK pathway. We found that ERK1/2 was phosphorylated by 3O-C12-HSL and that PD98059, an ERK pathway inhibitor, inhibited MUC5AC expression. These data indicate that the ERK pathway is important in 3O-C12-HSL-induced MUC5AC production and suggest that this pathway should be an attractive target for anti-inflammatory therapy.
Macrolide antibiotics have been shown to affect NF-κB activation (14). In our experiments, azithromycin inhibited the ERK1/2 phosphorylation induced by 3O-C12-HSL. These data indicate that azithromycin reduces MUC5AC production through an inhibitory effect on the ERK pathway. In addition, we also found that clarithromycin could inhibit the phosphorylation of ERK1/2 (data not shown), suggesting that clarithromycin should decrease MUC5AC production induced by 3O-C12-HSL.
In summary, the P. aeruginosa autoinducer 3O-C12-HSL was capable of up-regulating major mucin core protein MUC5AC. Azithromycin seemed to reduce MUC5AC production by interfering with intracellular signal transduction. Our results provide a possible explanation for the clinical efficacy of macrolides in chronic Pseudomonas airway infection.
Acknowledgments
We thank B. H. Iglewski for providing 3O-C12-HSL and F. G. Issa (Word-Medex, Sydney, Australia) for assistance with editing the manuscript.
REFERENCES
- 1.Charlton, T. S., R. de Nys, A. Netting, N. Kumar, M. Hentzer, M. Givskov, and S. Kjelleberg. 2000. A novel and sensitive method for the quantification of N-3-oxoacyl homoserine lactones using gas chromatography-mass spectrometry: application to a model bacterial biofilm. Environ. Microbiol. 2:530-541. [DOI] [PubMed] [Google Scholar]
- 2.Dabbagh, K., K. Takeyama, H. M. Lee, I. F. Ueki, J. A. Lausier, and J. A. Nadel. 1999. IL-4 induces mucin gene expression and goblet cell metaplasia in vitro and in vivo. J. Immunol. 162:6233-6237. [PubMed] [Google Scholar]
- 3.Davies, J. R., N. Svitacheva, L. Lannefors, R. Kornfalt, and I. Carlstedt. 1999. Identification of MUC5B, MUC5AC and small amounts of MUC2 mucins in cystic fibrosis airway secretions. Biochem. J. 344:321-330. [PMC free article] [PubMed] [Google Scholar]
- 4.de Kievit, T. R., and B. H. Iglewski. 2000. Bacterial quorum sensing in pathogenic relationships. Infect. Immun. 68:4839-4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DiMango, E., H. J. Zar, R. Bryan, and A. Prince. 1995. Diverse Pseudomonas aeruginosa gene products stimulate respiratory epithelial cells to produce interleukin-8. J. Clin. Investig. 96:2204-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dohrman, A., S. Miyata, M. Gallup, J. D. Li, C. Chapelin, A. Coste, E. Escudier, J. Nadel, and C. Basbaum. 1998. Mucin gene (MUC 2 and MUC 5AC) upregulation by gram-positive and gram-negative bacteria. Biochim. Biophys. Acta 1406:251-259. [DOI] [PubMed] [Google Scholar]
- 7.Gambello, M. J., S. Kaye, and B. H. Iglewski. 1993. LasR of Pseudomonas aeruginosa is a transcriptional activator of the alkaline protease gene (apr) and an enhancer of exotoxin A expression. Infect. Immun. 61:1180-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gendler, S. J., and A. P. Spicer. 1995. Epithelial mucin genes. Annu. Rev. Physiol. 57:607-634. [DOI] [PubMed] [Google Scholar]
- 9.Goswami, S. K., S. Kivity, and Z. Marom. 1990. Erythromycin inhibits respiratory glycoconjugate secretion from human airways in vitro. Am. Rev. Respir. Dis. 141:72-78. [DOI] [PubMed] [Google Scholar]
- 10.Hovenberg, H. W., J. R. Davies, A. Herrmann, C. J. Linden, and I. Carlstedt. 1996. MUC5AC, but not MUC2, is a prominent mucin in respiratory secretions. Glycoconj. J. 13:839-847. [DOI] [PubMed] [Google Scholar]
- 11.Jany, B., and C. B. Basbaum. 1991. Mucin in disease. Modification of mucin gene expression in airway disease. Am. Rev. Respir. Dis. 144:S38-S41. [DOI] [PubMed] [Google Scholar]
- 12.Kaliner, M., J. H. Shelhamer, B. Borson, J. Nadel, C. Patow, and Z. Marom. 1986. Human respiratory mucus. Am. Rev. Respir. Dis. 134:612-621. [DOI] [PubMed] [Google Scholar]
- 13.Kaneko, Y., K. Yanagihara, M. Seki, M. Kuroki, Y. Miyazaki, Y. Hirakata, H. Mukae, K. Tomono, J. Kadota, and S. Kohno. 2003. Clarithromycin inhibits overproduction of muc5ac core protein in murine model of diffuse panbronchiolitis. Am. J. Physiol. Lung Cell Mol. Physiol. 285:L847-L853. [DOI] [PubMed] [Google Scholar]
- 14.Kikuchi, T., K. Hagiwara, Y. Honda, K. Gomi, T. Kobayashi, H. Takahashi, Y. Tokue, A. Watanabe, and T. Nukiwa. 2002. Clarithromycin suppresses lipopolysaccharide-induced interleukin-8 production by human monocytes through AP-1 and NF-kappa B transcription factors. J. Antimicrob. Chemother. 49:745-755. [DOI] [PubMed] [Google Scholar]
- 15.Kim, Y. D., E. J. Kwon, D. W. Park, S. Y. Song, S. K. Yoon, and S. H. Baek. 2002. Interleukin-1beta induces MUC2 and MUC5AC synthesis through cyclooxygenase-2 in NCI-H292 cells. Mol. Pharmacol. 62:1112-1118. [DOI] [PubMed] [Google Scholar]
- 16.Kudoh, S., A. Azuma, M. Yamamoto, T. Izumi, and M. Ando. 1998. Improvement of survival in patients with diffuse panbronchiolitis treated with low-dose erythromycin. Am. J. Respir. Crit. Care Med. 157:1829-1832. [DOI] [PubMed] [Google Scholar]
- 17.Li, J. D., A. F. Dohrman, M. Gallup, S. Miyata, J. R. Gum, Y. S. Kim, J. A. Nadel, A. Prince, and C. B. Basbaum. 1997. Transcriptional activation of mucin by Pseudomonas aeruginosa lipopolysaccharide in the pathogenesis of cystic fibrosis lung disease. Proc. Natl. Acad. Sci. USA 94:967-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li, J. D., W. Feng, M. Gallup, J. H. Kim, J. Gum, Y. Kim, and C. Basbaum. 1998. Activation of NF-kappaB via a Src-dependent Ras-MAPK-pp90rsk pathway is required for Pseudomonas aeruginosa-induced mucin overproduction in epithelial cells. Proc. Natl. Acad. Sci. USA 95:5718-5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Longphre, M., D. Li, M. Gallup, E. Drori, C. L. Ordonez, T. Redman, S. Wenzel, D. E. Bice, J. V. Fahy, and C. Basbaum. 1999. Allergen-induced IL-9 directly stimulates mucin transcription in respiratory epithelial cells. J. Clin. Investig. 104:1375-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ochsner, U. A., and J. Reiser. 1995. Autoinducer-mediated regulation of rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 92:6424-6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olsen, K. M., G. San Pedro, L. P. Gann, P. O. Gubbins, D. M. Halinski, and G. D. Campbell, Jr. 1996. Intrapulmonary pharmacokinetics of azithromycin in healthy volunteers given five oral doses. Antimicrob. Agents Chemother. 40:2582-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pearson, J. P., K. M. Gray, L. Passador, K. D. Tucker, A. Eberhard, B. H. Iglewski, and E. P. Greenberg. 1994. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc. Natl. Acad. Sci. USA 91:197-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pearson, J. P., L. Passador, B. H. Iglewski, and E. P. Greenberg. 1995. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 92:1490-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saiman, L., B. C. Marshall, N. Mayer-Hamblett, J. L. Burns, A. L. Quittner, D. A. Cibene, S. Coquillette, A. Y. Fieberg, F. J. Accurso, and P. W. Campbell III. 2003. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA 290:1749-1756. [DOI] [PubMed] [Google Scholar]
- 25.Shim, J. J., K. Dabbagh, I. F. Ueki, T. Dao-Pick, P. R. Burgel, K. Takeyama, D. C. Tam, and J. A. Nadel. 2001. IL-13 induces mucin production by stimulating epidermal growth factor receptors and by activating neutrophils. Am. J. Physiol. Lung Cell Mol. Physiol. 280:L134-L140. [DOI] [PubMed] [Google Scholar]
- 26.Singh, P. K., A. L. Schaefer, M. R. Parsek, T. O. Moninger, M. J. Welsh, and E. P. Greenberg. 2000. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 407:762-764. [DOI] [PubMed] [Google Scholar]
- 27.Smith, R. S., E. R. Fedyk, T. A. Springer, N. Mukaida, B. H. Iglewski, and R. P. Phipps. 2001. IL-8 production in human lung fibroblasts and epithelial cells activated by the Pseudomonas autoinducer N-3-oxododecanoyl homoserine lactone is transcriptionally regulated by NF-kappa B and activator protein-2. J. Immunol. 167:366-374. [DOI] [PubMed] [Google Scholar]
- 28.Smith, R. S., S. G. Harris, R. Phipps, and B. Iglewski. 2002. The Pseudomonas aeruginosa quorum-sensing molecule N-(3-oxododecanoyl)homoserine lactone contributes to virulence and induces inflammation in vivo. J. Bacteriol. 184:1132-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith, R. S., R. Kelly, B. H. Iglewski, and R. P. Phipps. 2002. The Pseudomonas autoinducer N-(3-oxododecanoyl) homoserine lactone induces cyclooxygenase-2 and prostaglandin E2 production in human lung fibroblasts: implications for inflammation. J. Immunol. 169:2636-2642. [DOI] [PubMed] [Google Scholar]
- 30.Takeyama, K., K. Dabbagh, J. Jeong Shim, T. Dao-Pick, I. F. Ueki, and J. A. Nadel. 2000. Oxidative stress causes mucin synthesis via transactivation of epidermal growth factor receptor: role of neutrophils. J. Immunol. 164:1546-1552. [DOI] [PubMed] [Google Scholar]
- 31.Takeyama, K., K. Dabbagh, H. M. Lee, C. Agusti, J. A. Lausier, I. F. Ueki, K. M. Grattan, and J. A. Nadel. 1999. Epidermal growth factor system regulates mucin production in airways. Proc. Natl. Acad. Sci. USA 96:3081-3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tamaoki, J., K. Takeyama, I. Yamawaki, M. Kondo, and K. Konno. 1997. Lipopolysaccharide-induced goblet cell hypersecretion in the guinea pig trachea: inhibition by macrolides. Am. J. Physiol. Lung Cell Mol. Physiol. 272:L15-L19. [DOI] [PubMed] [Google Scholar]
- 33.Telford, G., D. Wheeler, P. Williams, P. T. Tomkins, P. Appleby, H. Sewell, G. S. Stewart, B. W. Bycroft, and D. I. Pritchard. 1998. The Pseudomonas aeruginosa quorum-sensing signal molecule N-(3-oxododecanoyl)-l-homoserine lactone has immunomodulatory activity. Infect. Immun. 66:36-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Temann, U. A., B. Prasad, M. W. Gallup, C. Basbaum, S. B. Ho, R. A. Flavell, and J. A. Rankin. 1997. A novel role for murine IL-4 in vivo: induction of MUC5AC gene expression and mucin hypersecretion. Am. J. Respir. Cell Mol. Biol. 16:471-478. [DOI] [PubMed] [Google Scholar]
- 35.Yanagihara, K., M. Seki, and P. W. Cheng. 2001. Lipopolysaccharide induces mucus cell metaplasia in mouse lung. Am. J. Respir. Cell Mol. Biol. 24:66-73. [DOI] [PubMed] [Google Scholar]
- 36.Yoon, J. H., K. S. Kim, H. U. Kim, J. A. Linton, and J. G. Lee. 1999. Effects of TNF-alpha and IL-1 beta on mucin, lysozyme, IL-6 and IL-8 in passage-2 normal human nasal epithelial cells. Acta Otolaryngol. 119:905-910. [DOI] [PubMed] [Google Scholar]