Abstract
Eritoran, a structural analogue of the lipid A portion of lipopolysaccharide (LPS), is an antagonist of LPS in animal and human endotoxemia models. Previous studies have shown that low doses (350 to 3,500 μg) of eritoran have demonstrated a long pharmacokinetic half-life but a short pharmacodynamic half-life. The present study describes the safety, pharmacokinetics and pharmacodynamics, and lipid distribution profile of eritoran during and after a 72-h intravenous infusion of 500, 2,000, or 3,500 μg/h into healthy volunteers. Except for the occurrence of phlebitis, eritoran administration over 72 h was safe and well tolerated. Eritoran demonstrated a slow plasma clearance (0.679 to 0.930 ml/h/kg of body weight), a small volume of distribution (45.6 to 49.8 ml/kg), and a relatively long half-life (50.4 to 62.7 h). In plasma, the majority (∼55%) of eritoran was bound to high-density lipoproteins. During infusion and for up to 72 h thereafter, ex vivo response of blood to 1- or 10-ng/ml LPS was inhibited by ≥85%, even when the lowest dose of eritoran (500 μg/h) was infused. Inhibition of response was dependent on eritoran dose and the concentration of LPS used as an agonist. Finally, in vitro analysis with purified lipoprotein and protein fractions from plasma obtained from healthy volunteers indicated that eritoran is inactivated by high-density but not low-density lipoproteins, very-low-density lipoproteins, or albumin. From these results, we conclude that up to 252 mg of eritoran can be safely infused into normal volunteers over 72 h and even though it associates extensively with high-density lipoproteins, antagonistic activity is maintained, even after infusion ceases.
Eritoran (E5564) is a synthetic lipid A analogue (10) that has been designed to antagonize the effects of lipopolysaccharide (LPS) (9) and has been found to do this by interacting with Toll-like receptor 4 (4), the recently-identified cell surface receptor for LPS (1, 5). Clinically, eritoran is being investigated for the treatment of severe sepsis, septic shock, and other endotoxin-mediated indications. Results from in vitro studies have found eritoran to be a highly active antagonist of the action of LPS on responsive cells, but low concentrations of eritoran have a relatively short pharmacodynamic (PD) half-life in blood or plasma that is observable in the absence of clearance or measurable metabolism. In vivo studies of low doses administered as short infusions into human volunteers (17) and animal model studies (11) further support this observation by demonstrating that eritoran is extremely active when coadministered with LPS, but its activity decreases shortly after ending infusion into humans and dogs. This occurs even though eritoran has a plasma elimination half-life of 40 to 50 h (11, 17). For this reason, it is likely that dosing of eritoran needs to be adjusted to overcome this loss in activity.
Studies of the association of lipophilic drugs such as amphotericin B and cyclosporine have found that interaction of these drugs with serum lipoproteins can affect their toxicity and activity (13), and studies with cyclosporine have suggested that an alteration in plasma lipid concentrations can modify pharmacological behavior (14) and nephrotoxicity (12, 13). Preliminary work by Wasan et al. using radiolabeled E5531, a similarly structured narrow-spectrum LPS antagonist (16), and, more recently, [14C]eritoran (15) suggests that these types of molecules predominantly bind to high-density lipoproteins (HDL) during incubation in human serum and that interaction with lipoproteins occurs within 5 min, with no measurable redistribution between lipoprotein fractions (16). Studies by Rose and coworkers have supported the importance of plasma lipoprotein binding in influencing the long-term effectiveness of E5531 (8). In these in vitro studies, E5531 was inactivated within several hours of being bound to HDL, while little or no loss in activity occurred when associated with low-density lipoprotein (LDL) or very-low-density lipoprotein (VLDL) (8). Preliminary studies with eritoran have yielded results suggesting that eritoran also was inactivated, but more slowly than E5531 (4), providing compelling evidence that eritoran may be a more pharmacologically effective antagonist of endotoxin.
While limited information is available about the lipoprotein distribution and PD of eritoran after in vivo infusion, in vitro research has determined that eritoran partitions into lipoproteins and is deactivated without being degraded. Our first phase I study (E5564-A001-001) established that after 30-min infusion of 500 to 3,500 μg of eritoran, activity is relatively short-lived, compared to its pharmacokinetic half-life (17).
In this phase I study, the plasma protein and lipoprotein distributions of eritoran were determined following a 72-h intravenous infusion in healthy volunteers. Another study of 72-h infusion into healthy volunteers followed by administration of endotoxin indicated that activity of eritoran against low-dose endotoxin could be retained for at least 72 h after ending eritoran infusion. However, human in vivo studies employing endotoxin administration to normal volunteers are obviously limited by the ability to perform multiple or high-dose endotoxin administrations, such as might be observed during severe sepsis and septic shock. For this reason, we have applied this model of ex vivo testing to determine how effectively eritoran blocks higher doses of endotoxin (up to 10 ng/ml) and for how long after test drug administration. The information obtained from these studies could be utilized to determine the in vivo lipoprotein distribution of eritoran, correlate the results to those obtained from PD assays, and compare them to results obtained in vitro. Combining these correlations with results from our previous in vivo endotoxin challenge studies should enable us to predict human eritoran dosing to protect against more toxic LPS exposures.
The PD activity of eritoran was determined by use of an ex vivo assay that measures the induction of tumor necrosis factor alpha (TNF-α) by LPS added to whole-blood samples drawn from placebo- and eritoran-treated subjects. After a 3-h incubation with 0, 1, or 10 ng of LPS per ml, plasma from these samples was tested for cellular activation by determining concentrations of TNF-α. LPS-induced TNF-α was measured in blood samples taken from the volunteers prior to drug administration to serve as controls for predose (baseline) LPS response. After eritoran infusion, blood samples generated less TNF-α, indicating eritoran inhibitory activity. This study confirms that eritoran demonstrates potent ex vivo activity during and after 72-h infusions of 36- to 252-mg doses.
Finally, in vitro studies measuring the ability of different plasma protein or lipoprotein fractions to inhibit the antagonistic action of eritoran confirm results previously reported for another lipid A antagonist (E5531). The results described here indicate that HDL inactivates eritoran, whereas long-term antagonistic activity can be obtained when eritoran associates with LDL, triglyceride-rich lipoproteins (TRL), and albumin.
(Portions of this study were presented at the 14th World Congress of Pharmacology, San Francisco, Calif., 7 to 12 July 2002.)
MATERIALS AND METHODS
Reagents.
Eritoran {CAS chemical name, α-d-glucopyranose, 3-O-decyl-2-deoxy-6-O-[2-deoxy-3-O-[(3R)-3-methoxydecyl]-6-O-methyl-2-[[(11Z)-1-oxo-11-octadecenyl]amino]-4-O-phosphono-β-d-glucopyranosyl]-2-[(1,3-dioxotetradecyl)amino-, 1-(dihydrogen phosphate), tetrasodium salt; CAS registry no. 185954-98-7} was synthesized at Eisai Research Institute Chemical Development, was >94% pure, and was formulated as described previously (3). LPS (from Escherichia coli O111:B4) from List Biological Laboratories and other reagents have been described previously (7, 8). Endotoxin-free albumin was purchased from Sigma Chemical (St. Louis, Mo.).
Origin of samples and study design.
This study was a single-center, randomized, double-blind, placebo-controlled, 72-h- infusion, sequential-group study of eritoran in healthy male volunteers (five eritoran treated and two placebo treated per dose group). Three doses of eritoran (500, 2,000, and 3,500 μg/h) were studied. Eritoran or matching placebo was administered via 72-h intravenous infusion.
Informed consent was obtained from all subjects, and human experimentation guidelines of the U.S. Department of Health and Human Services were followed in the conduct of this clinical research.
Twenty-two of 23 subjects (5 to 7 per treatment group) enrolled into this study completed the study and were discharged as scheduled. One subject in the placebo group withdrew consent. One subject in the 500-μg/h group was inadvertently administered an 8-h dose of drug over 10 min. Although there were no observable adverse effects of this overdose, this subject was discontinued from further dosing and was not included in this pharmacokinetic (PK) and PD analysis.
PK.
Healthy volunteers dosed with 500, 2,000, or 3,500 μg/h were assayed for eritoran content in EDTA-plasma samples collected up to 408 h, using a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) method (17), Briefly, plasma (0.5 ml) was mixed with internal standard (IS; E5531) solution and then extracted with methanol (MeOH). The supernatant was collected and evaporated to dryness at 50°C under a nitrogen stream and then reconstituted with MeOH. Each sample was analyzed on a Quattro LC-MS/MS system (Micromass, Inc., Beverly, Mass.), using electrospray ionization under the negative-ion mode. Eritoran was monitored at precursor ion m/z at 1,312 and product ion m/z at 159, and the IS was monitored at precursor ion m/z at 1,454 and product ion m/z at 159. The mobile phase was a mixture of 100% MeOH, 1% acetic acid, and 0.1% trifloroacetic acid. A 15-μl aliquot was injected onto a Luna phenyl-hexyl (3 μm, 2 by 100 mm; Phenomenex, Torrance, Calif.) column. The retention times for IS and eritoran were 2.8 and 3.4 min, respectively. Standard curves for eritoran were linear over the concentration range of 5 to 1,000 ng/ml. The accuracy of the quality control (QC) samples, based on a percent difference, ranged from −8.7 to 11.8%. The overall coefficients of variation of the QCs ranged from 5.5 to 13.6%. PK parameters for eritoran were estimated by noncompartmental methods with PC WINNONLIN.
The high- and low-dose volunteers (500 and 3,500 μg/h, respectively) were also assayed for eritoran content in different lipoprotein fractions. At 1, 4, 24, 48, 72 (end of infusion), 84, 96, and 144 h from the initiation of the infusion, EDTA-blood samples were collected and plasma was separated. In vitro pilot studies indicated that greatest stability of distribution of eritoran in lipoprotein fractions was obtained after storage at 4°C without freezing. For this reason, all samples for lipoprotein separation were stored at 4°C and shipped (on ice) within 48 h.
Plasma was separated into HDL, LDL, TRL (which contains VLDL plus chylomicrons), and lipoprotein-deficient plasma (LPDP) fractions by single-spin density gradient ultracentrifugation (13) and analyzed for eritoran content by LC-MS/MS as described above, using an external calibration curve for each fraction. For all experiments, cholesterol, triglyceride (TG), and protein concentrations were quantified in each fraction by established colorimetric, fluorometric, chromatographic, and radioactivity techniques (14).
LPS-inhibitory activity of eritoran in human whole blood after HDL, LDL, TRL, LPDP, and albumin pretreatment.
The HDL, LDL, TRL, and LPDP fractions were separated by density step-gradient ultracentrifugation (13). The cholesterol, TG, and protein contents of each fraction were determined by enzymatic colorimetric assays (ThermoDNA and Bio-Rad, respectively). Eritoran (50 nM) was preincubated in the respective lipoprotein or protein fraction for 18 h and diluted fivefold into fresh human blood prepared as described previously (8) and incubated with 10-ng/ml LPS with gentle shaking at 37°C and a 5% CO2 atmosphere. After 3 h, the samples were centrifuged at 1,000 × g for 10 min at 4°C. Plasma was then collected and stored at −80°C until analysis. The plasma samples were analyzed for TNF-α by an enzyme-linked immunosorbent assay (ELISA) as previously described (8). Normal saline served as the control for all studies. Controls for the presence of protein or lipoprotein in the LPS stimulation assays were performed by preincubating similar concentrations of the indicated protein or lipoprotein (no eritoran) for 18 h, diluting them fivefold into fresh human blood, and treating these dilutions with LPS as described above.
PD analysis of eritoran activity in whole blood.
Ex vivo activity was assessed as previously described (17) and was procedurally similar to in vitro analysis of eritoran activity (8). Briefly, samples of heparinized whole blood (0.40 ml) were dispensed to a 48-well cell culture cluster plate in triplicate, 50 μl of phosphate-buffered saline was added, and then 50 μl of LPS in D5W (5% dextrose in water) or D5W alone was added to generate samples containing 10, 1, 0.1, or 0-ng/ml LPS (final concentration). After 3 h of incubation at 37°C at 5% CO2 with gentle shaking (Belco plate shaker), all samples were reduced to plasma by centrifugation (900 × g) with an ELISA plate centrifuge. Samples (100 μl) were transferred into wells of duplicate 96-well microtiter plates, quick-frozen, and stored at −80°C until assay by ELISA.
Blood samples from each time point were evaluated for the possibility that they were contaminated during preparation by incubating a sample of each preparation in the absence of LPS (0 LPS data points). Of all samples assayed, two sets (0.9% of our tests) were excluded from our analyses. Samples at 108 h from two subjects and one sample at 76 h from one subject generated measurable amounts of TNF-α and were excluded from analysis of group means and the standard error of the means (SE).
A second blood sample at 76 h was 524% of the control value. (This value could be compared to the other three samples taken at this time that yielded results of 11, 0, and 0% response.). In addition, at the 144-h time point, results from one subject were omitted from analysis (781% of the control in this assay). The latter values are likely to represent an error in the assay or contamination of the samples.
Statistics.
All data are presented by dose group as mean ± standard deviation (SD) or SE. Protocol violators and subjects who received placebo were excluded from the PK analysis. The mean and SD were calculated with Excel 97 (Microsoft Corporation, Redmond, Wash.). Statistical analyses were performed with Unix-based SAS (version 7.0; SAS Institute, Cary, N.C.). A nonparametric (Kruskal-Wallis test) method was used to examine effects of dose on eritoran PK parameters. A multiple pair comparison was also performed, if the P value was < 0.05, and linearity of the relationship between dose and PK parameters was examined by using Spearman's test (17). A P value of < 0.05 was considered statistically significant. Correlation coefficients between the amount of eritoran recovered within the TRL, LDL, and HDL plasma fractions and the amounts of cholesterol, TG, and protein within these fractions were determined by using Pearson's test (18). Distribution of eritoran among different plasma groups was compared by analysis of variance (INSTAT, version 2.0; GraphPad Software). Critical differences were assessed by Newman-Keuls post hoc tests, considered significant with a P value of <0.05.
RESULTS
Safety.
Subjects were observed during the 72-h infusion and for up to 144 h following infusion. The only adverse events that occurred more than one time in these subjects were headache in three eritoran-treated subjects (19%) versus four subjects (19%) in the placebo group, rhinitis in two subjects (13%) in the eritoran-treated group, and a self-limiting phlebitis in 57% of the subjects in the placebo group versus 83% of the subjects in the 500-μg/h group and all subjects in the 2,000- and 3,500-μg/h groups. Differences in occurrence of phlebitis between subject groups were not statistically significant, and although there was a trend of worsening severity of phlebitis in the higher-dose groups, differences again did not reach statistical significance (Fisher's exact test).
PK.
As shown in Table 1, characterization of the PK of infused eritoran indicates that it appears to be confined to the vascular space, demonstrating a small volume of distribution (Vdss; 45.6 to 49.8 ml/kg). Eritoran is slowly cleared (0.679 to 0.930 ml/h/kg) and has a relatively long half-life (50.4 to 62.7 h). The maximum concentration of drug in serum (Cmax) and the area under the concentration-time curve from 0 h to infinity (AUC0-inf) were positively correlated with dose from 500 μg/h (total dose, 36 mg) to 3,500 μg/h (total dose, 252 mg). Clearance was negatively correlated with dose with a correlation coefficient of −0.81262 and a P value of <0.05. The clearance ranged from 0.930 (500 μg/h) to 0.808 (2.0 mg/h) to 0.679 (3,500 μg/h) ml/h/kg. The median time to maximum concentration of drug in serum (tmax) of eritoran was determined at the end of the 72-h infusion for all three groups. The elimination half-lives (t1/2) were 50.4 (500 μg/h), 56.9 (2,000 μg/h), and 62.7 (3,500 μg/h) and were positively correlated with dose.
TABLE 1.
Plasma levels and PK parameters of eritoran in healthy male volunteers following administration of a 72-h infusion of eritoran
| Parameter | 72-h infusion dose (μg/h)
|
||
|---|---|---|---|
| 500 | 2,000 | 3,500 | |
| Total dose (mg) | 36 | 144 | 252 |
| AUC | |||
| 0-408 h (μg/h/ml) | 460.6 ± 59.7cd | 2205.7 ± 296.9bd | 5082.1 ± 628.6bc |
| 0-infinity (μg/h/ml) | 462.4 ± 59.9cd | 2223.9 ± 307.0bd | 5142.8 ± 625.9bc |
| tmax in h (range)a | 72 (72-72) | 72 (72-76) | 72 (72-80) |
| Cmax (μg/ml) | 4.09 ± 0.564cd | 18.7 ± 2.01bd | 41.9 ± 5.86bc |
| t1/2 (h) | 50.4 ± 1.6cd | 56.9 ± 4.8bd | 62.7 ± 3.2bc |
| CL | |||
| ml/h | 78.8 ± 8.73cd | 65.6 ± 7.89bd | 49.5 ± 5.46bc |
| ml/h/kg | 0.930 ± 0.0502cd | 0.808 ± 0.122bd | 0.679 ± 0.0477bc |
| Vdss | |||
| Liters | 4.22 ± 0.559d | 4.04 ± 0.403 | 3.34 ± 0.582b |
| ml/kg | 49.8 ± 3.07 | 49.5 ± 3.99 | 45.6 ± 4.93 |
Values represent the median and the range.
Significantly different from the 500-μg/kg group.
Significantly different from the 2,000-μg/kg group.
Significantly different from the 3,500-μg/kg group.
PK analysis of interaction of eritoran with plasma lipoproteins.
Plasma protein and lipoprotein distributions for the 500- and 3,500-μg/h eritoran groups were determined at 1, 4, 24, and 72 h (during infusion) and at 84, 96, and 144 h (12, 24, and 72 h following intravenous infusion). These samples were separated into plasma protein and lipoprotein fractions by density gradient centrifugation and analyzed for eritoran content.
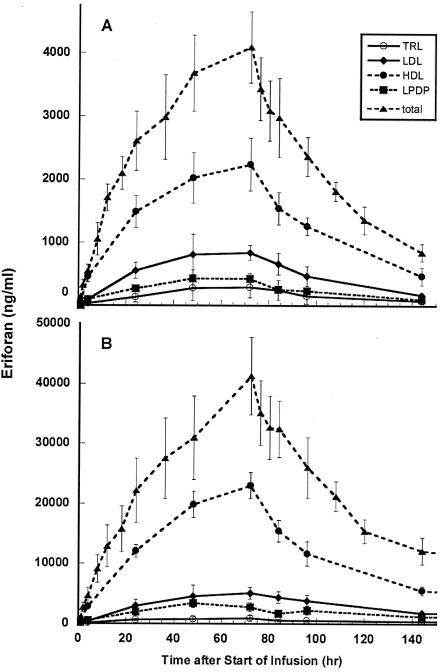
The amount of eritoran found in each lipoprotein or protein fraction during and following infusion of 500 μg of eritoran per h is shown in Fig. 1A. Eritoran was measurable in all samples, except for those obtained 1 h after beginning infusion, after which the eritoran content of samples was found to be remarkably consistent (Table 2).
FIG. 1.
Distribution of eritoran into lipoproteins during and after infusion of an eritoran dose of 500 μg/h for 72 h (A), or 3,500 μg/h for 72 h (B). Plasma samples taken from volunteers at the times indicated on the abcissa were separated into their lipoprotein and lipoprotein-deficient components and analyzed for eritoran content by LC-MS/MS as described in Materials and Methods. Total plasma eritoran content (▴) and eritoran found in the TRL (○), LDL (♦), HDL (•), and LPDP (▪) fractions are displayed. Results from samples taken at 408 h were at or below limits of detection in the low-dose group. In panel B, results from samples taken at 408 h were omitted for clarity.
TABLE 2.
Distribution of eritoran in different plasma fractions after infusion at 500 and 3,500 μg/h
| Time | % Eritoran found in fractiona:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| HDL
|
LDL
|
TRL
|
LPDP
|
|||||
| 500 μg/h | 3,500 μg/h | 500 μg/h | 3,500 μg/h | 500 μg/h | 3,500 μg/h | 500 μg/h | 3,500 μg/h | |
| 1 h | 84.8 (22.2) (n = 2) | 65.9 (15.3) | 21.1 (n = 1) | 12.5 (3.8) | bql | 4.3 (1.2) | bql | 10.8 (1.8) |
| 4 h | 84.2 (11.4) | 65.1 (12.9) | 17.5 (6.1) | 7.4 (1.2) | 5.3 (5.5) | 2.7 (1.0) | 17.5 (2.4) | 11.4 (2.5) |
| 24 h | 58.7 (16.3) | 57.8 (11.1) | 21.3 (3.4) | 13.3 (2.0) | 5.0 (2.1) | 3.0 (0.4) | 10.4 (0.7) | 8.8 (2.3) |
| 48 h | 55.4 (10.3) | 65.8 (9.5) | 21.5 (5.3) | 15.2 (7.4) | 7.1 (4.1) | 2.6 (1.3) | 11.6 (2.8) | 11.3 (2.1) |
| 72 h (end of infusion) | 55.4 (12.9) | 56.9 (11.3) | 20.6 (2.2) | 12.5 (2.7) | 7.0 (3.6) | 2.3 (1.4) | 10.4 (0.9) | 6.6 (0.8)* |
| 84 h | 52.9 (11.3) | 48.1 (6.7) | 22.1 (3.4) | 13.5 (2.6) | 7.9 (3.6) | 1.6 (0.6) | 8.7 (1.6)* | 5.2 (1.3)* |
| 96 h | 54.2 (10.8) | 46.0 (10.8) | 19.5 (4.5) | 14.7 (2.2) | 8.0 (5.2) | 1.9 (0.3) | 6.5 (5.5)* | 8.5 (1.6) |
| 144 h | 55.2 (12.2) | 50.1 (8.0) | 20.0 (2.9) | 16.6 (5.1) | 9.8 (6.8) | 3.6 (1.8) | 12.6 (2.3) | 10.9 (3.3) |
| Mean (SE) | 55.3 (0.786) | 54.1 (7.43) | 20.8 (0.400) | 14.3 (0.609) | 7.47 (0.642) | 2.5 (0.298) | 10.03 (0.886) | 8.55 (0.969) |
Shown is the percentage of total plasma drug concentration at the designated time point. The range of values is given; otherwise data are expressed as the mean (± SD) of samples from five subjects unless otherwise noted (i.e., the remainder from the five samples was below the quantifiable limit [bql]). The quantifiable limit was 10 ng/ml in all samples. *, P < 0.05 versus the 4-h point by Student's t test correlation analysis. LPDP, contains albumin and α-1-glycoprotein.
As depicted in Fig. 1A and Table 2, at 24 to 144 h from the initiation of the infusion, 53 to 59% of eritoran was recovered in the HDL fraction, 20 to 22% was recovered in the LDL fraction, 5 to 10% was recovered in the TRL fraction, and 6 to 13% was recovered in the LPDP fraction. At 408 h, total eritoran was 25.4 ± 4 ng/ml, with the eritoran content in individual lipoprotein fractions below detection limits.
Distribution of eritoran into lipoproteins during and after infusion of 3,500 μg/h was similar to that seen during infusion of the lower dose. Figure 1B and Table 2 display these results. At this dose, even the samples obtained at 1 h after beginning infusion contained measurable eritoran. At 1 to 144 h from the initiation of the infusion, 46 to 66% eritoran was recovered in the HDL fraction, 7 to 17% was recovered in the LDL fraction, 2 to 4% was recovered in the TRL fraction, and 5 to 11% was recovered in the LPDP fraction.
Is distribution of eritoran into lipoproteins dependent on lipoprotein concentration?
To determine if interindividual differences in lipoprotein distribution affect the amount of eritoran recovered within each lipoprotein fraction, as well as the amount of cholesterol (esterified and unesterified), TG and protein within each fraction were determined for all plasma samples from each of the five subjects in both the 500- and 3,500-μg/h groups.
At the end of infusion (500 μg/h), the amount of eritoran recovered in TRL was increased in subjects having higher TRL cholesterol and TRL TG levels (Table 3). Similarly, association of eritoran with HDL was correlated to HDL cholesterol concentration. At 144 h (72 h after ending infusion), the amount of eritoran recovered in TRL was correlated to levels of cholesterol, triglyceride, and protein found in the TRL fraction (Table 3).
TABLE 3.
Correlation between the percentage of eritoran recovered in each lipoprotein fraction and the amount of cholesterol, TG, or protein in each lipoprotein fraction at 72 and 144 h
| Infusion |
r for lipoprotein componenta:
|
|||||
|---|---|---|---|---|---|---|
| Eritoran-TRL
|
Eritoran-LDL
|
Eritoran-HDL
|
||||
| 72 h | 144 h | 72 h | 144 h | 72 h | 144 h | |
| 500 μg/h | ||||||
| Cholesterol | 0.90* | 0.97* | 0.06 | −0.07 | 0.82* | 0.58 |
| TG | 0.84* | 0.95* | 0.46 | −0.50 | −0.50 | 0.24 |
| Protein | NAb | 0.94* | 0.06 | −0.33 | −0.24 | 0.40 |
| 3,500 μg/h | ||||||
| Cholesterol | −0.06 | 0.61 | 0.51 | 0.53 | 0.87* | 0.36 |
| TG | 0.15 | 0.92* | 0.03 | −0.21 | −0.26 | −0.19 |
| Protein | 0.13 | 0.64 | 0.47 | 0.56 | 0.90* | 0.86* |
| 500 and 3,500 μg/h | ||||||
| Cholesterol | 0.81* | 0.95* | −0.31 | −0.06 | 0.50 | 0.16 |
| TG | 0.88* | 0.98* | −0.07 | 0.31 | −0.37 | 0.26 |
| Protein | 0.13 | 0.96* | 0.38 | 0.26 | 0.41 | 0.45 |
Results are expressed as r, the Pearson correlation coefficient between eritoran recovered in lipoproteins and the plasma lipoprotein lipid or protein amount and the indicated component measured in that fraction. Asterisks indicate values with significance (P < 0.05 as determined by Spearman correlation analysis).
NA, not available.
At the end of infusion (3,500 μg/h) the amount of eritoran recovered in HDL was correlated to HDL protein content (Table 3). At 144 h (72 h after ending infusion), the amount of eritoran recovered in TRL was found to be proportional to TG content of the TRL fraction (Table 3).
The magnitude of change in eritoran partitioning was greatest for TRL, which (as may be expected) was the only subfraction that changed dramatically between subjects. At 72 h, partitioning of eritoran into TRL ranged from 1.1 to 11.8% (10.7-fold difference) and correlated to the TG and cholesterol content of TRL. Partitioning into HDL varied by only 1.6-fold, and that into LDL varied by only 2.3-fold (Table 4).
TABLE 4.
Differences in partitioning into lipoprotein fractions due to variations in lipoprotein content
| Time | Fold eritoran distribution (range [%])a
|
||
|---|---|---|---|
| TRL | LDL | HDL | |
| 72 h | 10.7 (1.1-11.8) | 2.3 (10.1-22.9) | 1.6 (40.3-74.8) |
| 144 h | 9.8 (2.2-21.6) | 2.2 (11.1-23.9) | 1.5 (40.3-61.7) |
Data are presented as fold change between the lowest and highest values observed and the range of values (in parentheses).
To determine if distribution of eritoran into lipoprotein is dose dependent for eritoran, eritoran distributions into different lipoproteins and LPDP after infusion at 500 and 3,500 μg/h were compared. Distributions were roughly similar after infusion at both rates; however, the mean percentage of eritoran recovered in the LDL and TRL fractions increased less than proportionally, possibly distributing more into HDL. At the peak concentration of eritoran, after infusion of 7-fold more test drug (3,500 versus 500 μg/h), recovery in HDL increased 10.3-fold (2,237 versus 23,045 ng/ml) and incorporation into LDL increased 6.1-fold (838 versus 5,098 ng/ml). Incorporation into TRL was only 3.1-fold increased (293 versus 898 ng/ml).
Certain correlations between lipoprotein constitution and eritoran concentration persisted across both doses of eritoran. Pooled data from both dose groups were analyzed for correlation of eritoran incorporation into different lipoprotein fractions and the amount of cholesterol (esterified and unesterified), TG, and protein within each fraction. The results of these analyses are shown in Table 3. At 72 h after beginning infusion of eritoran, the amount of eritoran bound to TRL strongly correlated with TRL TG and cholesterol concentrations. At 144 h after beginning infusion of eritoran, the amount of eritoran bound to TRL strongly correlated to TRL TG, cholesterol, and protein (Table 3).
Ex vivo PD analysis of eritoran.
Examination of the activity of infused eritoran was tested by exposing blood samples drawn pre- and postdosing to LPS. Baseline values for LPS stimulation were determined with predose samples of blood drawn approximately 1 h prior (T−1) to beginning eritoran infusion and were mixed with 0, 1, or 10 ng of LPS per ml as agonist. Blood from each of the subjects drawn prior to dosing generated TNF-α levels of 973 to 9,130 pg of TNF-α per ml (mean, 4,095 ± 2,157 pg/ml) when 1-ng/ml LPS was used as the agonist and 1,556 to 21,623 pg of TNF-α per ml (mean, 6,843 ± 4,321 pg/ml) when 10-ng/ml LPS was used as the agonist. These baseline values provided each subject with their own control value by which to calculate inhibition of response.
Induction of TNF-α by LPS stimulation in ex vivo blood samples from placebo-administered subjects.
Blood from volunteers infused with placebo responded vigorously to LPS throughout the analysis period, with a mean response over the 144-h period of 133% ± 34% of predose (T−1) response for 1-ng/ml LPS and 143% ± 41% of predose response for stimulation by 10-ng/ml LPS). This indicates that response to LPS is robust but variable throughout the study period. Since the responses to 1-ng/ml LPS and 10-ng/ml LPS fluctuated similarly, this variability is not likely to be due to individual subject variation but to the different preparations of LPS used to make fresh solutions for each time point during the study.
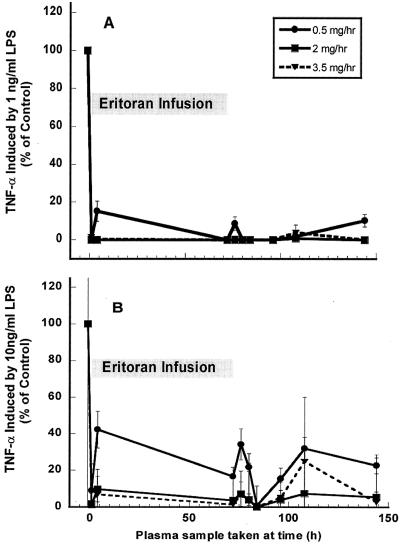
Infused eritoran inhibits response to 1-ng/ml LPS ex vivo.
As shown in Fig. 2, inhibitory activity of eritoran against 1 ng/ml of LPS was measurable by this assay within 1 h after beginning infusion. Inhibition was nearly complete for all doses of eritoran. At 500-μg/h eritoran, inhibition was nearly complete during and after infusion (>85% at all time points). A loss in inhibitory activity of eritoran may have occurred at the end of the study period (at 144 h), but the validity of this data point is unclear as only this last time point at this dose is incompletely inhibited. At infusion rates of 3,500 and 2,000 μg/h, inhibition was complete for the entire duration of the study, with activity persisting through the last time point assayed: 144 h, or 72 h after ending infusion (Fig. 2A).
FIG. 2.
Induction of TNF-α release by LPS at 1 ng/ml (A) or 10 ng/ml (B) in ex vivo blood samples from eritoran-infused volunteers. Blood samples from five volunteers each infused with 500-μg/h (•), 2,000-μg/h (▪), or 3,500-μg/h (▾) eritoran were drawn prior to beginning infusion (−1 h) or at the times after starting infusion, as indicated on the x axis, and were incubated in triplicate with 1-ng/ml LPS for 3 h at 37°C, and the plasma was assayed for release of TNF-α as described in Materials and Methods. Mean values were obtained from samples taken from five subjects for all time points except in panel B at 108 h (three subjects) and 144 h (four subjects). All mean values were significantly different from the predose control values (P < 0.005 or less) when compared by using a two-sample two-tailed t test assuming unequal variances.
Inhibition of response to 10-ng/ml LPS in eritoran-administered subjects.
Results for antagonism of 10-ng/ml LPS (Fig. 2B) demonstrate that inhibition of response was dose dependent for the agonist as well as the antagonist, especially for the lowest dose rate of eritoran infusion. At 500-μg/h eritoran, antagonism of this greater challenge dose was incomplete, averaging less than 80% through the time period. Antagonism by the two higher doses of eritoran remained 85 to 100% complete, even 72 h after ending infusion.
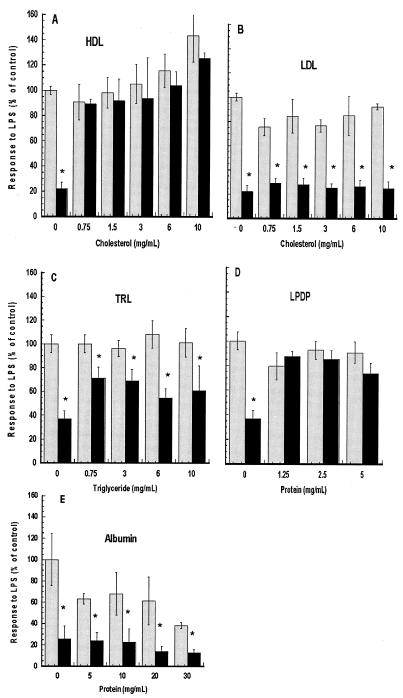
Eritoran activity is reduced by HDL and LPDP but not by LDL, TRL, or albumin.
The in vitro activity of eritoran was confirmed by treating whole blood with increasing concentrations of eritoran (0 to 0.2 μM). Plasma TNF-α concentrations were found to be reduced by greater than 50% compared to a control at an eritoran dose of 6.25 nM and greater than 95% inhibited at eritoran doses greater than 25 nM. In order to compare the inhibitory activities of different fractions, 50 nM eritoran was coincubated with various concentrations of each lipoprotein or lipoprotein-deficient fraction or albumin overnight at 37°C and diluted fivefold for assay. As shown in Fig. 3, after incubation with either LDL or TRL, eritoran retained its activity, blocking LPS-induced increases in plasma TNF-α by 50 to 75%, demonstrating similar activity to that seen after incubation with normal saline. However, when eritoran was preincubated with various concentrations of HDL (Fig. 3A) or LPDP fractions (Fig. 3D), the inhibitory activity of eritoran was blocked. In order to determine if eritoran was inactivated by protein in the LPDP fraction, the most common plasma protein, albumin was tested for its ability to inhibit activity of eritoran. Five to 30 mg of albumin per ml was preincubated with 50 nM E5546 as described above. The LPS response was inhibited 75% after preincubation in saline and 64% or more (relative to each control) after preincubation with albumin (Fig. 3E).
FIG. 3.
Effect of preincubation of eritoran with different plasma lipoprotein fractions on antagonistic activity. The indicated concentrations of HDL (A), LDL (B), TRL (C), LPDP (D), or albumin (E) were incubated with 50 nM eritoran (black bars) or in normal saline only (gray bars) for 18 h at 37°C and then diluted 1:5 into fresh human whole blood (final eritoran concentration = 10 nM). LPS was added to 10 ng/ml (final concentration) and incubated for 3 h at 37°C, and plasma was prepared and assayed for TNF-α as described in Materials and Methods. Induction of TNF-α by LPS in these assays was 1,100 to 1,300 pg/ml for panels A to D and 2,969 pg/ml for panel E. TNF-α was 45 pg/ml or less in the absence of LPS (HDL-only and blood-only controls). *, P < 0.001 versus LPS only.
DISCUSSION
During and after 72-h intravenous infusion into humans, eritoran was confined mainly to the systemic circulation, having a small Vdss (45.6 to 49.8 ml/kg). Clearance from this single compartment was slow (0.679 to 0.930 ml/h/kg), resulting in a relatively long half-life (50.4 to 62.7 h).
Maximum plasma drug concentration (Cmax) and total exposure (AUC0-inf) were positively correlated with dose from 500 μg/h (total dose, 36 mg) to 3,500 μg/h (total dose, 252 mg). Clearance was negatively correlated with dose, ranging from 0.930 (500 μg/h) to 0.679 ml/h/kg (3,500 μg/h), and elimination t1/2 increased with increasing dose from 50.4 (500 μg/h) to 62.7 h (3,500 μg/h). These results indicate that eritoran demonstrates a predictable single-compartment (blood) distribution with slow clearance. Studies in animal models indicate that eritoran is almost exclusively cleared by the liver (unpublished data), presumably through concomitant clearance of each lipoprotein fraction.
It is of interest that the pharmacokinetic half-life of eritoran in humans is similar to that observed in canine models (50.4 h) after single-bolus injection, whereas the half-life is considerably shorter in rats (∼5.2 h) (2). Despite this short half-life, eritoran demonstrated good efficacy both in LPS challenge studies and infection models in rodents (4), indicating that the PD half-life was not limiting.
As expected from in vitro studies (15), eritoran partitions predominantly (∼60%) into HDL in plasma. However, as described in Tables 3 and 4, the percentage of eritoran recovered within the TRL fraction proportionally increases with increasing TRL TG or cholesterol.
To determine if increased partitioning into TRL was an artifact of changes in non-TRL fractions, multiple regression analysis was carried out to determine if changes in other fractions (positive or inverse) were found between content of different lipoproteins. No relationships were observed between concentrations of HDL, LDL, or TRL. This indicates that increased partitioning of eritoran into TRL (as a function of TRL TG concentration) is not an artifact of decreased partitioning into other lipoproteins.
From this observation that eritoran partitions more into TRL as a function of TRL concentration, it follows that subjects having increased lipoprotein (TRL) content may have higher levels of eritoran in this fraction. The consequences of this difference are likely to be minimal, as clearance of eritoran appears to be largely unaffected by the lipoprotein to which it is bound (Fig. 1). However, extended PD activity of eritoran may be dependent on the amount present in this fraction as well as the LDL fraction (see below).
Relationship of PD activity to PK activity.
In a previous study, it was found that even though doses of eritoran of 350 to 3,500 μg administered as 30-min infusions demonstrated a long plasma elimination half-life, ex vivo PD activity decreased rapidly after ending infusion (17). In this study, activity of eritoran was similarly tested ex vivo by assay of peripheral blood. Results demonstrated that PD antagonistic activity of eritoran was readily measurable during and up to 72 h following infusion of eritoran, making it clear that long-term inhibitory activity could be attained after treatment with higher doses.
The small change in rate of drug clearance observed with increasing dose alone cannot explain the longer PD life. Rate of clearance (CL) changes (decreases) only 29% over doses of 350 μg to 72 mg (CL = 0.95 ml/h/kg after 350 μg versus 0.679 ml/h/kg after the 252-mg dose). PD activity of these same doses as measured in our ex vivo assay increased more than 70-fold: from a half life of less than 1 h for the 350-μg dose (17) compared to a PD half-life of greater than 72 h for the 252-mg dose.
Rather, longer-lasting activity may be explainable by our in vitro observation indicating that while low concentrations of eritoran were inactivated after overnight incubation with purified HDL, inactivation was not observed after incubation with LDL or TRL.
Based on these results, it is likely that long-lasting eritoran activity remains because of accumulation in the LDL and TRL subfractions. Clearly, the activity of this more minor fraction of drug is insignificant after infusion of low doses of eritoran, but it becomes important after higher-dose infusion. In vitro, the response of whole blood to 10-ng/ml LPS is inhibited 50% by 10 nM eritoran. After infusion of 36 mg of eritoran (72-h infusion of 500 μg/h), 28% of eritoran in plasma is present in the combined LDL-plus-TRL subfractions. This amounts to 1.12 μg/ml, or 790 nM. After higher-dose infusion, 15% is in LDL or TRL (6.3 μg/ml, or 4 μM). This indicates that there is sufficient eritoran in these fractions to account for this activity. Similarly, 72 h after ending infusion, the concentration of eritoran in these two fractions remains at 181 nM (36-mg infusion) and 1.7 μM (252-mg infusion), still adequate to block activity of 1-ng/ml LPS ex vivo. While we have not further studied activity after 72 h postinfusion, it is possible that at least some activity remains in the LDL or TRL fraction for as long as it is in circulation, resulting in inhibitory activity that correlates to PK activity.
Finally, it is of interest that studies in animals that have extremely high levels of HDL indicate that the period of PD activity of eritoran is short, even after high-dose infusion. In a canine infusion model using in vivo endotoxin challenges as well as ex vivo assays during and after infusion of eritoran (11), the results indicated that only partial inhibitory activity remained 24 h after infusion ended, even though levels of eritoran in plasma were as high as 15.9 μg/ml This dramatic loss in activity in the dog model may be due to the comparatively high level of HDL present in dog plasma (6).
Eritoran activity is inhibited after in vitro incubation in LPDP. However, albumin has no effect on eritoran activity. It is possible that other more minor proteins present in LPDP that also bind LPS may inactivate eritoran, but it is unlikely that they are present in sufficient quantity to quantitatively bind to and inactivate eritoran at the concentrations found in this study. Finally, it should be noted that this fraction contains a considerable amount of contaminating HDL, since HDL cholesterol in this fraction was estimated to be about 0.1 mg/ml, possibly explaining its ability to inactivate eritoran. Overall, however, the concentration of eritoran present in LPDP is extremely low, reducing any impact of eritoran in this fraction.
Conclusions.
Unlike low-dose, short-term infusions, higher-dose, long-term infusions of eritoran provide ex vivo LPS antagonistic activity that persists for at least 72 h after infusion, indicating that in vivo protection against LPS may be maintained after infusion has been discontinued. These results also suggest that dosing regimens may be optimized with knowledge of the eritoran-lipoprotein interactions.
REFERENCES
- 1.Du, X., A. Poltorak, M. Silva, and B. Beutler. 1999. Analysis of Tlr4-mediated LPS signal transduction in macrophages by mutational modification of the receptor. Blood Cells Mol. Dis. 25:328-338. [DOI] [PubMed] [Google Scholar]
- 2.Kaneko, K., R. Ueda, K. Kikuchi, Y. Sano, and T. Yoshimura. 1999. Quantitative determination of a potent lipopolysaccharide antagonist, E5564, in rat and dog plasma by high-performance liquid chromatography with fluorescence detection. J. Chromatogr. B Biomed. Sci. Appl. 736:67-75. [DOI] [PubMed] [Google Scholar]
- 3.Lynn, M., D. P. Rossignol, J. L. Wheeler, R. J. Kao, C. A. Perdomo, R. Noveck, R. Vargas, T. D'Angelo, S. Gotzkowsky, and F. G. McMahon. 2003. Blocking of responses to endotoxin by E5564 in healthy volunteers with experimental endotoxemia. J. Infect. Dis. 187:631-639. [DOI] [PubMed] [Google Scholar]
- 4.Mullarkey, M., J. R. Rose, J. Bristol, T. Kawata, A. Kimura, S. Kobayashi, M. Przetak, J. Chow, F. Gusovsky, W. J. Christ, and D. P. Rossignol. 2003. Inhibition of endotoxin response by E5564, a novel Toll-like receptor 4-directed endotoxin antagonist. J. Pharmacol. Exp. Ther. 304:1093-1102. [DOI] [PubMed] [Google Scholar]
- 5.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. V. Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 6.Ramaswamy, M., T. L. Wallace, P. A. Cossum, and K. M. Wasan. 1999. Species differences in the proportion of plasma lipoprotein lipid carried by high-density lipoproteins influence the distribution of free and liposomal nystatin in human, dog, and rat plasma. Antimicrob. Agents Chemother. 43:1424-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rose, J. R., W. J. Christ, J. R. Bristol, T. Kawata, and D. P. Rossignol. 1995. Agonistic and antagonistic activities of bacterially derived Rhodobacter sphaeroides lipid A: comparison with activities of synthetic material of the proposed structure and analogs. Infect. Immun. 63:833-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rose, J. R., M. A. Mullarkey, W. J. Christ, L. D. Hawkins, M. Lynn, Y. Kishi, K. M. Wasan, K. Peteherych, and D. P. Rossignol. 2000. Consequences of interaction of a lipophilic endotoxin antagonist with plasma lipoproteins. Antimicrob. Agents Chemother. 44:504-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossignol, D. P., W. J. Christ, L. D. Hawkins, S. Kobayashi, T. Kawata, M. Lynn, I. Yamatsu, and Y. Kishi. 1999. Synthetic endotoxin antagonists, p. 699-717. In H. Brade, S. M. Opal, S. N. Vogel, and D. C. Morrison (ed.), Endotoxin in health and disease. Marcel Dekker, Inc., New York, N.Y.
- 10.Rossignol, D. P., and M. Lynn. 2003. Antagonism of in vivo and ex vivo response to endotoxin by E5564, a novel synthetic lipid A antagonist. J. Endotoxin Res. 8:483-488. [DOI] [PubMed] [Google Scholar]
- 11.Suganuma, A., D. P. Rossignol, K. Kaneko, A. W. LaRochelle, and W. D. Kerns. 2000. Clinical pharmacology of E5564, a lipid A antagonist, in a canine lipopolysaccharide challenge model. J. Endotoxin Res. 6:115. [Google Scholar]
- 12.Wasan, K. M., and S. M. Cassidy. 1998. Role of plasma lipoproteins in modifying the biological activity of hydrophobic drugs. J. Pharm. Sci. 87:411-424. [DOI] [PubMed] [Google Scholar]
- 13.Wasan, K. M., S. M. Cassidy, M. Ramaswamy, A. Kennedy, F. W. Strobel, S. P. Ng, and T. Y. Lee. 1999. A comparison of step-gradient and sequential density ultracentrifugation and the use of lipoprotein deficient plasma controls in determining the plasma lipoprotein distribution of lipid-associated nystatin and cyclosporine. Pharm. Res. 16:165-169. [DOI] [PubMed] [Google Scholar]
- 14.Wasan, K. M., P. H. Pritchard, M. Ramaswamy, W. Wong, E. M. Donnachie, and L. J. Brunner. 1997. Differences in lipoprotein lipid concentration and composition modify the plasma distribution of cyclosporine. Pharm. Res. 14:1613-1620. [DOI] [PubMed] [Google Scholar]
- 15.Wasan, K. M., O. Sivak, R. Cote, A. M. MacInnes, K. D. Boulanger, M. Lynn, W. J. Christ, L. D. Hawkins, and D. P. Rossignol. 2003. Association of the endotoxin antagonist E5564 with high-density lipoproteins in vitro: dependence on low-density and triglyceride-rich lipoprotein concentration. Antimicrob. Agents Chemother. 47:2796-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wasan, K. M., F. W. Strobel, S. C. Parrott, M. Lynn, W. J. Christ, L. D. Hawkins, and D. P. Rossignol. 1999. Lipoprotein distribution of a novel endotoxin antagonist, E5531, in plasma from human subjects with various lipid levels. Antimicrob. Agents Chemother. 43:2562-2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong, Y. N., D. Rossignol, J. R. Rose, R. Kao, A. Carter, and M. Lynn. 2003. Safety, pharmacokinetics and pharmacodynamics of E5564, a lipid A antagonist, during an ascending single-dose clinical study. J. Clin. Pharmacol. 43:735-742. [PubMed] [Google Scholar]
- 18.Zar, J. H. 1984. Multiple regression and correlation in biostatistical analysis. Prentice-Hall, Inc., Englewood, N.J.