Abstract
In the present study we used a murine melanoma model to investigate the effect of the 25-kDa heat shock protein (Hsp25) on natural killer (NK) cytotoxicity. The melanoma lines K1735-Cl23 (low metastatic potential) and K1735-M2 (high metastatic potential) were transfected with hsp25 and a control plasmid. Highly purified interleukin (IL)-2–stimulated DX-5+ NK cells showed enhanced lysis of Hsp25-overexpressing K1735-Cl23 targets in comparison with controls. In contrast, there was no difference in susceptibility to lysis by purified IL-2–stimulated DX-5+ NK cells between Hsp25-overexpressing and control-transfected K1735-M2 targets. Fluorescence-activated cell sorter analysis revealed that Hsp25 is displayed on the cell surface independently of Hsp25 overexpression and metastatic phenotype. Thus, surface localization of Hsp25 does not correlate with the target cell susceptibility to killing. To sum up, a cytoplasmic overexpression of Hsp25 is associated with an increased susceptibility to lysis by DX-5+ NK cells in the low-metastatic murine melanoma model investigated.
INTRODUCTION
Heat shock proteins (Hsps) are a group of physiologically essential highly conserved proteins that are induced by heat shock as well as by other environmental and pathophysiological stresses. They play an essential role in protein folding, assembly, and transport, and are considered to act as molecular chaperones. Furthermore, Hsps play a role in the regulation of cell growth and differentiation (Hightower 1991; Morimoto et al 1994). There is evidence that these molecules are also involved in immune reactions resulting in auto- or tumor immunity (Melcher et al 1998; Van der Zee et al 1998; Schild et al 1999; Wells and Malkovsky 2000). Hsp25 is the murine homolog of human Hsp27 and belongs to the family of small Hsps (sHsps). This group consists of diverse intracytoplasmic proteins with molecular weights of 15–30 kDa and a variety of functions, including roles in signal transduction, growth arrest, and differentiation (Ciocca et al 1993; Kindas-Mügge and Trautinger 1994; Huot et al 1998).
We have previously reported that overexpression of Hsp27 by DNA transfer in a human melanoma cell line (A375) as well as in a human epidermal squamous carcinoma cell line (A431) leads to retardation of cell growth in vitro and a delay in tumor development in athymic BALB/c-nu+/nu+ mice (Kindas-Mügge et al 1996). Based on these findings the question arose whether the delayed tumor development observed was caused solely by the reduced proliferation rate of hsp27-transfected cells or whether it was also caused by immunological mechanisms. Innate immunity as represented by natural killer (NK) cells might have contributed to this in vivo effect as athymic mice lack an antigen-specific T-cell response.
NK cells are characterized by their capability to lyse a variety of tumor targets without prior sensitization. There is evidence that this population of lymphocytes contributes to the immune surveillance of malignant disease. These effector cells are involved in the control of tumor growth and progression (Van den Broek et al 1996; Schneeberger et al 1999) as well as in the formation of metastasis (Smith et al 1999). In cancer patients low levels of NK cytotoxicity of peripheral blood lymphocytes have been associated with progressive disease (Robertson and Ritz 1990). NK cells can recognize and kill freshly isolated autologous tumor cells (Uchida and Micksche 1983). They may kill target cells by the perforin-granzyme pathway or via Fas-FasL interaction (or both). The structures on the effector and target cells that are required for triggering NK killing are presently being studied intensively (Moretta et al 2000).
In the present study we used a mouse model consisting of immunocompetent C3H/HeN mice, and the syngeneic melanoma cell lines K1735-Cl23 (with low metastatic potential) and K1735-M2 (with high metastatic potential); both sublines were derived from the parental K1735 melanoma line by cloning of spontaneous metastases (Fidler 1996). This model was employed to elucidate the question of whether overexpression of Hsp25 in melanoma cell lines would result in an increased susceptibility to natural cytotoxicity and whether this effect was related to the metastatic potential of these tumor cells.
MATERIALS AND METHODS
Mice
C3H/HeN mice were obtained from Charles River Germany Laboratories, Sulzfeld, Germany, bred, and kept for experimental use at the Laboratory Animal Facility Vienna, Borschkegasse, University of Vienna, Austria. They were kept under standard laboratory animal conditions. Female mice at 8–14 weeks of age were sacrificed by cervical dislocation. Spleens were removed aseptically, and isolated lymphocytes were subsequently used as effector cells in cytotoxicity experiments.
Cells and cell culture
The K1735-Cl23 murine melanoma cell line with low metastatic potential and the K1735-M2 murine melanoma cell line with high metastatic potential were used (a kind gift from Prof Isaiah J. Fidler, MD Anderson Cancer Center, Houston, TX, USA). These adherent growing lines are sublines of the K1735 melanoma and have been generated by cloning of spontaneous metastases from syngeneic C3H/HeN mice (Fidler 1996).
The cells were cultivated, in accordance with the recommendations of Prof Isaiah J. Fidler, in Dulbecco modified Eagle medium (Life Technologies Inc, Paisley, Scotland, UK) supplemented with 5 % heat-inactivated fetal calf serum (PAA Laboratories, Linz, Austria), and 100 IU/mL penicillin and 100 μg/mL streptomycin (Biowhittaker, Verviers, Belgium) at 37°C in an incubator with 5 % CO2 and 100 % humidity. Subconfluent cells were split twice a week at a ratio of 1:20.
Creation of a vector carrying hsp25
The plasmid containing a 4.4-kb XbaI fragment consisting of the genomic sequence of murine hsp25, including a 5′ upstream region encoding endogenous promoter sequences, was kindly made available to us by Prof Roman Klemenz, University of Zurich, Switzerland (Fröhli et al 1993). The 4.4-kb XbaI fragment was ligated into the multiple cloning site of the retroviral vector-plasmid pLXSN, which contains a sequence conferring neomycin resistance under the control of the SV40 promoter. In pLXSN hsp25 is under transcriptional control of the retroviral long-terminal repeats promoter. This plasmid was named pHsp25. A corresponding antisense construct (pHsp25-AS) was used as a control.
Transfection and selection of target cells
Subconfluent cell cultures of K1735-Cl23 and K1735-M2 were transfected with 20 μg of the plasmid of interest using the calcium phosphate precipitation procedure (100-mm culture dish, Mammalian Transfection Kit; Stratagene, La Jolla, CA, USA). Cells were selected for neoresistance by Geneticin, a neomycin analog (0.5 mg/mL G418; Sigma, Munich, Germany). Single-cell colonies were isolated, cultivated in 60-mm culture dishes, and tested for overexpression of Hsp25 by Western blot analysis (polyclonal antibody to Hsp25; Stressgen, Victoria, BC, Canada), as previously described (Kindas-Mügge et al 1996). Stably transfected clones with — according to Western blot analysis — a strong overexpression of Hsp25 (K1735-Cl23: Cl23/6, Cl23/7, and Cl23/8; K1735-M2: M2/1, M2/3,and M2/5) were used as target cells in cytotoxicity assays. Antisense-hsp25–transfected targets (K1735-Cl23: Cl23/AS2 and Cl23/AS3; K1735-M2: M2/AS) were used as controls. Furthermore, clones were screened for the expression of Hsp60, Hsp70, and Hsp90 by Western blot analysis (polyclonal antibody to Hsp60: Stressgen; polyclonal antibodies to Hsp70, Hsp90α, and Hsp90β: Neomarkers, Fremont, CA, USA).
Separation of effector cell subpopulations
Lymphocytes
Lymphocytes were isolated from murine splenic cells (pooled from at least 4 age-matched female mice) by density gradient centrifugation using the Lympholyte-M density separation medium (Cedar Lane, Hornby, ON, Canada). B cells were removed by panning using affinity-purified goat anti-mouse immunoglobulins (DAKO A/S; Glostrup, Denmark). B-cell–depleted lymphocytes were used as effector cells in cytotoxicity assays or used in further purification steps.
Lymphokine-activated killer cells
To generate lymphokine-activated killer (LAK) cells, the B-cell–depleted populations were incubated at 37°C for 72 hours at a concentration of 1 × 106 cells/cm3 in 24-well tissue culture plates in Roswell Park Memorial Institute (RPMI)-1640 medium (Life Technologies) supplemented with 200 IU/mL recombinant murine interleukin (IL)-2 (R&D Systems Europe Ltd, Abingdon, UK) and 20 % heat-inactivated fetal calf serum. The LAK cells obtained were used as effector cells in cytotoxicity assays.
NK cell purification
For the purification of NK cells we used the new pan-NK marker DX-5, which — unlike the once common pan-NK marker NK1.1 — is expressed on almost all mouse strains including C3H/HeN (Ortaldo et al 1999). Lymphocytes, isolated and pooled from spleens of 10–15 age-matched female mice, were depleted of B cells, as described earlier, and subsequently incubated at +4°C for 15 minutes with anti-DX-5–conjugated microbeads (Miltenyi-Biotech, Bergisch-Gladbach, Germany). Cells were washed, and DX-5+ NK cells were positively selected by applying the suspension onto an LS+ column (Miltenyi-Biotech) placed in the magnetic field of a Vario-MACS® separator (Miltenyi-Biotech). To achieve high purity, both the DX-5+ fraction as well as the DX-5– fraction were applied a second time onto a new LS+ separation column. The populations were washed and used as effector cells in cytotoxicity assays after cultivation for 72 hours, at concentrations of 5 × 105 cells/cm3 (DX-5+ NK cells) and 1 × 106 cells/cm3 (NK-depleted fraction), in 24-well tissue culture plates in RPMI-1640 medium supplemented with 200 IU/mL recombinant murine IL-2 and 20 % heat-inactivated fetal calf serum.
Flow cytometric analyis
Prior to each experiment the purity of DX-5+ NK cells or NK-depleted cells was determined by fluorescence-activated cell sorter (FACS) analysis. Further, we determined the percentage of DX-5+ NK cells in experiments using LAK cells and unstimulated B-cell–depleted lymphocytes. Lymphocytes were stained with fluorescein isothiocyanate (FITC)-labeled anti-DX-5 antibodies (Pharmingen, San Diego, CA, USA) and matching isotype controls, and were analyzed using a FACScalibur® (Becton Dickinson, Heidelberg, Germany). The viability of effector cells was determined by flow cytometric measurement of propidium iodide uptake. The viability of effector cells was always more than 80 %.
Hsp25 cell-surface display by hsp25-transfected and antisense-hsp25–transfected clones of K1735-Cl23 and K1735-M2 sublines was evaluated by FACS analysis, using a rabbit polyclonal antibody to Hsp25 (Stressgen) and an FITC-labeled goat anti-rabbit IgG (H + L) second-step antibody (Jackson Immunoresearch Laboratories, West Grove, PA, USA). Second-step antibody alone was used as a negative control.
The expression of major histocompatibility complex (MHC) class I molecules on the cell surface of hsp25-transfected and antisense-hsp25–transfected cells was determined by FACS analysis using an FITC-labeled monoclonal antibody to murine H-2Dk (clone 15-5-5, Pharmingen) and H-2KK (clone 36-7-5, Pharmingen) MHC class I antigen. An FITC-labeled anti-mouse IgG2a antibody (clone R19-15, Pharmingen) was used as isotype control.
Lactate dehydrogenase–cytotoxicity assay
The Cytotox 96™ nonradioactive cytotoxicity assay (Promega, Madison, WI, USA) was used to investigate the susceptibility of hsp25-transfected and antisense-hsp25–transfected murine melanoma lines to lysis by various lymphocyte populations. This assay quantitatively measures lactate dehydrogenase (LDH), which is released upon cell lysis. The LDH content in culture supernatants is determined enzymatically. Because LDH is released in much the same way as 51Cr, this assay is a colorimetric alternative to the standard 51Cr release assay described earlier (Decker and Lohmann-Matthes 1988; Seidel et al 1998).
Hsp25-transfected and antisense-hsp25–transfected clones of K1735-Cl23 and K1735-M2 were used as targets. Target cells (5 × 103 per well) were plated in triplicate sets in U-bottom 96-well tissue culture plates and incubated at various effector to target (E:T) ratios at 37°C for 4 hours. LAK cells, IL-2–stimulated DX-5+ NK cells, and NK-depleted cells were used as effector cells. After incubation, 50 μL of supernatants from each well were transferred to a 96-well enzymatic assay plate, and 50 μL of the substrate mix was added to each well. After further incubation for 30 minutes in the dark at room temperature, 50 μL of stop solution was added to each well, and absorbance was measured at 490 nm.
Percent cytotoxicity was calculated applying the following formula: % cytotoxicity = (total LDH release − spontaneous LDH release) × 100/(target maximum LDH release − target spontaneous LDH release).
RESULTS
Expression of Hsp25
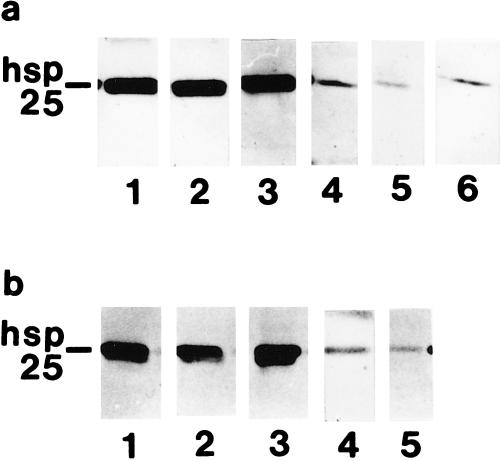
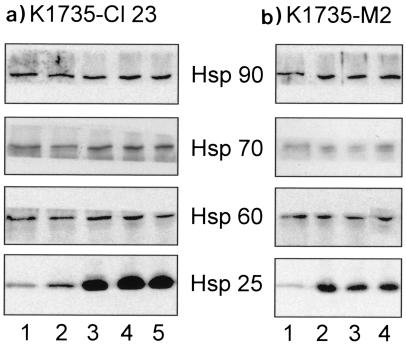
Low-metastatic (K1735-Cl23) and high-metastatic (K1735-M2) melanoma sublines were used to investigate the effects of Hsp25 overexpression on susceptibility to natural cytotoxicity. According to Western blot analysis, hsp25-transfected clones Cl23/6, Cl23/7, and Cl23/8 (Fig 1a), as well as M2/1, M2/3, and M2/5 (Fig 1b), exhibited strong expression of Hsp25. In contrast, antisense-hsp25–transfected control clones Cl23/AS2, Cl23/AS3 (Fig 1a), and M2/AS showed weak expression of Hsp25 (Fig 1b). In both the K1735-Cl23 and the K1735-M2 subline, we could detect no relevant difference with regard to Hsp25 expression between wildtype and antisense-hsp25–transfected clones (Fig 1 a,b). The expression of Hsp60, Hsp70, and Hsp90 showed some minor variations among the various clones without correlation with Hsp25 overexpression (Fig 2 a,b).
Fig 1.
Western blot analysis of Hsp25 in stably transfected (a) K1735-Cl23 and (b) K1735-M2 cell lines. Whole-cell extracts were equalized for total protein content (5 μg) and analyzed. (a) Lanes 1–3, hsp25-transfected K1735-Cl23 clones Cl23/6, Cl23/7, and Cl23/8; lanes 4–5, antisense-hsp25–transfected Cl23/AS2 and Cl23/AS3; lane 6, wildtype K1735-Cl23. (b) Lanes 1–3, hsp25-transfected K1735-M2 clones M2/1, M2/3, and M2/5; lane 4, antisense-hsp25–transfected M2/AS; lane 5, wildtype K1735-M2
Fig 2.
Western Blot analysis of Hsp25, Hsp60, Hsp70, and Hsp90 in stably transfected (a) K1735-Cl23 and (b) K1735-M2 lines. Whole-cell extracts were equalized for total protein content (10 μg) and analyzed. (a) Lanes 1 and 2, antisense-hsp25–transfected K1735-Cl23 clones Cl23/AS2 and Cl23/AS3; lanes 3–5, hsp25-transfected clones Cl23/6, Cl23/7, and Cl23/8. (b) Lane 1, antisense-hsp25–transfected K1735-M2 clone M2/AS; lanes 2–4, hsp25-transfected clones M2/1, M2/3, and M2/5
Low-metastatic K1735-Cl23 cells overexpressing Hsp25 show enhanced lysis by LAK cells and purified DX-5+ NK cells
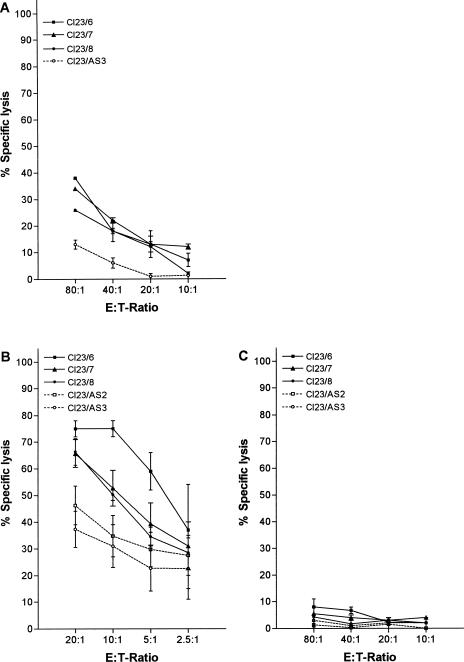
Using IL-2–stimulated lymphocytes (LAK) as effectors resulted in increased susceptibility to lysis of K1735-Cl23 clones overexpressing Hsp25, in comparison with antisense controls (Fig 3a).
Fig 3.
Lysis of K1735-Cl23 melanoma cells (low metastatatic potential) by lymphokine-activated killer (LAK) cells, by the interleukin (IL)-2–stimulated highly purified DX-5+ natural killer (NK) fraction, and by the NK-depleted fraction. (a) In 4-hour lactate dehydrogenase (LDH)-release assays, Hsp25-overexpressing clones of K1735-Cl23 (Cl23/6, Cl23/7, Cl23/8) showed higher susceptibility to LAK-mediated lysis compared with the antisense control (Cl23/AS3). (b) Four-hour LDH-release assays with the IL-2–stimulated DX-5+ NK fraction as effector population resulted in clearly enhanced lysis of clones overexpressing Hsp25 (Cl23/6, Cl23/7, Cl23/8) compared with the antisense control clones (Cl23/AS2, Cl23/AS3). (c) Four-hour LDH-release assays with the IL-2–stimulated NK-depleted fraction as effector population gave very low levels of specific lysis, with no relevant difference in susceptibility to lysis between hsp25 transfectants (Cl23/6, Cl23/7, Cl23/8) and controls (Cl23/AS2, Cl23/AS3) even at higher E:T ratios. (a)–(c) Each datapoint represents the percentage of specific lysis of at least 3 independent experiments (each carried out in triplicate). Error bars indicate standard error of the mean (SEM)
In order to determine which effector population mediated the effect described previously, DX-5+ NK cells were positively selected using an immunomagnetic method. FACS analysis revealed that the positively selected DX-5+ NK cells exhibited a purity of 90–95 %. The NK-depleted fraction showed a frequency of DX-5+ NK cells that was below 2 %.
DX-5–enriched and DX-5–depleted lymphocyte populations were incubated with recombinant murine IL-2 for 72 hours, and were consecutively used as effector cells.
Cytotoxicity tests with these highly purified IL-2–stimulated DX-5+ NK cells resulted in clearly enhanced susceptibility to lysis of K1735-Cl23 clones overexpressing Hsp25, compared with antisense-hsp25–transfected control cells (Fig 3b). In contrast, using IL-2–stimulated NK-depleted effector cells resulted in very low levels of cytotoxicity when compared with tests using purified DX-5+ NK cells as effectors. Furthermore, no relevant difference was observed between Hsp25-overexpressing clones and controls with regard to target cell lysis (Fig 3c).
Overexpression of Hsp25 in high-metastatic K1735-M2 cells has no effect on killing by LAK cells and DX-5+ NK cells
In the high-metastatic K1735-M2 line, no difference could be observed between hsp25-transfected clones and control cells with regard to susceptibility to lysis by LAK cells (Fig 4a).
Fig 4.
Lysis of K1735-M2 melanoma cells (high metastatatic potential) by lymphokine-activated killer (LAK) cells, by the interleukin (IL)-2–stimulated highly purified DX-5+ natural killer (NK) fraction, and by the NK-depleted fraction. (a) Hsp25-overexpressing clones of K1735-M2 (M2/1, M2/3, and M2/5) and the antisense control (M2/AS) show similar susceptibility to LAK-mediated lysis in 4-hour lactate dehydrogenase (LDH)-release assays. (b) 4-hour LDH-release assays with the IL-2–stimulated DX-5+ NK fraction as effector population resulted in no relevant difference between hsp25-transfected clones (M2/1, M2/3, and M2/5) and the antisense control clone (M2/AS). (c) Applying the IL-2–stimulated NK-depleted fraction gave no detectable lysis of hsp25 transfectants and controls even at higher E:T ratios. (a)–(c) Each datapoint represents percentage of specific lysis of at least 3 independent experiments (each carried out in triplicate). Error bars indicate standard error of the mean (SEM)
Likewise, cytotoxicity tests with IL-2–stimulated DX-5+ NK cells as effectors revealed no relevant difference in susceptibility to NK-mediated lysis between K1735-M2 cells overexpressing Hsp25 and antisense control cells (Fig 4b). Hsp25-overexpressing clones as well as the antisense control did not show susceptibility to lysis by IL-2–stimulated NK-depleted effectors (Fig 4c).
Hsp25 is displayed on the cell surface independently of Hsp25 overexpression and metastatic potential
FACS analysis was performed in order to investigate whether surface localization of Hsp25 would influence target cell susceptibility to killing. All clones showed surface display of Hsp25. No difference was detectable with regard to cell-surface expression of Hsp25 between clones overexpressing Hsp25 and antisense-hsp25–transfected cells (Fig 5 a,b).
Fig 5.
Cell-surface display of Hsp25 on (a) K1735-Cl23 cells (low metastatic potential) and (b) K1735-M2 cells (high metastatic potential) analyzed by fluorescence-activated cell sorter. Cells were labeled with a polyclonal antibody to Hsp25 or a control antibody. (a) Histograms of K1735-Cl23 cells. Clones overexpressing Hsp25 (Cl23/6, Cl23/7, Cl23/8) and clones transfected with antisense-hsp25 (Cl23/AS2, Cl23/AS3) express Hsp25 in equal amounts on their cell surface. (b) Histograms of K1735-M2 cells. No difference with regard to cell-surface display of Hsp25 between clones overexpressing Hsp25 (M2/1, M2/3, M2/5) and control cells (M2/AS) is detectable. (a,b) Analysis was repeated 3 times; a representative set of results is shown
Hsp25 expression does not influence cell-surface display of MHC class I
We used FACS analysis to investigate whether Hsp25 overexpression would alter the MHC class I expression on the cell surface of the low- and high-metastatic K1735 sublines. K1735-Cl23 as well as K1735-M2 cells showed a weak cell-surface expression of MHC class I antigens, with no detectable difference between Hsp25-overexpressing clones and control cells (Fig 6 a,b).
Fig 6.
Cell-surface expression of major histocompatibility complex (MHC) class I on K1735-M2 cells (high metastatic potential) and K1735-Cl23 cells (low metastatic potential) analyzed by fluorescence-activated cell sorter. Cells were labeled with a monoclonal antibody to the (a) H-2Dk and (b) H-2Kk MHC class I antigen or with a suitable isotype control. (a,b) We could find a weak MHC class I (H-2Dk, H-2Kk) expression on the cell surface of K1735-Cl23 and K1735-M2 cells, with no detectable difference between Hsp25-overexpressing clones (K1735-Cl23: Cl23/6, Cl23/7, Cl23/8; K1735-M2: M2/1,M2/3, M2/5) and control cells (K1735-Cl23: Cl23/AS2, Cl23/AS3; K1735-M2: M2/AS). Thin line represents isotype control; bold line represents H-2Dk or H-2Kk. (a,b) Analysis was repeated twice with essentially the same results
DISCUSSION
It has been reported by several authors that the expression of certain Hsps correlates with tumor immunogenicity. Menoret et al (1995) showed an association between the expression of inducible Hsp70 (=Hsp72) and the immunogenicity of rat carcinoma clones. Lukacs et al (1993) demonstrated that a murine macrophage tumor cell line lost tumorigenicity in athymic mice after transfection with mycobacterial hsp65. Additionally, when applied to immunocompetent mice, these hsp65 transfectants induced protection against tumor challenge. Multhoff et al (1995) and Botzler et al (1996) reported that cell-surface display of Hsp72 on human sarcoma cells and human lung carcinoma cells is associated with increased susceptibility to lysis mediated by NK cells.
In the present study we demonstrate for the first time that the expression of Hsp25 correlates with increased tumor cell lysis by lymphocytes. Investigation of DX-5 (a pan-NK marker)–enriched and -depleted effector populations led to the conclusion that DX-5+ NK cells are responsible for enhanced lysis of Hsp25-overexpressing K1735-Cl23 melanoma cells. These data indicate that overexpression of Hsp25 in murine melanoma cells can enhance lysis by DX-5+ NK cells. Interestingly, this effect is observed only in the low-metastatic K1735-Cl23 melanoma, whereas in the high-metastatic K1735-M2 subline, Hsp25 overexpression has no detectable effect on lysis by NK cells. As Hsp25 overexpression has no detectable influence on the expression of Hsp60, Hsp70, and Hsp90, we conclude that the observed enhanced NK susceptibility of hsp25-transfected K1735-Cl23 cells is not mediated by a possible induction of other Hsps through Hsp25 overexpression.
Our data indicating increased susceptibility of Hsp25-overexpressing cells to lysis by NK cells are in accordance with the findings of Beresford et al (1998) in the human system. They hypothesized that absence or low levels of Hsp27 in cytotoxic T lymphocytes (CTL) could contribute to their relative resistance to granule-mediated lysis. Mahvi et al (1993) investigated whether Hsp27 overexpression in MCF-7 breast cancer cells would have an effect on their susceptibility to lymphocyte-mediated killing. They report that overexpression of Hsp27 in MCF-7 breast cancer cells is associated with increased killing by γδ-T cells, but not by NK cells. In contrast, in our model we show that Hsp25 expression in melanoma cells enhances killing by DX-5+ NK cells. We could not detect significant cytotoxic activity in NK-depleted fractions, making it unlikely that γδ-T cells are responsible for the observed effect. It might be assumed that the effect of Hsp25 depends on the model and the characteristics of the particular cell line under investigation. In our model the metastatic potential of melanoma cells in vivo is associated with the different effects of Hsp25 on susceptibility to NK-mediated cytotoxicity in vitro.
Ample evidence exists that the expression of inducible Hsp70 (=Hsp72) is associated with enhanced tumor cell lysis by NK cells (Multhoff et al 1995, 1997; Botzler et al 1996, 1998; Blom et al 1997; Katschinski et al 1999). So far, in some, but not in all, the cell lines studied, a positive correlation between Hsp72 expression and lysis by NK cells has been established (Multhoff et al 1995; Blom et al 1997). Botzler et al (1996) showed that the amount of Hsp72 expressed on the cell surface, rather than the cytoplasmic Hsp72, is correlated with the enhanced target cell lysis by NK cells. Moreover, they demonstrated that the cell-surface display of Hsp72 is independent of the cytoplasmic amount of Hsp72.
In our present work we could detect Hsp25 on the cell surface of K1735-Cl23 and K1735-M2 murine melanoma cells. These data confirm the findings of Sapozhnikov et al (1999) who also observed cell-surface display of Hsp25 in mouse lymphoma cells, which increased when apoptosis was induced by heat shock or by actinomycin D. In contrast, Multhoff et al (1995) could not find significant amounts of Hsp27 on the cell surface of several human neoplastic cell lines and normal cells, neither untreated nor after heat shock. Also Mahvi et al (1993) did not detect Hsp27 on the cell surface of MCF-7 breast cancer cells overexpressing Hsp27. Further studies would be required to investigate whether these different results are species specific or whether they depend on the particular cell lines used. Because in our study Hsp25 surface display was identical in Hsp25-overexpressing and control clones, we may conclude that at least in this system Hsp25 cell-surface display is independent of Hsp25 overexpression. In analogy, Hsp72 cell-surface display is independent of the cytoplasmic amount of Hsp72 (Botzler et al 1996). To sum up, we conclude that in our system target cell recognition by NK cells is independent of the amount of Hsp25 displayed on the cell surface.
One might speculate that at least 3 mechanisms could have contributed to the enhanced susceptibility of Hsp25-overexpressing targets to lysis by NK cells. First, it might be that Hsp25 alters the cell-surface expression of ligands of activating and inhibiting receptors on NK cells. Second, Hsp25 could modulate the signaling pathways initated by NK cells, and third, Hsp25 could directly interact with the cell death–mediating molecules released by NK cells.
Recently, natural cytotoxicity receptors (NCR) on NK cells have been described (Moretta et al 2000). Binding of the as yet unidentified ligands to these receptors triggers NK cell cytotoxicity. Thus, the expression of these NCRs on effector cells and of their ligands on target cells determines the susceptibility of target cells to NK killing. In order to prevent damage to normal cells, NCRs are usually under the control of MHC class I–specific killer inhibitory receptors that provide signals down-regulating NCR function (Long et al 1997). As we could not find a detectable difference between hsp25 transfectants and controls with regard to MHC class I cell-surface expression, we conclude that the observed increased susceptibility of hsp25-transfected low-metastatic K1735-Cl23 melanoma targets to NK killing cannot be explained by the Hsp25-mediated down-regulation of MHC class I. Whether the as yet unidentified ligands for NCRs are up-regulated in target cells upon transfection with hsp25 remains to be investigated. Furthermore, comparison of the expression of NK-activating and NK-inhibiting cell-surface molecules on K1735-Cl23 vs K1735-M2 targets involved in triggering NK-mediated lysis might help to elucidate the molecular mechanisms responsible for a proposed “Hsp25-susceptible” and low-metastatic (K1735-Cl23), and an “Hsp25-resistant” and high-metastatic (K1735-M2) phenotype.
Hsp25 or Hsp27 as a phosphoprotein plays a role in signal-transduction pathways such as those involving stress-activated protein kinase-2/p38 (SAPK2/p38), which is a mitogen-activated protein kinase (Landry et al 1999). Activation of SAPK2/p38 has been correlated with cellular toxicity and with the onset of apoptosis. Phosphorylation of Hsp27 via a pathway involving SAPK2/p38 leads to F-actin reorganization, which regulates early membrane blebbing during stress-induced apoptosis (Huot et al 1998). Thus, it might be proposed that in contrast to low-metastatic K1735-Cl23 cells, high-metastatic K1735-M2 cells have lost sensitivity to the postulated modulating effects of Hsp25 on a cell death signal cascade triggered by DX-5+ NK cells. Although it has been reported that sHsps protect cells from apoptosis induced by various agents (Mehlen et al 1995; Garrido et al 1999), we cannot exclude the possibility that in the low-metastatic K1735-Cl23 cells, Hsp25 might have a promoting effect on cell death signaling induced by NK cells.
Binding of Hsp27 to granzyme A has been demonstrated in human CTLs (Beresford et al 1998). Thus, the direct interaction of Hsp25 with the granzyme-perforin pathway of cytotoxic effector cells might have contributed to the enhanced lysis of Hsp25-overexpressing low-metastatic K1735-Cl23 targets.
In this study we demonstrate that Hsp25 overexpression in murine melanoma cells enhances lysis by DX-5+ NK cells in the low-metastatic K1735-Cl23 subline, but it has no detectable effect in the high-metastatic K1735-M2 subline. Further studies on the molecular mechanisms responsible for this newly described effect of Hsp25 could provide insights into the proposed existence of “Hsp25-susceptible” (in our model: K1735-Cl23, low metastatic potential) and “Hsp25-resistant” (in our model: K1735-M2, high metastatic potential) melanoma phenotypes.
Acknowledgments
We thank Prof Isaac P. Witz, Tel-Aviv University, Tel-Aviv, Israel, for prereviewing the manuscript. We thank Ilse Fröhlich for excellent technical assistance. This work was supported in part by grant 7614 of the Jubiläumsfonds der Österreichischen Nationalbank.
REFERENCES
- Beresford PJ, Jaju M, Friedman RS, Yoon MJ, Liebermann J. A role for heat shock protein 27 in CTL-mediated cell death. J Immunol. 1998;161:161–167. [PubMed] [Google Scholar]
- Blom DJR, De Waard-Siebinga I, Apte RS, Luyten GPM, Niederkorn JY, Jager MJ. Effect of hyperthermia on expression of histocompatibility antigens and heat-shock protein molecules on three human ocular melanoma cell lines. Melanoma Res. 1997;7:103–109. doi: 10.1097/00008390-199704000-00003. [DOI] [PubMed] [Google Scholar]
- Botzler C, Issels R, Multhoff G. Heat-shock protein 72 cell-surface expression on human lung carcinoma cells is associated with an increased sensitivity to lysis mediated by adherent natural killer cells. Cancer Immunol Immunother. 1996;43:226–230. doi: 10.1007/s002620050326. [DOI] [PubMed] [Google Scholar]
- Botzler C, Li G, Issels RD, Multhoff G. Definition of extracellular localized epitopes of Hsp70 involved in an NK response. Cell Stress Chaperones. 1998;3(1):6–11. doi: 10.1379/1466-1268(1998)003<0006:doeleo>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocca DR, Oesterreich S, Chamness GC, McGuire WL, Fuqua AW. Biological and clinical implications of heat shock protein 27000 (Hsp27), a review. J Natl Cancer Inst. 1993;85:1558–1570. doi: 10.1093/jnci/85.19.1558. [DOI] [PubMed] [Google Scholar]
- Decker T, Lohmann-Matthes ML. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J Immunol Methods. 1988;15:61–69. doi: 10.1016/0022-1759(88)90310-9. [DOI] [PubMed] [Google Scholar]
- Fidler IJ. Critical determinants of melanoma metastasis. J Investig Dermatol Symp Proc. 1996;1(2):203–209. [PubMed] [Google Scholar]
- Fröhli E, Aoyama A, Klemenz R. Cloning of the mouse hsp25 gene and an extremely conserved hsp25 pseudogene. Gene. 1993;128:273–277. doi: 10.1016/0378-1119(93)90574-m. [DOI] [PubMed] [Google Scholar]
- Garrido C, Bruey J-M, Fromentin A, Hammann A, Arrigo AP, Solary E. Hsp27 inhibits cytochrome c-dependent activation of procaspase-9. FASEB J. 1999;13:2061–2070. doi: 10.1096/fasebj.13.14.2061. [DOI] [PubMed] [Google Scholar]
- Hightower LE. Heat shock proteins, chaperones and proteotoxicity. Cell. 1991;66:191–197. doi: 10.1016/0092-8674(91)90611-2. [DOI] [PubMed] [Google Scholar]
- Huot J, Houle F, Rousseau S, Deschesnes RG, Shah GM, Landry J. SAPK2/p38-dependent F-actin reorganisation regulates early membrane blebbing during stress-induced apoptosis. J Cell Biol. 1998;143(5):1361–1373. doi: 10.1083/jcb.143.5.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katschinski DM, Benndorf R, Wiedmann GJ, Mulkerin DL, Touhidi R, Robins HI. Heat shock protein antibodies in sarcoma patients undergoing 41.8°C whole body hyperthermia. J Immunother. 1999;22(1):67–70. doi: 10.1097/00002371-199901000-00009. [DOI] [PubMed] [Google Scholar]
- Kindås-Mügge I, Herbacek I, Jantschitsch C, Micksche M, Trautinger F. Modification of growth and tumorigenicity in epidermal cell lines by DNA-mediated gene transfer of 27,000 heat shock protein (Hsp27) Cell Growth Differ. 1996;7:1167–1174. [PubMed] [Google Scholar]
- Kindås-Mügge I, Trautinger F. Increased expression of the 27,000 heat shock protein (Hsp27) in in vitro differentiated normal human keratinocytes. Cell Growth Differ. 1994;5:777–781. [PubMed] [Google Scholar]
- Landry J, Huot J. Regulation of actin dynamics by stress-activated protein kinase 2 (SAPK2)-dependent phosphorylation of heat-shock protein of 27 kDa (Hsp27) Biochem Soc Symp. 1999;64:79–89. [PubMed] [Google Scholar]
- Long EO, Burshtyn DN, Clark WP, Peruzzi M, Rajagopalan S, Rojo S, Wagtmann N, Winter CC. Killer cell inhibitory receptors: diversity, specificity, and function. Immunol Rev. 1997;155:135–144. doi: 10.1111/j.1600-065x.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- Lukacs KV, Lowrie DB, Stokes RW, Colston MJ. Tumor cells transfected with a bacterial heat shock gene lose tumorigenicity and induce protection against tumors. J Exp Med. 1993;178:343–348. doi: 10.1084/jem.178.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahvi DM, Carper SW, Storm FK, Teal SR, Sondel PM. Overexpresion of 27-kDA heat-shock protein in MCF-7 breast cancer cells: effects on lymphocyte-mediated killing by natural killer cells. Cancer Immunol Immunother. 1993;37:181–186. doi: 10.1007/BF01525433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlen P, Preville X, Chareyron P, Briolay J, Klemenz R, Arrigo AP. Constitutive expression of human Hsp27, Drosophila Hsp27, or human αβ crystallin confers resistance to TNF- and oxidative stress-induced cytotoxicity in stably transfected murine L929 fibroblasts. J Immunol. 1995;215:363–374. [PubMed] [Google Scholar]
- Melcher A, Todryk S, Hardwick N, Ford M, Jacobson M, Vile RG. Tumor immunogenicity is determined by the mechanism of cell death via induction of heat shock protein expression. Nat Med. 1998;4(5):581–587. doi: 10.1038/nm0598-581. [DOI] [PubMed] [Google Scholar]
- Menoret A, Patry Y, Burg C, Le Pendu J. Co-segregation of tumor immunogenicity with expression of inducible but not constitutive Hsp70 in rat colon carcinomas. J Immunol. 1995;155(2):740–747. [PubMed] [Google Scholar]
- Moretta A, Biassoni R, Bottino C, Mingari MC, Moretta L. Natural cytotoxicity receptors that trigger human NK-cell-mediated cytolysis. Immunol Today. 2000;21(5):228–234. doi: 10.1016/s0167-5699(00)01596-6. [DOI] [PubMed] [Google Scholar]
- Morimoto RI, Tissieres A, and Georgopoulos C 1994 Progress and perspectives on the biology of heat shock proteins and molecular chaperones. In: The Biology of Heat Shock Proteins and Molecular Chaperones, ed Morimoto RI, Tissieres A, Georgopoulos C. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 1–30. [Google Scholar]
- Multhoff G, Botzler C, Jennen L, Schmidt J, Ellwart J, Issels R. Heat shock protein 72 on tumor cells—a recognition structure for natural killer cells. J Immunol. 1997;158:4341–4350. [PubMed] [Google Scholar]
- Multhoff G, Botzler C, Wiesnet M, Eißner G, Issels R. CD3-large granular lymphocytes recognize a heat-inducible immunogenic determinant associated with the 72-kD heat shock protein on human sarcoma cells. Blood. 1995;86(4):1374–1382. [PubMed] [Google Scholar]
- Ortaldo JR, Mason AT, Winkler-Picket R, Raziuddin A, Murphy WJ, Mason LH. Ly-49 receptor expression and functional analysis in multiple mouse strains. J Leukoc Biol. 1999;66:512–520. doi: 10.1002/jlb.66.3.512. [DOI] [PubMed] [Google Scholar]
- Robertson MJ, Ritz J. Biology and clinical relevance of human natural killer cells. Blood. 1990;76:2421–2438. [PubMed] [Google Scholar]
- Sapozhnikov AM, Ponomarev ED, Tarasenko TN, Telford WG. Spontaneous apoptosis and expression of cell surface heat-shock proteins in cultured EL-4 lymphoma cells. Cell Prolif. 1999;32(6):363–378. doi: 10.1111/j.1365-2184.1999.tb01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild HJ, Arnold-Schild D, Lammert E, Rammensee HG. Stress proteins and immunity mediated by cytotoxic T lymphocytes. Curr Opin Immunol. 1999;11:109–113. doi: 10.1016/s0952-7915(99)80019-3. [DOI] [PubMed] [Google Scholar]
- Schneeberger A, Koszik F, Schmidt W, Kutil R, Stingl G. The tumorigenicity of IL-2 gene-transfected murine M-3D melanoma cells is determined by the magnitude and quality of the host defense reaction: NK cells play a major role. J Immunol. 1999;162:6650–6657. [PubMed] [Google Scholar]
- Seidel MG, Freissmuth M, Pehamberger H, Micksche M. Stimulation of natural killer activity in peripheral blood lymphocytes of healthy donors and melanoma patients in vitro: synergism between interleukin (IL)-12 and IL-15 or IL-12 and IL-2. N-S Arch Pharmacol. 1998;358:382–389. doi: 10.1007/pl00005268. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Thia KYT, Cretney E, Kelly JM, Snook MB, Forbes CA, Scalzo A. Perforin is a major contributor to NK cell control of tumor metastasis. J Immunol. 1999;162:6658–6662. [PubMed] [Google Scholar]
- Uchida A, Micksche M. Lysis of fresh human tumor cells by autologous large granular lymphocytes from peripheral blood and pleural effusions. Int J Cancer. 1983;32:37–44. doi: 10.1002/ijc.2910320107. [DOI] [PubMed] [Google Scholar]
- Van den Broek MF, Kägi D, and Ossendorp F. et al. 1996 Decreased tumor surveillance in perforin-deficient mice. J Exp Med. 184:1781–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Zee R, Anderton SM, Prakken AB, Liesbeth Paul AG, Van Eden W. T cell responses to conserved bacterial heat-shock-protein epitopes induce resistance in experimental autoimmunity. Semin Immunol. 1998;10(1):35–41. doi: 10.1006/smim.1997.0103. [DOI] [PubMed] [Google Scholar]
- Wells AD, Malkovsky M. Heat shock proteins, tumor immunogenicity and antigen presentation: an integrated view. Immunol Today. 2000;21(3):129–132. doi: 10.1016/s0167-5699(99)01558-3. [DOI] [PubMed] [Google Scholar]