Abstract
Background
The diagnosis of subacromial pathology is limited by the poor accuracy of clinical tests for specific pathologies. The aim of this study was to estimate the diagnostic accuracy of clinical examination and imaging features for identifying subacromial pain (SAP) defined by a positive response to diagnostic injection, and to evaluate the influence of imaging findings on the clinical diagnosis of SAP.
Methods and Findings
In a prospective, diagnostic accuracy design, 208 consecutive patients presenting to their primary healthcare practitioner for the first time with a new episode of shoulder pain were recruited. All participants underwent a standardized clinical examination, shoulder x-ray series and diagnostic ultrasound scan. Results were compared with the response to a diagnostic block of xylocaineTM injected into the SAB under ultrasound guidance using ≥80% post-injection reduction in pain intensity as the positive anaesthetic response (PAR) criterion. Diagnostic accuracy statistics were calculated for combinations of clinical and imaging variables demonstrating the highest likelihood of a PAR. A PAR was reported by 34% of participants. In participants with no loss of passive external rotation, combinations of three clinical variables (anterior shoulder pain, strain injury, absence of symptoms at end-range external rotation (in abduction)) demonstrated 100% specificity for a PAR when all three were positive (LR+ infinity; 95%CI 2.9, infinity). A full-thickness supraspinatus tear on ultrasound increased the likelihood of a PAR irrespective of age (specificity 98% (95%CI 94, 100); LR+ 6.2; 95% CI 1.5, 25.7)). Imaging did not improve the ability to rule-out a PAR.
Conclusion
Combinations of clinical examination findings and a full-thickness supraspinatus tear on ultrasound scan can help confirm, but not exclude, the presence of subacromial pain. Other imaging findings were of limited value for diagnosing SAP.
Introduction
Shoulder pain is a common complaint in primary health care resulting in significant pain and disability, loss of productivity and health care costs [1]. The lifetime prevalence of shoulder pain in the general population is reported between 10% to 67% [2], and up to 6% of the population consult their general practitioner annually with an episode of shoulder pain [3, 4].
Subacromial disorders are the most common disorders accounting for up to 85% of shoulder conditions seen in primary care [5, 6]. Pain in the subacromial region can be caused by a number of pathological conditions including subacromial bursitis, rotator cuff tendinosis, calcific tendinosis and rotator cuff tears. There is a growing body of evidence for the specific management of these subacromial conditions including corticosteroid injections for pain relief [7], image-guided fenestration of calcific deposits [8, 9], physiotherapy and specific strengthening for non-calcific rotator cuff tendinosis and small rotator cuff tears [10–12] and surgery for large rotator cuff tears or lack of response to non-surgical measures [13]. Whether appropriate management interventions are applied is dependent upon the accurate diagnosis of the condition in the first instance [14].
In clinical practice, examination of the painful shoulder pain is typically aimed at identifying a specific pathoanatomic diagnosis to inform selection of appropriate treatment interventions [15]. This diagnostic approach relies upon the availability of clinical tests with high levels of accuracy for specific shoulder pathology. Many tests commonly used in clinical practice are reported to be diagnostic of specific pathologies including the various stages of impingement pathology (bursitis, partial and full thickness tears) [16, 17], large rotator cuff tears [18], superior labral tears [19, 20], posterior labral tears [21] and acromioclavicular joint pathology [19, 22]. However, it has been consistently demonstrated that commonly used clinical tests lack accuracy for specific pathoanatomic lesions of the shoulder when compared with imaging or surgical reference standards [15, 23, 24]. Previous diagnostic accuracy studies have shown that physical examination tests lack accuracy for, and are not able to differentiate between specific shoulder anatomic structures [15, 24, 25], or between specific pathologies of the rotator cuff (tendinosis versus a tear) [17, 26] and the glenohumeral joint (capsular versus labral pathology) [26]. One possible explanation for the poor accuracy of clinical tests lies in the complex anatomic and functional relationship between intra- and extra-articular shoulder structures making it difficult to isolate specific structures during clinical tests resulting in similar clinical presentations of different shoulder disorders.
The increasing use of diagnostic imaging such as x-ray and diagnostic ultrasound scans for shoulder pathology in primary health care settings may be related to low levels of practitioner confidence in making an accurate clinical diagnosis [27, 28]. While imaging investigations may provide evidence of pathological tissue changes, the high prevalence of asymptomatic pathology identified on imaging, particularly in ageing populations complicates the interpretation of imaging results with respect to symptomatic relevance [29–31]. This may result in the application of inappropriate treatment interventions if clinicians ascribe symptomatic causation to the reported imaging findings in the absence of clinical correlation. The majority of previous diagnostic accuracy studies used imaging or surgical visualisation of shoulder pathology as the reference standard test on the assumption that the visualised pathology was the source of pain. Asymptomatic shoulder pathology is common, and test accuracy, especially specificity values, are confounded in this design. This may provide another explanation for the poor reported accuracy of clinical tests in previous studies that used imaging or surgical visualization of shoulder pathology as the reference standard test.
Evidence is now clear that the pursuit of an accurate pathoanatomic diagnosis of shoulder pain using clinical tests alone is not possible in the primary care environment. Even when radiological imaging investigations are available, the high prevalence of asymptomatic pathology on imaging complicates the interpretation of imaging with respect to symptoms. The problems associated with the pathoanatomic approach to the clinical diagnosis of shoulder pain in primary care may lead the practitioner to an inaccurate diagnosis and/or inappropriate treatment interventions that may adversely affect patient outcome and result in inappropriate use of healthcare resources.
Instead of attempting to identify specific pathologies using clinical tests, the ability to first differentiate the source of the patients’ symptoms as arising from either the subacromial region, the glenohumeral joint or the acromioclavicular joint may be a more realistic clinical endeavour in the primary care setting. This would provide a sound basis from which to make clinical decisions regarding subsequent investigations and management. Having identified a primary subacromial pain source, additional imaging investigations could then be selectively and appropriately applied to further differentiate specific subacromial pathology where this may alter management decisions. The ability to clinically identify the likely pain source prior to diagnostic imaging investigations would also enhance the ability to interpret the symptomatic relevance of subsequent imaging findings, and facilitate the application of, or referral for appropriate treatment interventions for specific subacromial conditions such as fenestration of calcific deposits, physiotherapy for rotator cuff tendinosis or orthopaedic referral for acute, large rotator cuff tears.
Diagnostic injection of local anaesthetic (diagnostic block) is the accepted reference standard for identification of the tissue source of musculoskeletal pain [32, 33], with a marked reduction in, or complete abolition of post-injection pain being indicative of a positive response. Neer [34] described the ‘subacromial injection test’ for subacromial impingement pain [34, 35] using injection of local anaesthetic into the subacromial space to differentiate subacromial pain from other sources of shoulder pain and subacromial injections have since become a widely used diagnostic tool for painful shoulder conditions [36, 37]. While diagnostic injections into the subacromial space provide an accurate method for confirming the subacromial region as the source of symptoms, it is not clinically feasible to perform these invasive procedures on all patients. Injections also fall outside the scope of practice for many primary healthcare practitioners precluding their widespread use. The ability to identify clinical tests that accurately predict the response to subacromial diagnostic injection may provide a “proxy” for the subacromial injection test that would help the clinician to confirm or exclude a primary subacromial pain source without the need for invasive diagnostic injection procedures.
Little attention has been paid to identification of clinical examination features with the ability to predict the response to subacromial diagnostic injection. Previous analysis of our clinical examination data using stepwise regression analyses identified anterior shoulder pain, history of injury and the absence of symptoms in external rotation as the strongest predictors of a positive response following injection of local anaesthetic into the subacromial space [38]. However, stepwise regression analysis is known to be adversely affected by variable collinearity and coefficient bias [39, 40], and variable selection methods may fail to recognise variables that are of clinical and practical significance. Whether these same variables demonstrate higher diagnostic probability or likelihood than other clinical variables for accurately identifying subacromial pain has not been evaluated. In addition, despite the widespread use of diagnostic imaging in clinical practice, we are not aware of any other studies that have investigated the symptomatic relevance of specific imaging findings such as subacromial bursitis and rotator cuff tears in patients with subacromial pain by comparing them with the results of a diagnostic local anaesthetic injection.
The aim of this study was to identify individual, and combinations of clinical examination and imaging features with the highest levels of diagnostic accuracy for identifying subacromial pain (SAP) defined by a positive response to a diagnostic injection of local anaesthetic into the subacromial space, and estimate the influence of imaging findings on the ability to accurately identify SAP when combined with clinical findings.
Methods
In a prospective, diagnostic validity design, the results of clinical examination and diagnostic imaging tests (index tests) were compared with the results of a diagnostic injection of local anaesthetic into the subacromial space (reference standard procedure) to identify pain of subacromial origin. The study was designed according to existing guidelines for design of diagnostic accuracy studies [41]. Ethical approval was granted by the New Zealand Ministry of Health (Upper South) Ethics Committee and all participants provided written informed consent.
Participants
Participants were recruited consecutively from community-based medical and physiotherapy practices across Christchurch, New Zealand. Patients over the age of 18 years, presenting to their general practitioner or physiotherapist for the first time with a new episode of shoulder pain and with the ability to follow verbal instructions were eligible for inclusion in the study. Exclusion criteria were known fractures or dislocations around the shoulder complex, referred pain from the cervical spine, sensory or motor deficit involving the upper limb, previous surgery to the shoulder or cervical spine, or contraindications to study procedures.
Procedures
Clinical examination
All included participants received a standardized clinical examination including medical history, symptom chart, patient history and physical examination. Details were collected regarding the mechanism of pain onset and pain location (S1 Table). The physical examination consisted of the following tests that are used in the clinical diagnosis of subacromial conditions: assessment of passive glenohumeral joint range of motion (external rotation in neutral and 90 degrees of abduction) [42], resisted rotator cuff tests and orthopaedic tests performed as originally described; Hawkins-Kennedy test [43], drop-arm test [42], empty can test [44], external rotation lag sign [18].
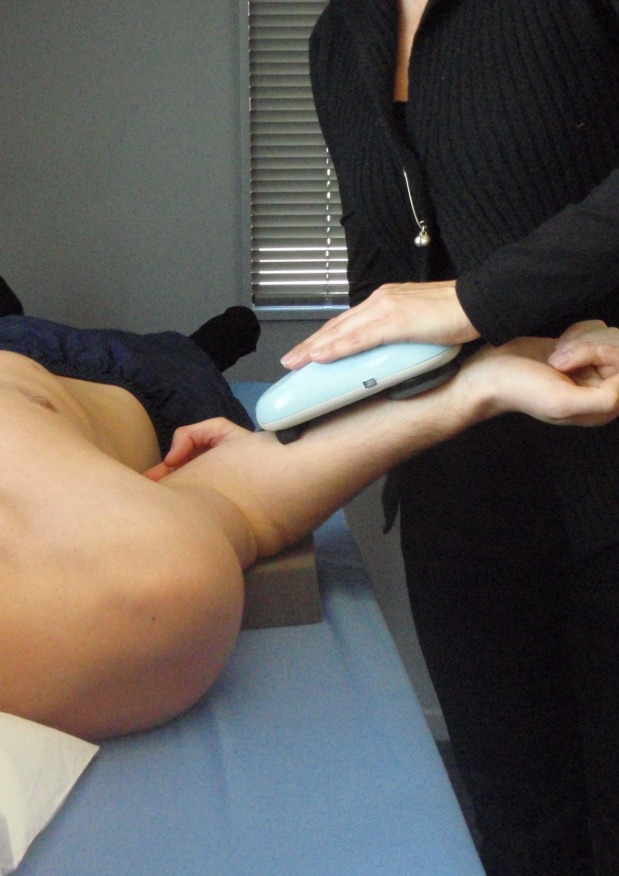
During the physical examination, those tests provocative of typical pain were identified for use in pre- and post-injection testing. Indeterminate results of clinical examination tests were recorded and coded as missing data. Passive range of motion was recorded using a hand-held dynamometer (Industrial Research Ltd, Christchurch, New Zealand) with the ability to standardize force overpressure at end-range of motion (Fig 1). Procedures for passive range of motion and strength testing have been described in detail elsewhere [45]. Intraobserver reliability for passive range of motion tests was subsequently evaluated with 95% limits of agreement within 10° [46] and intraclass correlation coefficients exceeding 0.80 for all tests. A passive external rotation deficit of ≥30 degrees compared with the unaffected side was used as the cut-point for the external rotation deficit variable. This value is consistently used to classify capsular restriction of glenohumeral joint range of motion associated with frozen shoulder and glenohumeral arthritis [47, 48]. The clinical examination was conducted by an experienced musculoskeletal clinician with 22 years of experience.
Fig 1. Measurement of passive external rotation.
Method used to measure passive range of motion in shoulder external rotation using a hand-held dynamometer (Industrial Research Ltd, Christchurch, New Zealand) with the ability to standardize force-overpressure at end range of motion.
X-ray and Diagnostic Ultrasound Scan
Within one week of the clinical examination, participants underwent a standardized series of shoulder radiographs (x-ray) consisting of anterior-posterior (AP) views in neutral, external and internal rotation, axial view and outlet view [49]. X-rays were reported by experienced musculoskeletal radiologists using a standardized report form that included specific abnormalities of the ACJ, acromion, GHJ and calcific deposits. Imaging diagnostic criteria are presented in Table 1.
Table 1. Radiologic diagnostic criteria.
| Pathology | Imaging Diagnostic Criteria |
|---|---|
| X-Ray | |
| Acromioclavicular joint: | |
| - arthropathy/degenerative change | joint space narrowing, subchondral sclerosis, subchondral cystic change or marginal osteophytes. |
| - Osteolysis | bony resorbtion or increased lucency in distal clavicle or acromion. |
| Glenohumeral joint: | |
| - arthropathy/degenerative change | joint space narrowing, subchondral sclerosis, subchondral cystic change or marginal osteophytes. |
| - other | loose bodies, joint calcifications. |
| Calcification of rotator cuff components: | calcific deposits with measurable dimensions only. Does not include ‘flecks’ of calcium. |
| - supraspinatus | calcific deposits adjacent to the greater tuberosity on AP-external rotation x-ray view. |
| - infraspinatus | calcific deposits adjacent to the greater tuberosity on AP-internal rotation x-ray view. |
| - subscapularis | calcific deposits in the anterior shoulder region on axial x-ray view. |
| Ultrasounda | |
| ACJ pathology | Capsular hypertrophy, cortical irregularity or osteophytes, capsular bulge, joint space narrowing or widening. |
| Glenohumeral joint effusion | more than 2mm between posterior glenoid labrum and posterior capsule. |
| Rotator cuff: | |
| - normal | normal contour, normal echogenicity. |
| - calcification | focal increase in echogenicity with or without shadowing. |
| - tendinosis | Rotator cuff or LHB: tendon thickening or decreased echogenicity. |
| - intrasubstance tear | hypoechoic change not extending to articular or bursal surface. |
| - partial thickness tear | SSp and ISp: hypoechoic change extending to either the articular or bursal surface. Subscapularis: partial fibre discontinuity. |
| - full thickness tear | SSp and ISp: hypoechoic region extends from bursal to articular surface. Subscapularis: complete fibre discontinuity. |
| Subacromial bursa: | |
| - bursitis | hypoechoic fluid or effusion present and >2mm thick. |
| - bursal thickening | ≥2mm measured from deep margin of deltoid to superficial margin of supraspinatus. |
| - “bunching” | Fluid distension of the SAB or ‘buckling’ of the rotator cuff during abduction |
Diagnostic ultrasound scans were performed by trained and experienced musculoskeletal sonographers and reported by fellowship trained musculoskeletal radiologists. Examinations were performed using a Philips IU22 machine with a 5–12MHz linear array probe using a standardized scan procedure: [50, 52] 1) patient sitting with palm face up on their knee (long head of biceps tendon); 2) elbow tucked into their side with external rotation of the shoulder (subscapularis); 3) arm resting on lap in neutral rotation with elbow behind body (supraspinatus); 4) hand in the small of the back with palm facing outwards to visualize (supraspinatus); 5) hand placed on the opposite shoulder (infraspinatus, ACJ, posterior labrum and glenohumeral joint). Scanning was conducted along the line of each tendon and at 90 degrees to the tendon.
The subacromial bursa was observed during dynamic abduction and ‘bunching’ under the acromion was recorded. Subacromial bursal dimensions were measured from the deep margin of deltoid muscle to superficial margin of supraspinatus tendon in all cases where this distance was measurable (dimensions exceeding 1mm).
Reference Standard Procedure
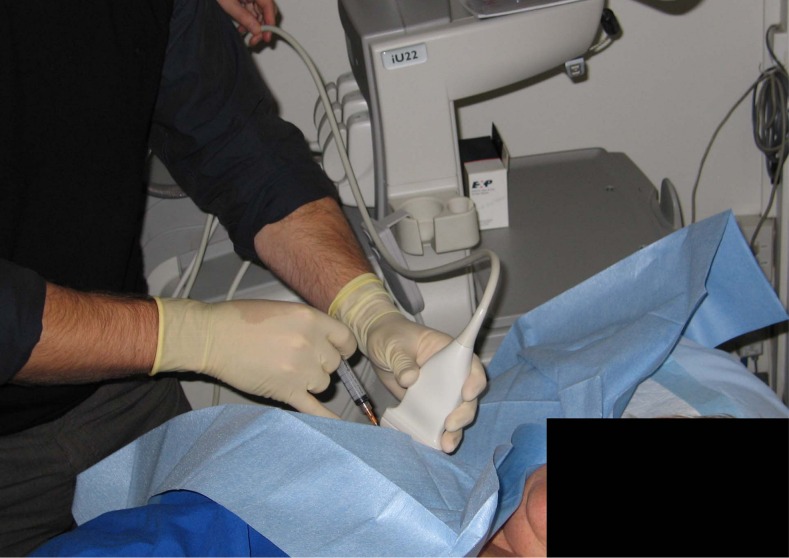
An ultrasound-guided injection of local anaesthetic into the subacromial bursa was used as the reference standard procedure (Fig 2). Participants were positioned supine with the arm in external rotation. Under aseptic conditions, a 22-gauge needle was used to inject 5mL of 1% lidocaine hydrochloride (xylocaineTM) into the SAB under ultrasound guidance using an anterior approach. When needle placement inside the subacromial bursa was confirmed by ultrasound, the contents of the syringe were emptied into the bursa. All injections were performed by a fellowship trained musculoskeletal radiologist.
Fig 2. Ultrasound guided diagnostic injection into the subacromial bursa.
Immediately prior to the injection, all participants were examined using up to six tests that had previously been identified during the clinical examination as being provocative of their typical symptoms. Pre-injection pain intensity was then recorded for each clinical test on a 100mm visual analogue scale (VAS) where 0mm indicated “no pain” and 100mm represented “worst imaginable pain”. Tests were repeated between 5 and 15 minutes following the diagnostic block and post-injection pain intensity VAS scores recorded again. The average change in pain intensity from all clinical tests was then calculated. A positive anaesthetic response was determined by 80% or more post-injection reduction in pain intensity (80% PAR). This is similar to the criteria for PAR used in other studies involving diagnostic blocks [53–55] and represents a high level of confidence that the target structure is a major contributor to symptoms.
The investigator performing the clinical examination and pre- and post-injection clinical tests was blinded to any diagnostic or treatment information from referring practitioners and to the results of imaging investigations. The radiologist who performed the SAB diagnostic block was blinded to any clinical information including the results of pre-injection provocative clinical testing.
Data Analysis
Sample size was estimated using methods for estimates for diagnostic accuracy studies described by Flahault et al. with the minimal acceptable lower confidence limit set at 0.75 and expected sensitivity/specificity both set at 0.90, with adjustment following sub-group analysis of the first 100 cases to maintain precision of confidence interval estimates [56].
Cross-tabulations of clinical examination and imaging results with an 80% PAR was carried out using Statistical Package for the Social Sciences (SPSS) version 22.0 (IBM Corporation®).[57] Diagnostic accuracy characteristics including sensitivity, specificity, predictive values, positive likelihood ratios (+LR) and negative likelihood ratios (-LR) and diagnostic odds ratios (DOR) with 95% confidence intervals (CI) were calculated for individual clinical examination and imaging variables for an 80% PAR following SAB diagnostic injection using Confidence Interval Analysis software [58]. Variables with the most favourable diagnostic characteristics based upon likelihood ratios were then combined and diagnostic characteristics re-calculated. Due to the well documented increase in rotator cuff tear prevalence in older populations, variable combinations were stratified across two age-groups using 50 years as the cut-point [29].
Results
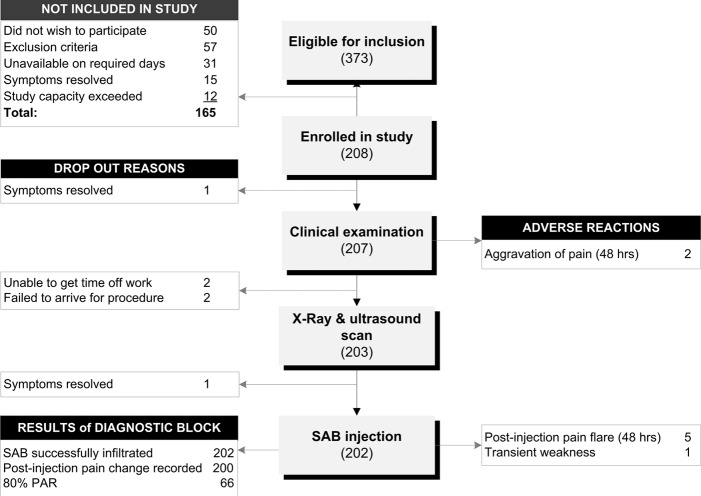
Three hundred and seventy three patients were referred to the study between July 2009 and June 2010 resulting in 208 participants being included in the study (Fig 3). Those excluded from the study reported shorter duration of symptoms (2 weeks; IQ range 4 weeks; p<0.001). Participant characteristics are presented in Table 2. No demographic variables were associated with a PAR (p>0.05). Frequency distributions for clinical examination variables in the PAR and negative anaesthetic response (NAR) groups are provided in the supplementary file (S1 Table). Missing or indeterminate data exceeded 5% for painful arc in abduction (11% had insufficient abduction range of motion to complete the test). Frequency distributions for imaging variables are presented in Table 3. Those aged over 50 years had a higher prevalence of full-thickness supraspinatus tears (13%) compared with those younger than 50 years (2%) (OR 9.7, 95%CI 2.0, 48.3; p = 0.001).
Fig 3. Flow chart of participants through the study.
Table 2. Participant characteristics.
| Characteristics | All participants | PAR Group | NAR Group | p-value | |
|---|---|---|---|---|---|
| (N = 202) | (n = 69) | (n = 133) | |||
| Mean (SD) | Range | Mean (SD) | Mean (SD) | ||
| Age (years) | 42 (14) | 18–81 | 42 (12) | 42 (15) | 0.834 |
| Height (cm) | 172 (10) | 147–199 | 171 (9) | 172 (10) | 0.120 |
| Weight (kg) | 80.6 (18.0) | 50.3–189.0 | 80.2 (21) | 81 (17) | 0.883 |
| Symptom duration (weeks)† | 7 (13)† | 0–175 | 7 (14) † | 7 (12) † | 0.851 |
| VAS (worst) | 62 (23) | 3–100 | 62 (22) | 64 (24) | 0.556 |
| VAS (average) | 37 (22) | 1–100 | 36 (18) | 37 (23) | 0.798 |
| SPADI pain score (%) | 50 (22) | 0–100 | 50 (21) | 51 (22) | 0.924 |
| SPADI disability score (%) | 30 (23) | 0–96 | 28 (22) | 31 (22) | 0.455 |
| SPADI total (%) | 38 (21) | 0–98 | 36 (20) | 39 (21) | 0.639 |
| Male gender | 102 (51%) | 31 (47%) | 71 (55%) | 0.365 | |
| Right hand dominant | 171 (87%) | 58 (88%) | 113 (87%) | 1.00 | |
| Dominant arm affected | 103 (53%) | 34 (52%) | 69 (53%) | 0.880 | |
| In paid employment | 158 (80%) | 54 (82%) | 104 (80%) | 0.850 | |
| Off work | 6 (3%) | 0 (0%) | 6 (5%) | 0.099 | |
| Co-existent medical conditions | 66 (34%) | 21 (32%) | 45 (35%) | 0.751 | |
| Smoker | 37 (19%) | 12 (19%) | 25 (19%) | 1.00 | |
Abbreviations: PAR, positive anaesthetic response (≥80% post-injection reduction in pain intensity); NAR, negative anaesthetic response (<80% reduction in post-injection pain intensity); VAS, 100mm visual analogue pain score in previous 48 hours; SPADI, Shoulder Pain & Disability Index; ACC, the Accident Compensation Corporation.
† Variable not normally distributed; median (interquartile range) are presented.
Table 3. Prevalence of imaged pathology in those reporting a positive and negative anaesthetic response to diagnostic injection into the subacromial bursa (N = 196).
| Pathology identified | PAR group | NAR group | OR | p-value | |
|---|---|---|---|---|---|
| (n = 66) | (n = 130) | ||||
| N | n (%) | n (%) | (95%CI) | ||
| X-ray | |||||
| Any ACJ pathology | 32 | 8 (12) | 24 (19) | 0.6 (0.3, 1.5) | 0.312 |
| ACJ arthropathy | 24 | 6 (9) | 18 (14) | 0.6 (0.2, 1.7) | 0.489 |
| ACJ osteolysis | 7 | 1 (2) | 6 (5) | 0.3 (0.0, 2.7) | 0.428 |
| GHJ pathology | 10 | 2 (3) | 8 (6) | 0.5 (0.1, 2.3) | 0.500 |
| Supraspinatus calcification | 16 | 9 (14) | 7 (5) | 2.8 (1.0, 8.0)* | 0.050 |
| Infraspinatus calcification | 7 | 2 (3) | 5 (4) | 0.8 (0.2, 4.2) | 1.000 |
| Subscapularis calcification | 6 | 0 (0) | 6 (5) | 1.5 (1.4, 1.7) | 0.181 |
| Ultrasound | |||||
| SAB pathology† | 135 | 46 (70) | 89 (69) | 1.1 (0.6, 2.0) | 1.000 |
| Bursal bunching (acromion) | 81 | 30 (46) | 51 (42) | 1.2 (0.6, 2.2) | 0.645 |
| Supraspinatus tear (any) | 45 | 18 (27) | 27 (21) | 1.4 (0.7, 2.8) | 0.369 |
| - intrasubstance tear | 23 | 9 (14) | 14 (11) | 1.3 (0.5, 3.2) | 0.640 |
| - PTT bursal surface | 4 | 0 (0) | 4 (3) | 1.5 (1.4, 1.7) | 0.302 |
| - PTT articular surface | 8 | 2 (3) | 6 (5) | 0.6 (0.1, 3.3) | 0.720 |
| - FTT | 10 | 7 (11) | 3 (2) | 5.0 (1.3, 20.1)* | 0.033 |
| Infraspinatus tear (any) | 3 | 1 (2) | 2 (2) | 1.0 (0.1, 11.1) | 1.000 |
| - PTT | 1 | 0 (0) | 1 (1) | 1.5 (1.4, 1.7) | 1.000 |
| - FTT | 1 | 0 (0) | 1 (1) | 1.5 (1.4, 1.7) | 1.000 |
| Subscapularis tear (any) | 10 | 3 (5) | 7 (5) | 0.8 (0.2, 3.3) | 1.000 |
| - PTT | 4 | 1 (2) | 3 (2) | 0.7 (0.1, 6.4) | 1.000 |
| - FTT | 1 | 0 (0) | 1 (1) | 1.5 (1.4, 1.7) | 1.000 |
| Supraspinatus tendinosis | 27 | 9 (14) | 18 (14) | 1.0 (0.4, 2.3) | 1.000 |
| Infraspinatus tendinosis | 1 | 0 (0) | 1 (1) | 1.5 (1.4, 1.7) | 1.000 |
| Subscapularis tendinosis | 4 | 0 (0) | 4 (3) | 1.5 (1.4, 1.7) | 0.302 |
| Supraspinatus calcification | 33 | 16 (24) | 17 (13) | 2.1 (1.0, 4.5)* | 0.050 |
| Infraspinatus calcification | 9 | 3 (5) | 6 (5) | 1.0 (0.2, 4.1) | 1.000 |
| Subscapularis calcification | 20 | 5 (8) | 15 (12) | 0.6 (0.2, 1.8) | 0.462 |
| LHB tear or tendinosis | 6 | 2 (3) | 4 (3) | 1.0 (0.2, 5.5) | 1.000 |
| LHB tendon sheath effusion | 26 | 10 (15) | 16 (12) | 1.3 (0.5, 3.0) | 0.657 |
Abbreviations: PAR, positive anaesthetic response; NAR, negative anaesthetic response; OR, unadjusted odds ratio; ACJ, acromioclavicular joint; GHJ, glenohumeral joint; SAB, subacromial bursa; CAL, coracoacromial ligament; PTT, partial thickness tear; FTT, full thickness tear; LHB, long head of biceps.
Note: Pathology subgroup totals may exceed composite pathology totals due to some cases identified in which multiple pathologies were present.
† SAB pathology included: thickening ≥2mm, calcification, bursal fluid or effusion.
*p≤0.05
Two hundred and two patients received the reference standard procedure (SAB diagnostic injection) (Fig 3). Two anaesthetic responses were not recorded as one participant was unable to understand VAS scales and one suffered transient post-injection weakness preventing post-injection clinical testing and clinical tests could not be repeated. Four anaesthetic responses were excluded from the analysis for which pre-injection pain intensity was low (<20mm) and this is known to affect the accuracy of post-injection change estimations using VAS scales [59]. This resulted in 196 cases being included in the analysis.
Diagnostic accuracy characteristics for the clinical examination variables are presented in Table 4 (S1 Table). Highest sensitivity (99%) and lowest LR- (0.14) were observed for full passive external rotation (ER). Highest specificity (97%) was observed for external rotation lag sign, and highest LR+ (2.6) for the lack of symptom provocation at end range abduction/external rotation (ABER). A loss of passive ER is the key diagnostic clinical feature for a ‘stiff’ shoulder due to frozen shoulder or glenohumeral arthritis. Cases with restriction of passive ER greater than 30° compared with the unaffected side (n = 15) were therefore excluded from further analysis to avoid the inclusion of competing clinical entities that would be managed differently in the clinical setting. Those with loss of ER who were excluded from further analysis were less likely to report a SAB PAR (OR 7.3; 7.3, 95%CI 0.9, 57.0) and had a higher prevalence of GHJ arthropathy on x-ray compared to those with full passive ER (17% vs 3% respectively) (OR 7.2, 95%CI 1.6, 33.1; p = 0.025). There were no other differences in demographic characteristics or other imaging features in those who were excluded from analysis due to a loss of passive ER (p>0.05).
Table 4. Diagnostic accuracy of clinical examination variables for a positive response to diagnostic injection into the subacromial bursa.
(N = 196).
| Clinical examination variables | Sens (95% CI) | Spec (95% CI) | PPV (95% CI) | NPV (95% CI) | LR+ (95% CI) | LR- (95% CI) | DOR (95% CI) |
|---|---|---|---|---|---|---|---|
| Age > 50 yrs | 29 (19, 41) | 69 (60, 76) | 32 (21, 44) | 65 (57, 73) | 0.91 (0.57, 1.42) | 1.04 (0.84, 1.25) | 0.9 (0.5, 1.7) |
| Traumatic onset† | 26 (17, 37) | 56 (48, 64) | 23 (15, 34) | 60 (51, 68) | 0.59 (0.37, 0.90) | 1.32 (1.06, 1.63) | 0.4 (0.2, 0.9) |
| Strain injury† | 55 (43, 66) | 65 (57, 73) | 44 (34, 55) | 74 (65, 81) | 1.58 (1.13, 2.17) | 0.70 (0.51, 0.91) | 2.3 (1.3, 4.1) |
| Lateral shoulder pain† | 20 (12, 31) | 80 (72, 86) | 33 (21, 49) | 66 (59, 73) | 0.99 (0.54, 1.75) | 1.00 (0.85, 1.15) | 1.0 (0.5, 2.1) |
| Anterior shoulder pain† | 42 (31, 54) | 73 (65, 80) | 44 (33, 57) | 71 (63, 78) | 1.58 (1.05, 2.33) | 0.79 (0.61, 0.98) | 2.0 (1.1, 3.7) |
| Night pain disturbs sleep | 58 (46, 69) | 51 (42, 59) | 37 (28, 47) | 71 (61, 79) | 1.18 (0.88, 1.53) | 0.83 (0.59, 1.14) | 1.4 (0.8, 2.6) |
| Painful arc in abduction | 56 (43, 67) | 39 (30, 48) | 35 (26, 44) | 60 (48, 71) | 0.91 (0.68, 1.17) | 1.14 (0.79, 1.63) | 0.8 (0.4, 1.5) |
| Resisted abd. or ER pain | 76 (64, 85) | 18 (12, 25) | 33 (26, 40) | 58 (42, 72) | 0.92 (0.77, 1.06) | 1.39 (0.78, 2.42) | 0.7 (0.3, 1.4) |
| Resisted int rotation pain | 50 (38, 62) | 52 (44, 61) | 36 (27, 46) | 67 (57, 75) | 1.05 (0.77, 1.41) | 0.96 (0.70, 1.26) | 1.1 (0.6, 2.0) |
| Full passive ER (0°)ǂ | 99 (92, 100) | 11 (7, 17) | 36 (30, 43) | 93 (70, 99) | 1.11 (1.02, 1.20) | 0.14 (0.02, 0.79) | 7.3 (0.9, 57.0) |
| No end-range pain passive ER (90°) | 40 (29, 52) | 84 (77, 90) | 57 (42, 70) | 74 (66, 80) | 2.56 (1.56, 4.21) | 0.71 (0.56, 0.86) | 3.6 (1.8, 7.2) |
| Full passive ER (90°)ǂ | 92 (84, 97) | 18 (13, 26) | 37 (30, 45) | 82 (64, 92) | 1.13 (1.00, 1.26) | 0.42 (0.17, 0.99) | 2.7 (1.0, 7.5) |
| Hawkins-Kennedy test + | 59 (46, 70) | 29 (22, 38) | 30 (23, 39) | 57 (45, 69) | 0.83 (0.64, 1.03) | 1.42 (0.95, 2.10) | 0.6 (0.3, 1.1) |
| Drop arm + | 12 (6, 22) | 90 (84, 94) | 40 (22, 61) | 66 (59, 73) | 1.26 (0.55, 2.84) | 0.97 (0.85, 1.07) | 1.3 (0.5, 3.4) |
| Empty Can test + (pain or weakness) | 86 (76, 93) | 14 (9, 21) | 35 (28, 43) | 65 (46, 81) | 1.00 (0.87, 1.12) | 0.99 (0.47, 2.03) | 1.0 (0.4, 2.4) |
| ERLS + | 5 (2, 13) | 97 (92, 99) | 43 (16, 75) | 66 (59, 73) | 1.44 (0.37, 5.59) | 0.99 (0.90, 1.05) | 1.5 (0.3, 6.7) |
Abbreviations: Sens, sensitivity; CI, confidence interval; Spec, specificity; PPV, positive predictive value; NPV, negative predictive value; +LR, positive likelihood ratio; -LR, negative likelihood ratio; DOR, diagnostic odds ratio; ER, external rotation; ER(0°), external rotation performed in neutral; ER(90°), external rotation performed at 90° abduction; +, positive test result; ERLS, external rotation lag sign.
† Operational definitions for history variables (onset and pain location) are provided in a supplementary file (S1 Table).
ǂ Full passive external rotation defined by less than 30° deficit compared with the unaffected side.
Diagnostic accuracy characteristics for the imaging variables with the highest odds (>2.0) of a PAR (supraspinatus calcification on x-ray, and supraspinatus calcification and a full-thickness supraspinatus tear (FTT) on ultrasound) are presented in Table 5 (S2 Table). A supraspinatus tear on ultrasound demonstrated the highest LR+ (6.2) regardless of age group and highest specificity for a PAR (100%) in the under-50 age group.
Table 5. Diagnostic accuracy of imaging variables for a positive response to diagnostic injection into the subacromial bursa.
| Imaging Findings | Sens (95% CI) | Spec (95% CI) | PPV (95% CI) | NPV (95% CI) | LR+ (95% CI) | LR- (95% CI) | DOR (95% CI) |
|---|---|---|---|---|---|---|---|
| All participants (n = 180) | |||||||
| SSp calcium (XR or USS) | 25 (16, 36) | 86 (79, 91) | 50 (34, 66) | 67 (59, 74) | 1.77 (0.95, 3.26) | 0.88 (0.73, 1.01) | 2.0 (0.9, 4.4) |
| SSp calcium (XR) | 14 (8, 25) | 95 (89, 98) | 60 (36, 80) | 67 (59, 73) | 2.70 (1.04, 6.97) | 0.91 (0.79, 1.00) | 3.0 (1.0, 8.8) |
| SSp calcium (USS) | 23 (15, 35) | 87 (80, 92) | 50 (33, 67) | 67 (59, 74) | 1.77 (0.93, 3.34) | 0.89 (0.74, 1.02) | 2.0 (0.9, 4.4) |
| SSp FTT (USS) | 11 (5, 21) | 98 (94, 100) | 78 (45, 94) | 66 (59, 73) | 6.19 (1.51, 25.69) | 0.91 (0.81, 0.98) | 6.8 (1.4, 33.9) |
| Age ≥ 50 yrs (n = 49) | |||||||
| SSp calcium (XR or USS) | 33 (16, 56) | 81 (64, 91) | 50 (25, 75) | 68 (52, 81) | 1.72 (0.66, 4.38) | 0.83 (0.53, 1.15) | 2.1 (0.6, 7.80 |
| SSp calcium (XR) | 11 (3, 33) | 94 (79, 98) | 50 (15, 85) | 64 (50, 77) | 1.72 (0.32, 9.09) | 0.95 (0.71, 1.15) | 1.8 (0.2, 14.1) |
| SSp calc (USS) | 33 (16, 56) | 84 (67, 93) | 55 (28, 79) | 68 (53, 81) | 2.07 (0.75, 5.60) | 0.80 (0.51, 1.09) | 2.6 (0.7, 10.2) |
| SSp FTT (USS) | 28 (13, 51) | 94 (79, 98) | 71 (36, 92) | 69 (54, 81) | 4.31 (1.06, 17.90) | 0.77 (0.52, 0.99) | 5.6 (1.0, 32.6) |
| Age < 50 yrs (n = 131) | |||||||
| SSp calcium (XR or USS) | 21 (12, 35) | 88 (80, 93) | 50 (30, 70) | 67 (58, 75) | 1.79 (0.81, 3.89) | 0.89 (0.73, 1.04) | 2.0 (0.8, 5.2) |
| SSp calcium (XR) | 15 (8, 28) | 95 (88, 98) | 64 (35, 85) | 67 (58, 75) | 3.20 (1.05, 9.77) | 0.89 (0.75, 1.00) | 3.6 (1.0, 13.0) |
| SSp calc (USS) | 19 (10, 33) | 88 (80, 93) | 47 (27. 68) | 66 (57, 74) | 1.61 (0.71, 3.58) | 0.92 (0.76, 1.06) | 1.8 (0.7, 4.7) |
| SSp FTT (USS) | 4 (1, 14) | 100 (96, 100) | 100 (34, 100) | 65 (57, 73) | ∞ (0.95, ∞) | 0.96 (0.99, ∞) | 2.9 (2.3, 3.6) |
Abbreviations: Sens, sensitivity; CI, confidence interval; Spec, specificity; PPV, positive predictive value; NPV, negative predictive value, LR+, positive likelihood ratio; LR-, negative likelihood ratio; DOR, diagnostic odds ratio; SSp, supraspinatus; XR, x-ray; USS, diagnostic ultrasound scan; FTT, full thickness tear.
Where LR include infinity, values for predictive values represent estimated post-test probability and 95% CI.
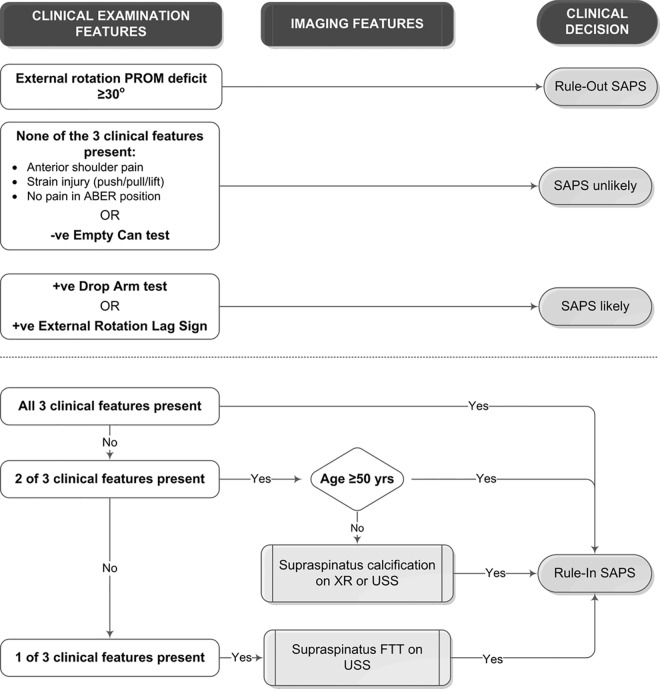
Three clinical variables were identified with the highest LR+ and DOR for an 80% PAR for which the lower confidence limits exceeded 1.0: strain injury, anterior shoulder pain and the inability to reproduce symptoms at end-range abduction/external rotation (ABER). These variables were combined and diagnostic characteristics recalculated (Table 6. Data in S3 Table). For combinations of clinical examination and imaging variables, highest sensitivity (85%) and lowest LR- (0.43) were observed when none of the clinical features were present for both age groups. Highest specificity (100) and LR+ (infinity) were observed when all three clinical features were present in both age groups. In the over-50 age group, two or more clinical features combined with supraspinatus calcium deposits on x-ray or USS yielded the highest specificity (100) and LR+ (infinity) for an 80% PAR. In the under-50 age group, one or more clinical features combined with supraspinatus FTT on USS yielded the highest specificity (100) and LR+ (infinity) for an 80% PAR. Sensitivity was low (<29%) for all combinations of clinical examination and imaging variables in both age groups. A diagnostic flow chart was developed providing possible clinical applications of the results. (Fig 4).
Table 6. Diagnostic accuracy of combinations of clinical examination variables alone, and when combined with diagnostic ultrasound scan reports of supraspinatus pathology.
| Combinations of Clinical Variables | Sens (95% CI) | Spec (95% CI) | PPV (95% CI) | NPV (95% CI) | LR+ (95% CI) | LR- (95% CI) | DOR (95% CI) |
|---|---|---|---|---|---|---|---|
| All Participants (n = 180) | |||||||
| 1 clinical feature† | 85 (74, 91) | 36 (28, 45) | 43 (34, 51) | 80 (68, 89) | 1.32 (1.10, 1.57) | 0.43 (0.23, 0.77) | 3.0 (1.4, 6.6) |
| 2 clinical features† | 45 (33, 57) | 85 (77, 91) | 63 (49, 76) | 73 (65, 90) | 3.00 (1.80, 5.00) | 0.65 (0.50, 0.80) | 4.6 (2.3, 9.4) |
| 3 clinical features† | 9 (4, 19) | 100 (97, 100) | 100 (61, 100) | 67 (59, 73) | ∞ (2.87, ∞) | 0.91 (0.94, ∞) | 3.0 (2.4, 3.7) |
| Age ≥50 years (n = 49) | |||||||
| 1 of 3 clinical features:† | 83 (61, 94) | 39 (24, 61) | 44 (29, 61) | 80 (55, 93) | 1.36 (0.93, 1.97) | 0.43 (0.14, 1.17) | (0.8, 13.3) |
| - With SSp calcium on XR or USS | 28 (13, 51) | 94 (79, 98) | 71 (36, 92) | 69 (54, 81) | 4.31 (1.06, 17.90) | 0.77 (0.52, 0.99) | (1.0, 32.6) |
| - With SSp FTT on USS | 28 (13, 51) | 94 (79, 98) | 71 (36, 92) | 69 (54, 81) | 4.31 (1.06, 17.90) | 0.77 (0.52, 0.99) | 5.6 (1.0, 32.6) |
| 2 of 3 clinical features:† | 44 (25, 66) | 97 (84, 99) | 89 (57, 98) | 75 (60, 86) | 13.78 (2.51, 81.36) | 0.57 (0.35, 0.79) | (2.7, 216.3) |
| - With SSp calcium on XR or USS | 22 (9, 45) | 100 (89, 100) | 100 (51, 100) | 69 (54, 81) | ∞ (1.91, ∞) | 0.78 (0.89, ∞) | (2.1, 5.0) |
| - With SSp FTT on USS‡ | |||||||
| 3 clinical features:† | 6 (1, 26) | 100 (89, 100) | 100 (21, 100) | 64 (50, 77) | § | § | (2.0, 4.1) |
| - With SSp calcium on XR or USS | 6 (1, 26) | 100 (89, 100) | 100 (21, 100) | 64 (50, 77) | § | § | (2.0, 4.1) |
| - With SSp FTT on USS‡ | |||||||
| Age <50 years (n = 131) | |||||||
| 1 of 3 clinical features:† | 85 (72, 93) | 35 (25, 45) | 42 (33, 52) | 81 (65, 90) | 1.30 (1.06, 1.59) | 0.43 (0.20, 0.87) | (1.2, 7.6) |
| - With SSp calcium on XR or USS | 21 (12, 35) | 93 (85, 97) | 63 (39, 82) | 68 (59, 76) | 2.98 (1.19, 7.45) | 0.85 (0.70, 0.97) | (1.2, 10.4) |
| - With SSp FTT on USS | 4 (1, 14) | 100 (96, 100) | 100 (34, 100) | 65 (57, 73) | ∞ (0.95, ∞) | 0.96 (0.99, ∞) | 2.9 (2.3, 3.6) |
| 2 of 3 clinical features:† | 45 (31, 59) | 81 (71, 88) | 57 (41, 71) | 72 (62, 80) | 2.32 (1.35, 3.98) | 0.69 (0.50, 0.88) | (1.5, 7.5) |
| - With SSp calcium on XR or USS | 15 (7, 28) | 98 (92, 99) | 78 (45, 94) | 67 (58, 75) | 6.18 (1.52, 25.45) | 0.87 (0.74, 0.96) | (1.4, 35.7) |
| - With SSp FTT on USS‡ | |||||||
| 3 clinical features:† | 11 (5, 23) | 100 (96, 100) | 100 (57, 100) | 67 (59, 75) | ∞ (2.44, ∞) | 0.89 (0.94, ∞) | (2.4, 3.9) |
| - With SSp calcium on XR or USS | § | § | § | § | § | § | (2.3, 3.7) |
| - With SSp FTT on USS‡ | |||||||
Abbreviations: Sens, sensitivity; Spec, specificity; PPV, positive predictive value; NPV, negative predictive value, LR+, positive likelihood ratio; LR-, negative likelihood ratio; DOR, diagnostic odds ratio; SSp, supraspinatus; XR, plain x-ray; USS, diagnostic ultrasound scan; FTT, full thickness tear.
† Represents the minimum number of positive clinical features (strain mechanism of injury, anterior shoulder pain and absence of symptom reproduction with passive range of motion external rotation (at 90° abduction)) required to fit the criterion.
‡ No cases identified fitting the criterion, invalid calculation.
§ Insufficient cases for valid calculation.
Where LR include infinity, values for predictive values represent estimated post-test probability and 95% CI.
Fig 4. Diagnostic algorithm.
Algorithm for identifying participants with subacromial pain defined by ≥80% post-injection pain relief following ultrasound guided injection of local anaesthetic into the subacromial bursa. Abbreviations: PROM, passive range of motion; SAP, subacromial pain; ABER, abduction/external rotation (external rotation performed at 90o abduction); -ve, negative; +ve, positive; XR, x-ray; USS, diagnostic ultrasound scan; FTT, full thickness tear
Discussion
In patients with no significant capsular restriction of the shoulder, combinations of three clinical examination features (anterior shoulder pain, strain injury and the absence of symptoms at end range abduction/external rotation (ABER)) helped confirm the presence of subacromial pain, defined by ≥80% pain relief following an injection of local anaesthetic into the subacromial bursa. The majority of imaging features were of limited diagnostic value, with the exception of supraspinatus pathology (calcification or full thickness tear) which increased the likelihood of SAP in this cohort irrespective of age. No individual or combinations of clinical examination and imaging findings were able to rule-out SAP with high levels of confidence.
A loss of passive external rotation is the key diagnostic feature for conditions causing capsular restriction of the glenohumeral joint including frozen shoulder and arthritis [47]. For this reason, full passive range of motion is reported as a precondition for the diagnosis of a rotator cuff tear [42, 60]. Our findings support this clinical construct indicating that those with more than 30° restriction of external rotation (performed in neutral) were 7 times less likely to have SAP (sensitivity 99%) and had a higher prevalence of glenohumeral joint arthropathy on x-ray. Confidence in this result is high, with narrow confidence limits for sensitivity and the negative likelihood ratio. To our knowledge, this is the first report of the diagnostic accuracy of “full passive external rotation” for painful subacromial conditions.
Clinical examination variables
The clinical examination variables that produced the highest likelihood of SAP were anterior shoulder pain, strain injury (push, pull or lifting injury) and the absence of symptom provocation in ABER. These are the same three variables previously identified as the strongest predictors of a PAR using multivariate analyses (adjusted odds ratio 2.3–3.9) [38]. However, the positive likelihood ratios for these variables were 1.6, 1.6 and 2.6 respectively indicating that, when used in isolation, these clinical features result in only a small increase in the likelihood of SAP. However, when used in combination, the three clinical features were able to rule-in a PAR with high levels of confidence when all three were present. In those older than 50 years, two of the three clinical features also provided confidence in identifying SAP. However, there was little change in the post-test probability of SAP when none of the three variables were identified, and the absence of these clinical features would therefore not allow SAP to be ruled-out with confidence.
The inability to reproduce typical symptoms at end range ABER increased the likelihood of SAP in this study. Pain provocation at end range ABER is reported to be indicative of glenohumeral joint pathology including capsulo-labral and articular surface supraspinatus lesions [61, 62]. Although symptom reproduction in this position does not permit identification of a specific pathoanatomic lesion, the absence of symptoms in this position may help exclude the GHJ as a source of pain. The three most common causes of shoulder pain are the GHJ, the acromioclavicular joint and subacromial conditions [5]. The ability to eliminate one or more of these sources of shoulder pain would thereby increase the probability of another (diagnosis by exclusion). Thus, the absence of symptoms at end range ABER may help eliminate the GHJ as a source of pain thereby increasing the likelihood of an alternate source of shoulder pain such as subacromial pain.
Overall, few individual clinical variables yielded highly accurate diagnostic estimates. Accuracy of the empty can test (sensitivity 86%), drop arm sign and external rotation lag signs (specificity 90–97%) was similar in this study compared with previous reports for rotator cuff integrity [16–18, 63, 64]. However, clinical tests previously reported to be diagnostic for subacromial “impingement” pathology including Hawkins-Kennedy test and painful arc in abduction [34] were of limited value for identifying SAP in the current study. Our results differ from others who used a subacromial local anaesthetic injection reference standard, yielding lower sensitivity for the Hawkins-Kennedy test (59% vs 92%) and lower specificity for arc pain in abduction (56% vs 81%). Previous studies reported higher sensitivity (69–88%) and specificity (45–81%) for the Hawkins-Kennedy test and painful arc in abduction for all stages of surgically visualized “impingement” pathology [17, 65]. Possible explanations for the differences in results include previous studies recruiting patients from rheumatology or orthopaedic clinics where the prevalence and severity of painful subacromial conditions is likely to differ from primary health care settings affecting the frequency of positive or negative clinical examination test results [66]. Differences in the reference standard tests (diagnostic injection vs surgically visualized pathology) may also account for differences in results. Some visualized pathology may not have been symptomatic in previous studies leading to a higher number of ‘true positive’ results and increased sensitivity. In our study, some intra-tendinous pathology may not have been exposed to anaesthetic in the presence of an intact bursa/cuff interface increasing the number of false negative results resulting in decreased sensitivity.
Imaging variables
A full-thickness supraspinatus tear on diagnostic ultrasound scan increased the likelihood of SAP by almost 7 times compared to those with an intact supraspinatus for all participants across all age groups. In the hands of skilled operators using modern equipment, as was the case in this study, the pooled sensitivity and specificity of ultrasound is 92% and 95% respectively, similar to magnetic resonance imaging [67]. In agreement with previous reports, full-thickness supraspinatus tears were more common in those over 50 years in our study (11% vs 2% in the under-50 age group) [29, 30, 68, 69]. However, in this study involving symptomatic patients, the presence of a full thickness supraspinatus tear in those older than 50 years resulted in high specificity (94%), LR+ (4.3) and odds (5.6) of a PAR, indicating that many of these tears may in fact be symptomatic. It is well known that the prevalence of supraspinatus tears increases with age in asymptomatic populations [29]. These results provide preliminary indications that full thickness supraspinatus tears may be of symptomatic relevance in symptomatic populations, irrespective of age. Studies involving larger numbers are required to confirm this finding.
Supraspinatus tendon calcification on x-ray or ultrasound scan also increased the probability of SAP in this study. Calcification is a cell-mediated process characterised by asymptomatic stages (formative and resting phase) and a highly symptomatic resorptive phase that most commonly affects those between the ages of 30 and 50 years [70]. Participants with calcification reported on x-ray were 3.0 times more likely to have SAP, and this likelihood increased in those younger than 50 years to 3.6 times. Our results provide an initial indication that calcific lesions may be of symptomatic relevance especially in those <50 years of age.
Pathology affecting the subacromial bursa (fluid, effusion, thickening or calcification) and bunching of the subacromial bursa under the lateral edge of the acromion was reported in approximately 40% of patients with shoulder pain in this cohort [71]. These findings were not associated with a PAR at the 80% threshold (p>0.05) and did not increase the odds of a PAR when present in this study. Care is therefore required when interpreting this finding with respect to the use of targeted pain relief interventions such as therapeutic corticosteroid injections in the clinical setting.
Combinations of clinical examination and imaging findings
When combined with the clinical examination findings, imaging features improved the ability to rule-in, but not to rule-out SAP. When a supraspinatus FTT or calcification was combined with one of the three clinical features a marked increase in specificity (94%) and post-test probability (71%) for a PAR was observed in both age groups. In the under-50 age group, the low prevalence of FTT affected the precision of diagnostic estimates and results should be interpreted accordingly in the clinical setting.
The x-ray or USS report of calcific deposits in supraspinatus also increased the likelihood of SAP by more than 5 times when combined with at least one clinical feature in the over-50 age group, and by more than 7 times when combined with two clinical features in the under-50 age group. We are not aware of any other studies in which the accuracy of combinations of clinical examination and imaging features have been combined using diagnostic injection as the reference standard test.
There were some limitations to consider. The false-positive rate for subacromial bursa diagnostic injection procedures is unknown, however all possible steps were taken to ensure accuracy of needle placement using imaging guidance. Analysis of post-injection pain response was undertaken according to international guidelines that recommend exclusion of cases from analysis with low pre-injection pain severity that may increase the rate of false positive or negative results.
In patients with no significant GHJ capsular restriction, combinations of patient history and physical examination features helped positively identify subacromial pain. The presence of supraspinatus calcification or a full thickness tear on ultrasound scan increased the likelihood of SAP regardless of age indicating these variables are likely to be of symptomatic relevance in the primary care setting.
Supporting Information
Operational definitions and contingency cell counts for clinical examination variables for a positive response to diagnostic injection of local anaesthetic into the subacromial bursa.
(PDF)
Contingency cell counts for imaging variables for a positive response to diagnostic injection of local anaesthetic into the subacromial bursa.
(PDF)
Contingency cell counts for combinations of clinical examination variables alone, and when combined with diagnostic ultrasound scan reports of supraspinatus pathology.
(PDF)
Acknowledgments
Christchurch Radiology Group for radiological expertise, resources and support in kind. Industrial Research Ltd (Christchurch, New Zealand) for their technical support for the hand-held dynamometer. Physiosouth, Active Health QEII, Barrington Physiotherapy Clinic and Christchurch South Health Centre for participant recruitment.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Health Research Council of New Zealand, Clinical Research Training Fellowship to AC, New Zealand Manipulative Physiotherapists Association to AC, and Physiotherapy New Zealand to AC. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Virta L, Joranger P, Brox J, Eriksson R. Costs of shoulder pain and resource use in primary health care: A cost-of-illness study in Sweden. BMC Musculoskelet Disord. 2012;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luime JJ, Koes BW, Hendriksen IJM, Burdorf A, Verhagen AP, Miedema HS, et al. Prevalence and incidence of shoulder pain in the general population: A systematic review. Scand J Rheumatol. 2004;33(2):73–81. [DOI] [PubMed] [Google Scholar]
- 3.Feleus A, Bierma-Zeinstra SM, Miedema HS, Bernsen RM, Berhaar JA, Koes BW. Incidence of non-traumatic complaints of arm, neck and shoulder in general practice. Man Ther. 2008;13:426–33. 10.1016/j.math.2007.05.010 [DOI] [PubMed] [Google Scholar]
- 4.Greving K, Dorrestijn O, Winters JC, Groenhof F, van der Meer K, Stevens M, et al. Incidence, prevalence, and consultation rates of shoulder complaints in general practice. Scand J Rheumatol. 2012;41(2):150–5. 10.3109/03009742.2011.605390 [DOI] [PubMed] [Google Scholar]
- 5.Ostor AJK, Richards CA, Prevost AT, Speed CA, Hazleman BL. Diagnosis and relation to general health of shoulder disorders presenting to primary care. Rheumatology (Oxford). 2005;44:800–5. [DOI] [PubMed] [Google Scholar]
- 6.van der Windt DAWM, Koes BW, De Jong BA, Bouter LM. Shoulder disorders in general practice: Incidence, patient characteristics, and management. Ann Rheum Dis. 1995;54(12):959–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchbinder R, Green S, Youd JM. Corticosteroid injections for shoulder pain. Cochrane Database Syst Rev. 2003;(1):Art. No.: CD004016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lam F, Bhatia D, van Rooyen K, de Beer JF. Modern management of calcifying tendinitis of the shoulder. Curr Orthop. 2006;20(6):446–52. [Google Scholar]
- 9.Louwerens JKG, Sierevelt IN, van Noort A, van den Bekerom MPJ. Evidence for minimally invasive therapies in the management of chronic calcific tendinopathy of the rotator cuff: A systematic review and meta-analysis. J Shoulder Elbow Surg. 2014;23(8):1240–9. 10.1016/j.jse.2014.02.002 [DOI] [PubMed] [Google Scholar]
- 10.Dong W, Goost H, Lin XB, Burger C, Paul C, Wang ZL, et al. Treatments for shoulder impingement syndrome a prisma systematic review and network meta-analysis. Medicine (United States). 2015;94(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holmgren T, Hallgren HB, Öberg B, Adolfsson L, Johansson K. Effect of specific exercise strategy on need for surgery in patients with subacromial impingement syndrome: Randomised controlled study. BMJ (Online). 2012;344(7846). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haik MN, Alburquerque-Sendín F, Moreira RFC, Pires ED, Camargo PR. Effectiveness of physical therapy treatment of clearly defined subacromial pain: a systematic review of randomised controlled trials. Br J Sports Med. 2016;50(18):1124–34 10.1136/bjsports-2015-095771 [DOI] [PubMed] [Google Scholar]
- 13.Ruotolo C, Nottage WM. Surgical and nonsurgical management of rotator cuff tears. Arthroscopy. 2002;18(5):527–31. 10.1053/jars.2002.31707 [DOI] [PubMed] [Google Scholar]
- 14.Baring T, Emery R, Reilly P. Management of rotator cuff disease: Specific treatment for specific disorders. Best Pract Res. 2007;21(2):279–94. [DOI] [PubMed] [Google Scholar]
- 15.Hegedus EJ, Goode A, Campbell S, Morin A, Tamaddoni M, Moorman CT, et al. Physical examination tests of the shoulder: A systematic review with meta-analysis of individual tests. Br J Sports Med. 2008;42:80–92. 10.1136/bjsm.2007.038406 [DOI] [PubMed] [Google Scholar]
- 16.Leroux J, Thomas E, Bonnel F, Blotman F. Diagnostic value of clinical tests for shoulder impingement syndrome. Revue du Rhumatisme (Engl Ed). 1995;62:423–8. [PubMed] [Google Scholar]
- 17.Park HB, Yokota A, Gill HS, el Rassi G, McFarland EG. Diagnostic accuracy of clinical tests for the different degrees of subacromial impingement syndrome. J Bone Joint Surg Am. 2005;87(7):1446–55. 10.2106/JBJS.D.02335 [DOI] [PubMed] [Google Scholar]
- 18.Hertel R, Ballmer FT, Lambert SM, Berber C. Lag signs in the diagnosis of rotator cuff rupture. J Shoulder Elbow Surg. 1996;5(4):307–13. [DOI] [PubMed] [Google Scholar]
- 19.O'Brien SJ, Pagnani MJ, Fealy S, McGlynn SR, Wilson JB. The active compression test: A new and effective test for diagnosing labral tears and acromioclavicular joint abnormality. Am J Sports Med. 1998;26(5):610–3. [DOI] [PubMed] [Google Scholar]
- 20.Parentis MA, Glousman RE, Mohr KS, Yocum LA. An evaluation of the provocative tests for superior labral anterior posterior lesions. Am J Sports Med. 2006;34(2):265–8. 10.1177/0363546505279911 [DOI] [PubMed] [Google Scholar]
- 21.Kim SH, Park JC, Jeong WK, Shin SK. The Kim test: A novel test for posteroinferior labral lesion of the shoulder—a comparison to the Jerk test. Am J Sports Med. 2005;33(8):1188–92. 10.1177/0363546504272687 [DOI] [PubMed] [Google Scholar]
- 22.Chronopoulos E, Kim TK, Park HB, Ashenbrenner D, McFarland EG. Diagnostic value of physical tests for isolated chronic acromioclavicular lesions. Am J Sports Med. 2004;32(3):655–61. [DOI] [PubMed] [Google Scholar]
- 23.Hanchard NCA, Handoll HHG. Physical tests for shoulder impingements and local lesions of bursa, tendon or labrum that may accompany impingement. Cochrane Database Syst Rev. 2008;(4):Art. No CD007427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hegedus EJ, Goode AP, Cook CE, Michener L, Myer CA, Myer DM, et al. Which physical examination tests provide clinicians with the most value when examining the shoulder? Update of a systematic review with meta-analysis of individual tests. Br J Sports Med. 2012;46(14):964–78. 10.1136/bjsports-2012-091066 [DOI] [PubMed] [Google Scholar]
- 25.Dinnes J, Loveman E, McIntyre L, Waugh N. The effectiveness of diagnostic tests for the assessment of shoulder pain due to soft tissue disorders: A systematic review. Health Technol Assess. 2003;7(29):1–178. [DOI] [PubMed] [Google Scholar]
- 26.Magarey ME, Jones MA, Cook CE, Hayes MG. Does physiotherapy diagnosis of shoulder pathology compare to arthroscopic findings? Br J Sports Med. 2016;50(18):1151–7 10.1136/bjsports-2014-094339 [DOI] [PubMed] [Google Scholar]
- 27.Awerbuch MS. The clinical utility of ultrasonography for rotator cuff disease, shoulder impingement syndrome and subacromial bursitis. Med J Aust. 2008;188(1):50–3. [DOI] [PubMed] [Google Scholar]
- 28.Johal P, Martin D, Broadhurst N, Johal P, Martin D, Broadhurst N. Managing shoulder pain in general practice—assessment, imaging and referral. Aust Fam Physician. 2008;37(4):263–5. [PubMed] [Google Scholar]
- 29.Milgrom C, Schaffler M, Gilbert S, van Holsbeeck M. Rotator-cuff changes in asymptomatic adults. The effect of age, hand dominance and gender. J Bone Joint Surg Br. 1995;77:296–8. [PubMed] [Google Scholar]
- 30.Moosmayer S, Smith HJ, Tariq R, Larmo A. Prevalence and characteristics of asymptomatic tears of the rotator cuff: An ultrasonographic and clinical study. J Bone Joint Surg Br. 2009;91(2):196–200. 10.1302/0301-620X.91B2.21069 [DOI] [PubMed] [Google Scholar]
- 31.Shubin Stein BE, Ahmad CS, Pfaff CH, Bigliani LU, Levine WN. A comparison of magnetic resonance imaging findings of the acromioclavicular joint in symptomatic versus asymptomatic patients. J Shoulder Elbow Surg. 2006;15(1):56–9. 10.1016/j.jse.2005.05.013 [DOI] [PubMed] [Google Scholar]
- 32.Hancock MJ, Maher CG, Latimer J, Spindler MF, McAuley JH, Laslett M, et al. Systematic review of tests to identify the disc, SIJ or facet joint as the source of low back pain. Eur Spine J. 2007;16(10):1539–50. 10.1007/s00586-007-0391-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.International Spine Intervention Society [ISIS]. Practice guidelines for spinal diagnostic & treatment procedures. San Francisco 2004 p. 61.
- 34.Neer CS. Impingement lesions. Clin Orthop. 1983;173:70–8. [PubMed] [Google Scholar]
- 35.Calis M, Akgun K, Birtane M, Karacan I, Calis H, Tuzun F. Diagnostic values of clinical diagnostic tests in subacromial impingement syndrome. Ann Rheum Dis. 2000;59(1):44–7. 10.1136/ard.59.1.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tallia AF, Cardone DA. Diagnostic and therapeutic injection of the shoulder region. Am Fam Physician. 2003;67(6):1271–8. [PubMed] [Google Scholar]
- 37.Wittich CM, Ficalora RD, Mason TG, Beckman TJ. Musculoskeletal injection. Mayo Clin Proc. 2009;84(9):831–7. 10.1016/S0025-6196(11)60493-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cadogan A, Laslett M, Hing W, McNair P, Taylor S. Clinical predictors of a positive response to guided diagnostic block into the subacromial bursa. J Rehabil Med. 2012;44(10):877–84. 10.2340/16501977-1049 [DOI] [PubMed] [Google Scholar]
- 39.Steyerberg EW, Eijkemans MJC, Habbema JDF. Stepwise selection in small data sets: A simulation study of bias in logistic regression analysis. J Clin Epidemiol. 1999;52(10):935–42. [DOI] [PubMed] [Google Scholar]
- 40.Yoo W, Mayberry r, Bae S, Singh K, He Q, Lillard J, J. W. A study of effects of multicollinearity in the multivariable analysis. Int J Appl Sci Technol. 2014;4(5):9–19. PMCID: PMC4318006. [PMC free article] [PubMed] [Google Scholar]
- 41.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, et al. STARD 2015: An updated list of essential items for reporting diagnostic accuracy studies. BMJ (Online). 2015;351:5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Codman EA. Rupture of the supraspinatus tendon and other lesions in or about the subacromial bursa 1st ed. Todd T, editor. Boston, MA: T. Todd Company; 1934. 123–77. [Google Scholar]
- 43.Hawkins R, Kennedy J. Impingement syndrome in athletes. Am J Sports Med. 1980;8:151–8. [DOI] [PubMed] [Google Scholar]
- 44.Jobe F, Moynes D. Delineation of diagnostic criteria and a rehabilitation program for rotator cuff injuries. Am J Sports Med. 1982;10:336–9. [DOI] [PubMed] [Google Scholar]
- 45.Cadogan A, Laslett M, Hing W, McNair P, Williams M. Reliability of a new hand-held dynamometer in measuring shoulder range of motion and strength. Man Ther. 2011;16(1):97–101. 10.1016/j.math.2010.05.005 [DOI] [PubMed] [Google Scholar]
- 46.Bland J, Altman D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–10. [PubMed] [Google Scholar]
- 47.ISAKOS Upper Extremity Committee. ISAKOS Upper Extremity Committee Consensus Statement: Shoulder Stiffness. Berlin: Springer-Verlag; 2014. [Google Scholar]
- 48.Ombregt L, Bisschop P, ter Veer HJ. Clinical examination of the shoulder In: Ombregt L, editor. System of Orthopaedic Medicine. 2nd ed. London: Churchill Livingstone; 2003. p. 291–302. [Google Scholar]
- 49.Anderson J, Read JW, Steinweg J. The shoulder and arm In: Anderson J, Steinweg J, Read JW, editors. Atlas of Imaging in Sports Medicine. Volume 1 1st ed. Sydney, Australia: McGraw-Hill Book Company Australia Pty Limited; 1998. p. 96–129. [Google Scholar]
- 50.McNally EG. Practical Musculoskeletal Ultrasound. 1st ed. London: Churchill Livingstone; 2004. [Google Scholar]
- 51.Stoller DW, Wolf EM, Li AE, Nottage WM, Tirman PFJ. The Shoulder In: Stoller DW, editor. Magnetic Resonance Imaging in Orthopaedics and Sports Medicine. 3rd ed. Baltimore: Lippincott Williams and Wilkins; 2007. p. 1131–462. [Google Scholar]
- 52.Backhaus M, Burmester GR, Gerber T, Grassi W, Machold KP, Swen WA, et al. Guidelines for musculoskeletal ultrasound in rheumatology. Ann Rheum Dis. 2001;60(7):641–9. 10.1136/ard.60.7.641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dreyfuss P, Michaelsen M, Pauza K, McLarty J, Bogduk N. The value of history and physical examination in diagnosing sacroiliac joint pain. Spine. 1996;21:2594–602. [DOI] [PubMed] [Google Scholar]
- 54.Laslett M, McDonald B, Aprill CN, Tropp H, Oberg B. Clinical predictors of screening lumbar zygapophyseal joint blocks: development of clinical prediction rules. Spine J. 2006;6(4):370–9. 10.1016/j.spinee.2006.01.004 [DOI] [PubMed] [Google Scholar]
- 55.Strobel K, Pfirrmann CWA, Zanetti M, Nagy L, Hodler J. MRI features of the acromioclavicular joint that predict pain relief from intraarticular injection. Am J Roentgenology. 2003;181:755–60. [DOI] [PubMed] [Google Scholar]
- 56.Flahault A, Cadilhac M, Thomas G. Sample size calculation should be performed for design accuracy in diagnostic test studies. J Clin Epidemiol. 2005;58(8):859–62. 10.1016/j.jclinepi.2004.12.009 [DOI] [PubMed] [Google Scholar]
- 57.IBM Corp Released 2013. SPSS for Windows. 22.0 ed. Armonk, NY: IBM Corporation; 2013. [Google Scholar]
- 58.Bryant TN. Confidence Interval Analysis In: Altman DG, Machin D, Bryant TN, Gardner MJ, editors. Statistics with Confidence. 2.1.2 ed. Bristol: British Medical Journal Books; 2000. [Google Scholar]
- 59.Bogduk N. Practice guidelines for spinal diagnostic & treatment procedures 1st ed. Bodguk N, editor. San Francisco: International Spine Intervention Society; 2004. [Google Scholar]
- 60.Eubank BH, Mohtadi NG, Lafave MR, Wiley JP, Bois AJ, Boorman RS, et al. Using the modified Delphi method to establish clinical consensus for the diagnosis and treatment of patients with rotator cuff pathology. BMC Med Res Methodol. 2016;16(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jobe FW, Kvitne RS. Shoulder pain in the overhand or throwing athlete: The relationship of anterior instability and rotator cuff impingement. Orthop Rev. 1989;18(9):963–75. [PubMed] [Google Scholar]
- 62.Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II slap lesions: Three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553–65. [DOI] [PubMed] [Google Scholar]
- 63.Itoi E, Kido T, Sano A, Urayama M, Sato K. Which is more useful, the full can test or the empty can test in detecting the torn supraspinatus tendon? Am J Sports Med. 1999;27(1):65–8. [DOI] [PubMed] [Google Scholar]
- 64.Walch G, Boulahia A, Calderone S, Robinson A. The 'dropping' and 'hornblowers' signs in evaluation of rotator cuff tears. J Bone Joint Surg Br. 1998;80(4):624–8. [DOI] [PubMed] [Google Scholar]
- 65.MacDonald PB, Clark P, Sutherland K. An analysis of the diagnostic accuracy of the Hawkins and Neer subacromial impingement signs. J Shoulder Elbow Surg. 2000;9(4):299–301. 10.1067/mse.2000.106918 [DOI] [PubMed] [Google Scholar]
- 66.Aerts M, Aertgeerts B, Bossuyt PM, Bruyninckx R, Butinx F, Dinant GJ, et al. The Evidence Base of Clinical Diagnosis: Theory and Methods of Diagnostic Research. Knottnerus JA, Buntinx F, editors. Oxford: Blackwell Publishing; 2009. 282 p. [Google Scholar]
- 67.de Jesus JO, Parker L, Frangos AJ, Nazarian LN, de Jesus JO, Parker L, et al. Accuracy of MRI, MR arthrography, and ultrasound in the diagnosis of rotator cuff tears: A meta-analysis. Am J Roentgenology. 2009;192(6):1701–7. [DOI] [PubMed] [Google Scholar]
- 68.Sher JS, Uribe JW, Posada A, Murphy BJ, Zlatkin MB. Abnormal findings on magnetic resonance images of asymptomatic shoulders. J Bone Joint Surg Am. 1995;77(1):10–5. [DOI] [PubMed] [Google Scholar]
- 69.Tempelhof S, Rupp S, Seil R. Age-related prevalence of rotator cuff tears in asymptomatic shoulders. J Shoulder Elbow Surg. 1999;8(4):296–9. [DOI] [PubMed] [Google Scholar]
- 70.Gartner J, Heyer A. Calcifying tendinitis of the shoulder joint. Orthopade. 1995;24(3):284–302. [PubMed] [Google Scholar]
- 71.Cadogan A, Laslett M, Hing WA, McNair PJ, Coates MH. A prospective study of shoulder pain in primary care: Prevalence of imaged pathology and response to guided diagnostic blocks. BMC Musculoskelet Disord. 2011;12:119 10.1186/1471-2474-12-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Operational definitions and contingency cell counts for clinical examination variables for a positive response to diagnostic injection of local anaesthetic into the subacromial bursa.
(PDF)
Contingency cell counts for imaging variables for a positive response to diagnostic injection of local anaesthetic into the subacromial bursa.
(PDF)
Contingency cell counts for combinations of clinical examination variables alone, and when combined with diagnostic ultrasound scan reports of supraspinatus pathology.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.