Abstract
Delonix regia (Boj. Ex. Hook) is a flowering plant in the pea family found in tropical areas and its leaves are used informally to treat diseases in folk medicine. However, the cardioprotective effects in this plant are still unclear. In this study, we found that the Delonix regia leaf extract (DRLE) (400 mg/kg/d) can reduce the mortality rate in an isoproterenol (ISO)-induced heart injury and hypertrophy mouse model. Decreased serum levels of creatine phosphokinase, LDH, GOT, TNF-alpha and increased nitric oxide levels were found in DRLE-treated ISO-injured mice. In the in vitro study, the porcine coronary artery exhibited vasodilation effect induced by DRLE in a dose-dependent manner. In the DRLE toxic test, overdose of DRLE showed the high safety in normal mice and may have the ability to remove the metabolic wastes in blood. In conclusion, we demonstrated for the first time that DRLE has the cardioprotective effects by activating the vasodilation through NO pathway and preventing the myocyte injury via inhibition of TNF-alpha pathway. We suggest that DRLE may act as a promising novel herbal medicine for cardioprotection.
Introduction
Delonix regia (Bojer ex. Hook.) Raf., also known as the Royal Poinciana or Flamboyant, is a species of flowering plants in the pea family, Fabaceae and subfamily Caesalponioideae. It’s widely planted as ornamental trees in tropical areas, such as Taiwan, India, Vietnam, Malaysia, and the central region of South America. In some countries, D. regia has folkloric used as a medicinal agent to treat some disorders, such as constipation, inflammation, rheumatoid arthritis, diabetes, pneumonia, and malaria [1–6]. Many biological activity substances in the extracts of D. regia were reported to have anti-inflammatory [5, 6], antioxidant [4, 7, 8], antimicrobial [4, 9], anti-diarrhoeal [6, 10], antidiabetic [3], hepatopretective [11, 12], wound healing [13], and gastroprotective activity [14]. These functional phytoconstituents exist in leaves, flowers, barks, and seeds of D. regia including flavonoids, alkaloids, saponins, sterols, β-sitosterol, lupeol, tannins, carotenoids, and phenolic acids. [4, 6, 8, 15–17].
Ischemic heart disease (IHD) is a severe cardiovascular disease with a high global mortality rate. Previous studies have indicated that inflammation and oxidation of heart cells are commonly seen in IHD and contributed to IHD formation [18]. Several traditional Chinese prescriptions such as cinnamon, paeonol-danshensu combination, and catalpol from Rehumannia glutinosa were reported to be beneficial for acute myocardial injury [19–21]. In this study, we followed the law of Chinese medicine judging theory “red foods nourish the heart” on plant morphology, speculated that the flaming red flowers of D. regia may act as an “activate blood and resolve stasis” reagent on heart diseases and may improve the heart functions. Leaf extracts of D. regia (DRLE) was used as a tool for its convenient to obtain and the higher anti-inflammatory and antioxidative properties.
This study is the first time we demonstrate that the DRLE has the vasodilation effect on heart vessels and can act as a cardioprotective agent to protect the heart from isoproterenol-induced damage. Our findings may provide a possible candidate drug or healthy food for clinical medical use to treat or prevent cardiovascular diseases in the future.
Materials and Methods
Plant collection and preparation
The leaves of D. regia were collected from Kaohsiung, Taiwan and were taxonomically identified by botanist professor of Institute of Natural Products, College of Pharmacy, Kaohsiung Medical University, Kaohsiung, Taiwan. The samples were washed twice using double-distilled H2O, dried in shade, and ground into fine powder for further processing.
Extraction of D. regia leaf
The D. regia leaf powder was mixed with 80% ethanol at a ratio of 1:5 (w/w) for 48 h, filtrated and collected the supernatant. The insoluble residue was then mixed with ethanol at a ratio of 1:3 (w/w) for 48 h and repeated again. The three collected supernatants were poured, ran for vacuum concentration and freeze-dried to give the product (yield 13.8 ± 2.4%; mean ± SD), then stored in dark bottles for further use.
Animals
The (12- to 16-wk-old) C57BL/6 female mice were obtained from National Laboratory Animal Center, Taipei, Taiwan. The mice received regular mouse chow and tap water ad libitum. All the experimental protocols were reviewed and approved by the Committee of Institutional Animal Care and Use of I-Shou University (Approval No: IACUC-ISU-103005) and all the methods in this study were carried out in accordance with the approved guidelines. We followed the Guiding Principles for the Care and Use of Vertebrate Animals in Research and Training of the American Physiological Society during mice maintenance and experimentation.
In vivo heart injury induction and DRLE treatment
The isoproterenol (ISO)-induced mice heart injury and hypertrophy model was performed in our experiment [22, 23] The leaf extract of D. regia (DRLE) was dissolved in ddH2O and administrated to mice orally in low and high doses (100 and 400 mg/kg/d, respectively) for consequent 9 days. The control group was given with water and no DRLE treatment. ISO was injected subcutaneously at a dose of 60 mg/kg/d at the Day 4 for consecutive 5 days. The mice serum at the beginning (Day 0) and the end before sacrificed (Day 9) were collected from the tail vein for advanced biochemical assay. Creatine phosphokinase (CPK), lactate dehydrogenase (LDH), glutamic oxaloacetic transaminase (GOT), and glutamic pyruvic transaminase (GPT) were evaluated by using a biochemical analyzer (ARKRAY SPOTCHEM EZ SP-4430). TNF-alpha and IL-10 were assayed by conducting ELISA (R&D Systems, Minneapolis, MN, USA). Nitric oxide concentration was measured using the Griess reaction [24]. The survival rate was recorded daily. At the end of the experiment, organs of heart, liver, and kidney were removed, fixed with 10% formaldehyde, embedded by paraffin and sliced with H&E staining for subsequent pathological examination.
Oral toxicity assay
Mice in this test were fed 2 g/kg/d of DRLE orally for 14 days. Serum samples were collected from the tail veins at the beginning and the end of the experiment for biochemical assay. The CPK, LDH, GOT, GPT, blood urea nitrogen (BUN), total protein, creatinine, and uric acid were evaluated using a biochemical analyzer (ARKRAY SPOTCHEM EZ SP-4430) and compared with the reference values [25]. TNF-alpha and IL-10 were measured by conducting ELISA (R&D Systems) and the NO concentration was tested using the Griess reaction method [24]. The mice were sacrificed at Day 15 and tissues were obtained for pathological study in H&E staining.
Isolation of porcine coronary artery rings
Explanted pig hearts, weighing approximately 110 kg, were obtained from a local abattoir. The heart was placed in an ice-cold Krebs–Henseleit buffer composed of the following (in mM): 122 NaCl, 4.7 KCl, 15.5 NaHCO3, 1.2 KH2PO4, 1.2 MgCl2, 1.8 CaCl2, and 11.5 glucose, with pH 7.4, and preserved on ice during transport to the laboratory approximately 60 min elapsed between the removal of the hearts and the arrival of the tissue in the laboratory. All procedures were performed in compliance with relevant laws and national guidelines.
The left anterior descending coronary artery (LAD) was excised, removed excess fat and connective tissue in an ice-cold buffer solution. Four 4-mm rings were obtained from each LAD, and were prepared for isometric contractile force recordings as described previously, but with minor modifications [26–30]. To control for the possible indirect effects of endothelium-derived vasoactive factors, the endothelium was removed physically by rubbing the luminal surface gently with a cotton swab. In preliminary experiments, removal of the endothelium was confirmed by the loss of relaxation in response to substance P (10 nM) [30].
In vitro relaxation of isolated porcine coronary arterial rings caused by DRLE
The porcine coronary arterial rings were mounted using a wireless triangular support (Radnoti, Monrovia, CA, USA) in conjunction with a rigid L-shaped support. The triangular support was attached to a force-displacement transducer (FORT 10g, World Precision Instruments Inc., Sarasota, FL, USA) connected to an amplifier and a computer recording system (BIOPAC systems, Santa Barbara, CA, USA). Next, the coronary arterial rings were suspended in an organ bath containing 5 mL Krebs–Henseleit buffer and gassed with 95% O2•5% CO2. The final pH at 37°C was 7.40 ± 0.05.
The coronary arterial ring preparations were equilibrated at a resting tension of 2.0 g. After initial equilibration, the preparations were exposed to 60 mM KCl for 4 min. The experiments commenced after a 45-min equilibration period. For the relaxation measurements in the contracted arterial rings, the DRLE was added in a non-cumulative manner to U46619 (100 nM)-contracted muscle strips 15 min after the addition of U46619. The relaxation responses were represented as a percentage of U46619-stimulated contraction. Only one single dose response was studied with each preparation.
Statistical analysis
Statistical analysis was conducted using GraphPad Prism 4 (San Diego, CA, USA). All data were expressed as mean ± standard deviation (SD). The differences between groups were performed using unpaired student’s t-test and one-way analysis of variance (ANOVA) with Tukey’s post hoc test. Significant difference was defined as P < 0.05, P < 0.01 or P < 0.001.
Results
DRLE enhances the survival rate in ISO-induced mice
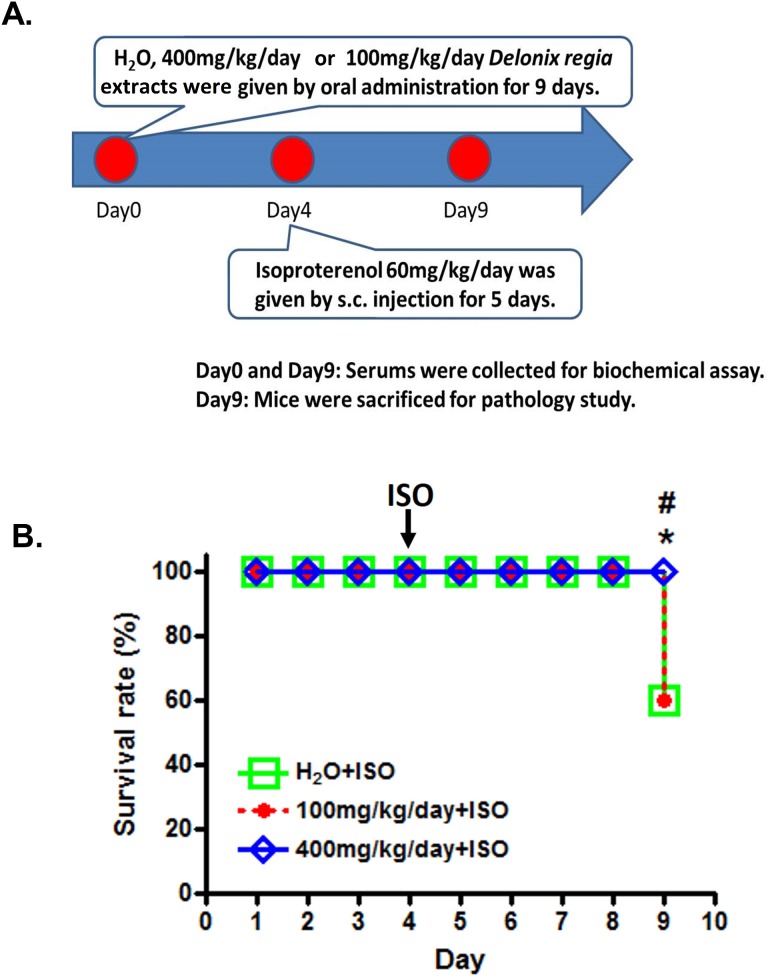
In this study, we first explored the survival rate of ISO-induced mice on DRLE treatment. The flowchart of the experimental design is shown in Fig 1A. The low, high doses of DRLE (100 and 400 mg/kg/d) and water were oral gavaged individually in ISO-treated mice for consecutive 9 days. At the 9th day, all ISO-induced mice treated with high dose of DRLE were alive (survival rate 100%), while the mice treated with low dose of DRLE and water displayed only 60% survival rates (Fig 1B). The results revealed that high dose (400 mg/kg/d) of DRLE can effective prevent ISO-induced mice from dying and enhance the survival chances.
Fig 1. DRLE had a cardioprotective effect.
(A) H2O and DRLE (100 mg/kg/d) and (400 mg/kg/d) were orally administered to C57BL/6 mice for 9 d. At Day 4, 60 mg/kg/d of isoproterenol was injected subcutaneously for 5 d. (B) The survival rate of ISO-injected mice treated with H2O and 100 mg/kg/d and 400 mg/kg/d of DRLE. *, P < 0.05 vs. H2O. #, P < 0.05 vs. 100 mg/kg/d (n = 5 per group).
DRLE reduces ISO-induced ventricular hypertrophy and heart injury
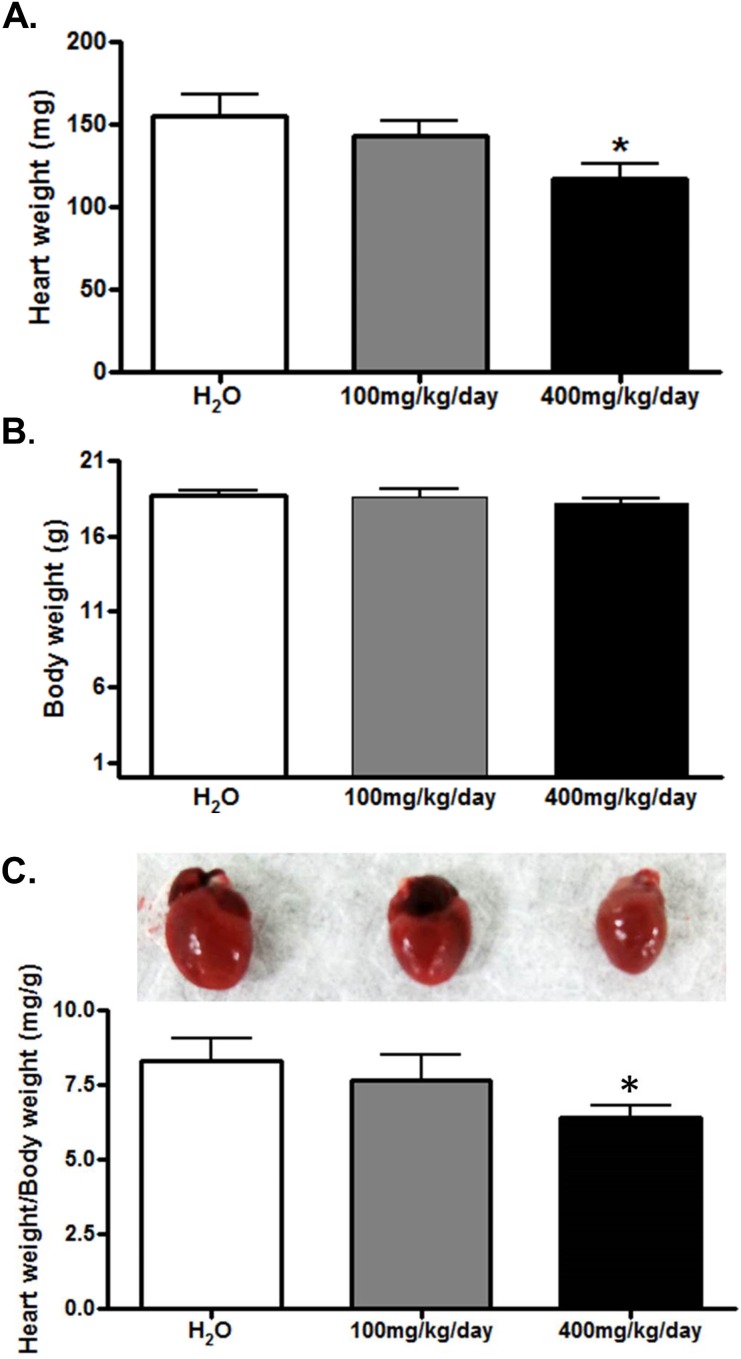
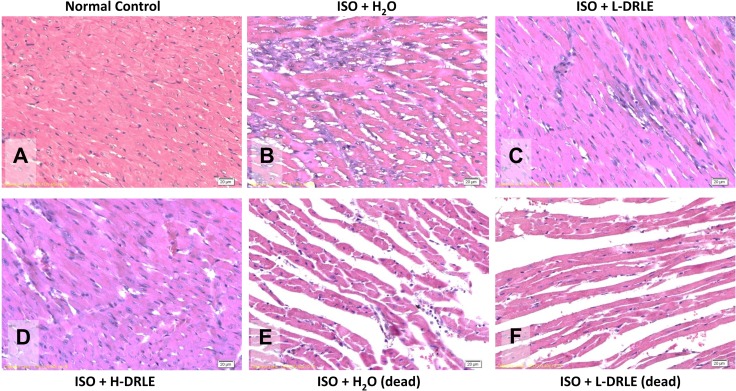
The heart weight and histopathological examination were performed to assess the protective effect of DRLE on ISO-induced heart injury [31, 32]. The heart-body weight ratio in mice treated with high dose DRLE (400 mg/kg) had the lowest proportion compared with the low dose (100 mg/kg) and water control groups (6.43 ± 0.82 vs. 7.65 ± 0.86 and 8.29 ± 1.50 kg, respectively, p< 0.05), and it displayed in a dose-dependent manner (Fig 2). In histological examination, normal cardiac myocyte was a single-nucleus, striated, and branched structure (Fig 3A). The injured myocytes by isoproterenol existed irregular and split arrangement, severe inflammation, infiltration of leukocytes, and lost the nucleus in the pathological morphology, which showed the sign of myocardial infarction (Fig 3B and 3E). The cell morphology in low dose DRLE-treated group was similar to the normal control and its inflammatory state was alleviated compared with non-treated heart injury group (Fig 3C and 3F). Surprisingly, the best protection was occurred in high dose DRLE-treated group (Fig 3D). It displayed effective protection against ISO-induced myocyte damage and inflammation, thus increased the survival rate. All these examinations revealed that the high dose DRLE can reduce ISO-induced ventricular hypertrophy and Injury, thus can restore heart to reach almost normal state.
Fig 2. DRLE reduced ISO-induced ventricular hypertrophy.
Heart and body weight in ISO-induced mice treated with H2O, 100 and 400 mg/kg/d DRLE (A) Heart weight. (B) Body weight. (C) The image of different sized mouse hearts and heart weight/body weight (mg/g) used to estimate the degree of ventricular hypertrophy. *, P < 0.05 vs. H2O (n = 5 per group).
Fig 3. DRLE alleviated ISO-induced heart injury.
Pathological examination of heart tissues in normal and ISO-induced mice fed with or without DRLE (H&E, 400X). (A) Normal control; (B) 60 mg/kg/d of ISO; (C) 60 mg/kg/d of ISO + 100 mg/kg/d of DRLE (L-DRLE); (D) 60 mg/kg/d of ISO + 400 mg/kg/d DRLE (H-DRLE); (E) 60 mg/kg/d of ISO (mice dead before sacrifice); and (F) 60 mg/kg/d of ISO + 100 mg/kg/d of DRLE (mice dead before sacrifice).
DRLE prevented ISO-induced elevation of CPK, LDH, and GOT
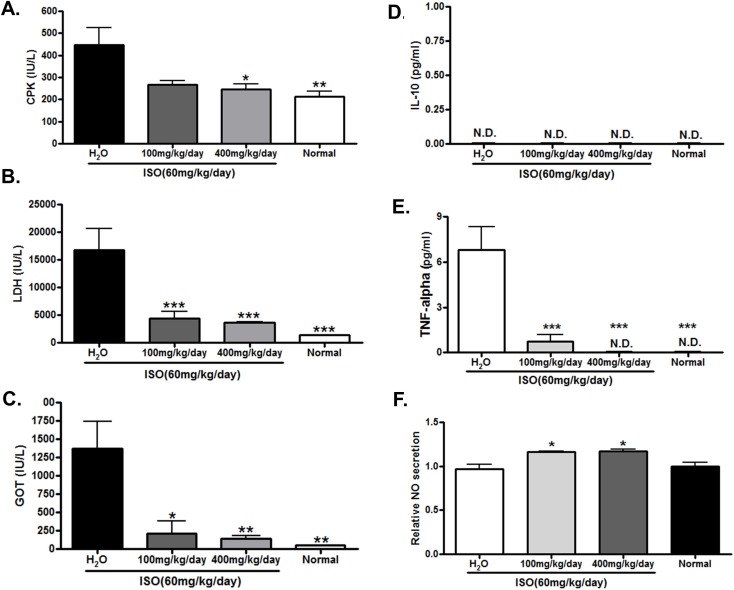
To confirm our pathological finding, several heart injury markers were evaluated in different groups. In the ISO-induced heart injury group, serum CPK, LDH, and GOT were substantial elevated (0.45 ± 0.11, 16.7 ± 6.88, and 1.37 ± 0.63 IU/mL, respectively), which denote the inflamed and damaged sign. In the DRLE-treated mice, elevated serum CPK, LDH, and GOT levels were significantly decreased and appeared in a dose-dependent manner (Fig 4A, 4B and 4C). These data revealed that DRLE can restrain the elevation of CPK, LDH, and GOT by ISO, and can effective prevent the heart injury in IHD.
Fig 4. DRLE prevented ISO-induced elevation of CPK, LDH, GOT, and TNF-α, and elevated serum NO level.
Serum levels of biomarkers and cytokines in normal and ISO-induced mice treated with H2O, 100 and 400 mg/kg/d DRLE. (A) CPK (B) LDH (C) GOT. (D) IL-10 (E) TNF-α (F) NO.*, P < 0.05 vs. H2O; **, P < 0.01 vs. H2O; ***, P < 0.001 vs. H2O (n = 3 to 5 per group).
DRLE reduced serum TNF-alpha and increased NO levels in ISO-induced mice in vivo
Previous researches showed that the inflammatory and cardiovascular change play critical roles in progressive heart injury and myocyte hypertrophy [21, 32–40]. In this study, we determined serum levels of IL-10, TNF-alpha, and NO to check whether the immunomodulation and vasodilation were involved in the protective effects of DRLE. As shown in Fig 4, serum TNF-alpha was decreased and serum NO was elevated in DRLE-treated mice, while the serum IL-10 was unable to detect because the very low levels in our study (Fig 4D, 4E and 4F). These evidences confirmed that the cardioprotective effect of DRLE may be accomplished through immunomodulation and vasodilation routes via TNF-alpha and NO secretion pathways.
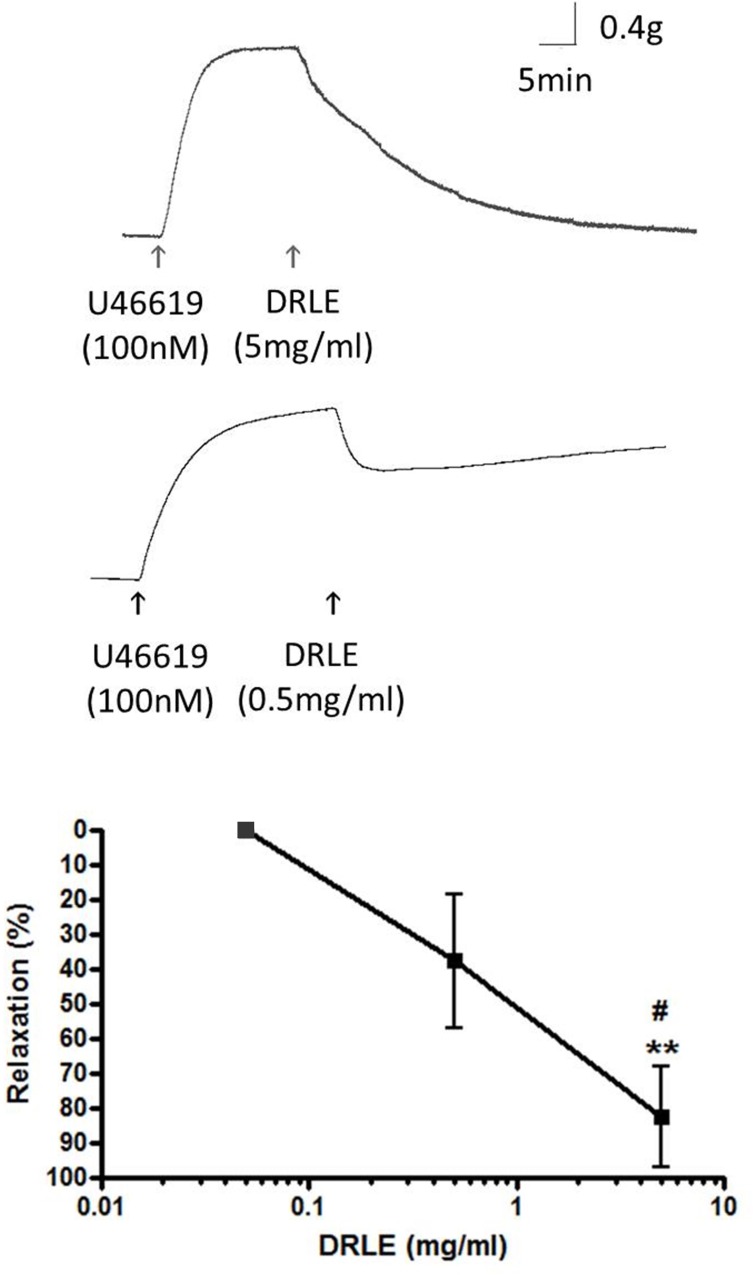
DRLE had a vasodilator effect in vitro
To determine the vessel relaxation potency of DRLE in vitro, we used porcine LAD as study material. Maximum vasoconstriction was first reached by U46619, an analog of endoperoxide prostaglandin PGH2, then added different doses of DRLE (5, 0.5 and 0.05 mg/mL, from high to low) to test the relaxation degree. The vessels displayed the largest relaxation in 5 mg/mL DRLE treatment (82.5 ± 28.7%), followed by 0.5 mg/mL DRLE (37.6 ± 27.2%), while the lowest dose of DRLE had no any vasodilator effect (0.0 ± 0.0%, in 0.05 mg/mL) (Fig 5). It showed a dose-dependent manner. The results verified that the DRLE had a direct effect on vasodilation in vitro and this was corresponding to the cardioprotective effect in vivo.
Fig 5. DRLE had a direct vasodilatory effect on porcine coronary arterial rings.
Porcine left arterial descending coronary arteries were treated with different DRLE doses of 0.05 mg/mL, 0.5 mg/mL, and 5 mg/mL to observe the direct vasodilatory effect. **, P < 0.01 vs. 0.05 mg/mL; #, P < 0.05 vs. 0.5 mg/mL (n = 3 to 5 per group).
DRLE exhibited a high safety profile
Shewale, et al. had reported that the DRLE was safe and non-toxic in the acute toxicity studies in vivo [5]. However, the chronic toxicity of DRLE remained unclear. In this study, a five times high dose of DRLE (2 g/kg) was administrated orally for consecutive 14 days and test the toxicity in mice. In the whole process, no lethality or any toxic reaction was found, and mice displayed normal in hair colour, activity and appearance. In addition, after overdose DRLE treatment, serum CPK, LDH, GOT, BUN, and uric acid levels were decreased while total protein level was increased compared with untreated control group. No change was detected in body weight, heart weight, heart-body weight ratio, GPT, albumin, creatinine, IL-10, TNF-alpha, and relative NO secretion levels (Table 1). All these biochemical data were in the normal range and not influenced by overdose DRLE, proved that DRLE is safe and harmless not only in acute state but also in chronic consumption.
Table 1. Body Weight and Serum Biochemical Markers in Oral Toxicity Test of DRLE.
| Normal | DRLE (2g/kg/d) | Reference values | |
|---|---|---|---|
| Body Weight (g) | 20.8 ± 1.3 | 20.8 ± 1.3 | 19.3–24.4 |
| Heart Weight (mg) | 124 ± 12 | 130 ± 18 | – |
| Heart Weight / Body Weight (mg/g) | 5.95 ± 0.36 | 6.41 ± 0.77 | 4–7 |
| CPK (IU/L) | 212 ± 71 | 150 ± 51* | 105–649 |
| LDH (IU/L) | 1334 ± 342 | 672 ± 94.6* | 293–3150 |
| GOT (IU/L) | 48.1 ± 16.5 | 25.0 ± 3.54* | 10–122 |
| GPT (IU/L) | 14.9 ± 0.5 | 15.2 ± 1.0 | 10–73 |
| Total protein (g/dl) | 4.20 ± 0.84 | 5.60 ± 0.54* | 3.5–8.3 |
| Albumin (g/dl) | 1.84 ± 0.65 | 1.90 ± 0.65 | 2.0–4.8 |
| BUN (mg/dl) | 32.0 ± 5.7 | 17.6 ± 2.5* | 10–58.1 |
| Creatinine (mg/dl) | 0.52 ± 0.19 | 0.56 ± 0.11 | 0.3–1 |
| Uric acid (mg/dl) | 4.70 ± 0.76 | 3.44 ± 0.78* | 2–12 |
Data are expressed as mean ± S.D.
* P < 0.05 vs. normal group
Discussion
Delonix regia leaves are informal used to treat disorders in folk, but few studies have reported to elucidate their potential mechanisms [6]. In this study, we tested the DRLE functions on ISO-induced heart injury mice. The ISO-induced heart injury mice model is a reliable, reproducible, and commonly used method for human myocardial infarction studies [19–21, 36, 41]. In this model, both male and female have been used in studies for the gender factors seldom affect the heart functions in young animals. Female mice were adopted in our study instead of male to avoid the stress fighting in cage and cause unnecessary injury and death. The ISO-injured myocyte was suffer from the threats of inflammation, reduced nitric oxide secretion and increased free radicals generation, thus induce the heart injury and hypertrophy [20–22, 34, 37, 42].
In this study, leaves of D. regia were used instead of flowers, barks or seeds for its easier to obtain and exhibit the higher biological activities [4, 6]. Previous studies have identified that four major bioactivity phytoconstituents of DRLE were involved: (1) β-sitosterol, (2) lupeol, (3) flavonoids, and (4) phenolic acid (gallic acid, protocatehuic acid, and salicylic acid) [4–6, 12] (S4 Table, S1 Fig). (1) β-Sitosterol is a sterol of plants that can reduce cholesterol serum levels, regulate inflammation, and has anticancer activity [43–45]. β-sitosterol can also provide the antioxidative and cardioprotective effect by attenuating TNF-alpha-induced monocyte adhesion on human aortic endothelial cells [46, 47]. (2) Lupeol, a triterpenoid compound, has anti-inflammatory activity and may act as a promising compound to protect heart from injury [48–50]. (3) Flavonoids are potent compounds that exhibit antioxidant, anti-tumour, anti-inflammatory, and cardioprotective effects [51–58]. (4) Phenolic acids is abundant in DRLE and it exhibits the high antioxidant, anticancer, antimicrobial, and cardioprotective activity [4, 59–63]. Based on these studies, we surmise that the cardiovascular protection of DRLE may be the combination of the four bioactivity compounds, but which compounds arouse the major cardioprotective effect remain to be further investigation.
In the choice of DRLE dosage, we followed the previous in vivo studies and set a high (400 mg/kg/d) and low (100 mg/kg/d) doses of DRLE to perform our study [1, 3, 5, 12]. The results revealed that the high dose of DRLE can effective protect mice from death elicited by ISO-induced heart injury and hypertrophy, but low dose and ISO-induced control mice showed little or no function on it. The heart-body weight ratio and morphology of cardiac myocytes in high dose DRLE group revealed similar and regular arrangement to those of normal mice, but the myocytes in low dose DRLE and ISO-induced control mice still showed severe hypertrophy and injury.
For the reason that if excessive DRLE can cause toxic damage on normal mice, an overdose (2 g/kg/d) of DRLE was oral administrated consequently for 14 days to test the toxicity and safety in mice. In the whole process, mice showed healthy and vitality state. Serum biochemical data were all in the normal range. These results prove that DRLE is safe by oral route in mice. According to the safety test, we can make sure that the 400 mg/kg/d dose DRLE in our test can’t make any change in the normal mice. Another benefit in the DRLE toxic test is the reduced levels of serum CPK, LDH, GOT, BUN, and uric acids. It means the DRLE may has the potential to lower the metabolic wastes in serum and can act as a heathy food or reagent for clinical use.
In the results in biochemical assays, serum CPK, LDH, and GOT levels were significant reduced after high dose DRLE treatment. This is agreed with the normalized myocyte morphology and elevated survival rates in DRLE treatment. It means that the DRLE may benefit for the body operations. In this present study, low dose of DRLE has slightly improved but incompletely protected on cardiac functions. The high dose of DRLE exhibited the effective functions to protect heart from injury and thus elevated the high survival rate. In the histopathological results, cardiac myocytes in low dose DRLE group was still in a bad condition but appeared to be much better than the untreated ISO-induced heart injury group. The regular and perfect morphology was seen in high dose DRLE group and similar to the normal tissue. It means that high DRLE can protect the heart from worsen induced by ISO and restore to the almost normal state.
To elucidate the possible mechanism of DRLE on cardioprotection, serum makers such as TNF-alpha, IL-10, and NO were evaluated, which may play a critical role in heart injury and hypertrophy [64]. In our current results, we found that serum NO level was increased in DRLE-treated heart-injury mice. Previous study in eNOS-overexpressed mouse model had proved that released NO can attenuate ISO-induced hypertrophy [42]. Serum NO level raised by cinnamic acid and cinnamic aldehyde in other study had also indicated the cardioprotective effects on ISO-induced acute myocardial ischemia rats [21]. The eNOS upregulation can also be incited by Matrine in ISO-induced myocardial ischemia rats [37]. The eNOS activity was undetected in our study for the technique shortage, but the serum NO was significant elevated in ISO-injury mice after high dose DRLE treatment. However, there was no elevated serum NO secretion observed in normal mice treated with DRLE. We suggest that DRLE-induced NO secretion was triggered by ISO-induced heart injury. In the porcine LAD test in vitro, we found the obvious vasodilation effect after DRLE treatment. Thus we can confirm that the cardioprotective effect of DRLE in ISO-induced heart injury may partially triggered via NO secretion pathway.
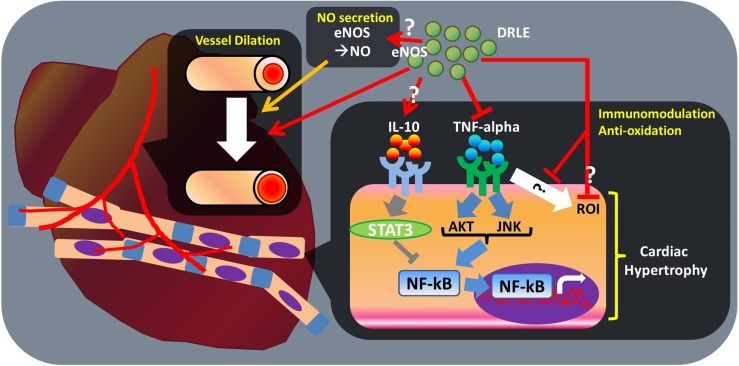
Some inflammatory cytokines play a critical role in heart injury and hypertrophy, such as TNF-alpha and IL-10 [32, 35, 38–40]. TNF-alpha secretion was elevated in myocyte hypertrophy by generating reactive oxygen intermediates and activating AKT, JNK and NF-kappaB (NF-kB) pathways, thus caused myocyte damage [35, 38, 39]. Previous study indicated that TNF-alpha expression was reduced by cinnamic acid and cinnamic aldehyde in ISO-induced heart injury rats [21]. In our present data, serum TNF-alpha levels were significant decreased in ISO-induced heart injury mice after DRLE treatment. TNF-alpha had the downregulation effect on eNOS expression in heart failure patients and obese mice [33, 65]. Combined with the elevated serum NO level and reduced TNF-alpha levels in our results, we can affirm that the protection mechanism of DRLE may functioned via TNF-alpha and NO pathways. In addition, serum IL-10 overexpression can attenuate ventricular hypertrophy via the STAT3 pathway [32]. Although the serum IL-10 level was too low to be detected in our experiment, we can’t exclude the actions of IL-10 in heart tissues. The outline of the possible mechanisms of DRLE on cardioprotection is shown in Fig 6.
Fig 6. Possible cardioprotective mechanism of DRLE.
Inflammation, oxidation, and NO secretion have been demonstrated to play a critical role in cardiac hypertrophy and myocardial injury. IL-10 can activate STAT3 to inhibit the NF-kB signalling pathway, thereby attenuating cardiac hypertrophy. TNF-alpha is associated with the AKT and JNK pathways in enhancing NF-kB activation to augment cardiac hypertrophy. TNF-alpha can also upregulate ROI expression to enhance myocyte hypertrophy. Another study reported that eNOS upregulation can attenuate myocardial injury. In our study, we first found that DRLE can attenuate TNF-alpha secretion, which might reduce AKT, JNK, and NF-kB signalling pathways, thereby inhibiting cardiac hypertrophy. ROI expression might also be inhibited by DRLE through TNF-alpha stimulation. We assumed that the direct inhibition of ROI is a potential mechanism for inhibiting cardiac hypertrophy, because the antioxidative effect of DRLE has also been reported. Second, we cannot confirm whether DRLE enhances IL-10 expression, because serum IL-10 level was undetected in our experiment. Third, the NO serum level increased in our study, but we could not determine the origin of the NO, and we postulated that eNOS is a possible candidate. Finally, we found that DRLE can dilate the porcine coronary artery directly in vitro.
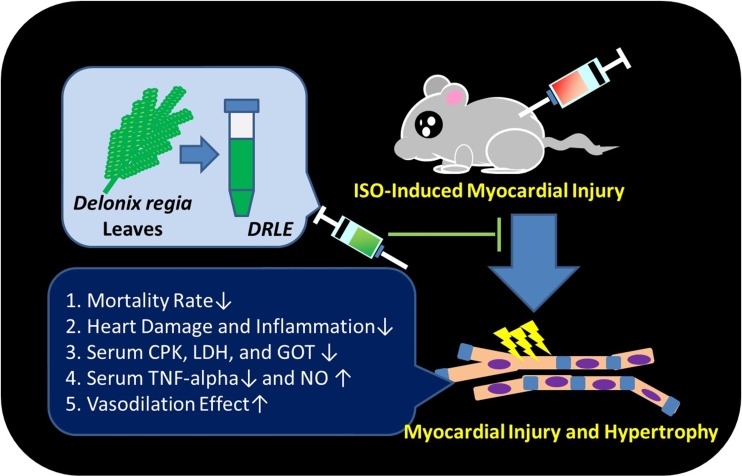
In summary, we found that the DRLE can reduce the mortality rate and heart injury in ISO-induced IHD mice. The cardioprotective mechanisms of DRLE may act via the anti-inflammatory and vasodilation effects on myocytes which were mediated by TNF-alpha and NO secretions (Fig 7). We also found that DRLE was safe by oral route for at least two weeks and it may possess the potential to improve the heart, liver or kidney functions. These results proved that the DRLE can act as a promising cardioprotective candidate agent or healthy food to prevent or improve the heart damage in IHD. Further studies will be explored to find out the real bioactive compounds in DRLE which were involved in the improvement of heart functions.
Fig 7. Cardioprotective effect of DRLE.
In this study, we found that DRLE can reduce the mortality rate, cardiac hypertrophy and heart injury in ISO-injured mice. Serum CPK, LDH, GOT, and TNF-alpha were also reduced by DRLE administration. Serum NO levels were induced by ISO injection in mice in vivo, and had the vasodilatory effects in vitro.
Supporting Information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
In previous studies, four major compounds in DRLE had been identified, including β-sitosterol, lupeol, flavonoids and phenolic acid. The background photograph of flowers was taken in Tainan, Taiwan, by Chun-Ting Lee, one of the co-authors in this article.
(TIF)
Acknowledgments
We are grateful to Professor Ming-Hong Yen for plant taxonomical identification, professor Ting-Ting Yang for slide histological examination, Valerio Puggioni for article editing and Sorrel Tilley for manuscript proofreading.
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
This study was sponsored by the Intramural Industry-University Collaboration Projects in I-Shou university and E-Da hospital, Kaohsiung, Taiwan (ISU-103-INT-05E). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Fatmawaty F, Astuti H. Antimalarial activity of Delonix regia on mice with Plasmodium berghei. J Nat Prod. 2013;6:61–6. [Google Scholar]
- 2.Modi A, Mishra V, Bhatt A, Jain A, Mansoori MH, Gurnany E, et al. Delonix regia: historic perspectives and modern phytochemical and pharmacological researches. Chin J Nat Med. 2016;14(1):31–9. 10.3724/SP.J.1009.2016.00031 [DOI] [PubMed] [Google Scholar]
- 3.Rahman M, Hasan N, Das AK, Hossain T, Jahan R, Khatun A, et al. Effect of Delonix regia leaf extract on glucose tolerance in glucose-induced hyperglycemic mice. Afr J Tradit Complement Altern Med. 2011;8(1):34–6. PubMed Central PMCID: PMCPMC3218438. [PMC free article] [PubMed] [Google Scholar]
- 4.Shabir G, Anwar F, Sultana B, Khalid ZM, Afzal M, Khan QM, et al. Antioxidant and antimicrobial attributes and phenolics of different solvent extracts from leaves, flowers and bark of Gold Mohar [Delonix regia (Bojer ex Hook.) Raf]. Molecules. 2011;16(9):7302–19. 10.3390/molecules16097302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shewale VD, Deshmukh TA, Patil LS, Patil VR. Anti-Inflammatory Activity of Delonix regia (Boj. Ex. Hook). Adv Pharmacol Sci. 2012;5(Suppl 3):1–4. PubMed Central PMCID: PMCPMC3206388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh S, Kumar SN. A review: introduction to genus Delonix. World J Pharm Pharm Sci. 2014;3(6):2042–55. [Google Scholar]
- 7.jancy Rani PM, Kannan P, Kumaravel S. Screening of antioxidant activity, total phenolics and gas chromatograph and mass spectrometer (GC-MS) study of Delonix regia. African journal of biochemistry research. 2011;2(12):341–7. [Google Scholar]
- 8.Shanmukha I, Patel H, Patel J, Riyazunnisa. Quantification of total phenol and flavonoid content of Delonix regia flowers. Int J Chem Tech Res. 2011;3:280–3. [Google Scholar]
- 9.Ragasa CY, Hofilena JG. Antimicrobial coumarin derivative from Delonix regia. Manila J Sci. 2011;7(1):7–11. [Google Scholar]
- 10.Shiramane RS, Biradar KV, Chivde BV, Shambhulingayya H, Goud V. In-vivo antidiarrhoeal activity of ethanolic extract of Delonix regia flowers in experimental induced diarrhoea in wistar albino rats. International Journal of Research in Pharmacy and Chemistry. 2011;1(3):2231–781. [Google Scholar]
- 11.Ahmed J, Nirmal S, Dhasade V, Patil A, Kadam S, Pal S, et al. Hepatoprotective activity of methanol extract of aerial parts of Delonix regia. Phytopharmacology. 2011;1(5):118–22. [Google Scholar]
- 12.Azab SS, Abdel-Daim M, Eldahshan OA. Phytochemical, cytotoxic, hepatoprotective and antioxidant properties of Delonix regia leaves extract. Med Chem Res. 2013;22(9):4269–77. [Google Scholar]
- 13.Khan MA, Saxena A, Fatima FT, Sharma G, Goud V, Husain A. Study of wound healing activity of Delonix regia flowers in experimental animal models. Am J PharmTech Res. 2012;2:380–90. [Google Scholar]
- 14.Shiramane R, Chivde B, Kamshetty M. Gastroprotective activity of ethanolic extract of Delonix regia flowers in experimental induced ulcer in wistar albino rats [J]. Int J Res in Pharm Chem. 2011;2(5):234–8. [Google Scholar]
- 15.Lakshmi V. Constituents of wood of Delonix regia. National Academy Science Letters. 1987;10(6):197. [Google Scholar]
- 16.Sama K, Raja A. Preliminary phytochemical screening of root bark of Delonix regia. Int Res J Pharm. 2011;2:42–3. [Google Scholar]
- 17.Jahan I, Rahman MS, Rahman MZ, Kaisar MA, Islam MS, Wahab A, et al. Chemical and biological investigations of Delonix regia (Bojer ex Hook.) Raf. Acta Pharm. 2010;60(2):207–15. 10.2478/v10007-010-0018-7 [DOI] [PubMed] [Google Scholar]
- 18.Mehta JL, Li DY. Inflammation in ischemic heart disease: response to tissue injury or a pathogenetic villain? Cardiovasc Res. 1999;43(2):291–9. [DOI] [PubMed] [Google Scholar]
- 19.Bi F-J, Zhang H, Xu Y-J, Hu J. Protective effect of catalpol on isoproterenol-induced myocardial injury in Wistar rats. Afr J Biotech. 2012;11(38):9270–5. [Google Scholar]
- 20.Li H, Xie YH, Yang Q, Wang SW, Zhang BL, Wang JB, et al. Cardioprotective effect of paeonol and danshensu combination on isoproterenol-induced myocardial injury in rats. PLoS One. 2012;7(11):e48872 PubMed Central PMCID: PMCPMC3490947. 10.1371/journal.pone.0048872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song F, Li H, Sun J, Wang S. Protective effects of cinnamic acid and cinnamic aldehyde on isoproterenol-induced acute myocardial ischemia in rats. J Ethnopharmacol. 2013;150(1):125–30. 10.1016/j.jep.2013.08.019 [DOI] [PubMed] [Google Scholar]
- 22.Takaki M. Cardiac mechanoenergetics for understanding isoproterenol-induced rat heart failure. Pathophysiology: the official journal of the International Society for Pathophysiology / ISP. 2012;19(3):163–70. Epub 2012/06/13. [DOI] [PubMed] [Google Scholar]
- 23.Boluyt MO, Long X, Eschenhagen T, Mende U, Schmitz W, Crow MT, et al. Isoproterenol infusion induces alterations in expression of hypertrophy-associated genes in rat heart. The American journal of physiology. 1995;269(2 Pt 2):H638–47. Epub 1995/08/01. [DOI] [PubMed] [Google Scholar]
- 24.Kleinbongard P, Rassaf T, Dejam A, Kerber S, Kelm M. Griess method for nitrite measurement of aqueous and protein-containing samples. Methods Enzymol. 2002;359:158–68. [DOI] [PubMed] [Google Scholar]
- 25.Rosenthal K. Rodent Reference Ranges [[Internet]]. 2003. Available from: http://cal.vet.upenn.edu/projects/ssclinic/refdesk/rodentrr.htm.
- 26.White RE, Han G, Dimitropoulou C, Zhu S, Miyake K, Fulton D, et al. Estrogen-induced contraction of coronary arteries is mediated by superoxide generated in vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2005;289(4):H1468–75. PubMed Central PMCID: PMCPMC1380187. 10.1152/ajpheart.01173.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee MC, Yang YC, Chen YC, Huang SC. Muscarinic receptor M3 mediates human gallbladder contraction through voltage-gated Ca2+ channels and Rho kinase. Scand J Gastroenterol. 2013;48(2):205–12. 10.3109/00365521.2012.741615 [DOI] [PubMed] [Google Scholar]
- 28.Huang CC, Hsu MC, Huang WC, Yang HR, Hou CC. Triterpenoid-rich extract from Antrodia camphorata improves physical fatigue and exercise performance in mice. Evid Based Complement Alternat Med. 2012;2012:364741 PubMed Central PMCID: PMCPMC3398672. 10.1155/2012/364741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang SC. Endothelin A receptors mediate relaxation of guinea pig internal anal sphincter through cGMP pathway. Neurogastroenterol Motil. 2010;22(9):1009–1, e264. 10.1111/j.1365-2982.2010.01513.x [DOI] [PubMed] [Google Scholar]
- 30.Huang SC. Dendroaspis natriuretic peptide is the most potent natriuretic peptide to cause relaxation of lower esophageal sphincter. Regulatory peptides. 2011;167(2–3):246–9. Epub 2011/02/19. 10.1016/j.regpep.2011.02.007 [DOI] [PubMed] [Google Scholar]
- 31.Xie J, Cha SK, An SW, Kuro OM, Birnbaumer L, Huang CL. Cardioprotection by Klotho through downregulation of TRPC6 channels in the mouse heart. Nat Commun. 2012;3:1238 PubMed Central PMCID: PMCPMC3526952. 10.1038/ncomms2240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verma SK, Krishnamurthy P, Barefield D, Singh N, Gupta R, Lambers E, et al. Interleukin-10 treatment attenuates pressure overload-induced hypertrophic remodeling and improves heart function via signal transducers and activators of transcription 3-dependent inhibition of nuclear factor-kappaB. Circulation. 2012;126(4):418–29. PubMed Central PMCID: PMCPMC3422741. 10.1161/CIRCULATIONAHA.112.112185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agnoletti L, Curello S, Bachetti T, Malacarne F, Gaia G, Comini L, et al. Serum from patients with severe heart failure downregulates eNOS and is proapoptotic: role of tumor necrosis factor-alpha. Circulation. 1999;100(19):1983–91. [DOI] [PubMed] [Google Scholar]
- 34.Anderson HD, Rahmutula D, Gardner DG. Tumor necrosis factor-alpha inhibits endothelial nitric-oxide synthase gene promoter activity in bovine aortic endothelial cells. J Biol Chem. 2004;279(2):963–9. 10.1074/jbc.M309552200 [DOI] [PubMed] [Google Scholar]
- 35.Condorelli G, Morisco C, Latronico MV, Claudio PP, Dent P, Tsichlis P, et al. TNF-alpha signal transduction in rat neonatal cardiac myocytes: definition of pathways generating from the TNF-alpha receptor. FASEB J. 2002;16(13):1732–7. 10.1096/fj.02-0419com [DOI] [PubMed] [Google Scholar]
- 36.Krenek P, Kmecova J, Kucerova D, Bajuszova Z, Musil P, Gazova A, et al. Isoproterenol-induced heart failure in the rat is associated with nitric oxide-dependent functional alterations of cardiac function. Eur J Heart Fail. 2009;11(2):140–6. PubMed Central PMCID: PMCPMC2639419. 10.1093/eurjhf/hfn026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li X, Wang X, Guo Y, Deng N, Zheng P, Xu Q, et al. Regulation of endothelial nitric oxide synthase and asymmetric dimethylarginine by matrine attenuates isoproterenol-induced acute myocardial injury in rats. J Pharm Pharmacol. 2012;64(8):1107–18. 10.1111/j.2042-7158.2012.01502.x [DOI] [PubMed] [Google Scholar]
- 38.Nakamura K, Fushimi K, Kouchi H, Mihara K, Miyazaki M, Ohe T, et al. Inhibitory effects of antioxidants on neonatal rat cardiac myocyte hypertrophy induced by tumor necrosis factor-alpha and angiotensin II. Circulation. 1998;98(8):794–9. [DOI] [PubMed] [Google Scholar]
- 39.Sack MN, Smith RM, Opie LH. Tumor necrosis factor in myocardial hypertrophy and ischaemia—an anti-apoptotic perspective. Cardiovasc Res. 2000;45(3):688–95. [DOI] [PubMed] [Google Scholar]
- 40.Sun M, Chen M, Dawood F, Zurawska U, Li JY, Parker T, et al. Tumor necrosis factor-alpha mediates cardiac remodeling and ventricular dysfunction after pressure overload state. Circulation. 2007;115(11):1398–407. 10.1161/CIRCULATIONAHA.106.643585 [DOI] [PubMed] [Google Scholar]
- 41.Wexler BC. Myocardial infarction in young vs old male rats: pathophysiologic changes. Am Heart J. 1978;96(1):70–80. [DOI] [PubMed] [Google Scholar]
- 42.Ozaki M, Kawashima S, Yamashita T, Hirase T, Ohashi Y, Inoue N, et al. Overexpression of endothelial nitric oxide synthase attenuates cardiac hypertrophy induced by chronic isoproterenol infusion. Circ J. 2002;66(9):851–6. [DOI] [PubMed] [Google Scholar]
- 43.Bouic PJ. Sterols and sterolins: new drugs for the immune system? Drug Discov Today. 2002;7(14):775–8. [DOI] [PubMed] [Google Scholar]
- 44.Bouic PJ, Lamprecht JH. Plant sterols and sterolins: a review of their immune-modulating properties. Altern Med Rev. 1999;4(3):170–7. [PubMed] [Google Scholar]
- 45.Miettinen TA, Puska P, Gylling H, Vanhanen H, Vartiainen E. Reduction of serum cholesterol with sitostanol-ester margarine in a mildly hypercholesterolemic population. N Engl J Med. 1995;333(20):1308–12. 10.1056/NEJM199511163332002 [DOI] [PubMed] [Google Scholar]
- 46.Loizou S, Lekakis I, Chrousos GP, Moutsatsou P. Beta-sitosterol exhibits anti-inflammatory activity in human aortic endothelial cells. Mol Nutr Food Res. 2010;54(4):551–8. 10.1002/mnfr.200900012 [DOI] [PubMed] [Google Scholar]
- 47.Ganapathy P, Rajadurai M, Ashokumar. Effect of β-Sitosterol on Cardiac Troponins, Marker Enzymes and Biochemical Parameters in Isoproterenol-Induced Myocardial Infarction. Journal of Academia and Industrial Research. 2014;3(5):209–14. [Google Scholar]
- 48.Arul V, Kumaraguru S, Dhananjayan R. Effects of aegeline and lupeol-the two cardioactive principles isolated from the leaves of Aegle marmelos Corr. Journal of Pharmacy and Pharmacology 1999;51:252. [Google Scholar]
- 49.Geetha T, Varalakshmi P. Anti-inflammatory activity of lupeol and lupeol linoleate in rats. J Ethnopharmacol. 2001;76(1):77–80. [DOI] [PubMed] [Google Scholar]
- 50.Maity P, Hansda D, Bandyopadhyay U, Mishra DK. Biological activities of crude extracts and chemical constituents of Bael, Aegle marmelos (L.) Corr. Indian J Exp Biol. 2009;47(11):849–61. [PubMed] [Google Scholar]
- 51.Williams RJ, Spencer JP, Rice-Evans C. Flavonoids: antioxidants or signalling molecules? Free Radic Biol Med. 2004;36(7):838–49. 10.1016/j.freeradbiomed.2004.01.001 [DOI] [PubMed] [Google Scholar]
- 52.Lotito SB, Frei B. Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: cause, consequence, or epiphenomenon? Free Radic Biol Med. 2006;41(12):1727–46. 10.1016/j.freeradbiomed.2006.04.033 [DOI] [PubMed] [Google Scholar]
- 53.Izzi V, Masuelli L, Tresoldi I, Sacchetti P, Modesti A, Galvano F, et al. The effects of dietary flavonoids on the regulation of redox inflammatory networks. Front Biosci (Landmark Ed). 2012;17:2396–418. [DOI] [PubMed] [Google Scholar]
- 54.Gomes A, Couto D, Alves A, Dias I, Freitas M, Porto G, et al. Trihydroxyflavones with antioxidant and anti-inflammatory efficacy. Biofactors. 2012;38(5):378–86. 10.1002/biof.1033 [DOI] [PubMed] [Google Scholar]
- 55.Romagnolo DF, Selmin OI. Flavonoids and cancer prevention: a review of the evidence. J Nutr Gerontol Geriatr. 2012;31(3):206–38. 10.1080/21551197.2012.702534 [DOI] [PubMed] [Google Scholar]
- 56.Siasos G, Tousoulis D, Tsigkou V, Kokkou E, Oikonomou E, Vavuranakis M, et al. Flavonoids in atherosclerosis: an overview of their mechanisms of action. Curr Med Chem. 2013;20(21):2641–60. [DOI] [PubMed] [Google Scholar]
- 57.Grassi D, Desideri G, Ferri C. Flavonoids: antioxidants against atherosclerosis. Nutrients. 2010;2(8):889–902. Epub 2010/08/01. PubMed Central PMCID: PMC3257706. 10.3390/nu2080889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fuhrman B, Aviram M. Flavonoids protect LDL from oxidation and attenuate atherosclerosis. Curr Opin Lipidol. 2001;12(1):41–8. [DOI] [PubMed] [Google Scholar]
- 59.Barros L, Duenas M, Ferreira IC, Baptista P, Santos-Buelga C. Phenolic acids determination by HPLC-DAD-ESI/MS in sixteen different Portuguese wild mushrooms species. Food Chem Toxicol. 2009;47(6):1076–9. 10.1016/j.fct.2009.01.039 [DOI] [PubMed] [Google Scholar]
- 60.Carocho M, Ferreira IC. The role of phenolic compounds in the fight against cancer—a review. Anticancer Agents Med Chem. 2013;13(8):1236–58. [DOI] [PubMed] [Google Scholar]
- 61.Alves MJ, Ferreira IC, Froufe HJ, Abreu RM, Martins A, Pintado M. Antimicrobial activity of phenolic compounds identified in wild mushrooms, SAR analysis and docking studies. J Appl Microbiol. 2013;115(2):346–57. 10.1111/jam.12196 [DOI] [PubMed] [Google Scholar]
- 62.Heleno SA, Martins A, Queiroz MJ, Ferreira IC. Bioactivity of phenolic acids: metabolites versus parent compounds: a review. Food Chem. 2015;173:501–13. 10.1016/j.foodchem.2014.10.057 [DOI] [PubMed] [Google Scholar]
- 63.Leifert WR, Abeywardena MY. Cardioprotective actions of grape polyphenols. Nutr Res. 2008;28(11):729–37. 10.1016/j.nutres.2008.08.007 [DOI] [PubMed] [Google Scholar]
- 64.Yang AL, Lo CW, Lee JT, Su CT. Enhancement of vasorelaxation in hypertension following high-intensity exercise. Chin J Physiol. 2011;54(2):87–95. [DOI] [PubMed] [Google Scholar]
- 65.Valerio A, Cardile A, Cozzi V, Bracale R, Tedesco L, Pisconti A, et al. TNF-alpha downregulates eNOS expression and mitochondrial biogenesis in fat and muscle of obese rodents. J Clin Invest. 2006;116(10):2791–8. PubMed Central PMCID: PMCPMC1564431. 10.1172/JCI28570 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
In previous studies, four major compounds in DRLE had been identified, including β-sitosterol, lupeol, flavonoids and phenolic acid. The background photograph of flowers was taken in Tainan, Taiwan, by Chun-Ting Lee, one of the co-authors in this article.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information file.