Abstract
Human diploid fibroblasts (HDFs) exposed to subcytotoxic stresses under H2O2, tert-butylhydroperoxide (t-BHP), and ethanol (EtOH) undergo stress-induced premature senescence (SIPS) characterized by many biomarkers of HDFs replicative senescence. Among these biomarkers are a growth arrest, an increase in the senescence-associated β-galactosidase activity, a senescent morphology, an overexpression of p21waf-1 and the subsequent inability to phosphorylate pRb, the presence of the common 4977-bp mitochondrial deletion, and an increase in the steady-state level of several senescence-associated genes such as apolipoprotein J (apo J). Apo J has been described as a survival gene against cytotoxic stress. In order to study whether apo J would be protective against cytotoxicity SIPS and replicative senescence in human fibroblasts, a full-length complementary deoxyribonucleic acid of apo J was transfected into WI-38 HDFs and SV40-transformed WI-38 HDFs. The overexpression of apo J resulted in an increased cell survival after t-BHP and EtOH stresses at cytotoxic concentrations. In addition, when WI-38 HDFs were exposed to 5 subcytotoxic stresses with EtOH or t-BHP, in conditions that were previously shown to induce SIPS, a lower induction of 2 biomarkers of SIPS was observed in HDFs overexpressing apo J. No effect of apo J overexpression was observed on the proliferative life span of HDFs, even if apo J overexpression triggered osteonectin (SPARC) overexpression, which was shown to decrease the mitogenic potential of platelet-derived growth factor but not of other common growth-inducing conditions. Apo J senescence–related overexpression is proposed to have antiapoptotic rather than antiproliferative effects.
INTRODUCTION
Human diploid fibroblasts (HDFs) exposed to subcytotoxic oxidative stress undergo stress-induced premature senescence (SIPS), which is characterized by several biomarkers of replicative senescence (for a review, see Toussaint et al 2000b). The exposure of WI-38 HDFs to 5 repeated stresses of 1 hour each under 30 μM tert-butylhydroperoxide (t-BHP) decreases their proliferative life span and increases the proportion of cells positive for the senescence-associated β-galactosidase (SA β-gal) activity. A senescent morphology appears, as well as the common 4977-bp mitochondrial deletion. The cyclin-dependent kinase inhibitor p21waf-1 is overexpressed, which inhibits the phosphorylation of the retinoblastoma protein (Dumont et al 2000). Subcytotoxic concentrations of H2O2 also trigger SIPS in IMR-90 HDFs. The cells become enlarged and flattened. Growth arrest occurs at phase G1 of the cell cycle. These cells also overexpress p21waf-1 concomitantly with the hypophosphorylation of pRb (Chen and Ames 1994; Chen et al 1998).
Cultures of WI-38 HDFs exposed to repeated subcytotoxic 2-hour stresses with 4 % ethanol (EtOH) (v/v) also display, 3 days after the treatment, an increase in the proportion of cells positive for the SA β-gal activity (Toussaint et al 2000a) and a senescent morphology (Toussaint et al 1992). Human fibroblasts can be sorted into 7 successive morphotypes based on cell shape, size, and nucleus-cytoplasm surface ratio: 3 mitotic morphotypes (MF I–III) followed by 4 postmitotic morphotypes (PMF IV–VII). PMF VII represent the degenerative state of PMF VI and die within a few hours. These morphotypes were biochemically characterized as distinctive cells by 2-dimensional gel electrophoresis. An expert computer software program allowing the sorting of fibroblasts into the cell morphotypes was developed. It couples an image analyzer to a software program capable of comparing the data to a repertoire of cell shapes (unpublished). Sublethal oxidative stresses under t-BHP were shown to induce a shift from the early fibroblast morphotypes to the later ones. Observations based on a time-lapse video of single cells after stress clearly showed that there is a transition from 1 morphotype to the next and not a selection of a given cell morphotype over the others (for a review, see Toussaint et al 2000b).
The steady-state messenger ribonucleic acid (mRNA) level of at least 7 genes is increased both in replicative senescence and in t-BHP- and H2O2-induced SIPS. Among these 7 genes, the genes of the most well-known functions are fibronectin, osteonectin (SPARC), and apolipoprotein J (apo J) (Dumont et al 2000). Apo J (also referred to as clusterin, sulfated-glycoprotein-2, testosterone-repressed message-2, glycoprotein 80, or SP-40) is a 70–80-kDa glycoprotein consisting of 2 disulfide-linked subunits named apo Jα (34–36 kDa) and apo Jβ (36–39 kDa) (de Silva et al 1990a, 1990b). Apo J is constitutively synthesized and secreted by many cell types. Apo J is overexpressed in several models of apoptosis (Lee and Sensibar 1987). Stimulation of LNCaP cells with tumor necrosis factor α (TNF-α) induces the expression of apo J (Sensibar et al 1995). High levels of apo J were shown to protect against TNF-α–mediated apoptosis in several cells lines, including PC3 (Sintich et al 1999), LNCaP (Sensibar et al 1995; Sintich et al 1999), and L929 (Humphreys et al 1997) cells. Osteonectin (SPARC, BM-40) is an extracellular matrix protein. It regulates the proliferation of different cell types. SPARC blocks the mitogenic effect of vascular endothelial growth factor (VEGF) in microvascular human endothelial cells. SPARC binds to VEGF, resulting in a reduced association of VEGF with its cell surface receptors (Kupprion et al 1998). SPARC reduces the mitogenicity of basic-fibroblast growth factor (bFGF) in endothelial cells (Hasselaar et al 1992) and of platelet-derived growth factor (PDGF) in mesangial cells (Pichler et al 1996). SPARC is expressed at high levels in human ovarian surface epithelial cells but at reduced levels in ovarian carcinoma cells in vitro and in vivo. In addition, the overexpression of SPARC into an ovarian carcinoma cell line results in a reduced growth rate in culture and a decreased tumorogenicity (Mok et al 1996).
In this work, we wished to know whether an increase in apo J expression could protect HDFs against cytotoxic stresses, SIPS, and normal replicative senescence. t-BHP, H2O2, and EtOH were used as stressors as the experimental conditions for them to induce SIPS are well established. No protective effect of apo J against EtOH stress had been shown earlier. As the overexpression of apo J was found to increase the mRNA steady-state level of osteonectin, we also tested whether overexpression of osteonectin would alter either the cellular survival after cytotoxic stress or the proliferative capacity of the fibroblasts.
MATERIALS AND METHODS
Cell cultures
Normal and SV40-transformed human WI-38 fibroblasts purchased from the American Type Culture Collection (USA) were cultivated in 75-cm2 culture flasks (Cel Cult, UK) containing 15 mL of basal medium Eagle (BME) or Dulbecco modified Eagle medium (DMEM) (Flow Laboratories, UK) supplemented with 10 % fetal calf serum (FCS) (Flow Laboratories). Confluent, cells were subcultivated as previously described (Hayflick and Moorhead 1961). In slowly proliferating cultures, the medium was changed every 4 days. The PT67-packaging cell line (Clontech, USA) was grown in high-glucose DMEM (Flow Laboratories) supplemented with 10 % FCS and 4 mM l-glutamine (Sigma, USA). All cultures were grown at 37°C in an atmosphere containing 5 % CO2.
Apo J retroviral expression vector construction and transfection
A 1477-bp complementary deoxyribonucleic acid (cDNA) fragment containing the entire open reading frame of dog apo J (gp80) (88.5 % identity with the human apo J) was released from the pBEH vector by restriction with BamH1 (Promega, USA). The purified fragment was inserted by T4 ligase (Promega) into the PLXSN retroviral vector (Clontech) in the sense orientation. The construct was verified by restriction profiles and sequencing (data not shown). The use of the dog apo J allowed differentiation between the endogenous apo J mRNA and the transfected apo J mRNA.
Subconfluent SV40 WI-38 cells, cultivated in 100-mm culture dishes containing 10 mL of DMEM without serum, were transfected for 8 hours with 30 μg of either the PLXSN/apo J expression vector or the PLXSN vector without insert, using the calcium phosphate precipitation method (Calphos mammalian transfection, Clontech). At 48 hours, the cells were plated in the selection medium containing 0.5 mg/mL G418 (GIBCO BRL, UK). Colonies were isolated from this medium 3 weeks later and expanded in DMEM + 10 % FCS supplemented with 0.5 mg/mL G418.
Overexpression of apo J in WI-38 HDFs between 35 % and 39 % of the replicative life span was achieved by retroviral infections. The PLXSN/apo J expression vector and the PLXSN vector without insert were transfected into the PT67-packaging cell line by calcium phosphate precipitation. After 15 days of selection under 0.5 mg/mL G418, stable virus-producing cell lines were obtained. At 18 hours before the infections, WI-38 HDFs were plated in 100-mm culture dishes at a density of 500 000 cells/dish. The culture medium from PT67 cells was collected, filtered through a 0.45 μM filter, and supplemented with 4 μg/mL of hexadimethrine bromide (Sigma). The retroviral supernatants were added (10 mL/dish) to the target cells for 24 hours. At day 2 after the infection, a 2-week selection under 0.5 mg/mL G418 was started.
SPARC retroviral expression vector construction and transfection
A 1040-bp human SPARC cDNA was inserted into the PLXSN retroviral vector (Clontech) and introduced in WI-38 HDFs by retroviral infections. The PLXSN/SPARC expression vector and the PLXSN vector without insert were transfected into the PT67-packaging cell line by the calcium phosphate precipitation method. After 15 days of selection under 0.5 mg/mL G418, stable virus-producing cells were obtained. Infections and selections in WI-38 HDFs between 35 % and 39 % of the replicative life span were performed as described in the previous section.
Determination of recombinant apo J, SPARC, and fibronectin expression relative levels
Assessment of dog apo J mRNA relative levels in SV40 WI-38 and WI-38 HDFs was done after G418 selection by semiquantitative reverse transcriptase–polymerase chain reaction (RT-PCR). Total RNA was extracted (RNA isolation kit, Promega). Prior to the RT-PCR, the RNA samples were treated with RNase-free DNase I (GIBCO BRL). Semiquantitative RT-PCRs were achieved in 1 step (Access RT-PCR kit, Promega) in the presence of 0.025 μCi of [α-32P] per reaction. RT-PCRs were performed in the exponential range of the PCR amplification, using 100 ng of total RNA in 50 μL reactions containing 50 pmol of both primers, 1 mM MgSO4, 0.2 mM of each deoxynucleoside triphosphate, and 5 U of both Tfl DNA polymerase and avian myeloblastosis virus reverse transcriptase. Negative controls were performed in the absence of RNA. The presence of DNA contamination was checked by performing a PCR without preliminary reverse transcription. Recombinant dog apo J expression was checked using primers 5′-GTG AGT AGT GGT AAG TAT CCT-3′ (position 226–246) and 5′-CCC TAA TAG AAC AGA CAA ACG-3′ (position 655–675) (Eurogentec, Belgium), giving a 449-bp amplicon. Endogenous apo J mRNA was amplified using the primers 5′-CGG GGT GAA ACA GAT AAA G-3′ (position 69–88) and 5′-TGC GGT CAC CAT TCA TCC A-3′ (position 373–392), giving a 322-bp amplicon. SPARC mRNA steady-state level was also determined by semiquantitative RT-PCR. SPARC mRNA was detected using the primers 5′-CTG TGG GAG CTA ATC CTG-3′ (position 181–199) and 5′-GGG TGC TGG TCC AGC TGG-3′ (position 765–783). The primers 5′-GAT TGC CTG TTC TGC TTC-3′ (position 360–378) and 5′-TTG GGT GAC TTT CCT ACT-3′ (position 502–520) were used to detect fibronectin mRNA. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA, amplified using the primers 5′-CGT CTT CAC CAT GGA GA-3′ (position 333–350) and 5′-CGG CCA TCA CGC CCA CAG TTT-3′ (position 601–622), was used as a reference level because its relative level did not change among all the situations tested. RT-PCR products were electrophoresed on 5 % polyacrylamide gels. The band radioactivity was quantified with an Instant Imager (Packard Instruments, USA). The autoradiographies of the corresponding gels were performed on β-max films (Amersham Life Science, UK).
Cytotoxic stresses under t-BHP, H2O2, and EtOH
SV40 WI-38 HDFs seeded in multidishes at 50 000 cells/well (wells of 2 cm2, Cel Cult) were exposed to a single stress of 2 hours under increasing concentrations of t-BHP (0–1.25 mM) (Merck, Germany), EtOH (0–10 %, v/v) (Merck), and H2O2 (0–1.5mM) (Merck) diluted in BME medium. After 2 washes with phosphate-buffered saline (PBS) (10 mM, pH 7.4), the cells were given fresh BME + 10 % FCS. WI-38 HDFs seeded in multidishes at 50 000 cells/well were exposed to a single stress of 2 hours under increasing concentrations of t-BHP (0–0.8 mM) and EtOH (0–10 %, v/v) diluted in PBS or BME, depending on the type of experiment, in order to test whether a protective effect would be maintained under these conditions. After 2 washes with BME, the cells were given fresh BME + 10 % FCS.
Cell survival was assessed at 24 hours after the stress, using either the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium salts (MTT) method (Mosmann 1983; Hansen et al 1989; Marshall et al 1995) or the classical method of measurement of cellular protein content (Lowry et al 1951). Measurement of the cellular protein content was previously shown to be representative of cell survival when compared with other methods (Toussaint et al 1994). The results are expressed as percentages of the control cells not incubated with the stressor, considered as 100 %. Mean values of 4 independent experiments ± standard deviations (SDs) are presented.
SIPS triggered by repeated EtOH and t-BHP stress
Confluent cultures of WI-38 HDFs at early cumulative population doublings (CPDs), in 75-cm2 culture flasks, were submitted to 5 repeated subcytotoxic stresses of 1 hour under 30 μM t-BHP or 2 hours under 4 % EtOH diluted in BME + 10 % FCS, with 1 stress per day for 5 days, according to previously described protocols (Toussaint et al 1992, 2000a; Dumont et al 2000). After 2 washes with BME, the cells were given fresh BME + 10 % FCS. Control cultures at the same early CPDs followed the same schedule of medium changes without stressing molecule.
Morphotypes determination and senescence-associated β-galactosidase activity
At 48 hours after stress, the cells were trypsinized and seeded in 60-mm2 culture dishes (Falcon, UK) containing 5 mL of BME + 10 % FCS at a low density of 700 cells/cm2. At 24 hours after cell seeding, the proportions of the different morphotypes as well as the proportions of SA β-gal positive cells were determined, as described by Toussaint et al (1992) and Dimri et al (1995), respectively. The proportions of cells positive for the SA β-gal activity and the proportions of the different morphotypes were assessed by counting 400 cells/dish, in 4 different dishes. To avoid staining as a result of confluency, SA β-gal staining was always performed on nonconfluent cells. The results are given as means of 4 independent experiments ± SD.
Mitogenic stimulations of WI-38 HDFs transfected with SPARC
Cells were seeded in 24-well plates at 25 000 cells/well. The next day, cells were rinsed twice with PBS and refed with BME. After 48 hours of serum deprivation, the cells were stimulated for 24 hours with the mitogens: bFGF, epidermal growth factor (EGF), insulin-like growth factor I (IGF-I) (Clonetics, USA), PDGF-AB (Calbiochem, USA), interleukin-1β (IL-1β), and tumor necrosis factor α (TNF-α) (R&D Systems, USA). Control cells were incubated in BME without growth factors. Concomitantly, 1 μCi [3H]-thymidine (specific activity: 2 Ci/mM; Du Pont NEN, USA) was added to each well. After the 24-hour stimulation, [3H]-thymidine–incorporation period, the cells were washed twice with 0.5 mL PBS, fixed for 5 minutes with 0.5 mL of ice-cold 10 % trichloro-acetic acid (TCA), and washed twice with 0.5 mL TCA, once with 70 % EtOH, and once with PBS. TCA precipitates were dissolved in 0.5 mL of 0.5 M NaOH. After neutralization with 0.5 M HCl, the incorporated radioactivity was quantified on a scintillation counter (Beckman Coulter Inc, USA). The growth stimulation induced by each mitogen was calculated as the ratio, in percent, of counts per minute (cpm)/25 000 stimulated cells to cpm/25 000 control cells. Results are presented as mean values of 3 independent experiments ± SD.
RESULTS
Recombinant apo J mRNA level in SV40 WI-38 HDFs
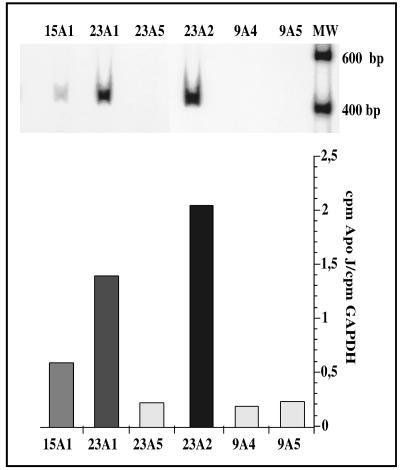
After 3 weeks of selection with G418, resistant clones were isolated and individually expanded. The level of apo J mRNA was determined in 6 clones by semiquantitative RT-PCR. Three clones had high levels of apo J mRNA (clones 23A2, 23A1, and 15A1) (Fig 1).
Fig 1.
Relative steady-state messenger ribonucleic acid (mRNA) level of dog apolipoprotein J (apo J) after transfection in SV 40 WI-38 fibroblasts. (A) Autoradiographies showing the steady-state level of mRNA of transfected dog apo J in different G418-resistant colonies of SV 40 WI-38 human diploid fibroblasts. (B) Quantifications using glyceraldehyde-3-phosphate dehydrogenase as a reference gene
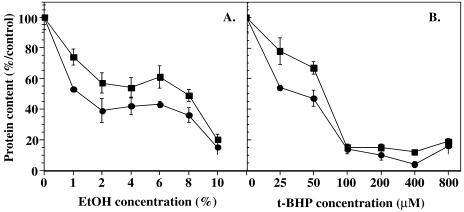
Apo J decreases the cytotoxicity of t-BHP and EtOH in SV40 WI-38 HDFs
SV40 WI-38 HDFs overexpressing apo J, as well as nontransfected HDFs and HDFs transfected with PLXSN control plasmid without insert, were exposed for 2 hours to increasing concentrations of EtOH and t-BHP. Cell survival was determined at 24 hours after stress by measurement of the cellular protein content. This methodology has proven earlier to give a reliable representation of cell survival after stress (Toussaint et al 1994). From 2 % to 6 % of EtOH, protective effects around 40 % were provided by apo J (Fig 2A). The protection afforded by apo J disappeared at the highest EtOH concentrations. Increased absolute values of cell survivals of 26 %, 27 %, 22 %, and 18 % were observed at 24 hours after stress under 250μM, 500μM, 750μM, and 1000 μM of t-BHP, respectively, in cells overexpressing apo J, when compared with the control cells (Fig 2B). Clone 23A1 gave a lower protective effect, whereas clone 15A1 did not protect at all. Similar results were obtained when cell survival was estimated using the MTT method (data not shown). Native cells and PLXSN-transfected cells gave very similar results (data not shown). No protective effect of apo J on cell survival was observed after exposures of the cells to increasing concentrations of H2O2 from 0 mM to 1 mM (data not shown).
Fig 2.
Role of apolipoprotein J (apo J) against cytotoxic stress with ethanol (EtOH) and tert-butylhydroperoxide (t-BHP) in SV 40 WI-38 human diploid fibroblasts (HDFs). (A) SV40 WI-38 HDFs exposed to increasing EtOH concentrations. Cells were exposed to a 2-hour stress with 0–10 % EtOH diluted in basal medium Eagle (BME). Cells were transfected with the PLXSN/apo J vector (▪) and the control PLXSN vector (•). Survival was estimated at 24 hours after stress by assay of the cellular protein content. Results are expressed as percentages of the protein content of control cells (no EtOH), considered as 100 %, and are mean values of 4 independent experiments ± SD. (B) SV40 WI-38 HDFs exposed to increasing t-BHP concentrations. Cells were exposed to a 2-hour stress with 0–1.25 mM t-BHP diluted in BME. Cells were transfected with the PLXSN/apo J vector (▪) and the control PLXSN vector (•). Survival was estimated at 24 hours after the stress by assay of the cellular protein content. Results are expressed as percentages of the protein content of control cells (without t-BHP), considered as 100 %, and are mean values of 4 independent experiments ± SD
Recombinant apo J mRNA relative level in WI-38 HDFs
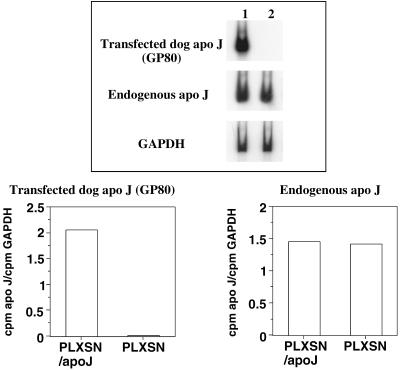
We tested whether apo J overexpression could also protect WI-38 HDFs against t-BHP and EtOH cytotoxicity. WI-38 HDFs have a limited proliferative life span. A long selection procedure after transfection would rapidly exhaust their proliferative potential and is therefore prohibited for studies on SIPS. Therefore, apo J cDNA was retrovirally transfected to allow the transfection of a high proportion of cells. We obtained from 50 % to 90 % of transfected cells, thus resulting in a minimal loss of replicative potential during the selection procedure. After 2 weeks with G418, endogenous and transfected apo J mRNA relative steady-state levels were analyzed. Despite a high homology between dog and human apo J, it was possible to differentiate between the transfected dog apo J mRNA and the endogenous human mRNA using specific primers. A high relative steady-state mRNA level of dog apo J was observed in cells transfected with the PLXSN/apo J vector. The expression of the endogenous apo J was similar in cells transfected either with the PLXSN/apo J vector or with the control plasmid (Fig 3).
Fig 3.
Relative steady-state messenger ribonucleic acid (mRNA) level of dog apolipoprotein J (apo J) after retroviral transfection in WI-38 human diploid fibroblasts (HDFs). (A) Autoradiographies showing the steady-state mRNA level of retrovirally transfected dog apo J, endogenous apo J, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in WI-38 HDFs. Lane 1: WI-38 HDFs transfected with the PLXSN/apo J expression vector; lane 2: WI-38 HDFs transfected with the control PLXSN vector. (B) Quantifications using GAPDH as a reference gene
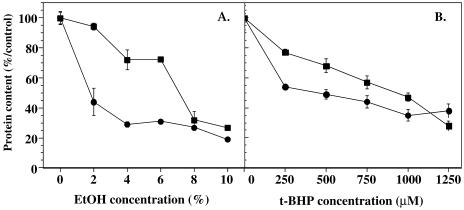
Apo J decreases the cytotoxicity of t-BHP and EtOH in WI-38 HDFs
Cells were exposed to a 2-hour stress with EtOH concentrations ranging from 1 % to 10 % (v/v). Between 1 % and 8 % EtOH, the survival of the cells overexpressing apo J was significantly higher (Fig 4A), with protective effects between 12 % and 21 %. When the cells were exposed to a 2-hour t-BHP stress (Fig 4B), a protective effect of 24 % and 20 % was observed after stress at 25 μM and 50 μM t-BHP, respectively. When the cells overexpressing apo J were exposed to various concentrations of H2O2 from 0 μM to 800 μM for 2 hours, which ranged from minimal to maximal cytotoxicity, no protection against the cytotoxicity of H2O2 was observed, when compared with cells transfected with the PLXSN without insert (data not shown).
Fig 4.
Role of retrovirally transfected apolipoprotein J (apo J) against cytotoxic stresses with ethanol (EtOH) and tert-butylhydroperoxide (t-BHP) in WI-38 human diploid fibroblasts (HDFs). (A) Survival curves of WI-38 HDFs exposed to increasing concentrations of EtOH. Cells were subcultivated in multiwell dishes at a density of 25 000 cells/cm2 and exposed, on the next day, to a stress of 2 hours with 0–10 % EtOH, diluted in phosphate-buffered saline (PBS). Cells were transfected with the PLXSN/apo J vector (▪) or the PLXSN control vector (•). Survival was determined at 24 hours after the stress. Results are expressed as percentages of the protein content of control cells (no EtOH), considered as 100 %, and are mean values of 4 independent experiments ± SD. (B) Survival curves of WI-38 HDFs exposed to increasing concentrations of t-BHP. Cells were subcultivated in multiwell dishes at a density of 25 000 cells/cm2 and exposed, on the next day, to a stress of 2 hours under 0–0.8 mM t-BHP, diluted in PBS. Cells were transfected with the PLXSN/apo J vector (▪) or the PLXSN control vector (•). Survival was determined at 24 hours after the stress. Results are expressed as percentages of the protein content of control cells (without EtOH), considered as 100 %, and are mean values of 4 independent experiments ± SD
Apo J overexpression protects against SIPS induced by t-BHP and EtOH stress
Exposures of HDFs at early CPDs to repeated subcytotoxic stresses with t-BHP and EtOH trigger SIPS (Toussaint et al 1992, 2000a, [for a review] 2000b; Dumont et al 2000). In order to avoid cell death, the subcytotoxic stresses are performed in medium plus serum as it was previously shown that cell viability is enhanced in the presence of substrates of the energy metabolism, such as d-glucose, pyruvate-malate, or glutamate-malate. In addition, the proteins of serum act as quenchers of reactive oxygen species (ROS), and growth factors can decrease cytotoxicity (Toussaint et al 1994).
Apo J overexpression and stress-induced changes in morphotype proportions
The morphology of the cells was already used as a reliable biomarker of SIPS (Toussaint et al 1992, 2000a; Dumont et al 2000). Along with their in vitro life span, HDFs evolve through a sequence of 7 morphological types, called morphotypes. Three mitotic morphotypes (MF I–III) and 4 postmitotic morphotypes (PMF IV–VII) are identified in HH8 skin HDFs (Bayreuther et al 1988, 1991, 1992) and in WI-38 human lung fibroblasts (Toussaint et al 1992, 1995). A software program was designed to sort the HDFs according to their morphotype. It was based on the analysis of pictures from a CCD camera by an expert system (unpublished). In addition, the proportions of the morphotypes shifted in favor of the latest morphotypes after various types of subcytotoxic stresses, such as UV light (Rodemann et al 1989), mitomycin C (Rodemann 1989), EtOH, and t-BHP (Toussaint et al 1992, 1995, 2000a).
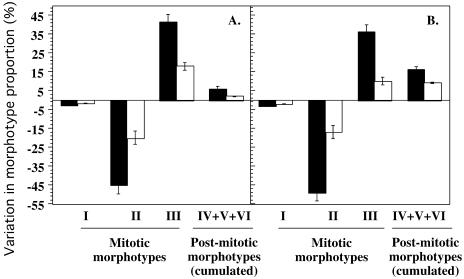
WI-38 HDFs at early CPDs retrovirally transfected with apo J cDNA were exposed to repeated concentrations of 4 % (v/v) EtOH or 30 μM t-BHP. The data were expressed as variations in morphotype proportions when compared with control nonstressed cells. In the cells transfected with the control vector, t-BHP and EtOH induced a dramatic decrease in the proportion of MF II, a large increase in the proportion of MF III, and a significant increase in PMF IV–VI (Fig 5A). Similar variations were previously obtained when WI-38 HDFs were exposed to t-BHP or EtOH under the same experimental conditions (Toussaint et al 2000a). These stress-induced transitions were greatly reduced in cells overexpressing apo J (Fig 5A). Repeated EtOH stresses also induced a drastic shift from MF II to III, PMF IV, V, and VI (Fig 5B). When compared with control cells transfected with the PLXSN vector without insert, this shift was greatly reduced in the cells overexpressing apo J (Fig 5B).
Fig 5.
Effect of retrovirally transfected apolipoprotein J (apo J) against SIPS induced by 5 repeated subcytotoxic stresses using morphotypes as criteria. The cells were exposed to 5 repeated subcytotoxic 1-hour stresses with 30 μM tert-butylhydroperoxide (A) or 2-hour stresses with 4 % ethanol (B). At 48 hours after the last stress, cells were seeded in 60-mm culture flasks at a low density of 700 cells/cm2. The next day, the morphotype proportions were determined. The results are presented as variations in the proportions of morphotype, recorded at 72 hours after the last stress, between the stressed and the nonstressed cells. Black columns: WI-38 human diploid fibroblasts (HDFs) transfected with the PLXSN control vector; white columns: WI-38 HDFs transfected with the PLXSN/apo J vector. Results are mean values of 4 independent experiments ± SD
Apo J overexpression and stress-induced increase in the proportions of SA β-gal positive cells
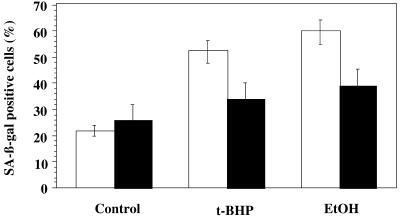
The proportion of HDFs positive for SA β-gal activity increases with the number of CPDs (Dimri et al 1995). The exposure of HDFs at early CPDs to repeated subcytotoxic stresses with EtOH or t-BHP results in a drastic increase in the proportion of cells positive for this activity (Dumont et al 2000; Toussaint et al 2000a). Five repeated exposures to t-BHP and EtOH at their respective subcytotoxic concentrations of 30 μM and 4 % (v/v) induced respective increases in SA β-gal–positive cells of 31 % and 38.5 % in the cells transfected with PLXSN vector without insert (Fig 6). These results were very similar to those obtained previously in nontransfected WI-38 HDFs (Dumont et al 2000; Toussaint et al 2000a). In the cells transfected with apo J cDNA, increases of only 9 % and 13.5 % were found after these stresses with t-BHP or EtOH, respectively, when compared with nonstressed cells transfected with apo J cDNA (Fig 6).
Fig 6.
Effects of retrovirally transfected apolipoprotein J (apo J) against SIPS induced by 5 repeated subcytotoxic stresses using senescence-associated β-galactosidase (SA β-gal) activity as criteria. The cells were exposed to 5 repeated subcytotoxic 1-hour stresses with 30 μM tert-butylhydroperoxide or 2-hour stresses with 4 % ethanol. At 48 hours after the last stress, cells were seeded in 60-mm culture flasks at a low density of 700 cells/cm2. The next day, the percentage of cells positive for the SA β-gal activity was determined. Results are expressed as percentages of the total cell population and are means of 4 independent experiments ± SD. Black columns: WI-38 human diploid fibroblasts (HDFs) transfected with the PLXSN control vector; white columns: WI-38 HDFs transfected with the PLXSN/apo J vector
Effect of apo J on the relative mRNA steady-state level of fibronectin and osteonectin
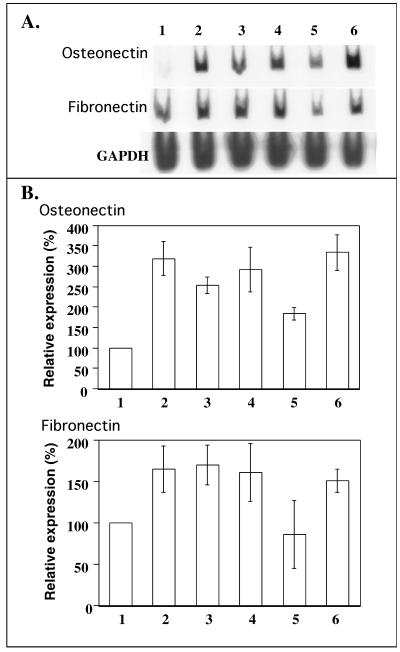
We described 7 genes whose relative mRNA steady-state level undergoes a senescence-associated increase as well as a SIPS-associated increase at 72 hours after t-BHP and H2O2 subcytotoxic stress (Dumont et al 2000). Two of these genes, eg, fibronectin and osteonectin, have well-known functions. We wished to know whether interactions take place between an increased apo J mRNA level and the level of transcripts of these 2 genes. For both genes, we first confirmed their senescence-associated increase in relative transcript levels in WI-38 HDFs. The relative steady-state levels of osteonectin and fibronectin mRNA were 3.2- and 1.7-fold higher, respectively, in senescent HDFs as compared with HDFs at early CPDs (Fig 7, lanes 1 and 2). Fibronectin and osteonectin relative transcript levels were increased in SIPS induced by t-BHP in cells transfected with the PLXSN vector without insert, with a respective 1.7- and 1.8-fold increase when compared with nonstressed cells transfected with the PLXSN control vector (Fig 7, lanes 5 [no t-BHP] and 6 [t-BHP]). This data allowed us to consider that the retroviral transfection of HDFs does not prevent the SIPS-induced increase in the transcript levels of these 2 genes, although an increase in the relative mRNA steady-state level of osteonectin cDNA was observed in the nonstressed controls. WI-38 HDFs retrovirally transfected with apo J cDNA behaved unexpectedly because both osteonectin and fibronectin relative transcript levels increased similarly by 1.4- and 2.0-fold, respectively, when compared with the respective relative transcript level observed in the control cells transfected with the PLXSN control vector (Fig 7, lanes 3 [no t-BHP] and 4 [t-BHP]). This suggests that apo J can induce an overexpression of fibronectin and osteonectin. After t-BHP–induced SIPS, no significant increase in osteonectin or fibronectin relative transcript levels was observed in the cells transfected with the PLXSN/apo J vector. These results indicate a protection against the SIPS-induced increase in the transcript level of these 2 genes. However, it could be possible that the transfection of apo J cDNA maximally induces an increase of these mRNA steady-state levels, leaving no possiblity for further SIPS-induced increase.
Fig 7.
Relative steady-state level of osteonectin and fibronectin messenger ribonucleic acid (mRNA) in senescent human diploid fibroblasts (HDFs) and after SIPS-inducing treatment under tert-butylhydroperoxide (t-BHP) performed on cells transfected with the PLXSN/apolipoprotein J (apo J) vector. (A) Autoradiographies showing the relative steady-state level of osteonectin, fibronectin, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA, as determined by semiquantitative reverse transcriptase–polymerase chain reaction. Cells were exposed to 5 subcytotoxic 1-hour stresses with 30 μM t-BHP. Total RNA was extracted at 72 hours after the last stress. WI-38 HDFs at early CPDs (45 % of proliferative life span) (lane 1). WI-38 HDFs at late cumulative population doublings (95 % of proliferative life span) (lane 2). WI-38 HDFs transfected with PLXSN/apo J exposed (lane 4) or not (lane 3) to five 1-hour stresses with 30 μM t-BHP. WI-38 HDFs transfected with the PLXSN control vector exposed (lane 6) or not (lane 5) to five 1-hour stresses with 30 μM t-BHP. (B) Quantifications using GAPDH as a reference gene
Effect of apo J on replicative senescence of WI-38 HDFs
We tested whether apo J overexpression affects the proliferative life span of WI-38 HDFs. HDFs transfected with the PLXSN/apo J or the PLXSN without insert were subcultivated until the exhaustion of the proliferative potential. No difference was found between the replicative life span of these transfected cells. Very similar numbers of cells per flask were found at each subcultivation in the HDFs transfected with the PLXSN/apo J, transfected with the PLXSN vector without insert, and nontransfected. The cells transfected with the PLXSN/apo J or the PLXSN without insert stopped growing at CPDs between 48 and 50, whereas the nontransfected cells stopped growing at CPDs between 49 and 52. Retroviral transfection allowed a high efficiency of transfection, avoiding an important decrease in proliferative potential, and gave similar results whether the cells were transfected with the PLXSN/apo J or the PLXSN control vector. No difference in the cell numbers was observed during the selection process of the cells transfected with the PLXSN/apo J or the PLXSN vector. As apo J overexpression did not affect the proliferative life span of HDFs, this suggests that apo J per se does not provoke senescence, although it is overexpressed in senescent HDFs and in HDFs in SIPS. The literature gives many indications that apo J is an antiapoptotic rather than an antiproliferative protein. Nevertheless, apo J overexpression in our system led to the overexpression of osteonectin. As the growth-inhibitory effects of osteonectin have been found in many cell types, we needed to know whether overexpression of osteonectin (SPARC) in our experimental models would affect the stress resistance or decrease the proliferative capacity of the cells.
Effect of SPARC overexpression
Determination of relative SPARC expression level
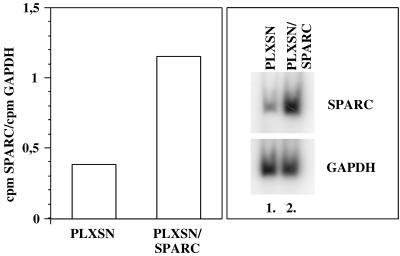
The SPARC cDNA cloned into the PLXSN vector was retrovirally introduced in WI-38 HDFs at early CPDs. After 2 weeks of selection with G418, the relative SPARC mRNA steady-state levels were analyzed in the cells transfected with the PLXSN/SPARC vector, and in the cells transfected with the PLXSN control vector without insert, by semiquantitative RT-PCR. A higher relative steady-state level of SPARC mRNA was observed in cells transfected with the PLXSN/SPARC as compared with cells transfected with the control PLXSN vector (Fig 8).
Fig 8.
Autoradiographies and quantifications showing the relative steady-state messenger ribonucleic acid level of SPARC in WI-38 human diploid fibroblasts transfected with the PLXSN/SPARC vector (lane 2) or the PLXSN control vector (lane 1)
Effects of SPARC overexpression on the survival of WI-38 HDFs against stress with t-BHP and H2O2
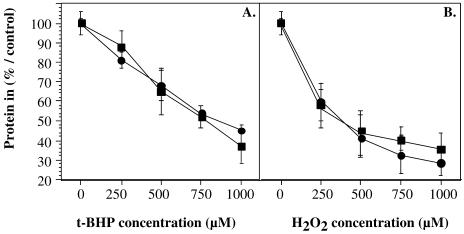
Cells were exposed to a 2-hour stress with either t-BHP or H2O2 at concentrations ranging from 0 mM to 1 mM. Similar survival rates were observed in the cells transfected with the PLXSN control vector or the PLXSN/SPARC vector (Fig 9). Thus, no protection against the cytotoxicity of H2O2 and t-BHP was observed in HDFs overexpressing SPARC.
Fig 9.
Survival curves of WI-38 human diploid fibroblasts transfected with either the PLXSN control vector (▪) or the PLXSN/SPARC vector (•) after exposures to cytotoxic stresses with tert-butylhydroperoxide (t-BHP) (A) and H2O2 (B). Cells plated in multiwell dishes at 25 000 cells/cm2 were exposed to a stress of 2 hours with increasing concentrations of t-BHP or H2O2 diluted in basal medium Eagle. Survival was determined at 24 hours after stress. Results are expressed as percentages of the protein content of control cells (no stressor), considered as 100 %. The results represent the mean value of 4 experiments ± SD
Effects of SPARC overexpression on the [3H]-thymidine incorporation after stimulation by mitogens
We tested the ability of several mitogens to induce DNA synthesis in WI-38 HDFs after a serum deprivation period of 48 hours. For each of the mitogens tested, the same number of cells were incubated with 50 ng/mL of mitogens, which represents the concentrations effective on WI-38 HDFs (Phillips and Cristofalo 1988; Owen et al 1989), in the presence of 1 μCi of [3H]-thymidine per well for 24 hours. These experiments were conducted in cells transfected with the PLXSN control vector and in cells transfected with the PLXSN/SPARC vector. Control cells (no mitogen added) nontransfected, transfected with the PLXSN control vector, or transfected with the the PLXSN/SPARC vector exhibited very similar levels of [3H]-thymidine incorporation.
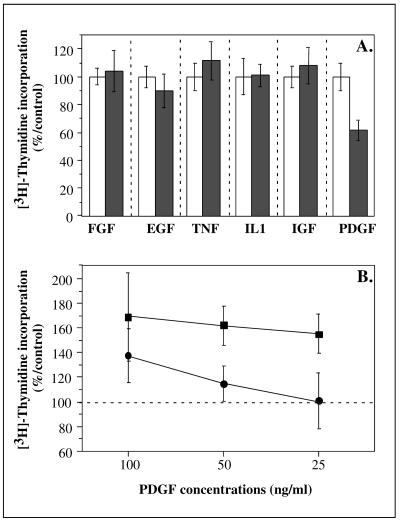
Whereas PDGF-AB at 50 ng/mL triggered a 62 % increase of the [3H]-thymidine incorporation level in cells transfected with the PLXSN control vector, no significant change (15 ± 14 %) was observed in the cells transfected with the PLXSN/SPARC vector. High levels of [3H]-thymidine incorporation were observed with IGF-I, IL-1β, FGF, EGF, TNF-α, and 10 % FCS, and showed great similarity between the cells transfected with the PLXSN control vector and the cells transfected with the PLXSN/SPARC. The results obtained after stimulation of HDFs transfected with the PLXSN control vector or the PLXSN/SPARC vector are compared in Figure 10A where the level of stimulation observed for each mitogen in HDFs transfected with the PLXSN control vector is considered to be 100 %.
Fig 10.
(A) Effects of SPARC on mitogenicity. WI-38 human diploid fibroblasts (HDFs) transfected with the PLXSN/SPARC vector (black columns) or the PLXSN control vector (white columns) were seeded in 24-well plates and serum deprived for 48 hours. The cells were then stimulated for 24 hours with 50 ng/mL of several mitogens (platelet-derived growth factor [PDGF]-AB, basic-fibroblast growth factor, epidermal growth factor, insulin-like growth factor I, interleukin-1β, and tumor necrosis factor α) in the presence of 1 μCi [3H]-thymidine. Control cells were incubated without mitogen. The level of [3H]-thymidine incorporation in the cells transfected with the PLXSN control vector was considered as 100 % for each mitogen. The results represent the mean value of 3 experiments ± SD. (B) Effects of PDGF-AB on [3H]-thymidine incorporation. WI-38 HDFs transfected with the PLXSN/SPARC vector (•) or the PLXSN control vector (▪) were seeded in 24-well plates and serum deprived for 48 hours. The cells were then stimulated for 24 hours with 25 ng/mL, 50 ng/mL, and 100 ng/mL of PDGF-AB in the presence of 1 μCi [3H]-thymidine. Control cells were incubated in the absence of mitogen. The level of [3H]-thymidine incorporation in the unstimulated control cells was considered as 100 %. The results represent the mean value of 3 experiments ± SD
We stimulated WI-38 HDFs transfected with the PLXSN control vector and with the PLXSN/SPARC vector with 25–100 ng/mL PDGF. Significant percentages of inhibitions of [3H]-thymidine incorporation, 76 % and 98 %, were observed at 50 ng/mL and 25 ng/mL PDGF, respectively, in cells transfected with the PLXSN/SPARC vector when compared with cells transfected with the PLXSN control vector (Fig 10B). At 100 ng/mL PDGF, the inhibition of [3H]-thymidine incorporation (45 % inhibition) caused by SPARC overexpression was much lower than that observed at 50 ng/mL (76 % inhibition).
DISCUSSION
Apo J is overexpressed in SIPS of HDFs triggered by H2O2 and t-BHP subcytotoxic stresses, as well as in the replicative senescence of HDFs (Dumont et al 2000). Apo J is overexpressed in response to stimulations with TNF-α and IL-1 or to stresses with UV light, H2O2, O2·(–), hyperoxia, and endotoxin (Clark and Griswold 1997; Schwochau et al 1998; Viard et al 1999). These agents lead to the production of ROS in cells, suggesting that apo J might be a survival factor against oxidative stress. Addition of exogenous apo J to a suspension of cells of the proximal tubular LLC-PK1 cell line increased cell survival after incubation, with either 1.5 mM H2O2 or the catalase inhibitor aminotriazole plus 1-chloro-2,4 dinitrobenzene, which depletes the cellular reduced glutathione (Schwochau et al 1998). Apo J antisense stable transfectants that fail to express apo J are much more sensitive to UVA and heat stress (Viard et al 1999). A heat shock factor 1 (HSF1) 14-bp element exists in the apo J–regulating sequences. This element regulates heat shock–mediated activation of transcription in transient expression assays (Michel et al 1997). In addition, the apo J–regulating sequences contain several binding sites for the transcription factor AP-1. These findings likely explain the high inducibility of apo J in conditions of stress (Michel et al 1997).
In this work, we showed that apo J overexpression greatly increased the survival of SV40 HDFs and WI-38 HDFs after exposure to cytotoxic concentrations of t-BHP and EtOH. t-BHP is a hydrophobic organic peroxide that generates deleterious oxidizing species such as hydroxyl, t-butoxy, and t-butylperoxy radicals. These ROS may be generated from t-BHP in aqueous medium by a Fenton reaction because of interaction of t-BHP with ferrous anions (Starke and Farber 1985; Masaki et al 1989). Given its high hydrophobicity, t-BHP critically affects the biological membranes. Low t-BHP concentrations induce the peroxidation of cellular lipids at levels causally related to cell death. t-BHP also inhibits glutathione peroxidase, which detoxifies lipid hydroperoxides (Ochi 1989). Additionally, t-BHP also damages proteins and DNA (Ochi and Miyaura 1989; Altman et al 1994). The exposure of cells to EtOH results in the destabilization of biological membranes with modifications of their fluidity (Brazeau and Fung 1990). Thus t-BHP and EtOH both drastically affect membrane integrity.
Many of the reported biological ligands of apo J are hydrophobic molecules (for a review, see Humphreys et al 1999). Apo J contains 3 amphipatic α-helical regions that can potentially mediate interactions with hydrophobic molecules, as well as a number of short hydrophobic regions (de Silva et al 1990a; Tsuruta et al 1990). Apo J is associated with the neural plaques found in Alzheimer's disease and exerts protection against the cytotoxicity of β-amyloid peptide. Apo J has the ability to form high–molecular weight complexes with damaged proteins. Therefore, like the small heat shock proteins, apo J may bind to the hydrophobic regions of partially unfolded proteins, thereby solubilizing them and protecting the cells from the cytotoxicity caused by protein precipitation (for a review, see Humphreys et al 1999). These data on apo J may explain its protective effect against cytotoxic stress with t-BHP and EtOH, as well as the absence of protection against H2O2 cytotoxicity because H2O2 is much more hydrophobic, which might therefore result in a lower level of oxidative damage in the hydrophobic cellular compartment.
Given its hydrophobicity, the secreted apo J could protect several critical targets of ROS, such as proteins of the extracellular matrix or extracellular domains of membrane proteins, participating thereby in membrane stabilization. Wilson and Easterbrook-Smith (2000) have proposed that apo J could sequester toxic compounds, thereby decreasing cytotoxicity.
We showed that overexpression of apo J decreases the appearance of 2 main biomarkers of SIPS, eg, senescent morphotypes III–VI and SA β-gal activity. In addition, we found that apo J overexpression results in an increase in fibronectin and osteonectin relative mRNA steady-state levels. An increase in the fibronectin level could protect the cells against apoptosis through the reinforcement of their anchorage to their substrate because fibronectin is a major component of the extracellular matrix that is responsible for anchorage of the cells.
Apo J overexpression did not affect the proliferative life span of the lung HDFs at early CPDs in normal culture conditions. Thereby, a simple conclusion of this work could be that the overexpression of apo J in senescence or SIPS could protect the cells against a subsequent apoptotic stimulus rather than provoke senescence. Nevertheless, apo J overexpression triggered SPARC overexpression, and it is known that SPARC can have antiproliferative effects. We showed herein that SPARC overexpression did not result in an overall inhibition of the proliferative response to several mitogens except PDGF-AB, confirming previous results (Raines et al 1992; Pichler et al 1996). The selective inhibition of the mitogenic effect of PDGF-AB explains why no growth inhibition took place in cells overexpressing apo J cultivated in normal culture conditions with FCS, where many other growth factors are present.
Stimulation of dermal human fibroblasts with transforming growth factor β1 (TGF-β1) results in increased expression of type I collagen and SPARC (Reed et al 1994). On the other hand, TGF-β1 induces the overexpression of fibronectin and SPARC, at both the mRNA and the protein levels, in human pulp cells (Shiba et al 1998). SPARC-null mesangial cells display significantly decreased levels of TGF-β1 mRNA and secreted TGF-β1 protein, as well as decreased steady-state levels of α1(I) procollagen mRNA and protein, as compared with cells expressing wild-type SPARC. Addition of recombinant SPARC to SPARC-null cells restores the expression of α1(I) procollagen and TGF-β1 mRNA (Francki et al 1999). Thus, in different systems, TGF-β1 regulates the expression of fibronectin, SPARC, and α1(I) procollagen mRNA, whereas, in return, SPARC protein levels regulate the expression of TGF-β1 mRNA. We recently showed that IMR-90 HDFs developing the phenotype of H2O2-induced SIPS have high steady-state levels of TGF-β1 mRNA between 24 hours and 72 hours after stress, and secrete high levels of TGF-β1. In addition, the stimulation of these HDFs by TGF-β1 is sufficient to trigger the appearance of biomarkers of SIPS as varied as SA β-gal activity, senescent morphology, and overexpression of the mRNA of the senescence-associated genes, fibronectin, SPARC, apo J, and SM22. Antibodies against TGF-β1 or TGF-β1 receptor II inhibited the overexpression of the genes observed after subcytotoxic H2O2 stress (Frippiat et al 2001).
In conclusion, our data favor the hypothesis that apo J exerts a protective effect against cytotoxicity caused by multiple stressing conditions, such as EtOH, which was previously unknown. In addition, we show for the first time that apo J protects HDFs against SIPS. Lastly, the overexpression of osteonectin triggered by apo J overexpression does not seem to affect the growth kinetics of cells used in this study, at least in normal culture conditions.
Acknowledgments
P.D. has a fellowship from the FRIA, Belgium. F.C. is a Research Assistant and O.T. is a Research Associate of the FNRS, Belgium. We thank the European Union Biomed and Health Research Programme, Shared-cost action “Genage” (BMH4-CT98-3149). We thank the IGBMC-LGME-U.184-ULP (Strasbourg, France) for providing SPARC cDNA.
REFERENCES
- Altman SA, Zastawny TH, Randers L, Lin Z, Lumpkin JA, Remacle J, Dizdaroglu M, Rao G. tert-Butyl hydroperoxide-mediated DNA base damage in cultured mammalian cells. Mutat Res. 1994;306:35–44. doi: 10.1016/0027-5107(94)90165-1. [DOI] [PubMed] [Google Scholar]
- Bayreuther K, Francz PI, Gogol J, Hapke C, Maier M, Meinrath HG. Differentiation of primary and secondary fibroblasts in cell culture systems. Mutat Res. 1991;256:233–242. doi: 10.1016/0921-8734(91)90014-3. [DOI] [PubMed] [Google Scholar]
- Bayreuther K, Francz PI, Gogol J, Kontermann K. Terminal differentiation, aging, apoptosis, and spontaneous transformation in fibroblast stem cell system in vivo and in vitro. Ann NY Acad Sci. 1992;663:167–179. doi: 10.1111/j.1749-6632.1992.tb38660.x. [DOI] [PubMed] [Google Scholar]
- Bayreuther K, Rodemann HP, Hommel R, Dittman K, Albiez M, Francz P. Human skin fibroblasts in vitro differentiate along a terminal cell lineage. Proc Natl Acad Sci U S A. 1988;85:5112–5116. doi: 10.1073/pnas.85.14.5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazeau GA, Fung H-L. Mechanisms of creatine kinase release from isolated rat skeletal muscles damaged by polypropylene glycol and ethanol. J Pharm Sci. 1990;79:393–397. doi: 10.1002/jps.2600790506. [DOI] [PubMed] [Google Scholar]
- Chen Q, Ames BN. Senescence-like growth arrest induced by hydrogen peroxide in human diploid fibroblast F65 cells. Proc Natl Acad Sci U S A. 1994;91:4130–4134. doi: 10.1073/pnas.91.10.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen QM, Bartholomew JC, Campisi J, Acosta M, Reagan JD, Ames BN. Molecular analysis of H2O2-induced senescent-like growth-arrest in human fibroblasts: p53 and Rb control G1 arrest but not cell replication. Biochem J. 1998;332:43–50. doi: 10.1042/bj3320043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AM, Griswold MD. Expression of clusterin/sulfated glycoprotein-2 under conditions of heat stress in rat Sertoli cells and a mouse Sertoli cell line. J Androl. 1997;18:257–263. [PubMed] [Google Scholar]
- de Silva HV, Harmony JAK, Stuart WD, Gil CM, Robbins J. Apolipoprotein J: structure and tissue distribution. Biochemistry. 1990a;29:5380–5389. doi: 10.1021/bi00474a025. [DOI] [PubMed] [Google Scholar]
- de Silva HV, Stuart WD, Park YB, Mao SJT, Gil CM, Wetterau SJ, Bush SJ, Harmony JAK. Purification and characterization of apolipoprotein J. J Biol Chem. 1990b;265:14292–14297. [PubMed] [Google Scholar]
- Dimri GP, Lee X, and Basile G. et al. 1995 A biomarker that identifies senescent human cells in culture and aging skin in vivo. Proc Natl Acad Sci U S A. 92:9363–9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont P, Burton M, and Chen QM. et al. 2000 Induction of replicative senescence biomarkers by sublethal oxidative stresses in human fibroblast. Free Radic Biol Med. 28:361–373. [DOI] [PubMed] [Google Scholar]
- Francki A, Bradshaw AD, Bassuk JA, Howe CC, Couser WG, Sage EH. SPARC regulates the expression of collagen type I and transforming growth factor-1 in mesangial cells. J Biol Chem. 1999;274:32145–32152. doi: 10.1074/jbc.274.45.32145. [DOI] [PubMed] [Google Scholar]
- Frippiat C, Chen QM, Zdanov S, Magalhaes J-P, Remacle J, Toussaint O. Sublethal H2O2 stress triggers a release of TGF-β1 which induces biomarkers of cellular senescence of human diploid fibroblasts. J Biol Chem. 2001;276:2531–2537. doi: 10.1074/jbc.M006809200. [DOI] [PubMed] [Google Scholar]
- Hansen MB, Nielsen SE, Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods. 1989;119:203–210. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- Hasselaar P, Sage EH. SPARC antagonizes the effect of basic fibroblast growth factor on the migration of bovine aortic endothelial cells. J Cell Biochem. 1992;49:272–283. doi: 10.1002/jcb.240490310. [DOI] [PubMed] [Google Scholar]
- Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- Humphreys DT, Carver JA, Easterbrook-Smith SB, Wilson MR. Clusterin has chaperone-like activity similar to that of small heat shock proteins. J Biol Chem. 1999;274:6875–6881. doi: 10.1074/jbc.274.11.6875. [DOI] [PubMed] [Google Scholar]
- Humphreys D, Hochgrebe TT, Easterbrook-Smith SB, Tenniswood MP, Wilson MR. Effects of clusterin overexpression on TNFα- and TGFβ-mediated death of L929 cells. Biochemistry. 1997;36:15233–15243. doi: 10.1021/bi9703507. [DOI] [PubMed] [Google Scholar]
- Kupprion C, Motamed K, Sage EH. SPARC (BM-40, osteonectin) inhibits the mitogenic effect of vascular endothelial growth factor on microvascular endothelial cells. J Biol Chem. 1998;273:29635–29640. doi: 10.1074/jbc.273.45.29635. [DOI] [PubMed] [Google Scholar]
- Lee C, Sensibar JA. Proteins of the rat prostate II: synthesis of new proteins in the ventral lobe during castration induced regression. J Urol. 1987;138:903–908. doi: 10.1016/s0022-5347(17)43413-6. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Marshall NJ, Goodwin CJ, Holt SJ. A critical assessment of the use of microculture tetrazolium assays to measure cell growth and function. Growth Regul. 1995;5:69–84. [PubMed] [Google Scholar]
- Masaki N, Kyle ME, Farber JL. tert-Butyl hydroperoxide kills cultured hepatocytes by peroxidizing membrane lipids. Arch Biochem Biophys. 1989;269:390–399. doi: 10.1016/0003-9861(89)90122-7. [DOI] [PubMed] [Google Scholar]
- Michel D, Chatelain G, North S, Brun G. Stress-induced transcription of the clusterin/apoJ gene. Biochem J. 1997;328:45–50. doi: 10.1042/bj3280045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok SC, Chan WY, Wong KK, Muto MG, Berkowitz RS. SPARC, an extracellular matrix protein with tumor-suppressing activity in human ovarian epithelial cells. Oncogene. 1996;12:1895–1901. [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–83. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Ochi T. Effect of iron chelators and glutathione depletion on the induction and repair of chromosomal aberrations by tert-butylhydroperoxide in cultured Chinese hamster cells. Mutat Res. 1989;213:243–251. doi: 10.1016/0027-5107(89)90156-5. [DOI] [PubMed] [Google Scholar]
- Ochi T, Miyaura S. Cytotoxicity of an organic hydroperoxide and cellular defense system against hydroperoxides in cultured mammalian cells. Toxicology. 1989;55:69–82. doi: 10.1016/0300-483x(89)90175-3. [DOI] [PubMed] [Google Scholar]
- Owen TA, Soprano DR, Soprano KJ. Analysis of the growth factor requirements of WI-38 cells after extended periods of density-dependent growth arrest. J Cell Physiol. 1989;139:424–431. doi: 10.1002/jcp.1041390227. [DOI] [PubMed] [Google Scholar]
- Phillips PD, Cristofalo VJ. Classification system based on the functional equivalency of mitogens that regulate WI-38 cell proliferation. Exp Cell Res. 1988;175:396–403. doi: 10.1016/0014-4827(88)90203-0. [DOI] [PubMed] [Google Scholar]
- Pichler RH, Bassuk JA, and Hugo C. et al. 1996 SPARC is expressed by mesangial cells in experimental mesangial proliferative nephritis and inhibits platelet-derived-growth-factor-mediated mesangial cell proliferation in vitro. Am J Pathol. 148:1153–1167. [PMC free article] [PubMed] [Google Scholar]
- Raines EW, Lane TF, Iruela-Arispe ML, Ross R, Sage EH. The extracellular glycoprotein SPARC interacts with platelet-derived growth factor (PDGF)-AB and -BB and inhibits the binding of PDGF to its receptors. Proc Natl Acad Sci U S A. 1992;89:1281–1285. doi: 10.1073/pnas.89.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed MJ, Vernon RB, Abrass IB, Sage EH. TGF-beta 1 induces the expression of type I collagen and SPARC, and enhances contraction of collagen gels, by fibroblats from young and aged donors. J Cell Physiol. 1994;158:169–179. doi: 10.1002/jcp.1041580121. [DOI] [PubMed] [Google Scholar]
- Rodemann HP. Differential degradation of intracellular proteins in human skin fibroblasts of mitotic and mitomycin C (MMC)-induced post-mitotic differentiation states in vitro. Differentiation. 1989;42:37–43. doi: 10.1111/j.1432-0436.1989.tb00605.x. [DOI] [PubMed] [Google Scholar]
- Rodemann HP, Bayreuther K, Francz PI, Dittman F, Albiez M. Selective enrichment and biochemical characterization of seven fibroblast cell types in vitro. Exp Cell Res. 1989;180:84–93. doi: 10.1016/0014-4827(89)90214-0. [DOI] [PubMed] [Google Scholar]
- Schwochau GB, Nath KA, Rosenberg ME. Clusterin protects against oxidative stress in vitro through aggregative and nonaggregative properties. Kidney Int. 1998;53:1647–1653. doi: 10.1046/j.1523-1755.1998.00902.x. [DOI] [PubMed] [Google Scholar]
- Sensibar JA, Sutkowski DM, Raffo A, Buttyan R, Griswold MD, Sylvester SR, Kozlowski JM, Lee C. Prevention of cell death induced by tumor necrosis factor-α in LNCaP cells by overexpression of sulfated glycoprotein-2 (clusterin) Cancer Res. 1995;55:2431–2437. [PubMed] [Google Scholar]
- Shiba H, Fujita T, and Doi N. et al. 1998 Differential effects of various growth factors and cytokines on the synthesis of DNA, type I collagen, laminin, fibronectin, osteonectin/secreted protein, acidic rich in cysteine (SPARC), and alkaline phosphatase by human pulp cells in culture. J Cell Physiol. 174:194–205. [DOI] [PubMed] [Google Scholar]
- Sintich SM, Steinberg J, Kozlowski JM, Lee C, Pruden S, Sayeed S, Sensibar JA. Cytotoxic sensitivity to tumor necrosis factor-α in PC3 and LNCaP prostatic cancer cells is regulated by extracellular level of SGP-2 (clusterin) Prostate. 1999;39:87–93. doi: 10.1002/(sici)1097-0045(19990501)39:2<87::aid-pros2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Starke PE, Farber JL. Ferric iron and superoxide ions are required for the killing of cultured hepatocytes by hydrogen peroxide. Evidence for the participation of hydroxyl radicals formed by an iron-catalyzed Haber–Weiss reaction. J Biol Chem. 1985;260:10099–10104. [PubMed] [Google Scholar]
- Toussaint O, Dumont P, and Dierick J-F. et al. 2000a Stress-induced premature senescence. Essence of life, evolution, stress and aging. Ann N Y Acad Sci. 908:85–98. [DOI] [PubMed] [Google Scholar]
- Toussaint O, Houbion A, Remacle J. Aging as a multi-step process characterized by a lowering of entropy production leading the cell to a sequence of defined stages. II. Testing some predictions on aging human fibroblasts in culture. Mech Ageing Dev. 1992;65:65–83. doi: 10.1016/0047-6374(92)90126-x. [DOI] [PubMed] [Google Scholar]
- Toussaint O, Houbion A, Remacle J. Effects of modulation of the energetic metabolism on the mortality of cultured cells. Biochem Biophys Acta. 1994;1186:209–220. doi: 10.1016/0005-2728(94)90180-5. [DOI] [PubMed] [Google Scholar]
- Toussaint O, Medrano EE, von Zglinicki T. Cellular and molecular mechanisms of stress-induced premature senescence (SIPS) of human diploid fibroblasts and melanocytes. Exp Gerontol. 2000b;35:927–945. doi: 10.1016/s0531-5565(00)00180-7. [DOI] [PubMed] [Google Scholar]
- Toussaint O, Michiels C, Raes M, Remacle J. Cellular aging and the importance of energetic factors. Exp Gerontol. 1995;30:1–22. doi: 10.1016/0531-5565(94)00038-5. [DOI] [PubMed] [Google Scholar]
- Tsuruta JK, Wong K, Fritz IB, Griswold MD. Structural analysis of sulphated glycoprotein 2 from amino acid sequence. Relationship to clusterin and serum protein 40,40. Biochem J. 1990;268:571–578. doi: 10.1042/bj2680571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viard I, Wehrli P, Jornot L, Bullani R, Vechietti J-L, Schifferli JA, Tschopp J, French LE. Clusterin gene expression mediates resistance to apoptotic cell death induced by heat shock and oxidative stress. J Invest Dermatol. 1999;112:290–296. doi: 10.1046/j.1523-1747.1999.00531.x. [DOI] [PubMed] [Google Scholar]
- Wilson MR, Easterbrook-Smith SB. Clusterin is a secreted mammalian chaperone. Trends Biochem Sci. 2000;25:95–98. doi: 10.1016/s0968-0004(99)01534-0. [DOI] [PubMed] [Google Scholar]