Abstract
In this study we characterized the chaperone functions of Xenopus recombinant Hsp30C and Hsp30D by using an in vitro rabbit reticulocyte lysate (RRL) refolding assay system as well as a novel in vivo Xenopus oocyte microinjection assay. Whereas heat- or chemically denaturated luciferase (LUC) did not regain significant enzyme activity when added to RRL or microinjected into Xenopus oocytes, compared with native LUC, denaturation of LUC in the presence of Hsp30C resulted in a reactivation of enzyme activity up to 80–100 %. Recombinant Hsp30D, which differs from Hsp30C by 19 amino acids, was not as effective as its isoform in preventing LUC aggregation or maintaining it in a folding-competent state. Removal of the first 17 amino acids from the N-terminal region of Hsp30C had little effect on its ability to maintain LUC in a folding-competent state. However, deletion of the last 25 residues from the C-terminal end dramatically reduced Hsp30C chaperone activity. Coimmunoprecipitation and immunoblot analyses revealed that Hsp30C remained associated with heat-denatured LUC during incubation in reticulocyte lysate and that the C-terminal mutant exhibited reduced affinity for unfolded LUC. Finally, we found that Hsc70 present in RRL interacted only with heat-denatured LUC bound to Hsp30C. These findings demonstrate that Xenopus Hsp30 can maintain denatured target protein in a folding-competent state and that the C-terminal end is involved in this function.
INTRODUCTION
Heat shock proteins (Hsps) function as molecular chaperones aiding in the folding and translocation of cellular proteins under normal conditions and protecting cellular proteins under stressed conditions (Arrigo and Landry 1994; Feige et al 1996; Waters et al 1996). The 3 major families of Hsps consist of the high–molecular weight Hsp90s, the Hsp70 family, and the small–molecular weight Hsps. Whereas the Hsp90 and Hsp70 families are conserved in a wide range of organisms, the small Hsps exhibit a very low degree of conservation, with the exception of an α-crystallin domain consisting of 80–100 amino acids. Small Hsps and α-crystallins can form highly polymeric structures that are believed to be necessary for function within the cell (Arrigo and Landry 1994; Waters et al 1996; Smykal et al 2000). Various in vivo functions have been suggested for small Hsps, including stress resistance, actin capping and decapping activity, cellular differentiation, modulation of redox parameters, and prevention of apoptosis (Lavoie et al 1993; Merck et al 1993; Arrigo and Landry 1994; Huot et al 1996; Ehrnsperger et al 1997a; Liang et al 1997; Mehlen et al 1997; Muchowski et al 1997; Arrigo 1998; Mehlen et al 1999; Morrow et al 2000; Samali et al 2001).
Small Hsps are developmentally regulated during animal embryogenesis in a range of organisms, including nematode, Drosophila, brine shrimp, mouse, and rat (Stringham et al 1992; Marin et al 1993; Tanguay et al 1993; Mirkes et al 1996; Liang and MacRae 1999). Our laboratory has been involved in studying the heat-inducible, developmental regulation of 2 members of the Xenopus small hsp30 gene family, hsp30C and hsp30D. Whereas hsp30C is first heat inducible at the early tailbud stage of development, hsp30D is not stress inducible until 1 day later at the midtailbud stage (Krone et al 1992; Ohan et al 1998). In situ hybridization studies revealed the presence of an unidentified hsp30 gene family member(s) that was expressed constitutively in the cement gland of early tailbud embryos (Lang et al 1999). Additionally, heat shock treatment of these embryos induced preferential accumulation of hsp30 message in selected tissues. At the protein level, Hsp30 isoforms were synthesized in a developmentally-regulated and tissue-specific pattern (Tam and Heikkila 1995; Lang et al 1999). Furthermore, it was determined that heat-induced Hsp30 protein formed high–molecular weight complexes (Ohan et al 1998). Recently, in an analysis of the functional role of these Xenopus small Hsps, we determined that recombinant Hsp30C (30C), which was recovered as multimeric complexes, was capable of acting as a molecular chaperone (Fernando and Heikkila 2000). Hsp30C protected citrate synthase (CS) and luciferase (LUC) against heat-induced aggregation by binding to these target proteins and maintaining them in a soluble state. Deletion mutant analysis revealed that the C-terminal end of 30C was required for optimal protection against heat-induced target protein aggregation.
In the present study, we have extended our analysis of the chaperone function of 30C and show that heat or chemical denaturation of LUC in the presence of 30C can maintain this target enzyme in a folding-competent state. Furthermore, Hsp30D, which differs from Hsp30C by 19 amino acids, was not as effective a chaperone as its isoform. Finally, we show that the C-terminal of 30C is required for its chaperone function. These analyses were performed using an in vitro rabbit reticulocyte lysate (RRL) refolding assay as well as a novel in vivo Xenopus oocyte microinjection assay.
MATERIALS AND METHODS
Preparation of 30C and 30D
Previously in our laboratory, the reading frames of Xenopus 30C and the end-terminal mutants, N-30C and C-30C (Fig 1), were amplified and cloned into pRSETB expression vectors (Fernando and Heikkila 2000). Recombinant 30C proteins were expressed in E coli and purified as previously described (Fernando and Heikkila 2000). The entire open reading frame of Xenopus hsp30D (Krone et al 1992) was amplified by polymerase chain reaction (PCR) such that a BamHI site was created 5′ to the start codon, and a HindIII site was created 3′ to the translational stop codon. The resulting fragment was gel purified, digested with HindIII/BamHI, inserted into the corresponding sites of a pRSETB expression vector (Invitrogen, Burlington, ON), and verified by deoxyribonucleic acid (DNA) sequencing. The resultant hsp30D-pRSETB vector was transformed into E coli BL21 (DE3) cells and grown at 37°C in M9 media overnight (Studier et al 1990). M9ZB media were inoculated with the overnight culture and grown to an OD600 of 0.6. Expression of hsp30D gene was induced by the addition of 0.4 mM isopropyl-β-d-thiogalactopyranoside (IPTG), and it was allowed to grow for 20 hours (Studier et al 1990; Kroll et al 1993). Recombinant protein was purified by means of nickel affinity column chromatography, as detailed in Fernando and Heikkila (2000). The concentrations of the proteins were calculated using a bicinchoninic acid (BCA) protein assay (Pierce, Rockford, IL, USA).
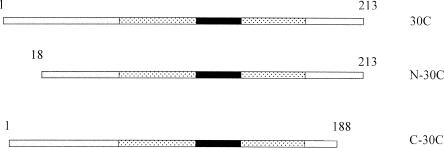
Fig 1.
A diagram of Xenopus Hsp30C and mutants. The N-terminal mutant N-30C lacking the first 17 amino acids and the C-terminal mutant C-30C with the last 25 amino acids deleted were created by polymerase chain reaction–directed mutagenesis. The textured region is the estimated α-crystallin domain, whereas the α-crystallin consensus sequence is contained within the black rectangle
Thermal aggregation assays
Aggregation assays were carried out as previously described (Fernando and Heikkila 2000). CS and LUC at 150 nM monomer concentrations were mixed with various molar amounts of 30D protein or incubated alone in a 50 mM N-2-hydroxythylpiperazine-N′-2-ethane-sulfonic acid (HEPES)-KOH (pH 7.5) buffer and heated at 42°C. Light scattering was determined at 10-minute intervals in a Beckman DU7 spectrophotometer at 320 nm. An increase in absorbance was indicative of protein aggregation.
Reticulocyte lysate LUC reactivation assay
LUC reactivation assays were carried out essentially as described by Lee et al (1997). LUC (0.2 μM) was incubated in refolding buffer (5 mM MgCl2, 10 mM KCl, 2 mM adenosine triphosphate (ATP), 125 mM HEPES-KOH (pH 7.5), and 2 mM dithiothreitol) in the presence or absence of 0.2–10 μM (monomer concentration) 30C, 30D, N-30C, or C-30C at 22°C or 42°C for 15 minutes. Five microliters of the mixtures were added to a solution of 9 μL RRL (Promega, Madison, WI, USA) and 1 μL of 0.1 M ATP equilibrated to 30°C. Samples (2 μm) were taken at various time points during incubation at 30°C and added to 48 μL of 50 mM HEPES-KOH (pH 7.5). The mixtures were then vortexed and diluted 10-fold into LUC reagent solution (Promega). LUC enzyme activity was monitored by a luminometer. In some experiments, LUC (0.05 μM) was incubated in the presence of 0.5 M GuHCl for 1 hour at 22°C in the presence or absence of 1.5 μM 30C, N-30C, or C-30C. Samples (1 μL) were removed and added to 24 μL refolding buffer. Five microliters samples were then taken and added to the RRL-ATP solution, as described previously.
Xenopus oocyte LUC reactivation assay
LUC was heat or chemically denatured as described in the preceding sections. Following denaturation, samples were diluted into 50 mM HEPES-KOH (pH 7.5). Microinjection needles were made from 7.7 cm capillary tubes (Drummond) using a microelectrode puller (Harvard, St. Laurent, Quebec). Heat-denatured LUC mixtures in HEPES-KOH (27.6 nL containing 1.38 fmol LUC) were microinjected into Xenopus oocytes using a Nanoject microinjection apparatus (Drummond, Broomall, PA, USA). Five oocytes were removed at various time points and added to 20 μL refolding and solubilization buffer (5 mM MgCl2, 10 mM KCl, 2 mM ATP, 2 mM DTT, 2 % protease inhibitor cocktail, in 125 mM HEPES-KOH). The oocytes were homogenized using a tissue homogenizer, and mixed by vortexing. The relative LUC activity was measured as described previously.
Immunoprecipitation analysis
Immunoprecipitation analysis was carried out as described by Lee and Vierling (2000). LUC (0.2 μM) in refolding buffer was heat denatured in the presence or absence of 6 μM 30C, N-30C, or C-30C, as described previously. Following heat denaturation, 25 μL of the mixtures was added to 45 μL RRL and 5 μL ATP, and incubated at 30°C. Samples (18 μL) were taken at various time points and added to 350 μL of immunoprecipitation buffer (1.0 M NaCl, 10 mM Tris-HCl [pH 8.0], 1 % Triton X-100, 0.5 % deoxycholic acid, 0.1 % sodium dodecyl sulfate [SDS], 100 μg/mL ovalbumin), followed by 20 μL of 10 mg/mL protein A suspension. After mixing either overnight at 4°C or for 1 hour at 22°C, the protein A beads were pelleted at 14 000 rpm for 10 minutes at 4°C. Six microliters of anti-LUC antibody (Cortex, Biochem Inc, San Leandro, CA) or rabbit anti-30C polyclonal antibody (Fernando and Heikkila 2000) were then added to the lysates and incubated at 4°C on a shaker overnight. The following day, 40 μL of 10 mg/mL protein A suspension was added to the mixtures and left to shake at 4°C overnight. Protein A beads were then removed by centrifugation at 14 000 rpm for 10 minutes at 4°C. The beads were washed 3 times with immunoprecipitation buffer for 10 minutes and twice with 50 mM Tris-HCl [pH 6.8], 300 mM NaCl, 1 % Triton X-100 for 15 minutes. The beads were then added to 5 μL of 5× protein loading dye (0.0625 M Tris [pH 6.8], 10 % glycerol, 2 % SDS, 5 % β-mercaptoethanol, 0.00125 % bromophenol blue) and stored at −20°C for up to 3 days. Samples were then subjected to SDS–polyacrylamide gel electrophoresis (PAGE) and immunoblot analysis, as described earlier.
PAGE and immunodetection
SDS-PAGE was performed in 12 % acrylamide gels using a BioRad Mini Protean II Gel system. Proteins were transferred to polyvinylidene difluoride membranes (Millipore, Nepean, ON) using a BioRad Mini Trans-Blot Transfer system according to the manufacturer's instructions. Immunodetection was carried out using an affinity-purified polyclonal anti-30C antibody (Fernando and Heikkila 2000) or a mouse monoclonal anti-Hsp70 antibody (Stressgen Biotech Corp, Victoria, BC). Blots were incubated with horseradish peroxidase–conjugated secondary IgG (Roche, Laval, Quebec) and detected using an ECL chemiluminescence kit (Amersham, Baie d'Urfé, Quebec), as described by the manufacturer. The resulting chemiluminescence was detected using a Fluorchem imager (Alpha Innotech Corp, San Leandro, CA, USA).
RESULTS
30C can maintain heat-denatured LUC in a folding-competent state
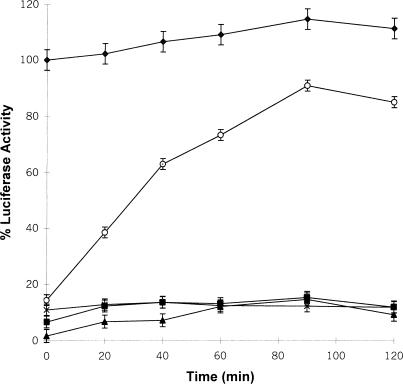
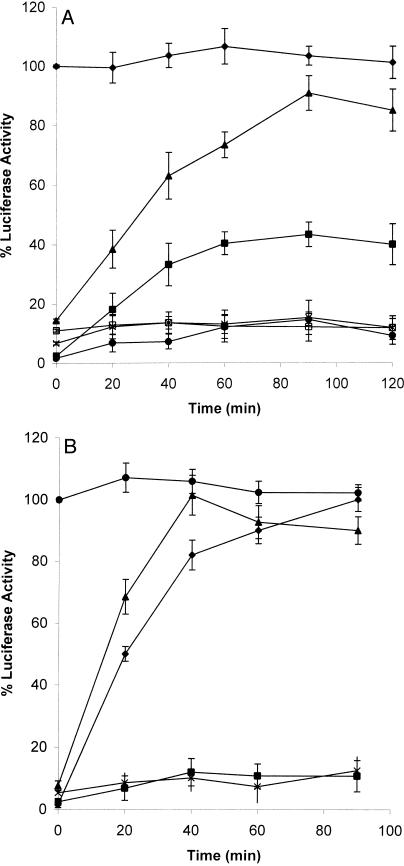
In the present study we determined whether LUC heat denatured in the presence of Xenopus 30C could be refolded in RRL, a source of chaperones including Hsp40, Hsc70, Hsp90, and TRiC (Nimmesgern and Hartl 1993; Lee et al 1997; Minami et al 2000). Whereas the enzyme activity of LUC previously maintained at 22°C increased slightly over time to approximately 116 % after the addition of RRL, LUC heat treated alone at 42°C for 15 minutes or in the presence of a 30-fold molar excess of bovine serum albumin (BSA) regained only about 10 % enzyme activity after 90 minutes (Fig 2). In contrast to these latter results, LUC heat denatured in the presence of 30C displayed 80–90 % enzyme reactivation after 90 minutes of incubation with RRL. The refolding and reactivation of LUC enzyme activity was ATP dependent because there was little LUC reactivation in the absence of exogenously added ATP. RRL was not able to reactivate LUC if 30C was added after heat treatment (data not shown).
Fig 2.
Luciferase (LUC) heat denatured in the presence of recombinant Hsp30C (30C) can be reactivated in vitro by reticulocyte lysate (RRL). LUC (0.2 μM) was incubated with 6.0 μM of 30C (○) or bovine serum albumin (x), and heat denatured at 42°C or kept at 22°C (♦) for 15 minutes. Samples were adjusted to 60 % RRL and 6 μM adenosine triphosphate (ATP), and incubated at 30°C for various periods of time. LUC activity was assayed as described in “Materials and Methods.” Some samples were heat treated without 30C (▪) or assayed without ATP (▴). The data are calculated as a percentage of the activity of unheated LUC at 0 minute, and expressed as the mean of 4–6 trials ± SE
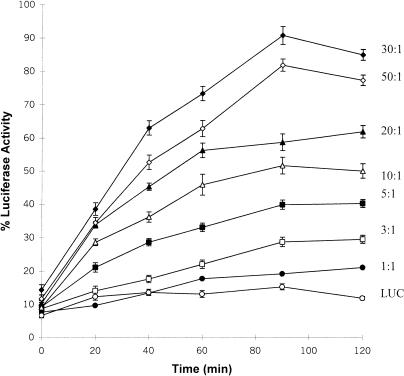
The effect of different monomer molar ratios of 30C to LUC on the ability of 30C to maintain the target enzyme in a folding-competent state is shown in Figure 3. Heat denaturation of LUC in the presence of 30C at a 30C-LUC molar ratio of 1:1 resulted in only 15 % LUC enzyme reactivation, a level only slightly more than LUC alone. LUC refolding increased with increasing 30C to LUC molar ratios from 3:1 to 30:1. A further increase in the 30C-LUC molar ratio to 50:1 caused a slight decline in the relative level of the LUC enzyme reactivation at 90 minutes, and compared with the 30:1 ratio.
Fig 3.
The effect of different amounts of recombinant Hsp30C (30C) during heat denaturation on luciferase (LUC) enzyme reactivation in vitro. LUC (0.2 μM) was heat denatured at 42°C with various molar quantities at 30C-LUC molar ratios, as indicated beside each curve. LUC activity was assayed after incubation with reticulocyte lysate and adenosine triphosphate, as described in “Materials and Methods.” All data are expressed as a percentage of the activity of native LUC and shown as the mean ± SE
LUC heat denatured in the presence of 30C can be refolded in vivo after microinjection into Xenopus oocytes
In the preceding experiments LUC heat denatured in the presence of 30C was refolded in RRL, a heterologous in vitro system. In order to examine this phenomenon in a homologous in vivo system, we developed a novel chaperone assay employing Xenopus oocytes. The large size of the Xenopus oocytes (1-mm to 1.1-mm diameter) has made them ideal for various microinjection studies (Heikkila 1990). Previously, it has been shown that Xenopus oocytes contain Hsp70 and Hsp90, but do not contain detectable levels of Hsp30 protein or messenger ribonucleic acid (mRNA) (Bienz 1984; Krone et al 1989; Uzawa et al 1995; Ali et al 1998; Tam and Heikkila, personal communication). To determine whether Xenopus oocytes could be used in an in vivo chaperone assay, LUC was heat denatured alone or in the presence of either BSA or 30C, followed by microinjection of 26.7 nL of the mixtures (containing 1.38 fmol LUC) into Xenopus oocytes and incubation at 22°C for up to 90 minutes. Whereas LUC heat treated alone or in the presence of BSA regained less than 10 % enzyme activity, LUC heat treated with 30C reactivated to almost 100 % enzyme activity after 40 minutes (Fig 4). This latter LUC reactivation level was higher than the value of 80–90 % routinely found with RRL after 90 minutes. Microinjection of higher amounts of LUC (10.12 fmol) plus 30-fold 30C resulted in only 35 % enzyme reactivation relative to unheated LUC alone (data not shown). It is likely that microinjecting these higher amounts of LUC and the accompanying 30C may overload the available chaperone folding machinery in the oocyte. Similar to the in vitro studies with the RRL system, the optimum 30C to LUC ratio for LUC refolding in Xenopus oocytes was 30:1 (Fig 4). A 1:1 30C-LUC molar ratio did not facilitate any detectable LUC refolding, whereas a ratio of 10:1 resulted in a reactivation of 45 % LUC enzyme activity.
Fig 4.
Luciferase (LUC) heat denatured in the presence of recombinant Hsp30C (30C) can be refolded in vivo after microinjection into Xenopus oocytes. LUC (0.2 μM) was incubated at 22°C (♦), or heat denatured alone at 42°C (X) or in the presence of either 6 μM bovine serum albumin (•) or 30C at 30C-LUC molar ratios of 1:1 (□), 10:1 (▴), or 30:1 (▵) for 15 minutes. Mixtures (containing 1.38 fmol of LUC in 26.7 nL) were microinjected into Xenopus oocytes, and LUC activity in the oocytes was monitored over time as described in “Materials and Methods.” Data are representative of 3–5 trials and shown as the mean ± SE
Chaperone activity of the isoform Hsp30D
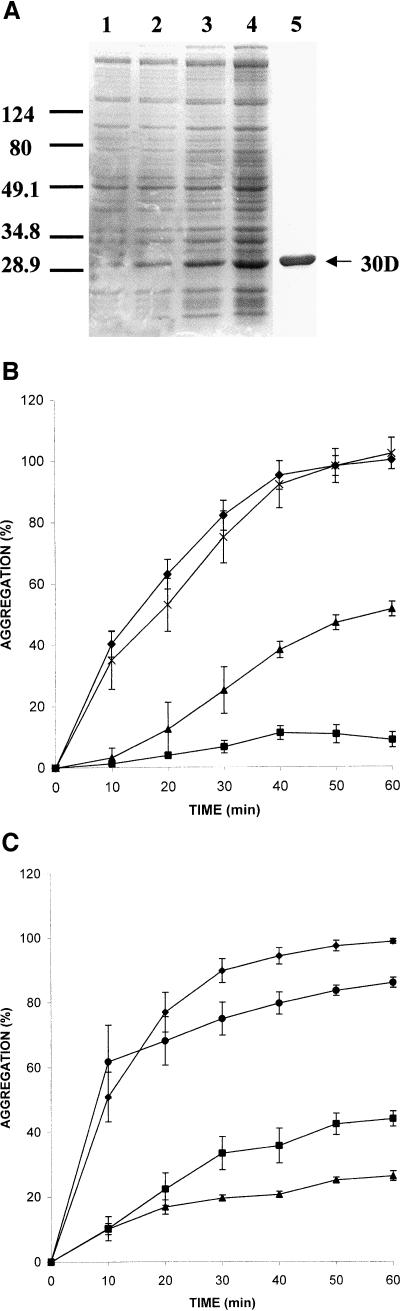
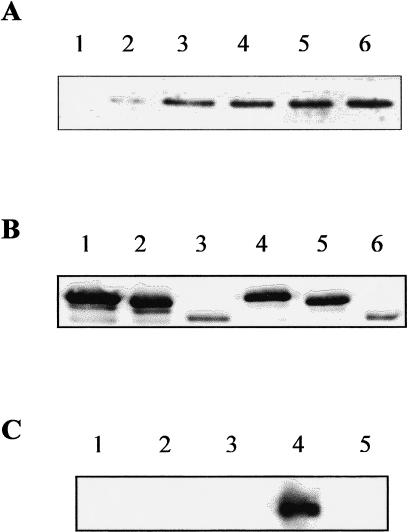
The complete open reading frame of the Xenopus hsp30D gene was amplified by PCR and inserted into the pRSETB-E coli expression vector. The Hsp30D-pRSETB vector was transformed into the E coli expression strain BL21(DE3)pLysS and induced with IPTG. Recombinant 30D was detected in bacterial lysates by SDS-PAGE after 5 hours of IPTG treatment (Fig 5A). Maximum accumulation of 30D occurred after 20 hours of induction. Bacterial lysates were subjected to nickel affinity column chromatography to purify 30D. Recombinant 30D was recovered as large multimeric complexes (data not shown) as found previously with 30C (Fernando and Heikkila 2000). The ability of 30D to function as a molecular chaperone was analyzed by means of CS and LUC thermal aggregation assays. Heat-induced aggregation of CS, as determined by the amount of light scattering at 320 nm, developed rapidly and irreversibly at 42°C (Fig 5B). Incubation with equimolar amounts of 30D resulted in a 50 % decrease in aggregation over the same time period. At a 5:1 molar ratio of 30D-CS, aggregation was reduced by at least 90 %. The chaperone activity of 30D was ATP independent because the addition of ATP to the assay had no effect (data not shown). In contrast to 30D, incubation of IgG with CS at a molar ratio of 5:1 did not inhibit CS aggregation. Moreover, incubation of LUC alone or in the presence of IgG at 42°C resulted in rapid aggregation to maximal levels after 50–60 minutes (Fig 5C). In contrast, inclusion of 30D with LUC at 5:1 and 10:1 molar ratios during heat treatment inhibited the heat-induced aggregation of LUC by 60 % and 75 %, respectively, after 60 minutes.
Fig 5.
Molecular chaperone activity of Xenopus Hsp30D. (A) Expression and purification of Hsp30D recombinant protein. Total bacterial protein from E coli BL21(DE3) cells containing the Hsp30D-pRSETB expression vector was collected before (lane 1) and after 5 hours, 14 hours, and 20 hours (lanes 2–4) of isopropyl-β-d-thiogalactopyranoside treatment. Recombinant protein was purified by means of nickel affinity column chromatography, as detailed in Fernando and Heikkila (2000). Protein was analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and visualized by Coomassie Brilliant Blue staining. Five micrograms of purified recombinant Hsp30D (30D) is shown in lane 5. Molecular mass markers in kDa are indicated on the left side of the figure. (B) Prevention of heat-induced citrate synthase (CS) aggregation by 30D. Aggregation assays were carried out using the protocols described previously (Fernando and Heikkila 2000). CS or luciferase (LUC) at 150 nM monomer concentrations was mixed with various molar amounts of 30D protein or incubated alone in a 50 mM N-2-hydroxythylpiperazine-N′-2-ethane-sulfonic acid–KOH (pH 7.5) buffer and heated at 42°C. Light scattering was determined at 10-minute intervals in a Beckman DU7 spectrophotometer at 320 nm. An increase in absorbance was indicative of protein aggregation. Data are representative of 4–6 trials and were calculated as a percentage of the maximum aggregation of CS or LUC after 60 minutes and expressed as the mean ± SE. CS was heat treated alone (♦, 0.1 μM) or in the presence of either 30D (▴, 0.1 μM; ▪, 0.5 μM) or IgG (x, 0.5 μM). (C) Inhibition of heat-induced aggregation of LUC by 30D. LUC was heat treated alone (♦, 0.1 μM) or in the presence of 30D (▪, 0.5 μM; ▴, 1.0 μM) or IgG (•, 0.5 μM)
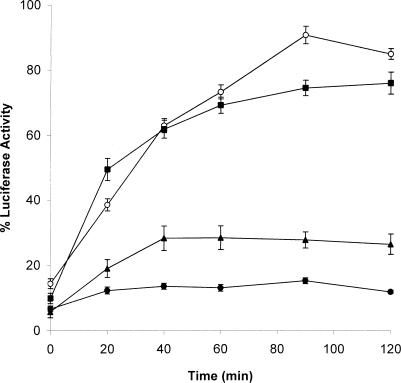
In RRL refolding assays LUC heat denatured with 30D at a 30D-LUC ratio of 30:1 displayed 43 % enzyme reactivation after 90 minutes of incubation with RRL (Fig 6A). In contrast, LUC heat denatured in the presence of 30C regained 80–90 % enzyme activity. Higher or lower 30D-LUC ratios did not result in enhanced LUC enzyme reactivation (data not shown). In microinjected Xenopus oocytes, LUC heat treated with 30D reactivated to almost 100 % enzyme activity after 90 minutes (Fig 6B). In comparison, heat treatment of LUC with 30C required only 40 minutes to reach this level in the microinjected oocytes. These results indicate that both Xenopus small Hsps can function as molecular chaperones, but that 30C is more efficient than 30D in preventing heat-induced aggregation of LUC as well as in maintaining it in a folding-competent state.
Fig 6.
In vitro and in vivo enzyme reactivation of luciferase (LUC) heat denatured in the presence of Hsp30D. LUC refolding assays were carried out as described in the legends of Figures 2 and 4. In the reticulocyte lysate (RRL) refolding assays (panel A), LUC (0.2 μM) was maintained at 22°C (♦) or combined with 6.0 μM of recombinant Hsp30D (30D) (▪), 30C (▴), or bovine serum albumin (BSA) (□), and heat denatured at 42°C for 15 minutes prior to incubation with RRL. Some LUC samples were heat treated alone (x) or without adenosine triphosphate (•). The data are calculated as a percentage of the activity of unheated LUC at 0 minute, and expressed as the mean of 4–6 trials ± SE. In the Xenopus oocyte refolding system (panel B), LUC (0.2 μM) was incubated at 22°C (•), or heat denatured alone at 42°C (▪) or in the presence of either 6 μM BSA (x) or 30D (♦) or 30C (▴) for 15 minutes prior to microinjection. Data are representative of 3–5 trials and shown as the mean ± SE
Carboxyl-terminal end of 30C is necessary for chaperone activity
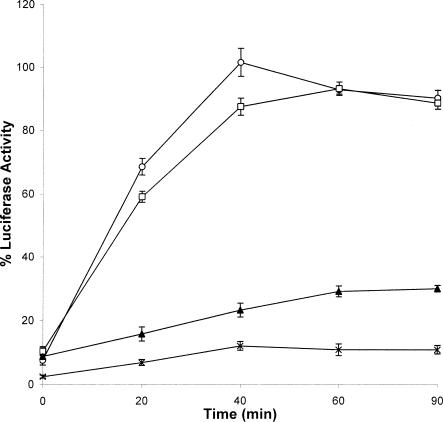
In a previous study we determined that the mutant N-30C, which had 17 amino acids deleted from the N-terminal end of 30C, behaved in a similar fashion to full-length 30C in inhibiting heat-induced aggregation of CS and LUC (Fernando and Heikkila 2000). However, removal of the last 25 amino acids from the carboxyl end severely affected the ability of 30C to prevent heat-induced aggregation of the target protein. In the current study these mutants were examined for their ability to maintain heat-denatured LUC in a folding-competent state using the RRL (Fig 7) and Xenopus oocyte systems (Fig 8). Whereas LUC heat treated in the presence of N-30C regained approximately 80 % of its enzyme activity, LUC heat denatured with C-30C recovered only 30 %. To determine whether the molecular chaperone functions of N-30C and C-30C mutants were affected by varying the Hsp-LUC molar ratios, LUC was heat denatured with various amounts of N-30C or C-30C, and then refolded with RRL. Similar to 30C, the optimum molar ratio for N-30C to LUC was 30:1 (data not shown). Finally, varying the molar ratio of C-30C to LUC from 5:1 to 50:1 resulted in LUC enzyme activity values of only 18–30 % (data not shown). The molecular chaperone functions of N-30C and C-30C were also analyzed in vivo using the Xenopus oocytes microinjection system. When LUC heat denatured with N-30C was injected into oocytes, almost all of the original LUC activity recovered within 1 hour (Fig 8). This level of LUC enzyme activity was much greater than the 25 % reactivation found with C-30C after 90 minutes. All these findings paralleled the results achieved with the in vitro RRL system.
Fig 7.
Ability of carboxyl- and amino-terminal deletion mutants of recombinant Hsp30C (30C) to maintain heat-denatured luciferase (LUC) in a reactivatable form. LUC (0.2 μM) was heat denatured alone (•) or with 6.0 μM 30C (○), N-30C (▪), or C-30C (▴) for 15 minutes, and then analyzed in the LUC refolding assay as described in “Materials and Methods.” Data are representative of 4 trials and expressed as the mean ± SE
Fig 8.
Luciferase (LUC) heat denatured in the presence of recombinant Hsp30C (30C) and N-30C, but not C-30C, can be refolded in Xenopus oocytes. LUC (0.2 μM) was heat denatured alone (X) or with 6.0 μM of 30C (○), N-30C (□), or C-30C (▴) at 42°C for 15 minutes. Samples were microinjected into Xenopus oocytes, and LUC activity was determined over time as indicated in “Materials and Methods.” Data are representative of 3–5 trials and shown as the mean ± SE
30C maintains LUC in a folding-competent state after chemical denaturation
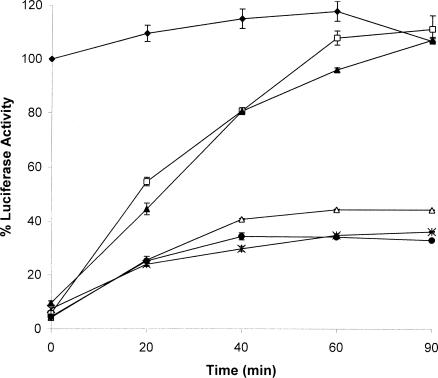
Additionally, this study assessed the ability of 30C, N-30C, and C-30C to maintain LUC in a folding-competent state after chemical denaturation. In this analysis LUC was treated with 0.5 M guanidinium hydrochloride (GuHCl) for 1 hour at 22°C in the presence or absence of 30C, and then incubated with RRL and ATP at 30°C. Whereas chemically denatured LUC regained only 33 % of its enzyme activity, LUC denatured in the presence of 30C was completely reactivated in RRL after 90 minutes (Fig 9). Only basal LUC enzyme activity was detected when 30C was added after chemical denaturation. This finding was similar to what was observed with heat inactivation of LUC. As shown in Figure 9, N-30C was able to maintain LUC in a folding-competent state, whereas LUC chemically denatured in the presence of C-30C regained less than 44 % enzyme activity. Similar results were found using the Xenopus oocyte refolding system (data not shown). Thus, the C-terminal end is required for the maintenance of LUC in a refoldable state after chemical denaturation.
Fig 9.
Luciferase (LUC) can be refolded in vitro when chemically denatured in the presence of recombinant Hsp30C (30C) and N-30C, but not C-30C. LUC (0.05 μM) was maintained in refolding buffer (♦) or incubated in 0.5 M guanidine hydrochloride in the absence (•) or presence of either 1.5 μM 30C (□) or N-30C (▴) or C-30C (▵) for 1 hour at 22°C. The samples were then assayed in the reticulocyte lysate refolding assay as described in “Materials and Methods.” In some samples 30C was added after the chemical treatment and prior to the refolding assay (X). Data are expressed as a percentage of native LUC activity and expressed as the mean of 3 trials ± SE
Association of 30C with heat-denatured LUC during enzyme reactivation
Another question addressed in this study was whether 30C remained associated with LUC during the refolding process. Because the LUC protein levels used with the Xenopus oocyte microinjection system were very low, this analysis was carried out with RRL. In these experiments LUC was maintained at either 22°C or 42°C for 15 minutes in the presence of 30C, followed by incubation with RRL and immunoprecipitation with an anti-LUC antibody. The protein complexes were recovered, separated by SDS-PAGE, blotted, and reacted with anti-30C antibody. As shown in Figure 10A, 30C did not associate with LUC after incubation at 22°C (Fig 10, lane 1), but did show minimal association during incubation with RRL at 30°C (Fig 10, lane 2). In contrast, 30C strongly associated with LUC after heat treatment at 42°C and then remained associated with LUC during the refolding process up to at least 90 minutes during which maximal enzyme reactivation occurred (Fig 10, lanes 3–6). N-30C demonstrated a similar association with LUC during enzyme reactivation (Fig 8B, compare lanes 2 and 4). Interestingly, C-30C displayed reduced binding ability to LUC, both prior to and during incubation with RRL (lanes 3 and 6).
Fig 10.
Association of recombinant Hsp30C (30C) and mutants with luciferase (LUC) and Hsc70 during refolding. (A) Association of 30C with LUC during refolding. LUC (0.2 μM) was maintained at 22°C for 15 minutes with 6 μM 30C, and then incubated with reticulocyte lysate (RRL) at 30°C for either 0 minute (lane 1) or 40 minutes (lane 2). Alternatively, LUC was heat denatured at 42°C with 30C, and then incubated with RRL for 0 minute, 40 minutes, 60 minutes, or 90 minutes (lanes 3–6). LUC and associated complexes were immunoprecipitated using an anti-LUC antibody and immunoblot analysis with an anti-30C antibody. (B) Comparison of 30C, N-30C, and C-30C association with LUC during refolding. LUC (0.2 μM) was heat denatured with 6 μM 30C (lanes 1 and 4), N-30C (lanes 2 and 5), or C-30C (lanes 3 and 6). Samples were then incubated with RRL at 30°C for either 0 minute (lane 1–3) or 40 minutes (lanes 4–6). LUC and associated complexes were immunoprecipitated using an anti-LUC antibody and immunoblot analysis with an anti-30C antibody. (C) LUC heat denatured with 30C associates with Hsc70 during refolding. Hsp30C (6 μM) was incubated alone at either 22°C (lane 1) or 42°C (lane 2), or with LUC (0.2 μM) at 22°C (lane 3) or at 42°C (lane 4) for 15 minutes. LUC incubated alone at 42°C is shown in lane 5. The samples were combined with RRL and adenosine triphosphate and kept at 30°C for 20 minutes. Samples were subjected to immunoprecipitation using rabbit polyclonal anti-30C and immunoblot analysis with mouse monoclonal anti-Hsp70 antibody
To determine whether the 30C-LUC complex interacted with Hsc70 in RRL, 30C-LUC complexes were immunoprecipitated from refolding reaction mixtures with anti-30C antibody and subjected to SDS-PAGE and immunoblot analysis utilizing a mouse monoclonal anti-Hsp70. In these experiments, Hsc70 did not coimmunoprecipitate with 30C that was previously kept at 22°C (Fig 10C, lane 1) or heated to 42°C (Fig 10C, lane 2) prior to incubation with RRL. Also, no Hsc70 band could be detected in reaction mixtures in which LUC was kept at 22°C (Fig 10C, lane 3) or when LUC was heat denatured without 30C (Fig 10C, lane 5) before RRL addition. Hsc70 association with 30C was observed only in RRL samples when LUC was previously heat denatured in the presence of 30C (Fig 10C, lane 4).
DISCUSSION
In the present study we demonstrated that LUC heat treated or chemically denatured in the presence of 30C recovered up to 90 % of the original enzyme activity, after incubation in a RRL refolding system. In contrast, LUC denatured alone or in the presence of BSA regained only 10 % enzyme activity. To investigate chaperone activity in an in vivo homologous system, we developed a novel Xenopus oocyte assay system. Because this large cell (equivalent to 200 000 somatic cells) is amenable to microinjection, it has been used in a wide variety of assays, including transcription of DNA, translation of mRNA, analysis of trans-acting factors, and protein biochemistry (Heikkila 1990). Interestingly, LUC enzyme reactivation in the presence of 30C occurred more rapidly after microinjection into Xenopus oocytes than in the RRL system. For example, almost 100 % of the injected LUC was refolded in vivo within 40 minutes, compared with 80–90 % after 1 hour in the RRL. One reason for this difference may be the better recognition of the 30C complexes by the homologous Xenopus protein–folding chaperones. Similar to plant and mammalian small Hsps (Lee et al 1995, 1997; Ehrnsperger et al 1997b), Xenopus 30C does not have the ability to refold heat-denatured LUC on its own. Hence, our observations indicate that 30C functions as a molecular chaperone not only by inhibiting irreversible protein aggregation (Fernando and Heikkila 2000) but also by maintaining target proteins in a folding-competent state.
The ability of 30C to function as a molecular chaperone and maintain LUC in refoldable form was optimal at a 30C to LUC molar ratio of 30:1 in the RRL and Xenopus oocyte systems. It is likely that 30C is interacting with LUC as a multimeric complex because size-exclusion chromatography indicated that 30C was present as 800–900 kDa complexes which may contain up to 30–35 molecules each (Fernando and Heikkila 2000). Interestingly, whereas a 30-fold molar excess of Xenopus 30C was required to maintain LUC in an optimal refolding-competent state, only a 5-fold molar excess of 30C was sufficient for maximal inhibition of heat-induced LUC aggregation (Fernando and Heikkila 2000). It is possible that individual 30C multimeric complexes are capable of maintaining only a limited number of LUC molecules in a folding-competent state, but can protect more target protein from aggregating. If this speculation is correct, then it may reflect the greater importance of preventing heat-induced protein aggregation and possible cell death over protein refolding.
Xenopus Hsp30D was more effective in the inhibition of heat-induced aggregation of CS than in protecting LUC. For example, a 5:1 molar ratio of 30D to target protein inhibited heat-induced aggregation of CS by 80–90 %, and of LUC by only 60 %. In contrast, our previous studies with 30C demonstrated that under the same conditions, LUC and CS were both protected by over 90 % (Fernando and Heikkila 2000). The ability of Xenopus 30D to maintain LUC in a folding-competent state was demonstrated in RRL and Xenopus oocyte microinjection refolding assays. In the RRL assay, LUC heat denatured alone displayed minimal enzyme reactivation, whereas LUC denatured with 30D recovered 43 % enzyme activity compared with 80–90 % with 30C. Whereas LUC heat denatured with 30D regained 100 % enzyme activity in the Xenopus oocyte assay, the time required was more than twice that of 30C. These results suggest that 30C may be a more effective molecular chaperone than 30D in preventing LUC thermal aggregation and in maintaining heat-denatured target proteins in a folding-competent state. It is likely that this difference in effectiveness resides at the amino acid sequence level. A comparison of 30D and 30C revealed a total of 19 amino acid differences, including 3 additional amino acids inserted in the N-terminal half of Hsp30D (Krone et al 1992). One future approach will be to gradually and selectively mutate the amino acids of 30D to those found in 30C and to monitor the ability of the mutated 30D to maintain heat-denatured target protein in a folding-competent state. This type of approach may be feasible because studies with αA- and αB-crystallin revealed that a single amino acid change reduced their chaperone activity (Kumar et al 1999).
In the present study we found that truncation of the N-terminal end of 30C did not have a major effect on its ability to maintain heat-treated or chemically denatured LUC in a folding-competent state. A similar result was obtained with murine Hsp25 in which removal of 33 amino acids from the N-terminal end did not alter its ability to act as a molecular chaperone in the refolding of chemically denatured CS (Guo and Cooper 2000). In contrast to N-30C, deletion of the last 25 amino acids from the C-terminal of Xenopus 30C severely reduced its ability to facilitate LUC refolding in RRL and Xenopus oocytes, regardless of the amount of C-30C added. Similarly, cleavage of the C-terminal end of αA-crystallin severely reduced its ability to protect target protein against heat-induced aggregation (Takemoto et al 1993). Most small Hsps including Xenopus Hsp30C contain a short, highly polar and flexible C-terminal extension that is variable in sequence and in length (Carver et al 1992, 1995; Ehrnsperger et al 1997b). Previously, we have shown that this C-terminal extension is required for the enhanced solubility of 30C and its target protein (Fernando and Heikkila 2000). The importance of the C-terminal end of small Hsps in vivo was shown by the finding that the enhanced stress resistance of monkey cells by transfected Drosophila Hsp27 was lost when the C-terminal was deleted (Mehlen et al 1993). In contrast to the present results, truncation of the C-terminal extension of C elegans Hsp16-2 had no effect on its chaperone activity (Leroux et al 1997). Also, although the removal of 18 amino acids from the C-terminal end of mouse Hsp25 reduced its chaperone activity in inhibiting α-lactalbumin precipitation following reduction with DTT, this mutant performed in a similar fashion to the wild-type small Hsp in a CS thermal aggregation assay (Lindner et al 2000). Although the reasons for these differences are not known, our results are consistent with a number of studies outlining the importance of the C-terminal tails of small Hsps for stabilization and solubilization of complexed and uncomplexed Hsps and α-crystallins (Smulders et al 1996; Ehrnsperger et al 1997b; Carver and Lindner 1998; Lindner et al 1998, 2000).
In this study, Xenopus 30C did not interact with native LUC, but did associate with heat-denatured LUC. Furthermore, this association between Xenopus 30C and LUC was detectable throughout the refolding period in RRL. This finding indicates that Xenopus 30C remains complexed with LUC as it undergoes folding by ATP-dependent folding chaperones in RRL. Whereas the N-terminal 30C mutant, N-30C, associated with heat-denatured LUC to a similar extent as 30C, C-30C had reduced affinity for this target protein. This may suggest the involvement of the C-terminal extension in complex formation with unfolded, unstable proteins. This hypothesis is supported by other studies showing that, upon interaction with substrates, the C-terminal tail of αB-crystallin loses its flexibility (Carver et al 1995; Lindner et al 1998). Finally, the present study found that Hsc70 was coimmunoprecipitated, using an anti-30C antibody, only from RRL mixtures containing LUC previously heated in the presence of 30C. Lee et al (2000) reported a similar result using plant small Hsp18.1. These findings support current models in which small Hsps bind to unfolded proteins, inhibit aggregation, and present them to other chaperones for ATP-dependent refolding (Carver et al 1995; Ehrnsperger et al 1997a; Lee et al 1997, 2000; Veinger et al 1998; Fernando and Heikkila 2000).
In conclusion, we have shown that Xenopus 30C and 30D act as molecular chaperones not only by inhibiting stress-induced protein aggregation but also by maintaining heat- or chemically denatured LUC in a folding-competent state. With Hsp30C these functions required the presence of the C-terminal end. Finally, this study demonstrates for the first time the ability of Xenopus oocytes to be used as an in vivo chaperone assay system. The ability to microinject proteins and mRNA (eg, Hsc70, Hsp40) into Xenopus oocytes may prove valuable in the study of in vivo chaperone–target protein interactions in the future.
Acknowledgments
This research was supported by a Natural Sciences and Engineering Research Council grant to J.J.H. The authors thank Garret Lee, University of Arizona, for his excellent advice.
REFERENCES
- Ali A, Bharadwaj S, O'Carroll R, Ovsenek N. Hsp 90 interacts with and regulates the activity of heat shock factor 1 in Xenopus oocytes. Mol Cell Biol. 1998;18:4949–4960. doi: 10.1128/mcb.18.9.4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo AP. Small heat shock proteins: chaperones that act as regulators of intracellular redox state and programmed cell death. J Biol Chem. 1998;379:19–26. [PubMed] [Google Scholar]
- Arrigo AP, Landry J 1994 Expression and function of the low-molecular weight heat shock proteins. In: The Biology of Heat Shock Proteins and Molecular Chaperones, ed Morimoto RI, Tissieres A, Georgopoulos C. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 335–373. [Google Scholar]
- Bienz M. Developmental control of the heat shock response in Xenopus. Proc Natl Acad Sci U S A. 1984;81:3138–3142. doi: 10.1073/pnas.81.10.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver JA, Aquilina JA, Truscott RJW, Ralston GB. Identification by H NMR spectroscopy of flexible C-terminal extensions in bovine lens alpha-crystallin. FEBS Lett. 1992;311:143–149. doi: 10.1016/0014-5793(92)81386-z. [DOI] [PubMed] [Google Scholar]
- Carver JA, Guerreiro N, Nicholls KA, Truscott RJW. On the interaction of α-crystallin with unfolded proteins. Biochem Biophys Acta. 1995;1252:251–260. doi: 10.1016/0167-4838(95)00146-l. [DOI] [PubMed] [Google Scholar]
- Carver JA, Lindner RA. NMR spectroscopy of α-crystallin. Insights into the structure, interactions and chaperone action of small heat shock proteins. Int J Biol Macromol. 1998;22:197–209. doi: 10.1016/s0141-8130(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Ehrnsperger M, Buchner J, and Gaestel M 1997a Structure and function of small heat shock proteins. In: Molecular Chaperones in the Life Cycle of Proteins. Structure, Function and Mode of Action, ed Fink AL, Goto Y. Marcell Dekker, New York, 533–575. [Google Scholar]
- Ehrnsperger M, Graber S, Gaestel M, Buchner J. Binding of non-native protein to Hsp25 during heat shock creates a reservoir of folding intermediates for reactivation. EMBO J. 1997b;16:221–229. doi: 10.1093/emboj/16.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feige U, Morimoto RI, Yahara I, and Polla BS 1996 Stress-inducible cellular responses. Birkhouser Verlag, Switzerland. [PubMed] [Google Scholar]
- Fernando P, Heikkila JJ. Functional characterization of Xenopus small heat shock protein, Hsp30C: the carboxyl end is required for stability and chaperone activity. Cell Stress Chaperones. 2000;5:148–159. doi: 10.1379/1466-1268(2000)005<0148:fcoxsh>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Cooper LF. An N-terminal 33-amino acid deletion variant of Hsp25 retains oligomerization and functional properties. Biochem Biophys Res Comm. 2000;270:183–189. doi: 10.1006/bbrc.2000.2401. [DOI] [PubMed] [Google Scholar]
- Heikkila JJ. Expression of cloned genes and translation of messenger RNA in microinjected Xenopus oocytes. Int J Biochem. 1990;22:1223–1228. doi: 10.1016/0020-711x(90)90302-j. [DOI] [PubMed] [Google Scholar]
- Huot JF, Houle F, Spitz DR, Landry J. Hsp27 phosphorylation-mediated resistance against actin fragmentation and cell death induced by oxidative stress. Cancer Res. 1996;56:273–279. [PubMed] [Google Scholar]
- Kroll DJ, Abdel-Malek , Abdel-Hafiz H, Marcell T, Simpson S, Chen C-Y, Gutierrez-Hartmann A, Lustbader JW, Hoeffler JP. A multifunctional prokaryotic protein expression system: overproduction, affinity purification and selective detection. DNA Cell Biol. 1993;12:441–453. doi: 10.1089/dna.1993.12.441. [DOI] [PubMed] [Google Scholar]
- Krone PH, Heikkila JJ. Expression of microinjected hsp70/cat and hsp30/cat chimeric genes in developing Xenopus laevis embryos. Dev Biol. 1989;106:271–281. doi: 10.1242/dev.106.2.271. [DOI] [PubMed] [Google Scholar]
- Krone PH, Snow A, Ali A, Pasternak JJ, Heikkila JJ. Comparison of the regulatory and structural regions of the Xenopus laevis small heat shock protein encoding gene family. Gene. 1992;110:159–166. doi: 10.1016/0378-1119(92)90643-4. [DOI] [PubMed] [Google Scholar]
- Kumar LVS, Ramakrishna T, Rao CM. Structural and functional consequences of the mutation of a conserved arginine residue in αA- and αB-crystallins. J Biol Chem. 1999;274:24137–24141. doi: 10.1074/jbc.274.34.24137. [DOI] [PubMed] [Google Scholar]
- Lang L, Miskovic D, Fernando P, Heikkila JJ. Spatial pattern of constitutive and heat-shock induced expression of the small heat shock protein gene family, hsp30, in Xenopus laevis tailbud embryos. Dev Genet. 1999;25:365–374. doi: 10.1002/(SICI)1520-6408(1999)25:4<365::AID-DVG10>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Lavoie JN, Gingras-Breton G, Tanguay RM, Landry J. Induction of the Chinese hamster hsp27 gene expression in mouse cells confers resistance to heat shock. Hsp27 stabilization of the microfilament organization. J Biol Chem. 1993;268:3420–3429. [PubMed] [Google Scholar]
- Lee GJ, Pokala N, Vierling E. Structure and in vitro molecular chaperone activity of cytosolic small heat shock proteins from pea. J Biol Chem. 1995;270:10432–10438. doi: 10.1074/jbc.270.18.10432. [DOI] [PubMed] [Google Scholar]
- Lee GJ, Roseman AM, Saibil HR, Vierling E. A small heat shock protein stably binds heat-denatured model substrates and can maintain a substrate in a folding-competent state. EMBO J. 1997;16:659–671. doi: 10.1093/emboj/16.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GJ, Vierling E. A small heat shock protein cooperates with heat shock protein 70 systems to reactivate a heat-denatured protein. Plant Physiol. 2000;122:189–198. doi: 10.1104/pp.122.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroux MR, Melki R, Gordon B, Batelier G, Candido EPM. Structure–function studies on small heat shock protein oligomeric assembly and interaction with unfolded polypeptides. J Biol Chem. 1997;272:24646–24656. doi: 10.1074/jbc.272.39.24646. [DOI] [PubMed] [Google Scholar]
- Liang P, Amons R, Clegg JS, MacRae TH. Purification, structure and in vitro molecular chaperone activity of Artemia p26, a small heat-shock/α-crystallin protein. Eur J Biochem. 1997;243:225–232. doi: 10.1111/j.1432-1033.1997.0225a.x. [DOI] [PubMed] [Google Scholar]
- Liang P, MacRae TH. The synthesis of small heat shock/α-crystallin protein in Artemia and its relationship to stress tolerance during development. Dev Biol. 1999;207:445–456. doi: 10.1006/dbio.1998.9138. [DOI] [PubMed] [Google Scholar]
- Lindner RA, Carver JA, and Ehrnsperger M. et al. 2000 Mouse Hsp25, a small heat shock protein. The role of the C-terminal extension in oligomerization and chaperone activity. Eur J Biochem. 267:1923–1932. [DOI] [PubMed] [Google Scholar]
- Lindner RA, Kapur A, Mariani M, Titmuss SJ, Carver JA. Structural alterations of alpha-crystallin during its chaperone action. Eur J Biochem. 1998;258:170–183. doi: 10.1046/j.1432-1327.1998.2580170.x. [DOI] [PubMed] [Google Scholar]
- Marin R, Valet JP, Tanguay RM. Hsp 23 and Hsp 26 exhibit distinct spatial and temporal patterns of constitutive expression in Drosophila adults. Dev Genet. 1993;14:69–77. doi: 10.1002/dvg.1020140109. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Briolay J, Smith L, Diaz-latoud C, Fabre N, Pauli D, Arrigo AP. Analysis of the resistance to heat and hydrogen peroxide stresses in COS cells transiently expressing wild type or deletion mutants of the Drosophila 27-kDa heat-shock protein. Euro J Biochem. 1993;215:277–284. doi: 10.1111/j.1432-1033.1993.tb18032.x. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Coronas V, Ljubic-Thibal V, Ducasse C, Granger L, Jourdan F, Arrigo AP. Small stress protein Hsp27 accumulation during dopamine-mediated differentiation of rat olfactory neurons counteracts apoptosis. Cell Death Differ. 1999;6:227–233. doi: 10.1038/sj.cdd.4400483. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Mehlen A, Godet J, Arrigo AP. Hsp27 as a switch between differentiation and apoptosis in murine embryonic stem cells. J Biol Chem. 1997;272:31657–31665. doi: 10.1074/jbc.272.50.31657. [DOI] [PubMed] [Google Scholar]
- Merck KB, Groenen PJTA, Voorter CEM, de Haard-Hoekman WA, Horwitz J, Bloemendal H, de Jong WW. Structural and functional similarities of bovine α-crystallin and mouse small heat shock protein. J Biol Chem. 1993;268:1046–1052. [PubMed] [Google Scholar]
- Minami Y, Kawasaki H, Minami M, Tanahashi N, Tanaka K, Yahara I. A critical role for the proteasome activator PA28 in the Hsp90-dependent protein refolding. J Biol Chem. 2000;275:9055–9061. doi: 10.1074/jbc.275.12.9055. [DOI] [PubMed] [Google Scholar]
- Mirkes PE, Little SA, Cornel L, Welsh MJ, Laney TNN, Wright FH. Induction of heat shock protein 27 in rat embryos exposed to hyperthermia. Mol Reprod Dev. 1996;45:276–284. doi: 10.1002/(SICI)1098-2795(199611)45:3<276::AID-MRD3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Morrow G, Inaguma Y, Kato K, Tanguay RM. The small heat shock protein Hsp22 of Drosophila melanogaster is a mitochondrial protein displaying oligomeric organization. J Biol Chem. 2000;275:31204–31210. doi: 10.1074/jbc.M002960200. [DOI] [PubMed] [Google Scholar]
- Muchowski PJ, Bassuk JA, Lubsen NH, Clark JI. Human αB-crystallin: small heat shock protein and molecular chaperone. J Biol Chem. 1997;272:2578–2582. doi: 10.1074/jbc.272.4.2578. [DOI] [PubMed] [Google Scholar]
- Nimmesgern E, Hartl FU. ATP-dependent protein refolding activity in reticulocyte lysate. Evidence for the participation of different chaperone components. FEBS Lett. 1993;331:25–30. doi: 10.1016/0014-5793(93)80290-b. [DOI] [PubMed] [Google Scholar]
- Ohan NW, Tam Y, Heikkila JJ. Heat shock-induced assembly of Hsp30 family members into high molecular weight aggregates in Xenopus laevis cultured cells. Comp Biochem Physiol. 1998;119B:381–389. doi: 10.1016/s0305-0491(97)00364-7. [DOI] [PubMed] [Google Scholar]
- Samali A, Robertson JD, and Peterson E. et al. 2001 Hsp27 protects mitochondria of thermotolerant cells against apoptotic stimuli. Cell Stress Chaperones. 6:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smulders RH, Carver JA, Lindner RA, van Boekel MA, Bloemedal H, de Jong WW. Immobilization of the C-terminal extension of bovine αA-crystallin reduces chaperone-like activity. J Biol Chem. 1996;271:29060–29066. doi: 10.1074/jbc.271.46.29060. [DOI] [PubMed] [Google Scholar]
- Smykal P, Hrdy I, Pechan PM. High-molecular-mass complexes formed in vivo contain small Hsps and Hsp70 and display chaperone-like activity. Eur J Biochem. 2000;267:2195–2207. doi: 10.1046/j.1432-1327.2000.01223.x. [DOI] [PubMed] [Google Scholar]
- Stringham EG, Dixon DK, Jones D, Candido EPM. Temporal and spatial expression of patterns of the small heat shock (Hsp16) genes in transgenic Caenorhabditis elegans. Mol Biol Cell. 1992;3:221–233. doi: 10.1091/mbc.3.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier FW, Rosenberg AH, Dunn JJ, Dubendorf JW. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Takemoto L, Emmons T, Horwitz J. The C-terminal region of α-crystallin: involvement in protection against heat-induced denaturation. Biochem J. 1993;294:435–438. doi: 10.1042/bj2940435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam Y, Heikkila JJ. Identification of members of the Hsp30 small heat shock protein family and characterization of their developmental regulation in heat shocked Xenopus laevis embryos. Dev Genet. 1995;17:331–339. doi: 10.1002/dvg.1020170406. [DOI] [PubMed] [Google Scholar]
- Tanguay RM, Wu Y, Khandjian EW. Tissue-specific expression of heat shock proteins of the mouse in the absence of stress. Dev Genet. 1993;14:112–118. doi: 10.1002/dvg.1020140205. [DOI] [PubMed] [Google Scholar]
- Uzawa M, Grams J, Madden B, Toft D, Salisbury JL. Identification of a complex between centrin and heat shock proteins in CSF-arrested Xenopus oocytes and dissociation of the complex following oocyte activation. Dev Biol. 1995;171:51–59. doi: 10.1006/dbio.1995.1259. [DOI] [PubMed] [Google Scholar]
- Veinger L, Diamant S, Buchner J, Goloubinoff P. The small heat-shock protein IbpB from Escherichia coli stabilizes stress-denatured proteins for subsequent refolding by a multichaperone network. J Biol Chem. 1998;273:11032–11037. doi: 10.1074/jbc.273.18.11032. [DOI] [PubMed] [Google Scholar]
- Waters E, Lee G, Veirling E. Evolution, structure and function of the small heat shock proteins in plants. J Exp Biol. 1996;47:325–338. [Google Scholar]