Abstract
Purpose:
To provide quantitative and qualitative image quality metrics and imaging dose for modern Varian On-board Imager (OBI) (ver. 1.5) and Elekta X-ray Volume Imager (XVI) (ver. 4.5R) cone-beam computed tomography (CBCT) systems in a clinical adaptive radiation therapy environment by accounting for varying patient thickness.
Methods:
Image quality measurements were acquired with Catphan 504 phantom (nominal diameter and with additional 10 cm thickness) for OBI and XVI systems and compared to planning CT (pCT) (GE LightSpeed). Various clinical protocols were analyzed for the OBI and XVI systems and analyzed using image quality metrics, including spatial resolution, low contrast detectability, uniformity, and HU sensitivity. Imaging dose measurements were acquired in Wellhofer Scanditronix i'mRT phantom at nominal phantom diameter and with additional 4 cm phantom diameter using GafChromic XRQA2 film. Calibration curves were generated using previously published in-air Air Kerma calibration method.
Results:
The OBI system full trajectory scans exhibited very little dependence on phantom thickness for accurate HU calculation, while half-trajectory scans with full-fan filter exhibited dependence of HU calculation on phantom thickness. The contrast-to-noise ratio (CNR) for the OBI scans decreased with additional phantom thickness. The uniformity of Head protocol scan was most significantly affected with additional phantom thickness. The spatial resolution and CNR compared favorably with pCT, while the uniformity of the OBI system was slightly inferior to pCT. The OBI scan protocol dose levels for nominal phantom thickness at the central portion of the phantom were 2.61, 0.72, and 1.88 cGy, and for additional phantom thickness were 1.95, 0.48, and 1.52 cGy, for the Pelvis, Thorax, and Spotlight protocols, respectively. The XVI system scans exhibited dependence on phantom thickness for accurate HU calculation regardless of trajectory. The CNR for the XVI scans decreased with additional phantom thickness. The uniformity of the XVI scans was significantly dependent on the selection of the proper FOV setting for all phantom geometries. The spatial resolution, CNR, and uniformity for XVI were lower than values measured for pCT. The XVI scan protocol dose levels at the central portion of the phantom for nominal phantom thickness were 2.14, 2.15, and 0.33 cGy, and for additional phantom thickness were 1.56, 1.68, and 0.21 cGy, for the Pelvis M20, Chest M20, and Prostate Seed S10 scan protocols, respectively.
Conclusions:
The OBI system offered comparable spatial resolution and CNR results to the results for pCT. Full trajectory scans with the OBI system need little-to-no correction for HU calculation based on HU stability with changing phantom thickness. The XVI system offered lower spatial resolution and CNR results than pCT. In addition, the HU calculation for all scan protocols was dependent on the phantom thickness. The uniformity for each CBCT system was inferior to that of pCT for each phantom geometry. The dose for each system and scan protocol in the interior of the phantom tended to decrease by approximately 25% with 4 cm additional phantom thickness.
Keywords: cone-beam CT, image quality, imaging dose, IGRT, Gafchromic film dosimetry, adaptive radiotherapy
I. INTRODUCTION
In the decade-plus following the clinical availability of linear accelerator-mounted cone-beam computed tomography (CBCT) systems,1 the use of CBCT as an image guidance tool has increased substantially. The need for volumetric imaging was coupled with the increasingly conformal radiation delivery offered by technologies such as 3D-CRT, IMRT, SBRT, and so on. Of course, three-dimensional imaging modalities such as CBCT offer improved soft tissue visualization as compared to planar imaging, making CBCT an attractive tool for targeting tumors in the abdomen,2,3 pelvis,4 and chest.5–8 The use of CBCT as an IGRT tool allows for reproducible patient setup and precise targeting of soft tissue targets. If multiple scans are acquired during a treatment session, the CBCT images can provide information on the intrafraction motion in addition to the interfraction motion data provided by the pretreatment images.
Currently, many centers use CBCT images for patient setup and target localization verification. However, there is considerable interest in the use of CBCT images for the purposes of adaptive radiation therapy (ART). Studies have looked at the process of ART for several disease sites: prostate,9–11 head and neck,12–14 lung,15,16 and so on. For each site, the use of CBCT images involves some advantages and difficulties. For ART to be feasible and effective, there is a significant need for high quality images with little-to-no correction needed for use in planning. In addition, it is vital that the additional radiation dose delivered to the patient via imaging procedures is kept as low as reasonably achievable (ALARA).
This study quantitatively and qualitatively analyzes the effects of changing phantom thickness on image quality and imaging dose for the latest versions of two clinical CBCT systems: the Varian OBI (ver. 1.5) and Elekta XVI (ver. 4.5R) CBCT systems.
II. METHODS AND MATERIALS
II.A. CBCT systems evaluated
CBCT scans were acquired with two clinical CBCT systems: Varian On-board Imager (OBI ver. 1.5) mounted on a Varian TrueBeam linear accelerator (SN 1176) and Elekta X-ray Volume Imager (XVI ver. 4.5R) mounted on an Elekta Synergy S linear accelerator with Beam Modulator (SN 151412). More detailed descriptions of the CBCT systems have been published for the OBI and XVI systems.17 The protocols used in this study are clinical protocols recommended by the vendor, and fit the following categories: pelvis, chest, head, and small field prostate. For image quality comparison, images were also acquired on a planning CT (pCT) simulator: GE Lightspeed 16 slice (GE Healthcare, Mickleton, NJ). The protocol parameters for the CBCT and pCT systems are shown in Table I.
TABLE I.
Protocol information for the Elekta XVI, Varian OBI, and GE LightSpeed system scans used in this study.
| Protocol | Collimator/filter settings | Field of view (cm) | Matrix size | Pixel size (mm) | Slice thickness (mm) | Tube voltage (kVp) | Total mAs | Frames | Scan angle (deg) |
|---|---|---|---|---|---|---|---|---|---|
| Elekta XVI Pelvis M20 | M20/F1 | 42.64 | 410 × 410 | 1.0 | 2.5 | 120 | 1056 | 660 | 360 |
| Elekta XVI Prostate Seed S10 | S10/F0 | 27.68 | 270 × 270 | 1.0 | 2.5 | 120 | 117.1 | 366 | 200 |
| Elekta XVI Chest M20 | M20/F1 | 42.64 | 410 × 410 | 1.0 | 2.5 | 120 | 1056 | 660 | 360 |
| Elekta XVI Head and Neck S20 | S20/F0 | 27.68 | 270 × 270 | 1.0 | 2.5 | 100 | 36.6 | 366 | 200 |
| Varian OBI Pelvis | Half-Fan | 46.0 | 512 × 512 | 0.90 | 2.5 | 125 | 1056 | 660 | 364 |
| Varian OBI Spotlight | Full-Fan | 26.0 | 512 × 512 | 0.51 | 2.5 | 125 | 1320 | 367 | 200 |
| Varian OBI Thorax | Half-Fan | 46.0 | 512 × 512 | 0.90 | 2.5 | 125 | 264 | 660 | 364 |
| Varian OBI Head | Full-Fan | 26.0 | 512 × 512 | 0.51 | 2.5 | 100 | 264 | 367 | 200 |
| GE LightSpeed Pelvis (Helical Mode) | … | 46.0 | 512 × 512 | 0.90 | 2.5 | 120 | 1056 | 660 | … |
| GE LightSpeed Head (Helical Mode) | … | 26.0 | 512 × 512 | 0.51 | 2.5 | 120 | 117.1 | 366 | … |
II.B. Image quality study
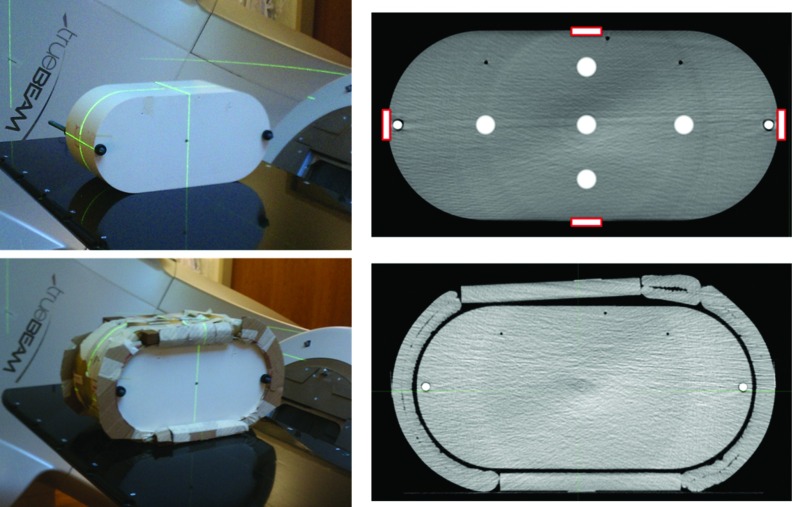
The image quality tests were designed to analyze the following parameters as recommended by Yoo et al.18 including: spatial resolution, low contrast resolution, uniformity, and HU sensitivity. To analyze image quality, the Catphan 504 phantom was used (Phantom Laboratory, Salem, NY). The Catphan 504 phantom is comprised of four modules: CTP528, CTP404, CTP515, and CTP486-2 (see Fig. 1).
FIG. 1.
(a) and (b) Cross-section images of the Catphan 504 phantom at nominal thickness (left, a) and with ring (right, b). (c) Catphan spatial resolution module (CTP528). The line pair objects are oriented in a circular fashion. (d) MTF bead with an indication of the finite bead diameter. (e) Catphan low contrast resolution module (CTP515). The low contrast objects are oriented in a circular fashion. The 1.0% low contrast object ROI is shown on the right and the background ROI is shown on the left. (f) Catphan uniformity module (CTP486-2). ROIs were generated at the center and each of the four cardinal directions to evaluate uniformity. (g) Catphan sensitometry module (CTP404). Each ROI was generated in the center of the insert with an 8 mm diameter. The insert materials are listed adjacent to the insert.
For each CBCT acquisition, images were acquired with and without additional phantom diameter to simulate the varying patient thickness seen clinically. The nominal thickness of the Catphan phantom is 20.5 cm. To increase the phantom diameter, an approximately 5 cm annulus composed of natural soy wax was used, resulting in a total phantom thickness of approximately 30.5 cm [Figs. 1(a) and 1(b)]. For this study, CBCT images acquired without additional thickness will be referred to as “Nominal” and CBCT images acquired with the additional thickness will be referred to as “With Ring.” Each image quality parameter listed below was analyzed for nominal CBCT scans and CBCT scans with ring.
Reporting of both qualitative and quantitative results in this study was done for two reasons. First, quantitative results represent an objective measure of the system's image quality, which has its own inherent value. Second, other investigations of CBCT image quality and many quality assurance (QA) procedures utilize a qualitative estimation of image quality, so the results can also be used for comparison with previously published data and existing clinical QA program data.
II.C. Spatial resolution
The modulation transfer function (MTF) can be used to completely characterize the spatial resolution of an imaging system, and can be determined through the response of the imaging system to a point-source input:
where the point-spread function (PSF) describes the system's response to a point source input.
A home-made script in Matlab (MathWorks, Inc., Natick, Massachusetts) was used to determine the MTF. First, the slice of the Catphan containing the MTF bead was localized and a ROI was generated with the MTF bead at its center. Intensity profiles along the horizontal (circumferential) and vertical (radial) directions were acquired and fit with a Gaussian function. The Fourier transform of this Gaussian curve represents the MTF (see Fig. 2).
FIG. 2.
Process of generating the MTF curve from the MTF bead intensity profile.
The Catphan phantom attempts to mimic a point source input with a finite-sized MTF bead, which has a diameter of 0.28 mm [Fig. 1(d)]. To account for the finite size of the bead, the following correction has been used:19
where J1 is the first-order Bessel function, u is the spatial frequency of interest, and d is the bead diameter. This correction factor was applied to the uncorrected MTF generated from the raw image intensity profile. In short, for each spatial frequency u, the uncorrected MTF(u) was multiplied by CF(u) to obtain the final MTF curve.
To qualitatively assess the spatial resolution of each system, the number of visible line pair objects was used. The corresponding resolution for the smallest visible line pair object was given a MTF value of 10% for comparison with the quantitative MTF curve data.20
II.D. Low contrast detectability
The low contrast detectability of an imaging system refers to the system's ability to accurately identify objects which have intensities close to the background value. To quantitatively assess low contrast detectability, the contrast-to-noise ratio (CNR) was used. For the CNR calculation, the largest 1.0% low contrast insert was used.19 The CNR can be calculated as follows:21,22
where mi is the mean HU value in the LC object ROI, mb is the mean HU value in the adjacent background ROI, σi is the standard deviation of HU values in the LC object ROI, and σb is the standard deviation of HU values in the background ROI [Fig. 1(e)]. The ROIs used for this calculation were averaged over three slices at the longitudinal center of the phantom module.
To qualitatively assess the low contrast resolution, the number of visible low contrast objects (1.0% and 0.5% supra-slice inserts) was used.
II.E. Image uniformity
The image uniformity of an imaging system refers to the system's response to a uniform input. To quantitatively assess the uniformity, the uniformity index (UI) was used. The UI can be calculated as the difference in maximum and minimum mean HU values between five ROIs (1.5 cm diameter) in the Catphan uniformity section (center, north, south, east, and west) [Fig. 1(f)]:
where the ROI mean HU values are averaged over the central three slices of the uniformity module.
To qualitatively assess the image uniformity, intensity profiles were generated across the uniformity module.
II.F. HU sensitivity
The HU sensitivity of an imaging system refers to the system's response to objects of a known electron density. To quantitatively assess HU sensitivity, CT-to-ED curves for the inserts of the Catphan sensitometry module [Fig. 1(g)] were constructed for each system and imaging protocol with and without additional phantom thickness.
II.G. Imaging dose study
Measurements were acquired with GafChromic XRQA2 film to evaluate the imaging dose for each system; further details on the film composition have been published.23–25 The film was scanned using an Epson XL10000 flatbed scanner. The use of this scanner for dosimetric measurements in the diagnostic imaging energy range has been thoroughly investigated.25
The GafChromic XRQA2 film contains high-atomic number constituents which make the film response sensitive to changes in energy. Therefore, a calibration curve must be generated for each tube peak voltage and filtration setting for each CBCT system. Because the film characteristics can vary from batch to batch, calibration curves must also be generated for each new batch of film. The calibration is performed with an instrument which contains a NIST-traceable calibration factor in the kV energy range. For this study, an ADCL-calibrated Farmer chamber (PTW N30010 SN 0444; University of Wisconsin-Madison ADCL, Exp. August, 2013) and electrometer (Cardinal Health C35040 SN 0000099460; University of Wisconsin-Madison ADCL, Exp. October, 2013) was used to measure Air Kerma at isocenter.
The protocol published by Tomic et al. based on Air Kerma-to-Dose calibration24 was used to generate calibration curves for each system. In summary, the Air Kerma is measured with Farmer chamber at isocenter for a wide range of Air Kerma levels. For this study, ten Air Kerma levels were used, ranging from approximately 0.27 cGy to approximately 13 cGy for each system. No buildup cap was used for Air Kerma measurements. The film was placed at isocenter for the same Air Kerma levels (same mAs) as used for the chamber measurements. Some postprocessing of the film data is required to convert the response of the film in pixel values to net reflectance. The details of the calculation of the net reflectance have been published.24
The film was cut into 2 × 3 cm pieces, with a 1 × 1 cm ROI used to evaluate pixel value. All films were handled according to the recommendations of AAPM Task Group 55. Care was taken to ensure consistent and reproducible setup of the films for irradiation and scanning. The film was scanned before and after irradiation to determine the change in pixel value. In addition, a group of three unirradiated films was scanned before and after each irradiation to account for changes in pixel value unrelated to radiation dose. To analyze the response of the film, only the red channel was used, following the recommendations of Devic et al. for measuring dose levels of 0–4 Gy with Gafchromic film.26
For dosimetric measurements, the phantom was composed of the superior-most 15 slices from the Wellhofer Scanditronix i'mRT Phantom (Fig. 3). GafChromic film pieces were placed on the surface and in the interior of the phantom. Previous studies have shown that the uncertainty of the XRQA2 film reaches a stable minimum of about 3%–5% for doses above approximately 3 cGy.23,24 To take advantage of this characteristic of the film, multiple scans were acquired for each scan type to deliver more than 3 cGy to the film when possible, thus reducing the uncertainty in dose measurement as much as possible. To simulate changing patient thickness, Superflab bolus and solid water slabs were used to increase the phantom diameter by 4 cm.
FIG. 3.
Wellhofer Scanditronix phantom used for dosimetric measurements. (Top left) Nominal phantom setup. (Top right) Example CBCT scan with nominal phantom setup and superimposed measurement locations on the surface and interior of the phantom. (Bottom left) Setup with additional phantom thickness. (Bottom right) Example CBCT scan with additional phantom thickness.
III. RESULTS
III.A. Image quality study
The data for each system (OBI and XVI) are reported in Secs. III B through III E. Each measured value represents the average of five scans for each setup and protocol selection.
III.B. Spatial resolution
The MTF was determined for the XVI and OBI systems. The MTF curves for the clinical Head and clinical Pelvis protocols for all imaging systems with nominal phantom thickness are shown below in Fig. 4. In addition, the number of visible line pair objects was also recorded for each system (Table II).
FIG. 4.
MTF data for nominal phantom thickness scans. (Left) MTF curve for the clinical head protocols for GE LightSpeed 16 slice and Varian OBI. The Elekta XVI HN S20 scan did not yield sufficient visualization of the MTF bead. Data points represent qualitative data, with the smallest visible line pair object given a MTF value of 10%, and the error bars representing half the increment of the line pair phantom. (Right) MTF curve for the clinical pelvis protocols for all three imaging systems.
TABLE II.
Qualitative spatial resolution data for the GE LightSpeed 16 slice, Elekta XVI, and Varian OBI systems. The uncertainty in each measurement is half of the smallest increment of the measurement scale (in lp/cm).
| GE | OBI | XVI | ||||||
|---|---|---|---|---|---|---|---|---|
| LP (cm) | LP (cm) | LP (cm) | LP (cm) | LP (cm) | LP (cm) | |||
| Protocol | (nominal) | (with ring) | Protocol | (nominal) | (with ring) | Protocol | (nominal) | (with ring) |
| Head | 8 ± 0.5 | 7 ± 0.5 | Head | 8 ± 0.5 | 7 ± 0.5 | Head and Neck S20 | 3 ± 0.5 | 2 ± 0.5 |
| Pelvis | 6 ± 0.5 | 6 ± 0.5 | Pelvis | 6 ± 0.5 | 5 ± 0.5 | Pelvis M20 | 4 ± 0.5 | 3 ± 0.5 |
| … | … | … | Thorax | 6 ± 0.5 | 5 ± 0.5 | Chest M20 | 4 ± 0.5 | 4 ± 0.5 |
| … | … | … | Spotlight | 8 ± 0.5 | 8 ± 0.5 | Prostate Seed S10 | 4 ± 0.5 | 3 ± 0.5 |
III.C. Low contrast detectability
The contrast-to-noise ratio was determined for each clinical CBCT protocol. In addition, the CNR was determined for the GE Lightspeed for two tube current values (300 and 600 mAs) to approximate the range of low contrast detectability for the conventional CT unit. The CNR results are shown in Fig. 5. The number of visible low contrast objects for each system is shown in Table III.
FIG. 5.
CNR data for each imaging system at nominal Catphan phantom thickness (20.5 cm) and additional phantom thickness (30.5 cm). Error bars represent one standard deviation.
TABLE III.
Qualitative analysis of low contrast detectability for each imaging system. The uncertainty in each measurement is half of the smallest increment of the measurement scale (number of low contrast objects visualized).
| Visual low-contrast object detection | ||||
|---|---|---|---|---|
| Nominal (20.5 cm) | With ring (30.5 cm) | |||
| Protocol | Supra 1.0% visualized | Supra 0.5% visualized | Supra 1.0% visualized | Supra 0.5% visualized |
| Varian OBI Head | 1 ± 0.5 | 0 ± 0.5 | 0 ± 0.5 | 0 ± 0.5 |
| Varian OBI Pelvis | 6 ± 0.5 | 3 ± 0.5 | 1 ± 0.5 | 0 ± 0.5 |
| Varian OBI Thorax | 4 ± 0.5 | 0 ± 0.5 | 0 ± 0.5 | 0 ± 0.5 |
| Varian OBI Spotlight | 5 ± 0.5 | 1 ± 0.5 | 0 ± 0.5 | 0 ± 0.5 |
| Elekta XVI HN S20 | 0 ± 0.5 | 0 ± 0.5 | 0 ± 0.5 | 0 ± 0.5 |
| Elekta XVI Pelvis M20 | 4 ± 0.5 | 0 ± 0.5 | 0 ± 0.5 | 0 ± 0.5 |
| Elekta XVI Chest M20 | 4 ± 0.5 | 0 ± 0.5 | 0 ± 0.5 | 0 ± 0.5 |
| Elekta XVI PS S10 | 1 ± 0.5 | 0 ± 0.5 | 0 ± 0.5 | 0 ± 0.5 |
| GE Head | 4 ± 0.5 | 2 ± 0.5 | 0 ± 0.5 | 0 ± 0.5 |
| GE Pelvis (300 mAs) | 6 ± 0.5 | 4 ± 0.5 | 2 ± 0.5 | 0 ± 0.5 |
| GE Pelvis (600 mAs) | 8 ± 0.5 | 5 ± 0.5 | 3 ± 0.5 | 0 ± 0.5 |
III.D. Image uniformity
The uniformity index was determined for each imaging system (Fig. 6, left). In addition, profiles across the uniformity slice were generated for visual representation of the data (Fig. 6, right).
FIG. 6.
(Left) Uniformity index for each system, including results for nominal Catphan phantom thickness (20.5 cm) and results with additional phantom thickness (30.5 cm total thickness). The error bars represent the standard deviation. (Right) Horizontal intensity profiles through the uniformity module (CTP486-2) of the Catphan phantom. The OBI CBCT system is susceptible to ring artifacts in both geometries, while the XVI system exhibits cupping artifact in the nominal scan.
III.E. HU sensitivity
The HU sensitivity data are presented in two ways: CT-to-ED curves for the each system and protocol with nominal thickness (Fig. 7), and comparing CT number calculation for each imaging system with varying phantom thickness (Fig. 8).
FIG. 7.
HU sensitivity data for each system with nominal phantom thickness: CT-to-ED curves have been constructed for each scan protocol. (Left) Varian OBI system. (Middle) Elekta XVI system. (Right) GE Lightspeed scanner.
FIG. 8.
HU sensitivity for each imaging system and scan protocol with varying phantom thickness. (Left) Varian OBI system. (Middle) Elekta XVI system. (Right) GE Lightspeed 16 slice scanner. Error bars represent one standard deviation.
For the OBI system, the average absolute value difference in CT number between the nominal phantom thickness and additional phantom thickness scans was (averaged over all inserts): 8.26 HU (Pelvis), 33.39 HU (Thorax), 178.98 HU (Head), and 108.20 HU (Spotlight). For the XVI system, the average absolute value difference in CT number between the nominal phantom thickness and additional phantom thickness scans was: 120.33 HU (Pelvis M20), 119.93 HU (Chest M20), 329.72 HU (Head and Neck S20), and 204.24 HU (Prostate Seed S10). For the GE LightSpeed scanner, the average absolute value difference in CT number between the nominal phantom thickness and additional phantom thickness scans was: 16.00 HU (Pelvis) and 19.85 HU (Head).
The OBI full trajectory scans (Pelvis and Thorax) show very little deviation in HU sensitivity. For half trajectory scans with the OBI system, such as the Head and Spotlight protocols in this study, there seems to be some deviations in HU value with varying patient thickness. This deviation in HU value does not improve by scanning in full trajectory mode; in fact, the deviation between calculated HU values for the nominal and additional thickness scans was larger with the full trajectory mode as compared to the half trajectory mode for OBI Head and OBI Spotlight (Fig. 9).
FIG. 9.
Comparison of half trajectory scans at nominal thickness and with ring to full trajectory scans with ring for OBI Head protocol (left) and OBI Spotlight Protocol (right). Error bars represent one average standard deviation.
III.F. Imaging dose study
Imaging dose was measured using three protocol types (clinical pelvis, clinical chest, and small FOV prostate protocols) for each CBCT system. Measurements were acquired to convert the pixel value of the film to dose and a calibration curve was generated. The previous study utilizing GafChromic XRQA2 film23 found minimal differences between the half-fan and full-fan calibration curves for 125 kVp setting on the Varian OBI system, so only one calibration curve was generated for this kVp setting. Otherwise, calibration curves were generated for each unique tube voltage and filtration setting. The calibration curves are shown in Fig. 10.
FIG. 10.
Calibration curves for each CBCT system.
For each scan protocol, imaging dose was measured at three locations: four points on the surface of the phantom (anterior, right, left, posterior), and five points on the interior of the phantom (central, anterior, right, left, posterior). The data for all measurement points are shown in Table IV (OBI) and Table V (XVI).
TABLE IV.
Imaging dose data for the Varian OBI system. Measurements were acquired for the full trajectory Pelvis protocol, full trajectory Thorax protocol, and half trajectory Spotlight protocol.
| Varian OBI | |||||||
|---|---|---|---|---|---|---|---|
| Pelvis | Thorax | Spotlight | |||||
| Additional 4 cm | Nominal | Additional 4 cm | Additional 4 cm | ||||
| Location | Nominal thickness(cGy) | diameter (cGy) | thickness(cGy) | diameter (cGy) | Nominal thickness(cGy) | diameter (cGy) | |
| Interior | Anterior | 2.73 ± 0.11 | 1.91 ± 0.08 | 0.66 ± 0.03 | 0.48 ± 0.02 | 0.92 ± 0.04 | 0.78 ± 0.03 |
| Posterior | 2.69 ± 0.11 | 2.07 ± 0.08 | 0.66 ± 0.03 | 0.48 ± 0.02 | 3.27 ± 0.13 | 2.67 ± 0.11 | |
| Left | 1.87 ± 0.08 | 1.42 ± 0.06 | 0.46 ± 0.02 | 0.33 ± 0.02 | 1.48 ± 0.06 | 1.10 ± 0.04 | |
| Right | 1.87 ± 0.08 | 1.50 ± 0.06 | 0.52 ± 0.02 | 0.37 ± 0.02 | 1.78 ± 0.07 | 1.56 ± 0.06 | |
| Center | 2.61 ± 0.11 | 1.95 ± 0.08 | 0.72 ± 0.03 | 0.48 ± 0.02 | 1.88 ± 0.06 | 1.52 ± 0.06 | |
| Surface | Anterior | 2.93 ± 0.12 | 2.46 ± 0.10 | 0.72 ± 0.03 | 0.56 ± 0.02 | 0.42 ± 0.02 | 0.22 ± 0.02 |
| Posterior | 2.94 ± 0.12 | 2.32 ± 0.09 | 0.71 ± 0.03 | 0.56 ± 0.02 | 3.88 ± 0.16 | 3.21 ± 0.10 | |
| Left | 1.68 ± 0.07 | 1.48 ± 0.09 | 0.40 ± 0.02 | 0.39 ± 0.02 | 1.07 ± 0.04 | 0.64 ± 0.02 | |
| Right | 1.60 ± 0.07 | 1.51 ± 0.06 | 0.42 ± 0.02 | 0.39 ± 0.02 | 1.79 ± 0.07 | 1.79 ± 0.07 | |
TABLE V.
Imaging dose data for the Elekta XVI system. Measurements were acquired for the full rotation Pelvis M20 protocol, full rotation Chest M20 protocol, and partial rotation Prostate Seed S10 protocol.
| Elekta XVI | |||||||
|---|---|---|---|---|---|---|---|
| Pelvis M20 | Chest M20 | Prostate Seed S10 | |||||
| Additional 4 cm | Additional 4 cm | Additional 4 cm | |||||
| Location | Nominal thickness (cGy) | diameter (cGy) | Nominal thickness (cGy) | diameter (cGy) | Nominal thickness (cGy) | diameter (cGy) | |
| Interior | Anterior | 2.32 ± 0.12 | 1.85 ± 0.07 | 2.49 ± 0.13 | 2.00 ± 0.10 | 0.45 ± 0.03 | 0.33 ± 0.03 |
| Posterior | 2.34 ± 0.12 | 1.76 ± 0.06 | 2.44 ± 0.12 | 1.80 ± 0.09 | 0.29 ± 0.03 | 0.17 ± 0.02 | |
| Left | 1.65 ± 0.08 | 1.40 ± 0.05 | 1.63 ± 0.08 | 1.43 ± 0.07 | 0.48 ± 0.03 | 0.34 ± 0.03 | |
| Right | 1.92 ± 0.10 | 1.54 ± 0.06 | 1.92 ± 0.10 | 1.56 ± 0.08 | 0.21 ± 0.02 | 0.14 ± 0.01 | |
| Center | 2.14 ± 0.11 | 1.56 ± 0.06 | 2.15 ± 0.11 | 1.68 ± 0.08 | 0.33 ± 0.03 | 0.21 ± 0.02 | |
| Surface | Anterior | 2.73 ± 0.14 | 2.21 ± 0.11 | 2.41 ± 0.12 | 2.22 ± 0.11 | 0.66 ± 0.03 | 0.65 ± 0.03 |
| Posterior | 2.57 ± 0.13 | 2.17 ± 0.11 | 2.74 ± 0.14 | 2.15 ± 0.11 | 0.33 ± 0.03 | 0.30 ± 0.03 | |
| Left | 1.50 ± 0.08 | 1.49 ± 0.08 | 1.64 ± 0.08 | 1.48 ± 0.07 | 0.55 ± 0.03 | 0.50 ± 0.03 | |
| Right | 1.71 ± 0.09 | 1.72 ± 0.09 | 1.66 ± 0.08 | 1.80 ± 0.07 | 0.02 ± 0.01 | 0.01 ± 0.01 | |
IV. DISCUSSION
This study addressed an important question in imaging using clinical CBCT systems: how are the image quality and dosimetric characteristics of clinical CBCT protocols for the OBI and XVI systems affected with changes in patient thickness? The measurements acquired to investigate this question also served as a comparison between the Varian OBI and Elekta XVI CBCT systems. Of course, the performance of each system provides a commentary on the system's appropriateness for use in conjunction with adaptive planning.
The image quality characteristics of the OBI and XVI systems were compared to those of a helical CT scanner used for clinical treatment planning. The OBI system offered comparable spatial resolution to the GE Lightspeed scanner for both small (∼26 cm) and large (∼46 cm) FOV settings. This is mainly attributable to the small detector size (approximately 194 μm spacing between elements) of the OBI flat panel array, as well as the similar image pixel size to the GE scanner (0.51 mm for Head protocols, 0.90 mm for Pelvis protocols). The XVI system offered less spatial resolution for small (27 cm) and large (41 cm) FOV settings. This is most likely due to the relatively larger detector size (400 μm spacing between elements) and pixel size (approximately 1 mm for each protocol regardless of FOV), in addition to the effects of the reconstruction filter (Hamming filter) for XVI CBCT images. Of the XVI scan protocols, only the Pelvis M20 and Chest M20 protocols allowed for adequate visualization of the MTF bead, which limited the spatial resolution analysis of the other protocols to a qualitative estimation. We believe the MTF bead was not well-visualized in the other protocols due to the absence of the bowtie filter for the Head and Neck S20 and Prostate Seed S10 scan protocols; other studies have noted the improvement in image quality with the use of the bowtie filter for the XVI system.27 The higher spatial resolution of the OBI system as compared to the XVI system can be attributed to the smaller detector and pixel size, use of a scatter grid, and the use of Ram-Lak reconstruction filter. Both CBCT systems tended to exhibit slightly lower spatial resolution characteristics with increasing phantom thickness. The additional phantom thickness in this experimental setup tended to decrease the spatial resolution by approximately 1 lp/cm.
The contrast-to-noise ratio (CNR) was used to evaluate the low contrast detectability of each system. The low contrast detectability of an imaging system is vital for ART, since it characterizes the system's ability to provide soft tissue contrast (e.g., the contrast between the target/OAR and surrounding tissue). The CNR as calculated here depends on two factors: the contrast between the two ROIs and the noise present in the ROIs. The contrast between the ROIs is largely determined by the system's ability to eliminate scattered radiation, while the noise is connected to the number of photons detected by the imaging panel. Since more photons are attenuated by the phantom with additional thickness, the relative noise increased and the CNR decreased for each system with increasing phantom thickness. This decrease in CNR was also reflective of degradation in the visibility of the low contrast objects with additional phantom thickness. With additional phantom thickness, only the GE LightSpeed scanner and OBI CBCT system allowed for visualization of the low-contrast objects. Overall, there was significant degradation in the detection of low contrast objects with additional phantom thickness, even in the GE LightSpeed scanner.
We also found that CNR and low-contrast object visibility do not necessarily give the same characterization of the imaging system's low-contrast detectability. For instance, both CBCT systems exhibited a larger CNR value than images with the 300 mAs planning CT scan acquisition, though the planning CT acquisition allowed for better visualization of the 1.0% and 0.5% low-contrast objects when compared to the OBI and XVI CBCT images. There are multiple reasons for this disagreement between metrics. First, the CNR is calculated using the largest 1.0% low-contrast object, which means that the CNR value does not contain information about smaller low-contrast objects in the phantom. Second, the nonuniformities present in CBCT images, of which ring and cupping artifacts are the most prominent, do not tend to affect the CNR calculation since the low-contrast and background ROI are in close proximity. However, the CBCT image nonuniformities do limit the visibility of smaller low-contrast inserts in the phantom. The cupping artifact in the nominal XVI images severely limited the visibility of the low-contrast objects.
The uniformity index (UI) was used to evaluate the uniformity of each system. The OBI system exhibited less nonuniformity than the XVI system, even with additional phantom thickness. The XVI system appears to be sensitive to the appropriate choice of FOV: the large FOV scan protocols (such as Pelvis M20 and Chest M20) exhibited improved uniformity with additional phantom thickness. For both the OBI and XVI systems, the uniformity of the clinical Head protocol was degraded with additional phantom thickness. For the OBI system, the relatively large UI value for the Head protocol scan with additional phantom thickness may be attributable to the low tube voltage used (100 kVp). Overall, the slight nonuniformities in the 125 kVp OBI images seemed to result from ring artifacts, while cupping or capping was kept to a minimum. However, the cupping in the XVI images was the most significant contribution to image nonuniformity, particularly when the phantom size did not match the FOV selection.
The accurate calculation of CT number was investigated for both CBCT systems. The HU calculation with varying patient thickness for the OBI full trajectory scans with half-fan filter (i.e., OBI Pelvis and Thorax protocols) was on par with that of pCT; little-to-no corrections for HU calculation are needed for these scan protocols. The OBI half trajectory scans with full-fan filter (i.e., OBI Head and Spotlight protocols) were found to be dependent on patient thickness for HU calculation. The HU calculation for the XVI system exhibited moderate-to-severe dependence on multiple scan parameters, including selection of scan protocol and varying patient thickness. The XVI scan protocols which utilized a bowtie filter (XVI Pelvis M20 and Chest M20 protocols) exhibited less dependence on patient thickness than the XVI scan protocols without bowtie filter (XVI Head and Neck S20 and Prostate Seed S10). Another study has proposed HU calculation corrections,28 which include the use of the planning CT HU data for certain regions of the patient anatomy and the surrounding air to generate a patient-specific CT-to-ED curve.
The accuracy of CBCT HU calculation has been investigated in a variety of settings.27–33 The most obvious use of HU values in radiation therapy is for dose calculation with kernel-based algorithms. On this note, previous studies have investigated the impact of CT number value variability on dose calculation using planning CT and CBCT.34–36 In addition, intensity-based deformable registration algorithms also use HU values. The additional uncertainty associated with variable HU calculation for these intensity-based algorithms warrants further investigation. Overall, the OBI system appears to be well-suited for intensity based algorithms with HU calculation that is not dependent on changes in patient thickness for full trajectory scans with half-fan bowtie filter.
Other studies23,24 have noted the decrease in imaging dose for a given scan protocol with each new version of the system. Our measurements with XRQA2 film agree with the previous study using Rando phantom for imaging dose measurements for the OBI and XVI systems.22 We also noted a decrease in imaging dose with increased patient thickness. This decrease in imaging dose with increased patient thickness was more pronounced for the measurement points in the interior of the phantom, though the imaging dose generally decreased for all measurement locations and imaging protocols with additional patient thickness.
Though the image quality was degraded to some degree with additional patient thickness for both CBCT systems, the imaging dose also decreased with additional phantom thickness. The low contrast detectability is arguably the most important parameter for visualization of anatomy on CT images. The low contrast detectability is related to the amount of noise in the image, which is related to the number of photons counted during acquisition. When increasing the tube current for OBI Pelvis protocols from the nominal value of 1064 mAs to the maximum programmable value of 1480 mAs, we noticed an increase in CNR with ring from 0.94 ± 0.10 to 1.18 ± 0.11. Thus, it may be feasible to increase the mAs for larger patients; this would maintain the same approximate dose levels for patients of various sizes, while improving image quality for larger patients.
V. CONCLUSIONS
The Varian OBI system provided similar spatial resolution and CT number accuracy to pCT. The uniformity and low-contrast detectability were slightly slightly lower for the OBI system as compared to planning CT. As evidenced by the HU calculation results with changing phantom thickness, virtually no corrections are needed for large FOV full-trajectory scans with the OBI system for use with dose calculation or adaptive planning. The CNR and spatial resolution decreased with increased phantom thickness. The average dose for the OBI protocols in the interior of the phantom decreased by 24%, 29%, and 18%, for the Pelvis, Thorax, and Spotlight protocols, respectively, with additional phantom thickness of 4 cm.
The Elekta XVI system provided lower spatial resolution, low-contrast detectability, HU calculation accuracy, and uniformity as compared to pCT measured values. The XVI system exhibited strong dependence on several factors for HU calculation, including protocol selection and patient thickness. It was also observed that the uniformity of the XVI system was strongly dependent on the correct choice of FOV. The visibility of low-contrast objects for the XVI system in the Catphan phantom was limited mainly by the cupping artifact in the image. The CNR and spatial resolution decreased with increased phantom thickness. The dose for the XVI system was lower than the OBI system for the pelvis and small FOV prostate scans. The average dose for the XVI protocols in the interior of the phantom decreased by 21%, 20%, and 33%, for the PelvisM20, ChestM20, and Prostate SeedS10 protocols, respectively, with additional phantom thickness of 4 cm.
ACKNOWLEDGMENTS
This work was carried out at Thomas Jefferson University Hospital and was funded, in part, under a grant with the Pennsylvania Department of Health. The Department of Health specifically declaims responsibility for any analyses, interpretations, or conclusions.
REFERENCES
- 1. Jaffray D. A. and Siewerdsen J., “Cone-beam computed tomography with a flat-panel imager: Initial performance characterization,” Med. Phys. 27, 1311–1323 (2000). 10.1118/1.599009 [DOI] [PubMed] [Google Scholar]
- 2. Zhong R., Wang J., Jiang X., He Y., Zhang H., Chen N., Bai S., and Xu F., “Hypofraction radiotherapy of liver tumor using cone beam computed tomography guidance combined with active breath control by long breath-holding,” Radiot. Oncol. 104, 379–385 (2012). 10.1016/j.radonc.2011.11.007 [DOI] [PubMed] [Google Scholar]
- 3. Nakagawa K., Yamashita H., Shiraishi K., Igaki H., Terahara A., Nakamura N., Ohtomo K., Saegusa S., Shiraki T., and Oritate T., “Verification of in-treatment tumor position using kilovoltage cone-beam computed tomography: A preliminary study,” Int. J. Radiat. Oncol., Biol., Phys. 69, 970–973 (2007). 10.1016/j.ijrobp.2007.08.026 [DOI] [PubMed] [Google Scholar]
- 4. Smitsmans M. H., De Bois J., Sonke J., Betgen A., Zijp L. J., Jaffray D. A., Lebesque J. V., and van Herk M., “Automatic prostate localization on cone-beam CT scans for high precision image-guided radiotherapy,” Int. J. Radiat. Oncol., Biol., Phys. 63, 975–984 (2005). 10.1016/j.ijrobp.2005.07.973 [DOI] [PubMed] [Google Scholar]
- 5. Purdie T. G., Moseley D. J., Bissonnette J., Sharpe M. B., Franks K., Bezjak A., and Jaffray D. A., “Respiration correlated cone-beam computed tomography and 4DCT for evaluating target motion in stereotactic lung radiation therapy,” Acta Oncol. 45, 915–922 (2006). 10.1080/02841860600907345 [DOI] [PubMed] [Google Scholar]
- 6. Sonke J., Rossi M., Wolthaus J., van Herk M., Damen E., and Belderbos J., “Frameless stereotactic body radiotherapy for lung cancer using four-dimensional cone beam CT guidance,” Int. J. Radiat. Oncol., Biol., Phys. 74, 567–574 (2009). 10.1016/j.ijrobp.2008.08.004 [DOI] [PubMed] [Google Scholar]
- 7. Wang Z., Wu Q. J., Marks L. B., Larrier N., and Yin F., “Cone-beam CT localization of internal target volumes for stereotactic body radiotherapy of lung lesions.” Int. J. Radiat. Oncol., Biol., Phys. 69, 1618–1624 (2007). 10.1016/j.ijrobp.2007.08.030 [DOI] [PubMed] [Google Scholar]
- 8. Bissonnette J., Purdie T. G., Higgins J. A., Winnie L., and Bezjak A., “Cone-beam computed tomographic image guidance for lung cancer radiation therapy,” Int. J. Radiat. Oncol., Biol., Phys. 73, 927–934 (2009). 10.1016/j.ijrobp.2008.08.059 [DOI] [PubMed] [Google Scholar]
- 9. Dong L., Lee A. K., Cheung R., Bonnen M. D., O’Daniel J., Wang H., Mohan R., and Kuban D., “An automatic CT-guided adaptive radiation therapy technique by online modification of multileaf collimator leaf positions for prostate cancer,” Int. J. Radiat. Oncol., Biol., Phys. 62, 154–163 (2005). 10.1016/j.ijrobp.2004.09.045 [DOI] [PubMed] [Google Scholar]
- 10. Wu Q. J., Thongphiew D., Wang Z., Mathayomchan B., Chankong V., Yoo S., Lee W. R., and Yin F., “On-line re-optimization of prostate IMRT plans for adaptive radiation therapy,” Phys. Med. Biol. 53, 673–691 (2008). 10.1088/0031-9155/53/3/011 [DOI] [PubMed] [Google Scholar]
- 11. Ghilezan M., Yan D., and Martinez A., “Adaptive radiation therapy for prostate cancer,” paper presented at Seminars in Radiation Oncology, 20(2), 130–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang T., Chi Y., Meldolesi E., and Yan D., “Automatic delineation of on-line head-and-neck computed tomography images: Toward on-line adaptive radiotherapy,” Int. J. Radiat. Oncol., Biol., Phys. 68, 522–530 (2007). 10.1016/j.ijrobp.2007.01.038 [DOI] [PubMed] [Google Scholar]
- 13. Castadot P., Lee J. A., Parraga A., Geets X., Macq B., and Grégoire V., “Comparison of 12 deformable registration strategies in adaptive radiation therapy for the treatment of head and neck tumors,” Radiother. Oncol. 89, 1–12 (2008). 10.1016/j.radonc.2008.04.010 [DOI] [PubMed] [Google Scholar]
- 14. Wu Q., Chi Y., Chen P. Y., Krauss D. J., Yan D., and Martinez A., “Adaptive replanning strategies accounting for shrinkage in head and neck IMRT,” Int. J. Radiat. Oncol., Biol., Phys. 75, 924–932 (2009). 10.1016/j.ijrobp.2009.04.047 [DOI] [PubMed] [Google Scholar]
- 15. Sonke J. and Belderbos J., “Adaptive radiotherapy for lung cancer,” presented at Seminars in Radiation Oncology, 20(2), 94–106. [DOI] [PubMed] [Google Scholar]
- 16. Ramsey C. R., Langen K. M., Kupelian P. A., Scaperoth D. D., Meeks S. L., Mahan S. L., and Seibert R. M., “A technique for adaptive image-guided helical tomotherapy for lung cancer,” Int. J. Radiat. Oncol., Biol., Phys. 64, 1237–1244 (2006). 10.1016/j.ijrobp.2005.11.012 [DOI] [PubMed] [Google Scholar]
- 17. Song W. Y., Kamath S., Ozawa S., Al Ani S., Chvetsov A., Bhandare N., Palta J. R., Liu C., and Li J. G., “A dose comparison study between XVI and OBI CBCT systems,” Med. Phys. 35, 480–486 (2008). 10.1118/1.2825619 [DOI] [PubMed] [Google Scholar]
- 18. Yoo S., Kim G., Hammoud R., Elder E., Pawlicki T., Guan H., Fox T., Luxton G., Yin F., and Munro P., “A quality assurance program for the on-board imager,” Med. Phys. 33, 4431–47 (2006). 10.1118/1.2362872 [DOI] [PubMed] [Google Scholar]
- 19. Nickoloff E. L., “Measurement of the PSF for a CT scanner: Appropriate wire diameter and pixel size,” Phys. Med. Biol. 33, 149–155 (1988). 10.1088/0031-9155/33/1/014 [DOI] [PubMed] [Google Scholar]
- 20. Garayoa J. and Castro P., “A study on image quality provided by a kilovoltage cone-beam computed tomography,” J. Appl. Clin. Med. Phys. 14, 239–57 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bissonnette J., Moseley D. J., and Jaffray D. A., “A quality assurance program for image quality of cone-beam CT guidance in radiation therapy,” Med. Phys. 35, 1807–1815 (2008). 10.1118/1.2900110 [DOI] [PubMed] [Google Scholar]
- 22. Stock M., Pasler M., Birkfellner W., Homolka P., Poetter R., and Georg D., “Image quality and stability of image-guided radiotherapy (IGRT) devices: A comparative study,” Radiot. Oncol. 93, 1–7 (2009). 10.1016/j.radonc.2009.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Giaddui T., Cui Y., Galvin J., Yu Y., and Xiao Y., “Comparative dose evaluations between XVI and OBI cone beam CT systems using Gafchromic XRQA2 film and nanoDot optical stimulated luminescence dosimeters,” Med. Phys. 40, 062102 (12pp.) (2013). 10.1118/1.4803466 [DOI] [PubMed] [Google Scholar]
- 24. Tomic N., Devic S., DeBlois F., and Seuntjens J., “Reference radiochromic film dosimetry in kilovoltage photon beams during CBCT image acquisition,” Med. Phys. 37, 1083–1092 (2010). 10.1118/1.3302140 [DOI] [PubMed] [Google Scholar]
- 25. Giaddui T., Cui Y., Galvin J., Chen W., Yu Y., and Xiao Y., “Characteristics of Gafchromic XRQA2 films for kV image dose measurement,” Med. Phys. 39, 842–850 (2012). 10.1118/1.3675398 [DOI] [PubMed] [Google Scholar]
- 26. Devic S., Tomic N., Soares C., and Podgorsak E., “Optimizing the dynamic range extension of a radiochromic film dosimetry system,” Med. Phys. 36, 429–437 (2009). 10.1118/1.3049597 [DOI] [PubMed] [Google Scholar]
- 27. Mail N., Moseley D., Siewerdsen J., and Jaffray D., “The influence of bowtie filtration on cone-beam CT image quality,” Med. Phys. 36, 22–32 (2009). 10.1118/1.3017470 [DOI] [PubMed] [Google Scholar]
- 28. Richter A., Hu Q., Steglich D., Baier K., Wilbert J., Guckenberger M., and Flentje M., “Investigation of the usability of conebeam CT data sets for dose calculation,” Radiat. Oncol. 3, 42–54 (2008). 10.1186/1748-717X-3-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chan M., Yang J., Song Y., Burman C., Chan P., and Li S., “Evaluation of imaging performance of major image guidance systems,” Biomed. Imaging Interv. J 7, 1–7 (2011). 10.2349/biij.7.2.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hatton J., McCurdy B., and Greer P. B., “Cone beam computerized tomography: The effect of calibration of the Hounsfield unit number to electron density on dose calculation accuracy for adaptive radiation therapy,” Phys. Med. Biol. 54, N329–N346 (2009). 10.1088/0031-9155/54/15/N01 [DOI] [PubMed] [Google Scholar]
- 31. Seet K. Y., Barghi A., Yartsev S., and Van Dyk J., “The effects of field-of-view and patient size on CT numbers from cone-beam computed tomography,” Phys. Med. Biol. 54, 6257–6262 (2009). 10.1088/0031-9155/54/20/014 [DOI] [PubMed] [Google Scholar]
- 32. Jarry G., Graham S. A., Moseley D. J., Jaffray D. J., Siewerdsen J. H., and Verhaegen F., “Characterization of scattered radiation in kV CBCT images using Monte Carlo simulations,” Med. Phys. 33, 4320–4329 (2006). 10.1118/1.2358324 [DOI] [PubMed] [Google Scholar]
- 33. Siewerdsen J. H. and Jaffray D. A., “Optimization of x-ray imaging geometry (with specific application to flat-panel cone-beam computed tomography),” Med. Phys. 27, 1903–1914 (2000). 10.1118/1.1286590 [DOI] [PubMed] [Google Scholar]
- 34. Ding G. X., Duggan D. M., Coffey C. W., Deeley M., Hallahan D. E., Cmelak A., and Malcolm A., “A study on adaptive IMRT treatment planning using kV cone-beam CT.” Radiot. Oncol. 85, 116–125 (2007). 10.1016/j.radonc.2007.06.015 [DOI] [PubMed] [Google Scholar]
- 35. Yoo S. and Yin F. F., “Dosimetric feasibility of cone-beam CT-based treatment planning compared to CT-based treatment planning.” Int. J. Radiat. Oncol., Biol., Phys. 66, 1553–1561 (2006). 10.1016/j.ijrobp.2006.08.031 [DOI] [PubMed] [Google Scholar]
- 36. Lee L., Le Q., and Xing L., “Retrospective IMRT dose reconstruction based on cone-beam CT and MLC log-file,” Int. J. Radiat. Oncol. Biol. Phys. 70, 634–644 (2008). 10.1016/j.ijrobp.2007.09.054 [DOI] [PubMed] [Google Scholar]