Abstract
Stressful stimuli can elicit 2 distinct reactive cellular responses, the heat shock (stress) response and the activation of cell death pathways. Most studies on the effects of hyperthermia on the mammalian nervous system have focused on the heat shock response, characterized by the transient induction of Hsps, which play roles in repair and protective mechanisms. This study examines the effect of hyperthermia on the induction of cell death via apoptosis, assayed by terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling and active caspase 3 cytochemistry, in the adult rat brain, testis, and thymus. Results show that a fever-like increase in temperature triggered apoptosis in dividing cell populations of testis and thymus, but not in mature, postmitotic cells of the adult cerebellum. These differential apoptotic responses did not correlate with whole-tissue levels of Hsp70 induction. We further investigated whether dividing neural cells were more sensitive to heat-induced apoptosis by examining the external granule cell layer of the cerebellum at postnatal day 7 and the neuroepithelial layers of the neocortex and tectum at embryonic day 17. These proliferative neural regions were highly susceptible to hyperthermia-induced apoptosis, suggesting that actively dividing cell populations are more prone to cell death induced by hyperthermia than fully differentiated postmitotic neural cells.
INTRODUCTION
Studies in tissue culture cells have established 2 types of reactive cellular responses to stress, termed the heat shock (stress) response and the cell death program (Samali and Orrenius 1998). In the heat shock response, a range of stressful stimuli activate the transient induction of a set of genes encoding Hsps, while ongoing gene expression is down-regulated (Lindquist and Craig 1988; Pardue et al 1992). These induced proteins play a role in cellular repair mechanisms by functioning as molecular chaperones, mediating the refolding or degradation of stress-damaged proteins, and thus promoting cell recovery (Georgopoulos and Welch 1993; Parsell and Lindquist 1993; Morimoto et al 1994). The accumulated Hsps also play a role in protective mechanisms by increasing cellular resistance to a subsequent stress, a phenomenon known as thermotolerance (Lowenstein et al 1991; Mailhos et al 1993, 1994; Li et al 1995; De Maio 1999).
In the cell death program, cells induce signaling pathways involving the coordinate action of multiple kinases and cysteine proteases, known as caspases, which cleave various target substrates, bringing about the cell's own demise (Dorstyn et al 1998; Earnshaw et al 1999; Wolf and Green 1999). This mode of programmed cell death is termed apoptosis, a genetically controlled suicide mechanism that does not produce an inflammatory response and is therefore considered a tidy method of cell elimination. One of the characteristic morphological changes associated with apoptosis is the cleavage of DNA, which can be detected in situ by the terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate (dUTP) nick-end labeling (TUNEL) method (Gavrieli et al 1992). Apoptosis is distinct from necrosis, which is a pathological form of cell death in response to extreme trauma or environmental disruption. Morphological alterations that are associated with necrosis include cell swelling, degeneration of organelles, membrane disruption, and cell lysis, causing inflammation (Buja et al 1993).
Previous studies in this laboratory have established that a physiologically relevant increase in body temperature is sufficient to activate the cellular stress response in the mammalian brain, such that populations of neural cells induce the heat shock proteins Hsp70, 32, and 27, whereas the levels of constitutive Hsps do not change (Brown 1994; Brown and Sharp 1999; Bechtold and Brown 2000). Our recent work has found that these inducible Hsps associate with synaptic elements where they may facilitate the repair of stress-induced damage and contribute to neuroprotective events at the synapse (Bechtold and Brown 2000; Bechtold et al 2000). Our collaborative studies demonstrate the protective effect of a prior heat shock on synaptic transmission from subsequent stress, at the Drosophila neuromuscular junction (Karunanithi et al 1999).
We now wish to investigate whether a physiologically relevant increase in body temperature activates the cell death program in neural cells of the adult rat brain. Cells in the adult brain are postmitotic and fully differentiated, whereas cells in other body tissues such as the testis and the thymus undergo cell division and differentiation processes throughout life (Westermann et al 1989; Russell et al 1990; Alam et al 1997). In addition, we explore if the effect of hyperthermia on cell death in the brain changes during development. That is, whether dividing neural cells are more prone to hyperthermia-induced cell death than fully differentiated, postmitotic neural cells.
Very few studies have examined the effect of hyperthermia on the mammalian brain with respect to the induction of cell death. Hyperthermia is a physiologically relevant phenomenon as clinical studies have demonstrated the deleterious effects of fever in young children and the teratogenic effects of hyperthermia during early embryonic development (Graham et al 1998). Even slight temperature elevations at crucial developmental stages can cause neural defects resulting in brain malformations (Edwards et al 1974, 1995; Walsh et al 1991; Mirkes et al 1997; Breen et al 1999). Adult, postnatal day 7 (P7), and embryonic day 17 (E17) rat brains have been selected for the present study, as representative stages of development, to assess the impact of a fever-like temperature shock on neural cell death in vivo.
MATERIALS AND METHODS
Induction of hyperthermia
The body temperatures of 30-day male and pregnant Wistar rats (E17) were elevated 3.5 ± 0.8°C above normal body temperature (∼37.8 ± 0.6°C and ∼37.3 ± 1.0°C, respectively) by placement in a dry-air incubator at 42°C. Body temperature was monitored using a rat rectal thermistor probe. Once peak rectal temperature was reached (usually 30 minutes after placement in the incubator for 30-day rats, and 45 minutes for pregnant rats on account of their increased size), the elevated temperature was maintained for 1 hour. Rats were then removed from the incubator, given water ad libitum, and allowed to recover at room temperature (RT). Adult (30 days) rats were sacrificed at 2.5 hours, 5 hours, 10 hours, 15 hours, and 24 hours from the onset of heating, whereas 17-day embryos were removed from their mothers at 2.5 hours, 5 hours, and 10 hours postheat.
P7 rat pups were removed from their mothers, and their body temperatures were elevated to 41.3 ± 0.8°C within 20 minutes of heating in a dry-air incubator at 42°C and maintained for 1 hour. Body temperature was monitored with a needle thermistor probe placed under the forelimb. Rat pups were removed from the incubator, cooled to normal temperature, and returned to their mothers. The pups were sacrificed at 5 hours, 10 hours, 15 hours, and 24 hours after the onset of heating.
Western blot analysis
Control and heated rats at 5 hours, 10 hours, 15 hours, and 24 hours were killed by decapitation. Tissue was dissected from cerebellum, thymus, and testis, homogenized in 0.32 M sucrose, and protein concentrations were determined using the BioRad protein assay. Homogenates were stored at −20°C. Protein samples were solubilized by boiling for 5 minutes with an equal volume of dissociation buffer (8 M urea, 2 % sodium dodecyl sulfate [SDS], 2 % β-mercaptoethanol, 20 % glycerol). SDS–polyacrylamide gel electrophoresis was performed on 10 % gels with a 5 % stacking gel, using the discontinuous buffer system of Laemmli (1970). Aliquots of 50 μg of protein were loaded per lane. Gels were stained with Coomassie Blue in order to test for equal protein loading in each lane.
Proteins were electrophoretically transferred onto a nitrocellulose membrane for 16–18 hours in a solution of 50 mM boric acid, 4 mM β-mercaptoethanol, and 2 mM ethylenediamine–tetraacetic acid at 400 mA. Blots were stained briefly with Ponceau S to verify equal loading and efficient transfer of protein, then washed 4 times for 5 minutes each in TBST buffer (10 mM Tris, 0.25 M NaCl, 0.5 % Tween-20, pH 7.5). Blots were then blocked for 2 hours at RT in 5 % Carnation milk powder in TBST buffer and incubated in primary antibody overnight. The C92 mouse monoclonal anti-human Hsp70, specific to stress-inducible Hsp70 proteins (StressGen, SPA810), was diluted 1:5000 in 1 % purified bovine serum albumin (BSA) in TBST with 0.02 % sodium azide. Blots were then washed 4 times for 10 minutes each in 1 % BSA (Sigma Grade) in TBST, incubated with horseradish-peroxidase–conjugated secondary antibody, anti-mouse IgG, diluted 1:5000 in 1 % BSA in TBST for 2 hours at RT, and washed 6 times for 5 minutes each in TBST. Immunoreactive bands were visualized using enhanced chemi-luminescence (ECL) Western blotting detection reagents (Amersham RPN 2106). Data shown are representative of independent experiments carried out on 3 sets of animals.
Tissue preparation for TUNEL and immunocytochemistry
Adult Wistar rats (30 days) and rat pups at P7 were anaesthetized with sodium pentobarbital (50 mg/kg) and perfused intracardially with 4 % paraformaldehyde in 0.1 M phosphate-buffered saline (PBS), pH 7.4. Brain, thymus, and testes were removed from the 30-day rats, whereas only the brain was removed from the P7 pups. Tissues were fixed in 4 % paraformaldehyde overnight at 4°C. Pregnant rats were sacrificed by decapitation, and embryos were removed, rinsed in 0.1 M PBS, and fixed in 4 % paraformaldehyde overnight at 4°C. For cryoprotection, tissue was equilibrated through a sucrose gradient series (5 %, 10 %, and 20 % sucrose in 0.1 M PBS), mounted in OCT embedding compound (Somagen Diagnositics Inc., Edmonton, Alberta, Canada), and stored at −70°C until use. Glass microscope slides coated with a solution of 1 % gelatin and 0.05 % chromium potassium sulphate were used to collect 20 μm cryostat sections floating on water (for adult thymus and brain, and for P7 brain). Cross-sections of testis and embryo were collected directly on the slide. Sections were air-dried for at least 2 hours before processing for TUNEL, immunocytochemistry, and staining with a 1 % solution of cresyl violet.
Cell death detection by TUNEL
For in situ cell death detection, the TUNEL method, based on Gavrieli et al (1992), was performed, with slight modifications, according to Sgonc et al (1994). The in situ cell death detection kit (Roche Diagnostics, Laval, Quebec, Canada) was used, and contents were diluted to half-strength. Tissue sections were cut on a cryostat at 20 μm, fixed with 4 % paraformaldehyde for 5 minutes and washed with 1× PBS (0.1 M) for 30 minutes. Endogenous peroxidase activity was blocked with 0.3 % H2O2 in methanol for 30 minutes, followed by a 5-minute rinse with PBS. For cell permeabilization, slides were immersed in solution (0.1 % Triton X-100 in 0.1 % sodium citrate) for 2 minutes on ice (4°C), and then rinsed 2 times for 5 minutes each in PBS. Slides were incubated for 1 hour at 37°C in a humid chamber, in a TUNEL reaction mixture containing a 1:10 ratio of terminal deoxynucleotidyl transferase and fluorescein-conjugated dUTP, and then rinsed 3 times for 5 minutes each in 1 % BSA in PBS. Incorporated fluorescein was detected by incubation with a sheep antifluorescein antibody conjugated with horse-radish peroxidase for 30 minutes at 37°C. After rinsing the slides 3 times for 5 minutes each in 1 % BSA in PBS, TUNEL labeling was visualized with a 10-minute diaminobenzidine (DAB) colorimetric reaction with nickel chloride (DAB substrate kit; Vector Labs, Burlingame, CA) and light microscopy. Images were captured using Northern Eclipse Software (Empix Inc., Mississauga, Ontario, Canada). Data shown are representative of independent experiments carried out on 3 animals per time point. For each set of animals, this experiment was performed in triplicate.
Quantitative analysis
TUNEL-positive cells were quantified for each tissue examined, using 3 animals per time point. The number of TUNEL-positive cells in the granule cell layer (gcl), molecular layer (ML), and deep white matter (dwm) of the 30-day cerebellum and in the external granule cell layer (egl) of the P7 cerebellum were counted within 10 randomly selected areas of 12 000 μm2 for each animal. For the thymus, counts were made of 10 randomly selected areas of 6 000 μm2 in the cortex and medulla to avoid overlapping of regions. For the testis, the number of TUNEL-positive cells within 60 seminiferous tubules (approximately 170 μm in diameter) were counted for each animal per time point, and the average number per tubule cross-section was determined. The number of TUNEL-positive cells within the neuroepithelium of the neocortex and tectum were counted within several regions, each spanning 800 μm in length along the entire neuroepithelium. For each set of animals, this experiment was performed in triplicate. The data plotted are representative of the mean number of TUNEL-positive cells within the designated area for each time point after heat shock. Analysis of variance (ANOVA) was performed, and data were considered significant when P < 0.05.
Immunocytochemistry
Tissue sections were rehydrated for 20 minutes at RT in PBS-G buffer (0.1 M PBS, pH 7.4, 0.2 % Triton-X 100, 0.1 % BSA), and then blocked in PBS-G buffer with 10 % normal goat serum for 1 hour for CM-1 immunocytochemistry and with 5 % horse serum for 2 hours for proliferating cell nuclear antigen (PCNA) immunocytochemistry. Sections were incubated for 1 hour at RT in primary antisera CM-1, rabbit polyclonal anti-human active caspase 3 (IDUN Pharmaceuticals, La Jolla, CA), diluted 1:3000 in PBS-G buffer with 0.02 % sodium azide, or overnight in mouse anti-human PCNA (Pharmingen, Mississauga, Ontario, Canada, 32551A), diluted 1:600 in the same buffer. After washing 2 times for 5 minutes each in PBS-G buffer, the sections were incubated in biotinylated goat–anti-rabbit IgG, diluted 1:1000 for 45 minutes at RT, or for 1.5 hours in biotinylated horse–anti-mouse IgG, diluted at 1:400. After again washing 2 times for 5 minutes each in buffer, endogenous peroxidase activity was blocked by immersing sections in 0.3 % H2O2 in methanol for 30 minutes. Tissue sections were washed for 20 minutes in PBS-G buffer and processed with the Vectastain Elite ABC kit (Vector Labs). DAB was used as the chromogen for active caspase 3 cytochemistry, resulting in a brown immunoreactive product. For PCNA, DAB was used with nickel chloride to give a black immunoreactive product. Data shown are representative of independent experiments carried out on 3 animals per time point. For each group, immunocytochemical studies were performed in triplicate.
RESULTS
The effect of hyperthermia on the induction of cell death in the adult brain, testis, and thymus
We first examined the effect of a fever-like increase in temperature on the induction of cell death in neural cells of the adult brain, which are postmitotic and fully differentiated. Secondly, in the same animals, hyperthermia-induced cell death was investigated in the testis and thymus, 2 nonneural tissues, which, unlike the brain, contain dividing and differentiating cells at the adult stage. The TUNEL method was used throughout the study for cytochemical detection of deoxyribonucleic acid (DNA) fragmentation, a hallmark of apoptosis (Gavrieli et al 1992).
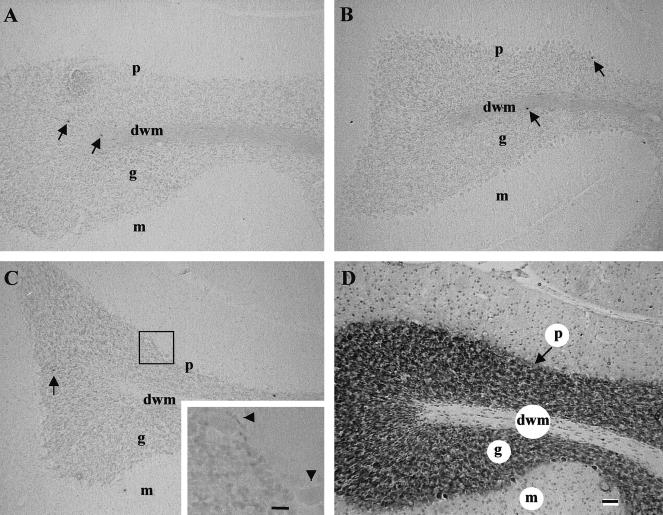
The response of the adult brain to hyperthermia was investigated in the cerebellum, a multilayered structure with distinct neuronal and glial cell populations that are easily identifiable. During the heat shock time course examined (0–24 hours postheat), whole-body hyperthermia (3.5 ± 0.8°C) did not induce cell death in any cellular layer of the adult cerebellum. This was determined by the lack of an increase in TUNEL labeling (black precipitate) in the neuronal-enriched gcl (g) and in the glial-enriched dwm as shown at 5 hours and 15 hours postheat (Fig 1, panels B and C, respectively), compared with the controls (Fig 1, panel A). Similarly, no increase in cell death was observed in cells of the cerebellar ML (m). Higher magnification of the Purkinje cellular layer (p) (Fig 1C, inset) revealed the same result, ie, no induction of cell death after heat shock in large Purkinje neurons (Fig 1, arrowheads). Figure 1 (panel D) shows a cresyl violet–stained sagittal section of the adult cerebellum, demonstrating the morphology of the cellular layers discussed previously.
Fig 1.
The effect of hyperthermia on the induction of cell death in the adult rat cerebellum. Panels A–C: Sagittal sections of the adult cerebellum from control, 5 hours, and 15 hours after hyperthermia were processed with the TUNEL method. There was no evidence of an increase in TUNEL-positive cells in any layer of the cerebellum from control to 15 hours postheat. In the granule cell layer and deep white matter of both control and hyperthermic brain, a few scattered TUNEL-positive cells were observed (indicated by arrows). At 15 hours (panel C), the inset showed no cell death in large Purkinje neurons (arrowheads). Panel D: A cerebellar section stained with cresyl violet to show the morphology of the cellular layers. dwm, deep white matter; g, granule cell layer; m, molecular layer; p, Purkinje cellular layer; TUNEL, terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling. Bar = 55 μm in panels A–D. Bar = 13.8 μm in panel C, inset
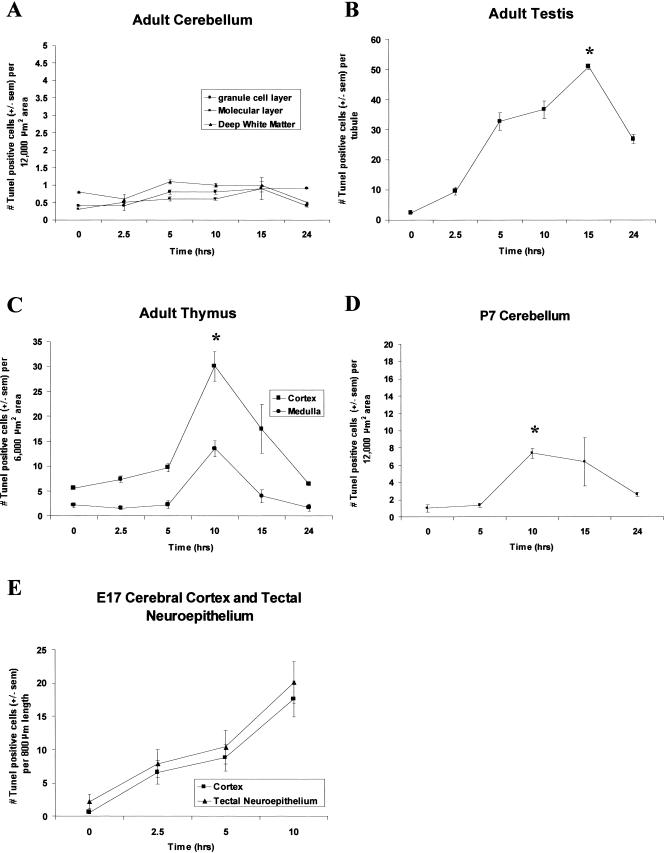
A quantitative analysis of the number of TUNEL-positive cells per designated area in each of the cerebellar layers was performed at specific time points following hyperthermia. Figure 2A shows no significant increase (P > 0.05) in the number of TUNEL-positive cells in the gcl, ML, or dwm. In addition, other regions of the adult brain, namely the cerebral cortex and hippocampus, did not show an increase in TUNEL-positive cells after hyperthermia (results not shown).
Fig 2.
Quantitative time-course analysis of the effect of hyperthermia on adult, postnatal day 7 (P7), and embryonic day 17 (E17) tissues. The average number of the terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling (TUNEL)-positive cells is shown for each tissue over the heat shock time course. Error bars indicate standard error of the mean (sem). Asterisks (*) indicate the time point of maximal cell death. Statistical analysis was performed using analysis of variance, and data were considered significant when P < 0.05. (A) Cerebellum. The mean number of TUNEL-positive cells per 12 000 μm2 area were plotted for 3 cerebellar layers, namely, the granule cell layer, molecular layer, and deep white matter. No significant change (P > 0.05) in the number of TUNEL-positive cells was observed in any cerebellar layer over the hyperthermic time course. TUNEL counts for the Purkinje cellular layer are not shown as none of these cells were observed to be TUNEL positive under control or hyperthermic conditions. (B) Testis. A significant increase (P < 0.001) in the number of TUNEL-positive cells per tubule (average diameter 170 μm) was observed, peaking at 15 hours after hyperthermia (asterisk) and subsequently declining at 24 hours. (C) Thymus. An increase in TUNEL-positive cells per 6 000 μm2 area of cortex and medulla was detected over the heat shock time course (P < 0.001), peaking at 10 hours postheat (asterisk) and declining almost to basal levels by 24 hours, with the greater incidence of cell death in the cortex. (D) The external granule layer (egl) of the cerebellum at P7 showed a significant increase in the mean number of TUNEL-positive cells (P < 0.01), peaking at 10 hours postheat and declining thereafter toward basal levels. (E) The developing cerebral cortex and tectal neuroepithelium at E17 were susceptible to heat-induced cell death, as demonstrated by the significant increase in the number of TUNEL-positive cells (P < 0.001)
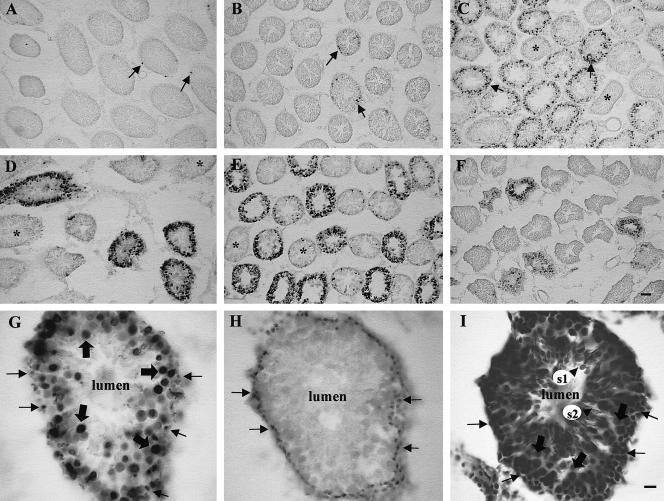
The effect of hyperthermia on cell death in the adult testis was examined next. A time-course analysis of hyperthermia-induced cell death in the testis is shown at the cellular level in Figure 3 and at the quantitative level in Figure 2B. In a cross-section of the seminiferous tubules in the control testis (Fig 3, panel A), a few TUNEL-positive cells were detected that were localized at the periphery of the tubule (indicated by arrows), consistent with the mitotic spermatogonia undergoing apoptosis (Burkitt et al 1993). The incidence of cell death was most prevalent at 15 hours postheat (Fig 3, panel E), and had greatly subsided by 24 hours (Fig 3, panel F) after hyperthermia. It was evident, by the lack of TUNEL staining in certain cross-sections, that not all testis tubules undergo cell death to the same extent. These resistant tubules are indicated by asterisks in Figure 3, panels C–E. These tubules may be representative of specific spermatogenic stages, which have been demonstrated to be relatively protected from heat-induced apoptosis (Lue et al 1999). The average number of TUNEL-positive cells per tubule (∼170 μm in diameter) was plotted against time in Figure 2B, confirming that a significant increase in cell death was observed following hyperthermia (P < 0.001).
Fig 3.
Hyperthermia induces cell death in the adult testis. Panels A–F: Time-course analysis of heat-induced cell death in cross-sections of adult rat seminiferous tubules. Terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling (TUNEL) staining was detected in a few peripheral cells of the seminiferous tubules in the unstressed control rat (panel A, arrows). The number of TUNEL-positive cells increased slightly at 2.5 hours (panel B, arrows), and a further increase, both in the number of tubules being affected and in the number of TUNEL-positive cells per tubule, was observed at 5 hours postheat (panel C). Asterisks (*) indicate tubules that did not appear to undergo heat-induced cell death. The number of TUNEL-positive cells continued to increase at 10 hours (panel D) and up to 15 hours after hyperthermia (panel E), which was the time point of maximal cell death, followed by a decline at 24 hours (panel F). Bar = 55 μm. Panels G–I: Adjacent sections of a 15-hour testis tubule at high magnification, processed with TUNEL (G), PCNA (proliferating cell nuclear antigen) immunocytochemistry (H), and cresyl violet stain (I), respectively. TUNEL labeling (G) shows that certain cell types were more sensitive to the heat than others. No cell death was observed in cells near the lumen. To aid in the identification of these cells, PCNA immunocytochemistry was performed (panel H), which selectively labels the mitotically active spermatogonia. Comparative analysis shows that type A spermatogonia (indicated by arrows) were susceptible to heat-induced cell death. The next layer of TUNEL-positive cells proceeding toward the lumen (large arrows) corresponded to primary spermatocytes, as was evident by cresyl violet staining (panel I). It was evident by the lack of TUNEL labeling in the lumen (panel G) that the round (s1) and elongating (s2) spermatids in panel I, located near the lumen, did not undergo cell death. Bar = 13.8 μm
To identify the cell types in the testis that were sensitive to heat, adjacent sections of a 15-hour tubule (the time of maximal cell death) were processed for TUNEL (Fig 3, panel G), and PCNA immunocytochemistry, to identify the dividing cells (Fig 3, panel H) (Bravo et al 1987; Iatropoulos and Williams 1996), or stained with cresyl violet, to identify morphologically the cell types, as shown in Figure 3, panel I. The PCNA method identified the mitotically active cells at the periphery of the tubule, which were type A spermatogonia (Chandra et al 1997). A comparison between TUNEL-labeled tubules and PCNA-stained tubules revealed that the mitotically active type A spermatogonia were heat sensitive (Fig 3, arrows). The next layer of cells proceeding toward the central lumen consists of primary spermatocytes, characterized by their large size and abundant cytoplasm. These cells, which are undergoing the first stage of meiosis, were also heat sensitive as demonstrated by the TUNEL labeling (Fig 3, large arrows). The absence of TUNEL staining in adluminal spermatids (s1 and s2) suggests that these testis cell types are not heat sensitive (refer to the cresyl violet–stained section, Fig 3I, for the location of the spermatids).
Another adult tissue undergoing cell division and differentiation even in the adult is the thymus. Thymus tissue was isolated at time points following hyperthermia and examined for the presence of TUNEL-positive cells. As shown in Figure 4A, scattered TUNEL-positive cells were present in the cortex (c) and medulla (m) of control thymus (Fig 4, arrows). However, a major transient increase in TUNEL-positive cells was observed at 10 hours postheat (Fig 4D). A quantitative time-course analysis (Fig 2C) revealed that the number of TUNEL-positive cells in the thymus that increased significantly (P < 0.001) peaked 10 hours after hyperthermia and was greater in the cortex of the thymus than in the medulla.
Fig 4.
Hyperthermia induces cell death in the adult rat thymus. Panels A–E: Terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling (TUNEL) assay performed on sections of control (A), 2.5-hour (B), 5-hour (C), 10-hour (D), and 15-hour (E) thymus, after hyperthermia. A few scattered TUNEL-positive cells were present in the control thymus (indicated by arrows). After hyperthermia, there was a gradual increase to 5 hours postheat, peaking at 10 hours (panel D) and declining by 15 hours. TUNEL-positive cells (indicated by arrows) were evident in both the cortex (c) and the medulla (m); however, the majority were present in the cortex. Panel F: A cresyl violet–stained thymus section showing the morphology of the cortex and the medulla. Bar = 55 μm
Does time course of heat shock–induced cell death correlate to the time course of Hsp70 induction in the adult cerebellum, testis, and thymus?
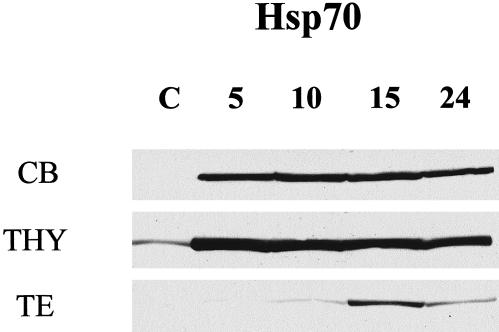
To correlate the sensitivity to heat of the adult cerebellum, testis, and thymus to the whole-tissue levels of Hsp70 induction after hyperthermia, a Western blot analysis was performed. Basal Hsp70 levels were highest in the thymus (THY) (Fig 5) and not detectable in the cerebellum (CB) or testis (TE). After hyperthermia, these tissues induced Hsp70, but to different extents. A prominent induction of Hsp70 in the thymus and cerebellum was evident by 5 hours, and the levels were maintained up to 15 hours after hyperthermia, declining slightly at 24 hours in the cerebellum. The testis displayed a less robust and delayed induction of Hsp70, which did not peak until 15 hours after hyperthermia. Whole-tissue levels of Hsp70 inducibility correlated with resistance to hyperthermia-induced cell death in the cerebellum, but not in the thymus, which showed high levels of cell death, yet a robust induction of Hsp70.
Fig 5.
Time-course analysis of Hsp70 induction in adult rat tissues following hyperthermia. The induction of the Hsp70 proteins was analyzed in control (C) animals and at 5 hours, 10 hours, 15 hours, and 24 hours following hyperthermia. Western blot analysis of cerebellum (CB), thymus (THY), and testis (TE) showed high basal levels of Hsp70 in the control thymus, but not in the cerebellum or the testis. Following hyperthermia, an accumulation of stress-inducible Hsp70 proteins was observed in the cerebellum and thymus at 5 hours, and levels were maintained up to 15 hours, after which they declined slightly in the cerebellum. A delayed and less robust induction of Hsp70 was seen in the testis, peaking at 15 hours postheat and declining thereafter
Is the early postnatally developing brain susceptible to hyperthermia-induced apoptosis?
Dividing and differentiating cells in the adult testis and thymus were demonstrated to induce cell death in response to hyperthermia, as assayed by increased TUNEL labeling. In contrast, cells of the adult cerebellum, which are neither dividing nor differentiating but postmitotic, were resistant to hyperthermia-induced cell death. We next determined how dividing neural cells at earlier stages of development respond to hyperthermia.
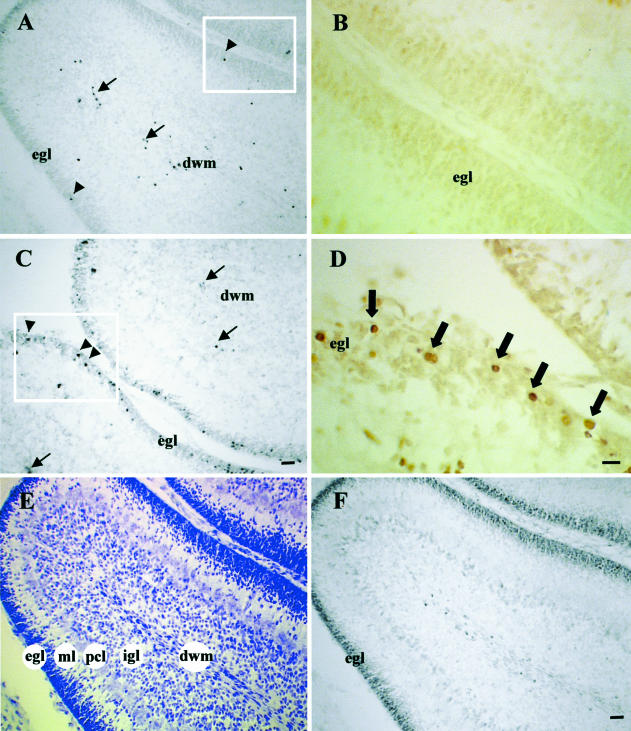
The egl of the cerebellum at P7 consists of actively proliferating cells that migrate through the ML and Purkinje cellular layer (pcl) to their final destination at the internal granule cell layer (igl), where they achieve terminal differentiation (Fig 6, panel E) (Altman 1997). The dividing cells of the egl in P7 rats (Fig 6, arrowheads) were susceptible to heat-induced cell death, as evidenced by TUNEL-positive staining (compare Fig 6A [control] to C [10 hours postheat]). These results were confirmed by quantitative analysis in Figure 2D, which indicates a significant increase in TUNEL-positive cells observed in the egl 10 hours after hyperthermia, with a subsequent decline at 24 hours (P < 0.01). In addition to the egl, TUNEL-positive cells were also detected in the dwm in both control (Fig 6A) and 10-hour (Fig 6B) cerebellar sections (Fig 6, arrows). This was not an effect of hyperthermia because a previous study showed that in the unstressed P7 rat cerebellum, the dwm contains several TUNEL-positive cells, which were determined to be astrocytes naturally undergoing cell death (Krueger et al 1995).
Fig 6.
Hyperthermia induces cell death in the egl of the postnatal day 7 (P7) cerebellum. Adjacent sections of control (A and B) and 10-hour (C and D) P7 cerebellum are processed for TUNEL (A and C) and active caspase 3 immunocytochemistry (B and D). Panels A and C reveal the increase in TUNEL-positive cells (arrowheads) in the egl at 10 hours after hyperthermia (C), compared with the control (A). Arrows in panels A and C indicate that cells undergoing cell death in the dwm in both the control and the 10-hour cerebellum were likely astrocytes. Panels B and D are higher magnifications of adjacent sections of the boxed areas in panels A and C, respectively. There was no evidence of caspase 3 activation in the control egl (B). At 10 hours postheat, however, caspase 3 immunoreactive cells were observed in the egl (D), indicated by large arrows. Panel E: P7 cerebellar section stained with cresyl violet, demonstrating the morphology of the cellular layers. Panel F: Adjacent section processed with PCNA immunocytochemistry to label actively dividing cells revealed that cells of the egl are actively dividing at P7. dwm, deep white matter; egl, external granule cell layer; igl, internal granule cell layer; ML, molecular layer; P7, postnatal day 7; pcl, Purkinje cellular layer; PCNA, proliferating cell nuclear antigen; TUNEL, terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling. Bar = 27.5 μm for panels A, C, E, and F. Bar = 13.8 μm for panels B and D
As previously mentioned, the TUNEL method was used for in situ detection of DNA fragmentation, one of the hallmarks of apoptosis. Recent data suggest, however, that the TUNEL assay does not exclusively label apoptotic cells, and it may label cells in the latter stages of necrosis, which also undergo random breakdown of DNA (Gold et al 1994). These limitations warranted the use of an additional biochemical marker of apoptosis at the single-cell level in order to confirm that the mode of hyperthermia-induced cell death was indeed apoptosis. Caspase 3, one of the effectors of apoptosis, which targets many cellular substrates to bring about apoptotic cell death, is activated by cleavage of its proenzyme (32 kDa) to yield 2 active subunits p18 and p12, which together form the active enzyme (Raff 1998). Caspase 3 has been shown to be activated in heat-induced cell death in vitro (Chan et al 1998) and is specific for apoptosis, not necrosis (Armstrong et al 1997). Thus the activation of caspase 3 was used as an improved index of apoptosis. The CM-1 antibody was characterized by Srinivasan et al (1998) and demonstrated to be useful for in situ detection of activated caspase 3.
Adjacent sections of the control and the 10-hour P7 cerebellum, labeled with TUNEL (Fig 6, panels A and C), were immunocytochemically stained with the CM-1 antibody to detect the presence of activated caspase 3, and shown at higher magnification (Fig 6, panels B and D). It was evident by the presence of CM-1 immunoreactivity in cells of the egl, that caspase 3 is activated following hyperthermia (Fig 6, arrows). Thus the mode of cell death induced by this level of hyperthermia was apoptosis. It is important to note that caspase 3 immunocytochemistry and TUNEL would not necessarily be expected to label the same cell because activation of caspase 3 occurs upstream of DNA fragmentation, which is detected by TUNEL. To confirm that cells in the egl were mitotically active at P7, PCNA immunocytochemistry was performed. This antigen selectively labeled cells in the egl compared with other cerebellar layers (Fig 6, panel F).
The effect of hyperthermia on dividing cells in the cortical neuroepithelium and tectal neuroepithelium at E17
At E17, the cells, which are destined to form the neocortex, are in a dividing state, and their response to hyperthermia was next examined. Under control conditions (Fig 7, panel A), TUNEL-positive cells were not visible in the neocortex at E17. Following hyperthermia, there was a major increase in TUNEL reactivity at the cellular level 10 hours after heat shock, particularly in the neuroepithelium (ne) (Fig 7C). This was confirmed by a quantitative analysis in Figure 2E, demonstrating a significant increasing trend of cell death following maternal hyperthermia (P < 0.001). Activation of caspase 3 was observed in cells of the same region, at 10 hours postheat (compare Fig 7, panels C and D), which confirmed the mode of cell death to be apoptosis.
Fig 7.
The effect of hyperthermia on dividing cells in the neocortex at E17. Adjacent sections of control (A and B) and 10-hour (C and D) cortical neuroepithelium at E17 were processed by the TUNEL method (A and C) and active caspase 3 immunocytochemistry (B and D). A major increase in TUNEL-positive cells was detected in the neuroepithelium at 10 hours after hyperthermia (C) compared with the control (A), indicated by arrowheads. Fewer TUNEL-positive cells were evident in the subventricular zone (panel C, arrows). Active caspase 3 immunoreactivity demonstrated a similar induction in the neuroepithelium at 10 hours postheat (panel D, arrowheads) with a negligible signal in the control (B). Scattered CM-1–positive cells were seen in the subventricular zone (panel D, arrows). Panel E: A cresyl violet–stained section of the developing cortex demonstrating the morphology of the cellular layers. Panel F: Adjacent section to (E) processed with PCNA immunocytochemistry showing actively dividing cells (black precipitate) in the ne and sv regions. E17, embryonic day 17; cp, cortical plate; iz, intermediate zone; lv, lateral ventricle; mz, mitotic zone; ne, neuroepithelium; PCNA, proliferating cell nuclear antigen; sv, subventricular zone; sz, synthetic zone; TUNEL, terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling; wm, white matter. Bar = 27.5 μm
A histologically stained section of the developing cerebral cortex at E17 (Fig 7E) shows that the neuroepithelium consists of a spatially segregated mitotic zone (mz), where cells are predominantly in the mitotic phase, and a synthetic zone (sz), where most cells are duplicating their DNA. At this stage of cortical development (E17), the neuroepithelium is maximal in thickness, and almost all proliferating cells are confined to this region (Bayer and Altman 1991). PCNA immunoreactivity, which identifies dividing cells (Fig 7F), was localized primarily to cells within the neuroepithelium (ne), whereas the intermediate zone (iz), white matter (wm), and cortical plate (cp), which contains young postmitotic cells of future cortical layers V and VI, were not immunoreactive.
Given the histology of the neocortex, it appeared that cells in both the mitotic (mz) and synthetic zones (sz) of the neuroepithelium were more susceptible to heat-induced apoptosis than the nonproliferating cells of the cortical plate and the intermediate zone at E17 (Fig 7 C,D). In addition to the neuroepithelium, some scattered cells in the subventricular zone also underwent apoptosis (Fig 7 C,D, arrows).
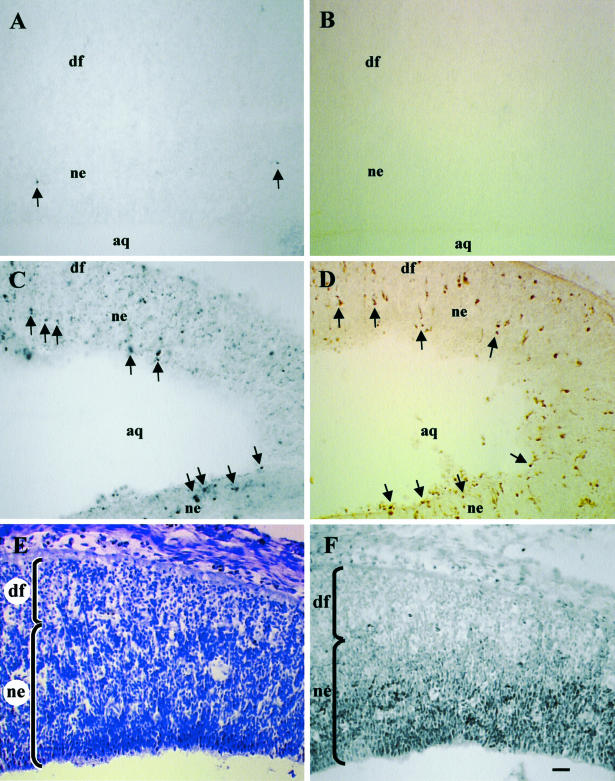
Examination of the E17 neocortex revealed another neuroepithelial layer in the developing midbrain, which labeled strongly with TUNEL. This region, identified as the tectal neuroepithelium, later becomes the inferior and superior colliculi of the brain. The cells in this neuroepithelial region are also mitotically active at E17 and undergo DNA synthesis as indicated by PCNA immunoreactivity (Fig 8F), compared with cells in the differentiating field (df), which are not dividing, and yet some of them are still susceptible to heat-induced cell death. A significant increase (P < 0.001) was observed in the number of TUNEL-positive cells, parallel to that seen in the cortical neuroepithelium (Fig 2E). Cellular observations demonstrate this increase in TUNEL-positive (Fig 8 A,C) and active caspase 3 immunoreactive cells (Fig 8 B,D) within the same region of adjacent sections. Thus, the tectal neuroepithelium provided another example of heat-induced apoptosis in mitotically active neural cells.
Fig 8.
Hyperthermia induces apoptosis in the tectal neuroepithelium at E17. Panels A–D: Adjacent sections of control (A and B) and 10-hour (C and D) tectal neuroepithelium were processed for terminal TUNEL and active caspase 3 immunocytochemistry. Hyperthermia-induced cell death in the neuroepithelium at 10 hours (C) compared with the control (A) as shown by the presence of TUNEL-positive cells (arrows). Similarly, active caspase 3 immunoreactivity was strongly detected at 10 hours postheat (D) and was negligible in the control (B). The majority of TUNEL-positive and active caspase 3 immunoreactive cells were present in the neuroepithelium, compared with the differentiating field. Panel E: A cresyl violet–stained section of developing tectum showing the neuroepithelium (ne) and the differentiating field (df). Panel F: Adjacent section to (E) processed for PCNA immunocytochemistry, demonstrating that actively dividing cells are present in the ne. aq, aqueduct; df, differentiating field; E17, embryonic day 17; ne, neuroepithelium; PCNA, proliferating cell nuclear antigen; TUNEL, terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling. Bar = 27.5 μm
DISCUSSION
Our previous in vivo studies have focused on the neural expression of heat shock genes in mammals under normal and hyperthermic conditions (Brown 1994; Brown and Sharp 1999). In this study, we investigate whether a similar fever-like increase in temperature could induce cell death in neural cells, which are fully differentiated and postmitotic in the adult, compared with the nonneural tissues, testis, and thymus, which contain populations of actively dividing and differentiating cells throughout life. The differences in heat sensitivities between these tissues prompted an investigation into the susceptibility of the embryonic and postnatally developing brain to hyperthermia-induced cell death.
TUNEL staining revealed that hyperthermia induced cell death in the cortex and medulla of the thymus and in dividing cells of the testis but not in any glial or neuronal cell types in the adult cerebellum. Although, neuronal cell death via apoptosis occurs developmentally, and in response to environmental and pathological stress (Pettmann and Henderson 1998), a fever-like temperature shock did not activate apoptotic pathways in the adult rat brain.
The few studies that have examined the effect of hyperthermia on cell death in the mammalian brain have demonstrated negligible effects, unless very high temperatures are employed. Hyperthermia (42°C for 1 hour) did not result in any neurological deficits to dogs, nor damage to the brain or spinal cord as assessed histologically 1 week after heating (Takahashi et al 1999). Similarly, no cell death was observed in the rat brain after a bacterial endotoxin, lipopolysaccharide-induced fever (Mouihate and Pittman 1998) or following a temperature shock of 41°C (McCabe and Simon 1993). In the mouse brain (body temperature 37°C), an increased level of hyperthermic stress (43.5°C for 30 minutes) was reported to induce Hsp70 and apoptosis, detected by internucleosomal DNA fragmentation analysis (Leoni et al 2000). The maximum induction of Hsp70 coincided with maximal DNA damage; however, no distinction is made between the responses of different regions or cell types (or both) to the heat shock. Overall, it appears that temperatures greater than 43°C can cause cell death in the mammalian brain, which is normally maintained at 37–38°C. This is in contrast to the thermal exposure used in the present study (3.5 ± 0.8°C, 1 hour), which is not detrimental to adult neural cells compared with the thymus and testis cells of the same animals.
Hyperthermia is a classic inducer of the heat shock response. The major Hsps induced after heat shock in the mammalian brain are the Hsp70 proteins that belong to a multigene family of highly conserved proteins, including both constitutively expressed and stress-inducible members (Kiang and Tsokos 1998). To correlate the effect of hyperthermia on cell death with the induction of Hsp70 proteins in the 3 tissues, a time-course analysis of the Hsp70 induction profile was performed. The robust induction of Hsp70 observed in the cerebellum correlated with the lack of heat-induced cell death observed in this tissue. Likewise, the delayed, less robust induction in the testis correlated with its sensitivity to hyperthermia-induced cell death. Cells in the thymus, however, readily underwent apoptosis despite the organ's high basal levels of Hsp70 and robust Hsp70 induction. Thus, the differences in heat sensitivities of these tissues could not simply be attributed to varied induction levels of the Hsp70 proteins. A better index of the correlation of Hsp70 induction and apoptosis is immunocytochemistry, which gives results at the cellular level.
In the seminiferous tubules of the adult testis, a few TUNEL-positive spermatogonia were detected in the control rat. Spontaneous spermatogenic cell death by apoptosis is common in mammals and is found most frequently in spermatogonia (Allan et al 1987; Bartke 1995; Mori et al 1997). The mouse testis, which is normally maintained at 32°C (Rockett et al 2001), is very sensitive to heat, and slight temperature elevation can inhibit spermatogenic cell function, causing infertility (Chowdhury and Steinberger 1964; Mieusset et al 1987). Following hyperthermia, cell death was observed in primary spermatocytes (meiotic) and mitotic spermatogonia, but not in postmeiotic spermatids. Early studies have shown that heat induces cell death in the testis; however, only recently has apoptosis been defined as the mode of cell death (Yin et al 1997; Blanco-Rodriguez and Martinez-Garcia 1998; Lue et al 1999). The most heat-sensitive cells in these studies were spermatogonia, primary spermatocytes, and round spermatids, whereas advanced spermatids and Sertoli cells remained unaffected. Thus, the testis results indicate that cells actively undergoing mitosis (spermatogonia) and meiosis (primary spermatocytes) are prone to heat-induced cell death.
The thymus is a tissue that exhibits naturally occurring cell death during the process of negative selection, whereby thymocytes with receptors that are capable of recognizing self-antigens are selected and destroyed (von Boehmer et al 1989). In addition to differentiation processes, extensive cell proliferation also occurs in the adult thymus, providing lymphocytes for export to the immune system (Westermann et al 1989). These factors render the thymus sensitive to heat-induced apoptosis, as demonstrated in the mouse, where hyperthermia was shown to accelerate the normal process of cellular differentiation, causing a decrease in the number of immature thymocytes (Sellins and Cohen 1991; Mansoor et al 1992; Mosser et al 1993). Similarly, hyperthermia has been shown to induce apoptosis in the rat thymus (Sakaguchi et al 1995). It was suggested that cells, which are in a high-turnover state, are programmed for apoptosis and thus easily activate this mode of cell death in response to lethal stimuli.
Numerous studies on the effects of temperature on cultured cells have demonstrated that rapidly proliferating cells are more temperature sensitive than slowly proliferating cells (Johnson and Pavelec 1972). Heat causes mitotic delay and cell cycle arrest (Edwards et al 1974; Maldonado-Codina et al 1993), damage to mitotic spindle apparatus (Debec and Marcaillou 1997), plasma membrane damage (Coss et al 1979), decay of topoisomerase II (required for DNA replication) (Goswami et al 1996), and cell death (Edwards et al 1974; Breen et al 1999). Moreover, mitosis and the S-phase have been determined to be the most heat-sensitive phases of the cell cycle (Westra and Dewey 1971; Bhuyan et al 1977; Kühl and Rensing 2000). As heat shock induces mitotic delay and cell death, particularly in dividing cells, and heat-induced cell death was observed in the testis and thymus, which undergo cell division and differentiation processes in the adult, it was logical to explore the effect of hyperthermia on cell death in the developing brain where neural cells are dividing.
Previous studies on the effect of hyperthermia on the developing brain have focused on embryonic development (Edwards et al 1974, 1995; Walsh et al 1991). The present study demonstrates that proliferating cells in the egl of the cerebellum at P7 are also sensitive to hyperthermia-induced apoptosis. Naturally occurring programmed cell death is a feature of postnatal cerebellar development (Lewis 1975; Krueger et al 1995; Tanaka and Marunouchi 1998); however, no studies to date have shown the effect of heat on these dividing cells. The susceptibility of cells in the egl to other apoptotis-inducing stimuli, such as exposure to irradiation and the cytotoxic agent methylazoxymethanol, is known (Ferrer et al 1993; Lafarga et al 1997). In contrast, the results in this study revealed that neither the cells in the Purkinje cell layer nor the igl underwent heat-induced apoptosis. On P7, the cells in these regions are no longer mitotically active (Altman 1997).
The activation of caspase 3 in the dividing cells of the egl provides evidence that the mode of cell death is apoptosis. The exact pathway of heat shock–induced apoptosis is as yet undefined; however, it is known that it occurs via activation of the stress-activated protein kinase–c-Jun N-terminal kinase pathway (Kyriakis and Avruch 1996; Verheij et al 1996). This, in turn, triggers activation of the caspase cascade, which targets several proteins, to bring about apoptotic cell death. Heat has also been reported to induce changes such as a drop in mitochondrial membrane potential and release of cytochrome c, which activates caspase 9 and, in turn, caspase 3 (Mirkes and Little 2000). Caspase 3 activation is involved in apoptosis stimulated by traumatic brain injury in rats (Yakovlev et al 1997), K+/serum deprivation (Armstrong et al 1997), optic nerve transection (Kermer et al 1999), cerebral ischemia (Namura et al 1998), and heat shock (Chan et al 1998). Caspase 3 successfully identified dying cells in the egl and was thus an effective marker of apoptosis.
The neuroepithelia of the embryonic neocortex and developing tectum were also susceptible to hyperthermia-induced cell death. There was a significant increase in TUNEL-positive cells, concomitant with active caspase 3 labeling in the same regions of the brain at E17, indicating apoptosis. The lethal effects of maternal hyperthermia on the developing embryo have been studied in guinea pigs (Edwards et al 1974), embryonic rats at gestational day 10 (GD10), and mice (E9) (Walsh et al 1991; Mirkes et al 1997; Mirkes and Little 1998; Breen et al 1999). These studies have shown that the embryo at the stage of neural tube closure is very sensitive to heat stress. The induction of cell death and mitotic delay in the neuroectoderm at this stage (which consists of rapidly proliferating cells) results in abnormal forebrain, optic cup, and somite development. Our results indicate that even later stages of embryonic development (E17) are sensitive to maternal hyperthermia, particularly in the neuroepithelium where cells are actively dividing and replicating DNA. These regions also undergo extensive, naturally occurring programmed cell death during the period of neurogenesis (Blaschke et al 1996; Thomaidou et al 1997). Thus, their increased susceptibility to heat-induced apoptosis may be caused by an active cell cycle and a natural disposition to cell death (Ross 1996). Evidence to support the theory that developing neurons are more susceptible to apoptotic-inducing stimuli exists in studies demonstrating increased NMDA- or kainate-induced cell death in the neonatal vs adult rat brain (van Lookeren Campagne et al 1995). Embryonic proliferating cortical neurons also have a high vulnerability to apoptotic stimuli because of their expression of Fas/Apo-1 receptors (Cheema et al 1999), which play a critical role in limiting cell proliferation by apoptosis (Nagata and Goldstein 1995). It has been suggested that such pathways are absent or down-regulated in postmitotic neurons, in keeping with the need to preserve cells that are irreplaceable (Marks and Berg 1999). Furthermore, although Hsp70 induction could provide protection and resistance to surviving cells against further stressful insults, it may be more beneficial to induce apoptosis in proliferating cells to prevent the perpetuation of DNA that may have been mutated by the stress.
To sum up, the present report demonstrates hyperthermia-induced cell death in dividing cells of the adult rat testis and thymus, but not in the adult cerebellum where cell division and differentiation processes have ceased. The differential apoptotic responses of these tissues to hyperthermia did not correlate with the whole-tissue levels of Hsp70 induction. Future studies will aim at determining, using immunocytochemistry, if a spatial correlation of the stress-inducible Hsp70s to apoptosis exists. Apoptosis was observed in the developing rat brain in response to hyperthermia, specifically in neural regions undergoing high proliferative activity. Adult neural cells may be resistant to hyperthermia-induced cell death because they are irreplaceable and nondividing. These results suggest that the level of cell maturity (ie, dividing vs postmitotic) contributes to the decision of whether or not heat induces apoptosis in neural cells.
Acknowledgments
We thank IDUN Pharmaceuticals for the generous donation of the CM-1 antibody and Sheila Rush for critical reading of the manuscript. This research was supported by grants to IRB from the Canadian Institutes for Health Research.
Footnotes
This paper is dedicated to the memory of Professor David A. Walsh (1945–2000), of the University of New South Wales, Australia, who pioneered research on the heat shock response during early mammalian development
REFERENCES
- Alam A, Braun MY, Hartgers F, Lesage S, Cohen L, Hugo P, Denis F, Sekaly RP. Specific activation of the cysteine protease CPP32 during the negative selection of T cells in the thymus. J Exp Med. 1997;186:1503–1512. doi: 10.1084/jem.186.9.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan DJ, Harmon BV, and Kerr JFR 1987 Cell death in spermatogenesis. In: Perspectives on Mammalian Cell Death, ed Potten CS. Oxford University Press, Oxford, 229–258. [Google Scholar]
- Altman J 1997 Development of the Cerebellum: In Relation to Its Evolution, Structure, and Functions. CRC Press, Boca Raton, 783 p. [Google Scholar]
- Armstrong RC, Aja TJ, and Hoang KD. et al. 1997 Activation of the CED3/ICE-related protease CPP32 in cerebellar granule neurons undergoing apoptosis but not necrosis. J Neurosci. 17:553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke A. Apoptosis of male germ cells, a generalized or a cell type-specific phenomenon? Endocrinology. 1995;136:3–4. doi: 10.1210/endo.136.1.7828545. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Altman J 1991 Neocortical Development. Raven Press, New York, 255 p. [Google Scholar]
- Bechtold DA, Brown IR. Heat shock proteins hsp27 and hsp32 localize to synaptic sites in the rat cerebellum following hyperthermia. Mol Brain Res. 2000;75:309–320. doi: 10.1016/s0169-328x(99)00323-x. [DOI] [PubMed] [Google Scholar]
- Bechtold DA, Rush SJ, Brown IR. Localization of the heat-shock protein hsp70 to the synapse following hyperthermic stress in the brain. J Neurochem. 2000;74:641–646. doi: 10.1046/j.1471-4159.2000.740641.x. [DOI] [PubMed] [Google Scholar]
- Bhuyan BK, Day KJ, Edgerton CE, Ogunbase O. Sensitivity of different cell lines and of different phases in the cell cycle to hyperthermia. Cancer Res. 1977;37:3780–3784. [PubMed] [Google Scholar]
- Blanco-Rodriguez J, Martinez-Garcia C. Apoptosis pattern elicited by several apoptogenic agents on the seminiferous epithelium of the adult rat testis. J Androl. 1998;19:487–497. [PubMed] [Google Scholar]
- Blaschke AJ, Staley K, Chun J. Widespread programmed cell death in proliferative and postmitotic regions of the fetal cerebral cortex. Development. 1996;122:1165–1174. doi: 10.1242/dev.122.4.1165. [DOI] [PubMed] [Google Scholar]
- Bravo R, Frank R, Blundell PA, Macdonald-Bravo H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-δ. Nature. 1987;362:515–517. doi: 10.1038/326515a0. [DOI] [PubMed] [Google Scholar]
- Breen JG, Claggett TW, Kimmel GL, Kimmel CA. Heat shock during rat embryo development in vitro results in decreased mitosis and abundant cell death. Reprod Toxicol. 1999;13:31–39. doi: 10.1016/s0890-6238(98)00056-2. [DOI] [PubMed] [Google Scholar]
- Brown IR 1994 Induction of heat shock genes in the mammalian brain by hyperthermia and tissue injury. In: Heat Shock Proteins in the Nervous System, ed Mayer RJ, Brown IR. Academic Press, London, 31–53. [DOI] [PubMed] [Google Scholar]
- Brown IR, Sharp FR 1999 The cellular stress gene response in brain. In: Stress Proteins, Handbook of Experimental Pharmacology,ed Latchman DS. Springer Verlag, New York, 243–263. [Google Scholar]
- Buja LM, Eigenbrodt ML, Eigenbrodt EH. Apoptosis and necrosis. Arch Pathol Lab Med. 1993;117:1208–1214. [PubMed] [Google Scholar]
- Burkitt HG, Young B, and Heath JW 1993 Wheater's Functional Histology. A Text and Colour Atlas, 3rd ed. Churchill Livingstone, New York, 407. [Google Scholar]
- Chan WH, Yu JS, Yang SD. Heat shock stress induces cleavage and activation of PAK2 in apoptotic cells. J Protein Chem. 1998;17:485–494. doi: 10.1023/a:1022578820147. [DOI] [PubMed] [Google Scholar]
- Chandra AMS, Qualls CW, Campbell GA. Testicular effects of 1,3,5-trinitro-benzene (TNB). II. Immunolocalization of germ cells using proliferating cell nuclear antigen (pcna) as an endogenous marker. J Toxicol Environ Health. 1997;50:379–387. [PubMed] [Google Scholar]
- Cheema ZF, Wade SB, Sata M, Walsh K, Sohrabji F, Miranda RC. Fas/Apo (apoptosis)-1 and associated proteins in the differentiating cerebral cortex: induction of caspase-dependent cell death and activation of NF-κB. J Neurosci. 1999;19:1754–1770. doi: 10.1523/JNEUROSCI.19-05-01754.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury AK, Steinberger E. A quantitative study of the effect of heat on germinal epithelium of rat testes. Am J Anat. 1964;115:509–524. doi: 10.1002/aja.1001150307. [DOI] [PubMed] [Google Scholar]
- Coss RA, Dewey WC, Bamburg JR. Effects of hyperthermia (41.5°C) on chinese hamster ovary cells analyzed in mitosis. Cancer Res. 1979;39:1911–1918. [PubMed] [Google Scholar]
- Debec A, Marcaillou C. Structural alterations of the mitotic apparatus induced by the heat shock response in Drosophila cells. Biol Cell. 1997;89:67–78. doi: 10.1016/s0248-4900(99)80082-3. [DOI] [PubMed] [Google Scholar]
- DeMaio A. Heat shock proteins: facts, thoughts, and dreams. Shock. 1999;11:1–12. doi: 10.1097/00024382-199901000-00001. [DOI] [PubMed] [Google Scholar]
- Dorstyn L, Kinoshita M, and Kumar S 1998 Caspases in cell death. In: Results and Problems in Cell Differentiation: Apoptosis Mechanisms and Role in Disease, ed Kumar S. Springer-Verlag, New York, 1–23. [DOI] [PubMed] [Google Scholar]
- Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Ann Rev Biochem. 1999;68:383–424. doi: 10.1146/annurev.biochem.68.1.383. [DOI] [PubMed] [Google Scholar]
- Edwards MJ, Mulley R, Ring S, Wanner RA. Mitotic cell death and delay of mitotic activity in guinea-pig embryos following brief maternal hyperthermia. J Embryol Exp Morphol. 1974;31:593–602. [PubMed] [Google Scholar]
- Edwards MJ, Shiota K, Smith MS, Walsh DA. Hyperthermia and birth defects. Reprod Toxicol. 1995;9:411–425. doi: 10.1016/0890-6238(95)00043-a. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Serrano T, Rivera R, Olive M, Zujar MJ, Graus F. Radiosensitive populations and recovery in X-ray-induced apoptosis in the developing cerebellum. Acta Neuropathol. 1993;86:491–500. doi: 10.1007/BF00228585. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labelling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos C, Welch WJ. Role of the major heat shock proteins as molecular chaperones. Ann Rev Cell Biol. 1993;9:601–634. doi: 10.1146/annurev.cb.09.110193.003125. [DOI] [PubMed] [Google Scholar]
- Gold R, Schmied M, Giegerich G, Breitschopf H, Hartung HP, Toyka KV, Lassmann H. Differentiation between cellular apoptosis and necrosis by the combined use of in situ tailing and nick translation techniques. Lab Invest. 1994;71:219–225. [PubMed] [Google Scholar]
- Goswami PC, Roti Roti JL, Hunt CR. The cell cycle-coupled expression of topoisomerase IIα during S phase is regulated by mRNA stability and is disrupted by heat shock or ionizing radiation. Mol Cell Biol. 1996;16:1500–1508. doi: 10.1128/mcb.16.4.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JM Jr,, Edwards MJ, Edwards MJ. Teratogen update: gestational effects of maternal hyperthermia due to febrile illnesses and resultant patterns of defects in humans. Teratology. 1998;58:209–221. doi: 10.1002/(SICI)1096-9926(199811)58:5<209::AID-TERA8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Iatropoulos MJ, Williams GM. Proliferation markers. Exp Toxic Pathol. 1996;48:175–181. doi: 10.1016/S0940-2993(96)80039-X. [DOI] [PubMed] [Google Scholar]
- Johnson HA, Pavelec M. Thermal injury due to normal body temperature. Am J Path. 1972;66:557–564. [PMC free article] [PubMed] [Google Scholar]
- Karunanithi S, Barclay JW, Robertson RM, Brown IR, Atwood HL. Neuroprotection at Drosophila synapses conferred by prior heat shock. J Neurosci. 1999;19:4360–4369. doi: 10.1523/JNEUROSCI.19-11-04360.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermer P, Klocker N, Labes M, Thomsen S, Srinivasan A, Bahr M. Activation of caspase-3 in axotomized rat retinal ganglion cells in vivo. FEBS Lett. 1999;453:361–364. doi: 10.1016/s0014-5793(99)00747-4. [DOI] [PubMed] [Google Scholar]
- Kiang JG, Tsokos GC. Heat shock protein 70 kDa: molecular biology, biochemistry and physiology. Pharmacol Ther. 1998;80:183–201. doi: 10.1016/s0163-7258(98)00028-x. [DOI] [PubMed] [Google Scholar]
- Krueger BK, Burne JF, Raff MC. Evidence for large-scale astrocyte death in the developing cerebellum. J Neurosci. 1995;15:3366–3374. doi: 10.1523/JNEUROSCI.15-05-03366.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühl NM, Rensing L. Heat shock effects on cell cycle progression. Cell Mol Life Sci. 2000;57:450–463. doi: 10.1007/PL00000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Sounding the alarm: protein kinase cascades activated by stress and inflammation. J Biol Chem. 1996;271:24313–24316. doi: 10.1074/jbc.271.40.24313. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lafarga M, Lerga A, Andres MA, Polanco JI, Calle E, Berciano MT. Apoptosis induced by methylazoxymethanol in developing rat cerebellum: organization of the cell nucleus and its relationship to DNA and rRNA degradation. Cell Tissue Res. 1997;289:25–38. doi: 10.1007/s004410050849. [DOI] [PubMed] [Google Scholar]
- Leoni S, Brambilla D, Risuleo G, de Feo G, Scarsella G. Effect of different whole body hyperthermic sessions on the heat shock response in mice liver and brain. Mol Cell Biochem. 2000;204:41–47. doi: 10.1023/a:1007053504960. [DOI] [PubMed] [Google Scholar]
- Lewis PD. Cell death in the germinal layers of the postnatal rat brain. Neuropathol Appl Neurobiol. 1975;1:21–29. [Google Scholar]
- Li GC, Mivenchi NF, Weitzel G. Heat shock proteins, thermotolerance, and their relevance to clinical hyperthermia. Int J Hyperthermia. 1995;11:459–488. doi: 10.3109/02656739509022483. [DOI] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Lowenstein DH, Chan PH, Miles MF. The stress protein response in cultured neurons: characterization and evidence for a protective role in excitotoxicity. Neuron. 1991;7:1053–1060. doi: 10.1016/0896-6273(91)90349-5. [DOI] [PubMed] [Google Scholar]
- Lue Y, Sinha Hikim AP, Swerdloff S, Im P, Taing KS, Bui T, Leung A, Wang C. Single exposure to heat induces stage-specific germ cell apoptosis in rats: role for intratesticular testosterone on stage specificity. Endocrinology. 1999;140:1709–1717. doi: 10.1210/endo.140.4.6629. [DOI] [PubMed] [Google Scholar]
- Mailhos C, Howard MK, Latchman DS. Heat shock protects neuronal cells from programmed cell death by apoptosis. Neuroscience. 1993;55:621–627. doi: 10.1016/0306-4522(93)90428-i. [DOI] [PubMed] [Google Scholar]
- Mailhos C, Howard MK, Latchman DS. Heat shock proteins hsp90 and hsp70 protect neuronal cells from thermal stress but not from programmed cell death. J Neurochem. 1994;63:1787–1795. doi: 10.1046/j.1471-4159.1994.63051787.x. [DOI] [PubMed] [Google Scholar]
- Maldonado-Codina G, Llamazares S, Glover DM. Heat shock results in cell cycle delay and synchronisation of mitotic domains in cellularised Drosophila melanogaster embryos. J Cell Sci. 1993;105:711–720. doi: 10.1242/jcs.105.3.711. [DOI] [PubMed] [Google Scholar]
- Mansoor S, Spano M, Baschieri S, Cividalli A, Mosiello L, Doria G. Effect of in vivo hyperthermia on thymocyte maturation and selection. Int Immunol. 1992;4:227–232. doi: 10.1093/intimm/4.2.227. [DOI] [PubMed] [Google Scholar]
- Marks N, Berg MJ. Recent advances on neuronal caspases in development and neurodegeneration. Neurochem Int. 1999;35:195–220. doi: 10.1016/s0197-0186(99)00061-3. [DOI] [PubMed] [Google Scholar]
- McCabe T, Simon RP. Hyperthermia induces 72kDa heat shock protein expression in rat brain in non-neuronal cells. Neurosci Lett. 1993;159:163–165. doi: 10.1016/0304-3940(93)90824-5. [DOI] [PubMed] [Google Scholar]
- Mieusset R, Bujan L, Mondinat C, Mansat A, Pontonnier F, Grandjean H. Association of scrotal hyperthermia with impaired spermatogenesis in infertile men. Fertil Steril. 1987;48:1006–1111. doi: 10.1016/s0015-0282(16)59600-9. [DOI] [PubMed] [Google Scholar]
- Mirkes PE, Cornel LM, Park HW, Cunningham ML. Induction of thermotolerance in early postimplantation rat embryos is associated with increased resistance to hyperthermia-induced apoptosis. Teratology. 1997;56:210–219. doi: 10.1002/(SICI)1096-9926(199709)56:3<210::AID-TERA4>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Mirkes PE, Little SA. Teratogen-induced cell death in postimplantation mouse embryos: differential tissue sensitivity and hallmarks of apoptosis. Cell Death Diff. 1998;5:592–600. doi: 10.1038/sj.cdd.4400390. [DOI] [PubMed] [Google Scholar]
- Mirkes PE, Little SA. Cytochrome c release from mitochondria of early postimplantation murine embryos exposed to 4-hydroperoxycyclophosphamide, heat shock, and staurosporine. Toxicol Appl Pharmacol. 2000;162:197–206. doi: 10.1006/taap.1999.8849. [DOI] [PubMed] [Google Scholar]
- Mori C, Nakamura N, Dix DJ, Fujioka M, Nakagawa S, Shiota K, Eddy EM. Morphological analysis of germ cell apoptosis during postnatal testis development in normal and hsp70-2 knockout mice. Dev Dyn. 1997;208:125–136. doi: 10.1002/(SICI)1097-0177(199701)208:1<125::AID-AJA12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Morimoto RI, Tissieres A, and Georgopoulos C 1994 The Biology of the Heat Shock Proteins and Molecular Chaperones. Cold Spring Harbor Laboratory Press, New York, 610 p. [Google Scholar]
- Mosser DD, Duchaine J, Bourget L, Martin LH. Changes in heat shock protein synthesis and heat sensitivity during mouse thymocyte development. Dev Genet. 1993;14:148–158. doi: 10.1002/dvg.1020140209. [DOI] [PubMed] [Google Scholar]
- Mouihate A, Pittman QJ. Lipopolysaccharide-induced fever is dissociated from apoptotic cell death in the rat brain. Brain Res. 1998;805:85–103. doi: 10.1016/s0006-8993(98)00675-1. [DOI] [PubMed] [Google Scholar]
- Nagata S, Goldstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- Namura S, Zhu J, Fink K, Endres M, Srinivasan A, Tomaselli KJ, Yuan J, Moskowitz MA. Activation and cleavage of caspase-3 in apoptosis induced by experimental cerebral ischemia. J Neurosci. 1998;18:3659–3668. doi: 10.1523/JNEUROSCI.18-10-03659.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardue ML, Ballinger DG, Hogan NC. The heat shock response. Cells coping with transient stress. Ann N Y Acad Sci. 1992;663:125–138. doi: 10.1111/j.1749-6632.1992.tb38656.x. [DOI] [PubMed] [Google Scholar]
- Parsell DA, Lindquist S. The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu Rev Genet. 1993;27:437–496. doi: 10.1146/annurev.ge.27.120193.002253. [DOI] [PubMed] [Google Scholar]
- Pettmann B, Henderson CE. Neuronal cell death. Neuron. 1998;20:633–647. doi: 10.1016/s0896-6273(00)81004-1. [DOI] [PubMed] [Google Scholar]
- Raff M. Cell suicide for beginners. Nature. 1998;396:119–122. doi: 10.1038/24055. [DOI] [PubMed] [Google Scholar]
- Rockett JC, Mapp FL, Garges JB, Luft JC, Mori C, Dix DJ. Effects of hyperthermia on spermatogenesis, apoptosis, gene expression, and fertility in adult male mice. Biol Reprod. 2001;65:229–239. doi: 10.1095/biolreprod65.1.229. [DOI] [PubMed] [Google Scholar]
- Ross ME. Cell division and the nervous system: regulating the cycle from neural differentiation to death. Trends Neurosci. 1996;19:62–68. doi: 10.1016/0166-2236(96)89622-6. [DOI] [PubMed] [Google Scholar]
- Russell LD, Etllin RA, Sinha Hikim AP, and Clegg ED 1990 Histological and Histopathological Evaluation of the Testis. Cache River Press, Clearwater, FL, 286 p. [Google Scholar]
- Sakaguchi Y, Stephens LC, Makino M, Kaneko T, Strebel FR, Danhauser LL, Jenkins GN, Bull JMC. Apoptosis in tumors and normal tissues induced by whole body hyperthermia in rats. Cancer Res. 1995;55:5459–5464. [PubMed] [Google Scholar]
- Samali A, Orrenius S. Heat shock proteins: regulators of stress response and apoptosis. Cell Stress Chaperones. 1998;3:228–236. doi: 10.1379/1466-1268(1998)003<0228:hspros>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellins KS, Cohen JJ. Hyperthermia induces apoptosis in thymocytes. Radiat Res. 1991;126:88–95. [PubMed] [Google Scholar]
- Sgonc R, Boeck G, Dietrich H, Gruber J, Recheis H, Wick G. Simultaneous determination of cell surface antigens and apoptosis. Trends Genet. 1994;10:41–42. doi: 10.1016/0168-9525(94)90140-6. [DOI] [PubMed] [Google Scholar]
- Srinivasan A, Roth KA, Sayers RO, Shindler KS, Wong AM, Fritz LC, Tomaselli KJ. In situ immunodetection of activated caspase-3 in apoptotic neurons in the developing nervous system. Cell Death Differ. 1998;5:1004–1016. doi: 10.1038/sj.cdd.4400449. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Tanaka R, Watanabe M, Takahashi H, Kakinuma K, Suda T, Yamada M, Takahashi H. Effects of whole-body hyperthermia on the canine central nervous system. Int J Hyperthermia. 1999;15:203–216. doi: 10.1080/026567399285729. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Marunouchi T. Immunohistochemical analysis of developmental stage of external granular layer neurons which undergo apoptosis in postnatal rat cerebellum. Neurosci Lett. 1998;242:85–88. doi: 10.1016/s0304-3940(98)00032-9. [DOI] [PubMed] [Google Scholar]
- Thomaidou D, Mione MC, Cavanagh JFR, Parnavelas JG. Apoptosis and its relation to the cell cycle in the developing cerebral cortex. J Neurosci. 1997;17:1075–1085. doi: 10.1523/JNEUROSCI.17-03-01075.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lookeren Campagne M, Lucassen PJ, Vermeulen JP, Balazs R. NMDA and kainate induce internucleosomal DNA cleavage associated with both apoptotic and necrotic cell death in the neonatal rat brain. Eur J Neurosci. 1995;7:1627–1640. doi: 10.1111/j.1460-9568.1995.tb01158.x. [DOI] [PubMed] [Google Scholar]
- Verheij M, Bose R, and Lin XH. et al. 1996 Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature. 380:75–79. [DOI] [PubMed] [Google Scholar]
- von Boehmer H, Teh HS, Kisielow P. The thymus selects the useful, neglects the useless and destroys the harmful. Immunol Today. 1989;10:57–61. doi: 10.1016/0167-5699(89)90307-1. [DOI] [PubMed] [Google Scholar]
- Walsh DA, Li K, Crowther C, Marsh D, and Edwards M 1991 Thermotolerance and heat shock response during early development of the mammalian embryo. In: Heat Shock and Development, vol 17, ed Hightower LE, Nover L. Berlin Springer-Verlag, Heidelberg, 58–70. [DOI] [PubMed] [Google Scholar]
- Westermann J, Ronneberg S, Fritz FJ, Pabst R. Proliferation of lymphocyte subsets in the adult rat: a comparison of different lymphoid organs. Eur J Immunol. 1989;19:1087–1093. doi: 10.1002/eji.1830190619. [DOI] [PubMed] [Google Scholar]
- Westra A, Dewey WC. Variation in sensitivity to heat shock during the cell-cycle of chinese hamster cells in vitro. Int J Radiat Biol. 1971;19:467–477. doi: 10.1080/09553007114550601. [DOI] [PubMed] [Google Scholar]
- Wolf BB, Green DR. Suicidal tendencies: apoptotic cell death by caspase family proteinases. J Biol Chem. 1999;274:20049–20052. doi: 10.1074/jbc.274.29.20049. [DOI] [PubMed] [Google Scholar]
- Yakovlev AG, Knoblach SM, Fan L, Fox GB, Goodnight R, Faden AI. Activation of CPP32-like caspases contributes to neuronal apoptosis and neurological dysfunction after traumatic brain injury. J Neurosci. 1997;17:7415–7424. doi: 10.1523/JNEUROSCI.17-19-07415.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Hawkins KL, DeWolf WC, Morgentaler A. Heat stress causes testicular germ cell apoptosis in adult mice. J Androl. 1997;18:159–165. [PubMed] [Google Scholar]