Abstract
The unique potential of chloroplasts/thylakoids to harness light energy to transport electrons from H2O to various entities was exploited for reduction of Ag+ to generate nanoparticles (NPs). Spinach thylakoids/chloroplasts turned AgNO3 solutions brown in light, but not in dark. Besides showing Ag-NPs specific surface plasmon resonance band, these brown solutions showed presence of 5–30 nm crystalline NPs composed of Ag. Powder X-ray diffraction (PXRD) analysis revealed that Ag-NPs were biphasic composed of face-centered cubic Ag0 and cubic Ag2O. X-ray photoelectron spectroscopy (XPS) data further corroborated the presence of Ag2O in Ag-NPs. Limited formation of Ag-NPs in dark and increased generation of Ag0/Ag2O–NPs with increase in light intensity (photon flux density) by thylakoids/chloroplasts, established the role of light-harvesting photosynthetic machinery in generation of Ag0/Ag2O-NPs. Potential of thylakoids/chloroplasts to generate Ag-NPs from Ag+ on exposure to red and blue wavelength regions of visible light of electromagnetic spectrum, further confirmed the involvement of photosynthetic electron transport in reduction of Ag+ and generation of Ag-NPs. While light energy mediated photosynthetic electron transport donates energized electrons extracted from H2O to Ag+ to form Ag0-NPs, O2 released as a by-product during photolysis of H2O oxidizes Ag0 to form Ag2O-NPs. Our findings furnish a novel, simple, economic and green method that can be exploited for commercial production of Ag0/Ag2O-NPs.
Introduction
Owing to unique properties, silver nanoparticles (Ag-NPs) find immense applications in engineering, medicine, agriculture and environment [1]. Due to excellent antimicrobial properties, there is a growing demand in use of Ag-NPs in hospitals, textiles, paints, emulsions, cosmetics etc. [1–3]. In spite of development of a number of green methods using biological systems or their components (which include extracts from various microbes/plants), none of the methods could replace chemical methods used for commercial production of NPs [4,5]. Although extracts of various plants/microbes are effective in generation of Ag-NPs, their use has been limited due to vast heterogeneity (in terms of size, shape and composition) of NPs generated. The vast heterogeneity results due to the presence of wide variety/range of biomolecules such as sugars, organic acids including amino acids, phenolics, soluble proteins etc., most of which have potential to generate metal nanoparticles [1–7].
Generation of Ag-NPs from Ag+ basically relies on reduction by reducing agents that have higher negative redox potential compared to Ag+ [6]. It is well established that light-harvesting photosynthetic machinery has immense potential to extract, energize and transport electrons from water to reduce various entities, which include NO2-, SO42- and O2, besides the key terminal acceptor, NADP+ [7,8]. This prompted us to evaluate if this potential of light-harvesting photosynthetic machinery to extract, energize and donate electrons can be exploited for reduction of Ag+. Here, we are reporting for the first time that light-harvesting photosynthetic machinery not only promotes reduction of Ag+ to Ag0 but also supports rapid oxidation of Ag0 to Ag2O by O2 released during photolysis of water to generate biphasic Ag-NPs.
Materials and Methods
Isolation of Thylakoids/Chloroplasts
Plants of spinach (Spinacia oleracea L.) were raised from seeds purchased from Agricultural Technology Information Centre (Pusa, New Delhi, India). Chloroplasts/thylakoids were isolated from spinach leaves as described earlier [7]. Leaves of S. oleracea were sliced into small pieces and incubated in isolation buffer (400 mM phosphate buffer with 5 mM NaCl, 1 mM MgCl2, 2 mM EDTA; pH 7.6) for 60 min in dark at 4°C. The leaves were then homogenized with chilled isolation buffer and filtered through 4 layers of Mira cloth. The filtered extract was centrifuged at 5000 xg at 4°C for 10 min and the resultant pellet containing thylakoids/chloroplast was suspended in distilled water instead of phosphate buffer as AgNO3 forms white precipitate with phosphate buffer. Thylakoids/Chloroplasts were washed 4 times with distilled water, through repeated suspension and centrifugation, to remove biomolecules like phenolics, sugars, free amino acids etc., which are known to promote generation of NPs [6,7,9]. The levels of chlorophyll, phenolics, sugars and amino acids were determined as described earlier [8,10].
Evaluating Potential of Thylakoids/Chloroplasts to Generate Ag-NPs
4 mL reaction mixture consisting of 0, 0.1, 0.25 and 0.5 mM AgNO3 and thylakoids/chloroplasts equivalent to ~200 μg Chl was incubated under a photon flux density (PFD) of ~600 μmol m-2s-1 for different time intervals at 24±2°C. Another set of thylakoids/chloroplasts with AgNO3 was kept under similar conditions in dark.
For evaluating impact of light intensities on generation of Ag-NPs, thylakoids/chloroplasts were incubated with 0.5 mM AgNO3 under different PFDs (0, 60, 300, and 600 μmol m-2s-1), for different time intervals at 24±2°C. The absorbance of the samples was measured at 410 nm at regular time intervals.
For further confirming the involvement of light harnessing photosynthetic electron transport of isolated thylakoids/chloroplasts in reduction of Ag+ to generate Ag-NPs, the reaction mixture consisting of 0.5 mM AgNO3 and thylakoids equivalent to ~200 μg Chl were exposed to ~600 μmol m-2s-1 of red and blue light at 24±2°C. Blue and red light were supplied using 465 nm and 650 nm broad band filters [Swarovski Optik, USA (delivered by Advanced Research Scientific, India)], respectively.
Characterization of NPs
NPs were characterized using UV-Vis spectrophotometer, transmission electron microscope (TEM) [coupled with energy dispersive X-ray (EDX) and selected area electron diffraction (SAED)] and powder X-ray diffraction (PXRD) as described earlier [7]. Chemical state of Ag-NPs was analyzed through X-ray photoelectron spectroscopy (XPS; Phi 5000 VersaProbe, Ulvac-Phi, Chigasaki, Japan).
Establishing the Release of O2 by Thylakoids/Chloroplasts and its Role in generation of Ag2O-NPs
Potential of thylakoids to evolve O2 (i.e. PS II activity) was measured polarographically using Oxygraph enabled Clark Type liquid phase Oxygen electrode (Hansatech, UK). Reaction mixture (1 ml) containing thylakoids (equivalent to ~200 μg Chl) and 1μmole p-benzoquinone was illuminated with PFD of 600 μmol m-2s-1 and the rate of O2 evolution was measured. PS II activity was represented as μmol O2 evolved mg Chl-1 h-1.
To confirm that the O2 released by light-harvesting photosynthetic machinery of thylakoids/chloroplasts leads to oxidation of Ag0/Ag0-NPs to form Ag2O-NPs, investigations were carried under controlled anaerobic conditions. For achieving controlled anaerobic conditions (i) AgNO3 and other solutions used in this set of investigations were bubbled with saturated levels of N2 gas; (ii) vials containing the reaction mixture were capped and placed in a desiccator; (iii) air in this desiccator was displaced with N2 gas. Subsequently, the desiccator containing reaction the mixtures as well as respective control solutions was exposed to light of ~600 μmol m-2 s-1 PFD at 24±2°C.
All experiments were carried out independently at least six times and the data was subjected to Duncan’s multiple range test [11].
Results and Discussion
Photosynthetic machinery has unique potential to harness light energy for extracting and energizing electrons from H2O for not only fixing CO2 but also for reduction of various entities associated with nitrogen and sulfur metabolism [6]. Isolated spinach thylakoids/chloroplasts turned AgNO3 solutions brown on exposure to light of ~600 μmol m-2 s-1 PFD within 60 min (Fig 1A). It is established that alteration in color of AgNO3 solution to brown is due to generation of Ag-NPs [2,3,12]. However, AgNO3 solutions incubated with thylakoids/chloroplasts in dark remained green (Fig 1). Absorption spectra of thylakoids/chloroplasts incubated in (i) light in absence of AgNO3 (control); and in (ii) dark in absence or presence of AgNO3, showed peaks specific for photosynthetic pigments [13,14] (Fig 1B and 1C). However, absorption spectra of AgNO3 solutions incubated with thylakoids/chloroplasts in light showed a prominent peak at ~410 nm (Fig 1C), which overlapped with peaks of photosynthetic pigments in this region. This specific peak around 410 nm, which arises due to surface plasmon oscillations of Ag-NPs [2,3,12], intensified with increase in AgNO3 concentration.
Fig 1. Potential of isolated thylakoids/chloroplasts to generate Ag-NPs.
Variation in color of reaction mixtures containing different levels of AgNO3 (mM) incubated with isolated spinach thylakoids/chloroplasts in light (L) and dark (D) for 60 min (A). Absorption spectra of AgNO3 containing reaction mixtures incubated with spinach thylakoids/chloroplasts in dark (B) and light (C). Absorption band at ~410 nm in (C) corresponds to surface plasmon oscillations of Ag0/Ag2O-NPs. Insets show absorbance spectra of AgNO3 containing reaction mixtures incubated with thylakoids/chloroplasts in dark or in light on a magnified X-axis ranging from 350–630 nm. Absorption bands in the blue region at ~440 and 477 nm correspond to Chl a and Chl b, respectively. Absorption band at 470 nm correspond to carotenoids. Absorption bands at ~677 and 650 nm correspond to Chl a and Chl b, respectively.
AgNO3 solutions incubated without thylakoids/chloroplasts under identical conditions remained colorless both in light as well as dark (Fig 2). Since generation of Ag-NPs relies on reduction of Ag+, we believed that light energized photosynthetic machinery of spinach thylakoids/chloroplasts donates electrons to Ag+ to form Ag0 and Ag0-NPs. Heat killed thylakoids/chloroplasts failed to turn colorless AgNO3 solutions brown on incubation under identical conditions both in light as well as dark, further establishing involvement of photosynthetic machinery of live/active thylakoids/chloroplasts in generation of Ag-NPs (Fig 2).
Fig 2. Thylakoids/chloroplasts must be photosynthetically active for generation of Ag-NPs in presence of light.
Variation in color of reaction mixtures containing different levels of AgNO3 (mM) incubated without (-) and with (+) heat killed isolated spinach thylakoids/chloroplasts under a photon flux density of 600 μmol photons m-2 s-1 for 4 h.
TEM investigations confirmed the presence of distinct NPs in brown solutions that resulted due to incubation of AgNO3 with thylakoids/chloroplasts in presence of light. Ag-NPs generated by thylakoids/chloroplasts were mostly spherical and in the size range of 10–30 nm (Fig 3A and 3B). EDX spectra showed the presence of distinct peaks which correspond to Ag (Fig 3C), conforming that NPs were composed of Ag. The PXRD pattern showed Bragg reflections (111), (200) and (311) revealing the face centered cubic structure and crystalline nature of Ag-NPs (Fig 3E). SAED pattern further corroborated that these NPs were crystalline in nature (inset in Fig 3E). Additional peaks seen in the PXRD spectra also corresponded to Bragg reflections (111)*, (211)*, (220)*, (221)* of cubic Ag2O (Fig 3E). Thus, PXRD studies clearly revealed that Ag-NPs were biphasic, composed of both Ag0 and Ag2O. It is well-known that Ag0 and Ag0-NPs are prone to oxidation [3,15,16] and thylakoids/chloroplasts generate O2 as a byproduct during extraction of electrons from water [7,8]. Potential of isolated spinach thylakoids/chloroplasts to evolve O2 is depicted in Fig 4. We also characterized Ag-NPs through XPS to further confirm the oxidation state of Ag. The high resolution XPS spectra showed two peaks arising due to emission of 3d5/2 and 3d3/2 photoelectrons at binding energies of ~367.7 and ~373.7 eV, respectively (Fig 3D). These peaks at the respective binding energies correspond to the values reported for Ag2O [17]. Thus, the XPS data clearly showed that Ag exists in +1 oxidation state (Ag+) i.e., in the form of Ag2O in these Ag-NPs, which is in corroboration with the PXRD data.
Fig 3. Characterization of Ag-NPs generated by thylakoids/chloroplasts in presence of light.
TEM pictures (A-B); EDX spectrum (C); High resolution XPS spectrum (D); and PXRD pattern (E) of Ag0/Ag2O-NPs generated by spinach thylakoids/chloroplasts in presence of light. Inset in E represents selected area diffraction pattern of Ag0/Ag2O-NPs.
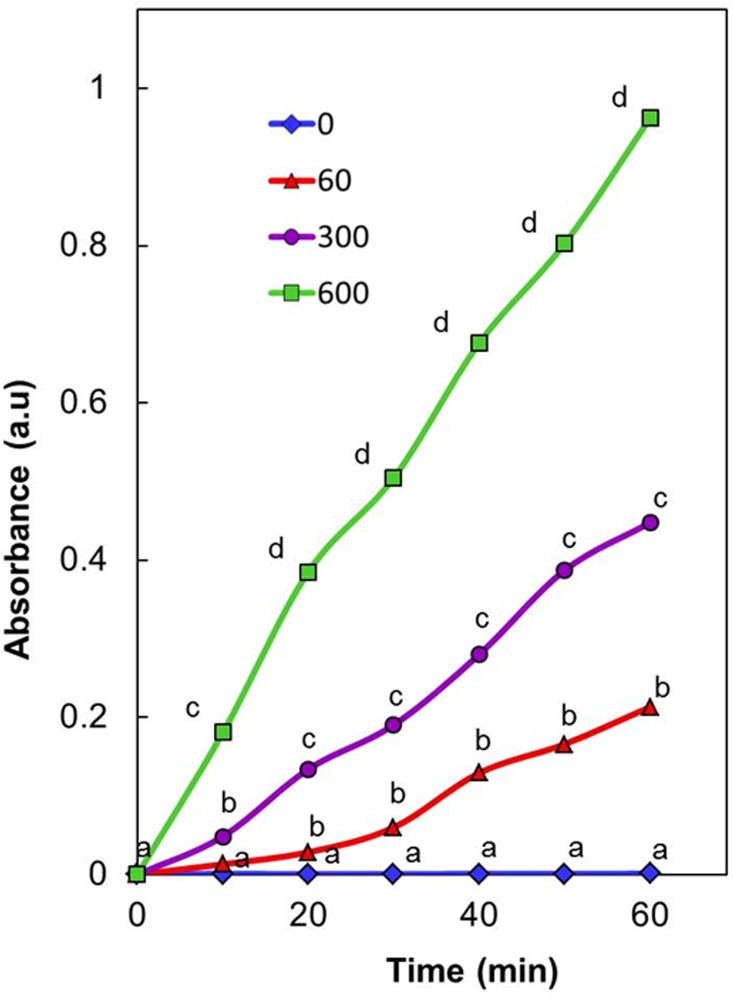
Fig 4. Impact of light intensity on potential of thylakoids/chloroplasts to generate Ag-NPs.
Generation of Ag0/Ag2O-NPs by isolated spinach thylakoids/chloroplasts incubated in 0.5 mM AgNO3 exposed to light of varying photon flux density (μmol m-2s-1) for different time intervals. Values represent mean of data collected from six independent experiments. Values designated by different small letters are significantly different at P≤0.05 (Duncan’s multiple range test).
As photosynthetic electron transport is light intensity dependent, potential of thylakoids/chloroplasts to generate Ag0/Ag2O-NPs was evaluated under different PFDs, namely 60, 300, and 600 μmol photons m-2s-1 for different time intervals. The potential of thylakoids/chloroplasts to generate Ag0/Ag2O-NPs increased with increase in (i) PFD; and (ii) exposure duration (Fig 4). Increase in generation of Ag0/Ag2O-NPs with increase in PFD further established the involvement of photosynthetic electron transport in generation of Ag-NPs.
It is well documented that red and blue wavelengths of the visible part of the electromagnetic spectrum are most effective for photosynthesis. Photosynthetic pigments absorb blue and red light more efficiently than the other spectral regions of photosynthetically active radiation [18]. Hence, overall photosynthetic electron transport linked to PS II quantum efficiency would be superior in red and blue regions of visible light. In order to further establish that photosynthetic electron transport of thylakoids/chloroplasts is involved in reduction of Ag+ and generation of Ag-NPs, we used broad band red (650 nm) and blue (465 nm) filters. As anticipated, we recorded alteration in color of green colored thylakoids/chloroplasts incubated with 0.5 mM AgNO3 to brown due to reduction of Ag+ and generation of Ag-NPs on exposure to red and blue wavelengths of light (Fig 5). These results further corroborated that light harnessing photosynthetic machinery of thylakoids is responsible for reduction of Ag0 and generation of Ag0/Ag2O-NPs.
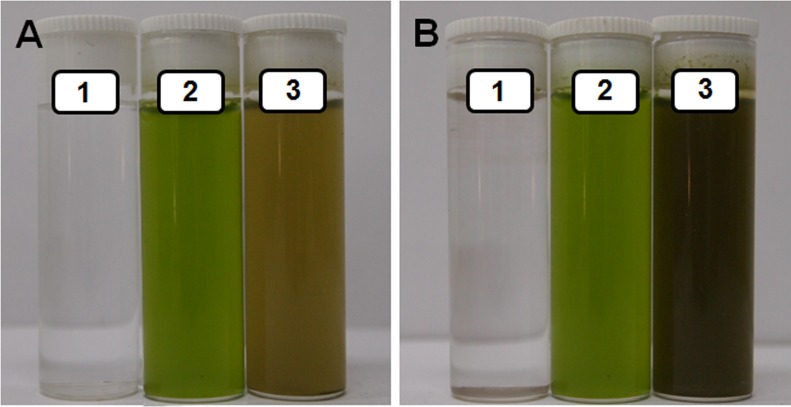
Fig 5. Potential of isolated thylakoids/chloroplasts to generate Ag-NPs in presence of red and blue light.
Capacity of isolated spinach thylakoids/chloroplasts to generate Ag0/Ag2O nanoparticles from 0.5 mM AgNO3 on exposure to red (A) and blue (B) regions of visible light. 0.5 mM AgNO3 (1), thylakoids/chloroplasts incubated in absence (2) and presence (3) of 0.5 mM AgNO3 were exposed to ~600 μmol photons m-2 s-1 of red (650) and blue (465) wavelengths of visible light of electromagnetic spectrum.
It is well-known that light harvesting photosynthetic machinery of thylakoids/chloroplasts generate O2 as a byproduct during extraction of electrons from water [7,8]. We believe that Ag0 and Ag0-NPs, which are highly susceptible to oxidation [13,15,16], are readily oxidized by O2 released during photolysis of water, by light harvesting photosynthetic machinery of thylakoids. Potential of isolated spinach thylakoids/chloroplasts to evolve O2 in presence of light is depicted in Fig 6. In order to establish/confirm that photosynthetic machinery of thylakoids/ chloroplasts is the key source of O2 involved in oxidation of Ag0 and Ag0-NPs to form Ag2O-NPs, thylakoids incubated with AgNO3 solutions were maintained under near controlled anaerobic conditions in a desiccator (Fig 7A). Even under nearly anaerobic conditions, we recorded formation of NPs. PXRD analysis showed sharp Bragg reflections (111)*, (211)*, (220)* and (221)*, which revealed that the majority of these NPs were composed of Ag2O (Fig 7B). These results further established that it is the O2 released during photolysis of water by the light harvesting machinery of thylakoids/chloroplasts that plays key role in oxidation of Ag0 and Ag0-NPs to form Ag2O-NPs. It is well known that the light harvesting photosynthetic machinery is the key source of oxygen in the biosphere.
Fig 6. Potential of isolated thylakoids/chloroplasts to evolve O2.
Potential of isolated spinach thylakoids/chloroplasts to evolve O2 rapidly on exposure to light of 600 μmol photons m-2s-1. Rate of photosystem (PS) II dependent O2 evolution was measured polarographically according to Shabnam et al. [8], using Oxygraph system enabled Clark type liquid phase O2 electrode (Hansatech Ltd., UK). Thylakoids/Chloroplasts equivalent to 20 μg of chlorophyll were used for each assay. It is evident that isolated thylakoids/chloroplasts evolve O2 only in presence of light.
Fig 7. Thylakoids/chloroplasts are the primary source of O2 for generation of Ag2O-NPs in presence of light.
Potential of isolated thylakoids/chloroplasts to generate Ag0/Ag2O-NPs under near controlled anaerobic conditions rapidly in presence of light of 600 μmol photons m-2s-1. Note variation in color of reaction mixtures containing different levels of AgNO3 (mM) incubated without (-) and with (+) isolated spinach thylakoids/chloroplasts (A). PXRD pattern (B) of Ag-NPs generated by spinach thylakoids/chloroplasts in presence of light. Bragg reflections (111)*, (211)*, (220)*, (221)* confirmed that large proportion of these NPs are composed of Ag2O.
Mechanism of Ag0/Ag2O-NPs Generation by Isolated Thylakoids/Chloroplasts
Based on ultrastructural investigations that revealed prevalence of NPs in abundance in chloroplasts, earlier researchers believed the probable involvement of chloroplasts in generation of noble metal NPs [6,19,20]. Beattie and Haverkamp [6] believed that the reducing sugars produced in chloroplasts are responsible for the generation of noble metal-NPs. Although, we do believe that reducing sugars could have a role in generation of noble metal NPs in living cells, during present investigations, no detectable levels of sugars were seen in association with the isolated thylakoids/chloroplasts. Similarly, no traces of phenolics or amino acids were found in the isolated thylakoids/chloroplasts preparations used during present investigations. These observations convincingly demonstrated that the potential of isolated thylakoids/chloroplasts to generate Ag-NPs in presence of light is governed by the photosynthetic electron transport. Although, Dahoumane et al. [20] presumed participation of NAD(P)H-dependent reducing enzymes, like nitrate reductase, for generation of NPs in chloroplasts, we are strongly of the opinion that enzymes like nitrate reductase require specific substrates. Zhang et al. [21] reported generation of Au-NPs by isolated chloroplasts by stirring them in Au3+ solutions in water bath for 24–36 h and believed that proteins associated with chloroplasts were responsible for generation of Au-NPs. Contrary to earlier reports, our present investigations have clearly revealed the potential of thylakoids/chloroplasts to generate Ag-NPs in a short duration only in presence of light, clearly revealing the involvement of photosynthetic electron transport in generation of NPs through reactions depicted in Fig 8.
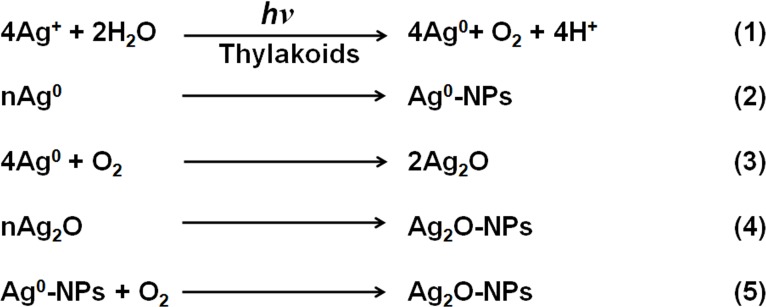
Fig 8. Steps involved in the synthesis of Ag0/Ag2O-NPs by isolated thylakoids/chloroplasts in presence of light.
Basic reactions involved in generation of Ag0/Ag2O-NPs by isolated spinach thylakoids/chloroplasts of spinach using light energy.
As photosynthetic electron transport machinery associated with thylakoids/chloroplasts, besides energizing and transporting the electrons (extracted from H2O) to the terminal acceptor NAD(P)+ by using light energy, can also transport electrons to other entities like O2, NO2-, SO42-, etc. to bring about their reduction [7], it is clear that light driven photosynthetic electron transport initially promotes reaction 1, which releases O2 in addition to reduction of Ag+ to Ag0. It is widely believed that Ag0 generate Ag0-NPs (reaction 2) through steps involving nucleation and agglomeration [22] and may also get oxidized by the prevailing O2 to form Ag2O (reaction 3). Latter agglomerates to generate Ag2O-NPs (reactions 4 and 5). It is known that Ag0 and Ag0-NPs are highly prone to oxidation and form Ag2O-NPs under oxygenic conditions [3,14,15]. Therefore, we believe that O2 produced in the thylakoids/chloroplasts during photolysis of water readily oxidize Ag0 and Ag0-NPs to form Ag2O-NPs. A hypothetical model illustrating mechanisms of generation of Ag0/Ag2O-NPs from thylakoids/chloroplasts from Ag+ is depicted in Fig 9.
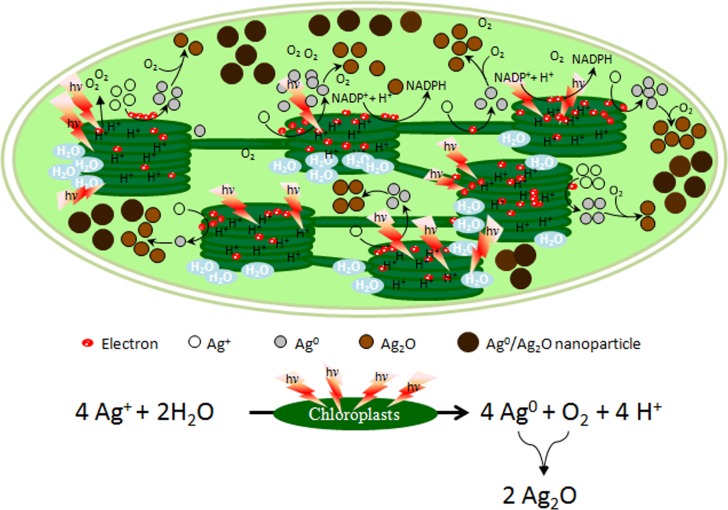
Fig 9. Mechanism of generation of Ag0/Ag2O-NPs by isolated thylakoids/chloroplasts in presence of light.
A hypothetical model depicting mechanism of light mediated generation of Ag0/Ag2O-NPs by isolated spinach thylakoids/chloroplasts. Electrons extracted from water are energized and transported by light mediated photosynthetic electron transport system to Ag+ to reduce them and generate Ag0 and Ag0-NPs. O2, which is released as a byproduct during photolysis of water, readily oxidizes Ag0 as well as Ag0-NPs to generate Ag2O-NPs.
Conclusions
Our findings clearly established that isolated thylakoids/chloroplasts can effectively generate Ag0/Ag2O-NPs using light energy. Light driven generation of Ag0/Ag2O-NPs by thylakoids/chloroplasts involves simple photochemical reactions involving (i) energization, transport and donation of electrons extracted from H2O through electron transport chain to Ag+ for its reduction to Ag0; (ii) spontaneous generation of Ag0-NPs through nucleation from Ag0; (iii) oxidation of Ag0 and Ag0-NPs to Ag2O-NPs by O2 released as a by-product during extraction of electrons from water. Light mediated generation of Ag0/Ag2O-NPs by thylakoids/chloroplasts is a novel, simple, economic and green method which can be exploited for large scale production of Ag-NPs, demand of which is growing rapidly.
Data Availability
All relevant data are within the paper.
Funding Statement
Support was provided by: the Department of Biotechnology, Ministry of Science and Technology (Award Number: BT/Bio-CARe/02/604/2011-12, Recipient: P. Sharmila); University of Delhi (IN) (Award Number: RC/2015/9677, Recipient: P. Pardha-Saradhi, Ph.D.); Korea Environmental Industry and Technology Institute (Award Number: 2015001790002, Recipient: Hyunook Kim); and University Grants Commission (Award Number: 3625(NET-DEC.2012), Recipient: Nisha Shabnam). The funding received during this specific study had no role in study design, data collection and analysis, decision to publish, or preparation of this manuscript.
References
- 1.Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2009; 27: 76–83. 10.1016/j.biotechadv.2008.09.002 [DOI] [PubMed] [Google Scholar]
- 2.Yamal G, Sharmila P, Rao KS, Pardha-Saradhi P. Inbuilt potential of YEM medium and its constituents to generate Ag/Ag2O nanoparticles. PLoS ONE. 2013; 8: e61750 10.1371/journal.pone.0061750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pardha-Saradhi P, Yamal G, Peddisetty T, Sharmila P, Nagar S, Singh J, et al. Reducing strength prevailing at root surface of plants promotes reduction of Ag+ and generation of Ag0/Ag2O nanoparticles exogenously in aqueous phase. PLoS ONE. 2014; 9: e106715 10.1371/journal.pone.0106715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma VK, Yngard RA, Lin Y. Silver nanoparticles: green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009; 145: 83–96. 10.1016/j.cis.2008.09.002 [DOI] [PubMed] [Google Scholar]
- 5.Song JY, Kim BS. Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioproc Biosyst Eng. 2009; 32: 79–84. [DOI] [PubMed] [Google Scholar]
- 6.Beattie IR, Haverkamp RG. Silver and gold nanoparticles in plants: sites for the reduction to metal. Metallomics. 2011; 3: 28–632. 10.1039/c1mt00044f [DOI] [PubMed] [Google Scholar]
- 7.Shabnam N, Pardha-Saradhi P. Photosynthetic electron transport system promotes synthesis of Au-nanoparticles. PLoS ONE. 2013; 8: e71123 10.1371/journal.pone.0071123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shabnam N, Sharmila P, Sharma A, Strasser RJ, Govindjee, Pardha-Saradhi P. Mitochondrial electron transport protects floating leaves of long leaf pondweed (Potamogeton nodosus Poir) against photoinhibition: comparison with submerged leaves. Photosynth Res. 2015; 125: 305–319. 10.1007/s11120-014-0051-3 [DOI] [PubMed] [Google Scholar]
- 9.Shabnam N, Pardha-Saradhi P, Sharmila P. Phenolics impart Au3+-stress tolerance to cowpea by generating nanoparticles. PLoS ONE. 2014; 9: e85242 10.1371/journal.pone.0085242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shabnam N, Pardha-Saradhi P. Floating and submerged leaves of Potamogeton nodosus exhibit distinct variation in antioxidant system as an ecophysiological adaptive strategy. Func Plant Biol. 2016; 43: 346–355. [DOI] [PubMed] [Google Scholar]
- 11.Duncan DB. Multiple range and multiple F tests. Biometrics. 1955; 39: 205–207. [Google Scholar]
- 12.Mohan S, Oluwafemi OS, George SC, Jayachandran VP, Lewu FB, Songca SP, et al. Completely green synthesis of dextrose reduced silver nanoparticles, its antimicrobial and sensing properties. Carbohydr Polym. 2014; 106: 469–474. 10.1016/j.carbpol.2014.01.008 [DOI] [PubMed] [Google Scholar]
- 13.Atal N, Pardha Saradhi P, Mohanty P. Inhibition of chloroplast photochemical reactions by treatment of wheat seedlings with low concentrations of cadmium: Analysis of electron transport activity and changes in fluorescence yield. Plant Cell Physiol. 1991; 32: 943–951. [Google Scholar]
- 14.Merzlyak MN, Chivkunova OB, Zhigalova TV, Naqvi KR. Light absorption by isolated chloroplasts and leaves: effects of scattering and ‘packing’. Photosynth Res. 2009; 102: 31–41. 10.1007/s11120-009-9481-8 [DOI] [PubMed] [Google Scholar]
- 15.Lowry GV, Espinasse BP, Badireddy AR, Richardson CJ, Reinsch BC, Bryant LD, et al. Long-term transformation and fate of manufactured Ag nanoparticles in a simulated large scale freshwater emergent wetland. Environ Sci Technol. 2012; 46: 7027–7036. 10.1021/es204608d [DOI] [PubMed] [Google Scholar]
- 16.Sharma VK, Siskova KM, Zboriln R, Gardea-Torresdey JL. Organic-coated silver nanoparticles in biological and environmental conditions: Fate, stability and toxicity. Adv Colloid Interface Sci. 2014; 204: 15–34. 10.1016/j.cis.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 17.Sarkar D, Ghosh CK, Mukherjee S, Chattopadhyay KK. Three dimensional Ag2O/TiO2 type-ii (p-n) nanoheterojunctions for superior photocatalytic activity. ACS Appl Mater Interfaces. 2013; 5: 331–337. 10.1021/am302136y [DOI] [PubMed] [Google Scholar]
- 18.Terfa MT, Solhaug KA, Gislerød HR, Olsen JE, Torre S. A high proportion of blue light increases the photosynthesis capacity and leaf formation rate of Rosa × hybrida but does not affect time to flower opening. Physiol Plantarum. 2013; 148: 146–159. [DOI] [PubMed] [Google Scholar]
- 19.Sicard C, Brayner R, Margueritat J, Hémadi M, Couté A, Yéprémian C, et al. Nano-gold biosynthesis by silica-encapsulated micro-algae: a “living” bio-hybrid material. J Mater Chem. 2010; 20: 9342–9347. [Google Scholar]
- 20.Dahoumane SA, Djediat C, Yepremian C, Coute´ A, Fiévet F, Coradin T, et al. Species selection for the design of gold nanobioreactor by photosynthetic organisms. J Nanopart Res. 2012; 14: 1–17.22448125 [Google Scholar]
- 21.Zhang YX, Zheng J, Gao G, Kong YF, Zhi X, Wang K, et al. Biosynthesis of gold nanoparticles using chloroplasts. Int J Nanomed. 2012; 6: 2899–2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thanh NTK, Maclean N, Mahiddine S. Mechanisms of nucleation and growth of nanoparticles in solution. Chem Rev. 2014; 114: 7610–7630. 10.1021/cr400544s [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.