Abstract
Although prior heat stress (HS) inhibits apoptosis in adenosine phosphate (ATP)-depleted renal epithelial cells (REC), the specific stress protein(s) responsible for cytoprotection have not been identified. The present study evaluated the hypothesis that Hsp72, the major inducible member of the Hsp70 family, protects REC against ATP depletion injury. In the presence of isopropyl-β-d-thiogalactoside (IPTG), a stable line of transfected opossum kidney cells was induced to overexpress human Hsp72 tagged with the flag epitope. Transfected cells from 2 clones that expressed Hsp72 at a level comparable with wild-type cells were subjected to transient heat stress (43°C for 1 hour). To assess the cytoprotective effect of Hsp72, transfected cells were subjected to transient ATP depletion followed by recovery in the presence vs the absence of IPTG. ATP depletion resulted in nuclear chromatin condensation without cell membrane injury (ie, minimal leak of lactate dehydrogenase) and activation of caspase-3, confirming that apoptosis is the major cause of cell death. In both clones cell survival 1–3 days after ATP depletion was significantly improved in the presence of IPTG. Selective overexpression of Hsp72 reproduced nearly 60% of the protective effect on the survival afforded by prior heat stress. In transfected cells subjected to ATP depletion, Hsp72 overexpression significantly inhibited caspase activation. In native renal cells brief ATP depletion markedly induced the expression of native Hsp72, a finding identical to that observed after renal ischemia in vivo. These studies are the first to directly show that Hsp72 per se mediates acquired resistance to ischemic injury in REC.
INTRODUCTION
Heat stress, sufficient to induce Hsps, increases the resistance of cells as well as organs and intact organisms to a variety of noxious events, including hyperthermia, ischemia, and toxin exposure (Welch 1992). The most abundant and well characterized of the inducible heat stress proteins is Hsp72, a molecular chaperone in the Hsp70 family that refolds and repairs damaged proteins (Rassow et al 1997). Although elegant studies using Hsp72 deletion mutants provided important insights into the relationship between protein structure and function (Milarski and Morimoto 1989; Freeman et al 1995), the target proteins and the cell functions that are protected by Hsp72 are not well characterized.
In renal epithelial cells (REC) indirect evidence suggests that Hsp72 itself is responsible for improving survival after adenosine triphosphate (ATP) depletion. In an earlier report the degree of protection against ATP depletion positively correlated with the cellular content of the Hsp72 induced by heat stress (Wang and Borkan 1996). In nonrenal cells evidence of cytoprotection by Hsp72 is more direct, suggesting that this protein mediates at least part of the acquired resistance associated with heat stress (Jaattela et al 1992; Williams et al 1993; Heads et al 1994; Mestril et al 1994; Bellmann et al 1996; Ravagnan et al 2001). Surprisingly, a direct comparison between the protection conferred by Hsp72 and heat stress, a potent inducer of several stress proteins, has not been reported.
Apoptosis is an important cause of cell death after renal ischemia in vivo (Schumer et al 1992) and in REC subjected to ATP depletion in vitro (Lieberthal and Levine 1996; Lieberthal et al 1998; Wang et al 1999). Several laboratories have suggested that Hsp72 is responsible for improving cell survival by exerting antiapoptotic effects at specific points in the cell death pathway (Gabai et al 1997; Meriin et al 1999; Wang et al 1999; Li et al 2000). Using a model identical to that reported in the present study, our laboratory recently demonstrated that heat stress, sufficient to induce Hsp72 (as well as other heat inducible proteins), inhibited apoptosis in ATP-depleted REC (Wang et al 1999). Although these data suggest that Hsp72 is likely to mediate protection against ATP depletion injury, direct evidence is lacking.
To evaluate the hypothesis that Hsp72 improves cell survival after ATP depletion, opossum kidney (OK) cells were stably transfected with human Hsp72 (hHsp72) under the control of a lac-inducible promoter. ATP depletion induced changes in nuclear morphology (in the absence of cell membrane injury) and caspase activation that are characteristic of apoptosis. Selective induction of hHsp72 significantly improved cell survival and inhibited caspase-3 activation after transient ATP depletion. Hsp72 per se reproduced most of the cytoprotection conferred by prior heat stress. These results demonstrate, for the first time, that Hsp72 per se protects REC against injury caused by ATP depletion.
MATERIALS AND METHODS
Materials
All reagents were obtained from Sigma (St Louis, MO, USA) unless otherwise indicated.
Cell culture
OK cells, an immortalized line derived from the proximal tubule, were obtained from the American Type Culture Collection (ATCC #CRL-1840, Rockville, MD, USA) and were grown in Dulbecco modified Eagle medium (DMEM, GIBCO BRL, Grand Island, NY, USA) supplemented with 10% fetal calf serum. Unless otherwise indicated, cells were used within 72 hours of achieving confluence, as assessed by visual inspection with a phase contrast microscope.
ATP depletion
REC derived from OK were subjected to ATP depletion (0 mm glucose, 5 mm cyanide, and 5 mM of 2-deoxy-d-glucose for 1.5–2.0 hours). This maneuver reduces ATP content to <10% of the normal within 10 minutes. ATP content remains at this low level during the entire period of ATP depletion (Wang and Borkan 1996). Cells were permitted to recover in the presence of DMEM containing 5 mM dextrose.
Constructs and transfection
Complementary deoxyribonucleic acid (cDNA) coding for hHsp72 was removed from pRc/CMV-Hsp72 at the HindIII site (Knowlton 1999). Not 1 adapters (Stratagene, La Jolla, CA, USA) were used to convert the HindIII sites flanking Hsp72 to Not 1 sites. The pOPRSVICAT vector containing a neomycin-resistant gene (LAC-Switch™ Inducible Mammalian Expression System; Stratagene) was digested with Not 1 (New England Biolabs, Beverly, MA, USA) to remove the CAT insert. Hsp72 was then subcloned into this site. The correct orientation of Hsp72 was confirmed by restriction digestion. The resulting construct contained the full-length hHsp72 with a flag epitope (a residue of 8 amino acids) attached to the carboxyl terminus under the control of the RSV-LTR promoter (Knowlton 1999; Knowlton et al 2000). The RSV-LTR promoter contains a binding site for the lac-repressor protein. A second eukaryotic plasmid p3′SS contained the gene for the lac-repressor protein and a gene conferring resistance to hygromycin. Under basal conditions, the lac-repressor protein (coded for by the p3′SS plasmid) binds as a homotetramer to the lac-operator site (located on the pOPRSVICAT plasmid), blocking transcription of the downstream hHsp72-flag gene. To induce hHsp72 expression, isopropyl-β-d-thiogalactoside (IPTG) was used to decrease the affinity of the lac-repressor protein for the lac-operator site. To introduce these 2 plasmids, OK cells were subjected to a 2-step transfection using the calcium phosphate method (Chen and Okayma 1987). Up-regulation of Hsp72 caused by the expression of the plasmid cDNA was performed by immunoblot analysis using a specific antibody directed against the flag epitope (Eastman Kodak, Rochester, NY, USA). Transfected cells were sequentially selected by treatment with hygromycin and G-418. Under basal conditions and in the absence of IPTG, there was minimal expression of Hsp72. After adding IPTG (10 μM for 18 hours), increased expression of hHsp72 was observed.
Detection of Hsp72 and Hsc70
Steady-state Hsp72 (72 kDa) and Hsc70 (73 kDa) contents were measured in whole-cell lysates. To obtain cell lysates, harvested cells were resuspended in phosphate-buffered saline, pH 7.4, at 4°C, containing protease inhibitors (25 μM benzamidine, 0.5 mM phenylmethylsulfonyl-fluoride, and 0.2 IU/mL aprotinin, as described previously [Wang and Borkan 1996]). The cells were sonicated 10 times at 4°C (Bramson Cell Disrupter, Danbury, CT, USA) and centrifuged at 10 000 × g for 10 minutes. The supernatant was designated as the whole-cell lysate. Wild-type Hsp72 was detected by immunoblot, using a commercially available monoclonal antibody specific for Hsp72 (72 kDa) (Amersham, Arlington Hts, IL, USA, as previously reported [Wang and Borkan 1996]) or for Hsc70 (StressGen, Victoria, British Columbia, Canada). Hsp72 arising from the plasmid cDNA was detected with an antibody directed against the flag II epitope (Kodak, Rochester, NY, USA). The intensity of immunoreactive bands was analyzed with NIH image software after scanning 3 representative blots with a densitometer (Hewlett-Packard, Desk Scan II).
Cell survival
The number of cells surviving ATP depletion was estimated from the measurements of the total DNA in subconfluent cells before and for 1–3 days after ATP depletion, using an established spectrophotometric method (Wang and Borkan 1996). The data are expressed in micrograms of DNA per plate, using purified salmon DNA as the standard.
Lactate dehydrogenase release
Membrane injury that is characteristic of cell necrosis was estimated from the release of intracellular lactate dehydrogenase (LDH) from fresh samples maintained at 4°C. Enzyme activity was then measured at 37°C by a kinetic, spectrophotometric assay, as described previously (Wang and Borkan 1996). This assay measures the stoichiometric conversion of the reduced form of nicotinamide adenine dinucleotide to nicotinamide adenine dinucleotide, as pyruvate is converted to lactate. Cell LDH release is expressed as a percentage of the total enzyme activity after exposing the cells to 0.3 mg/mL digitonin (Wang and Borkan 1996).
Apoptosis assays
Chromatin condensation and the formation of apoptotic bodies were confirmed by exposing cells to Hoechst dye #33342, as described previously (Wang et al 1999). Changes in the appearance of chromatin visualized with this DNA-binding dye correlate with the morphological and biochemical evidences of apoptosis in this experimental model (Wang et al 1999).
Caspase activity
Caspase-3 enzyme activity was measured using a commercially available fluorometric assay (ApoAlert Caspase-3 fluorescence assay; Clontech Laboratories, Palo Alto, CA, USA). Cells grown on 60-mm2 dishes were lysed with 200 μL of chilled cell lysis buffer (Clontech Laboratories) on ice for 10 minutes. After centrifugation (12 000 rpm for 3 minutes at 4°C), 50 μL of the supernatant, 50 μL of 2× reaction buffer containing 1 mM dithiothreitol, and 5 μL caspase substrate (DEVD-AFC; 50 μM) were added to each well of a 96-well plate. After incubating at 37°C for 1 hour, fluorescence was determined using a plate reader with a 400-nm excitation filter and a 505-nm emission filter (Spectra Max Gemini, Molecular Devices Corp, Sunnyvale, CA, USA). The reported caspase activity was corrected for background fluorescence determined in the absence of the caspase-3 substrate. To confirm assay specificity, parallels were performed in the presence of a specific caspase-3 inhibitor (1 μL DEVD-CHO; Clontech Laboratories).
Protein assay
Protein concentrations were determined with a colorimetric dye-binding assay (BCA Assay, Pierce, Rockford, IL, USA). Results are expressed in milligrams of protein per milliliter.
Statistical analysis
Data are expressed as mean ± SE. Two groups were compared using an unpaired, 2-tailed Student's t-test. Results involving more than 1 group were compared using analysis of variance (ANOVA) and were then analyzed with Fisher's post hoc test. A result was considered significant if the P value was less than 0.05.
RESULTS
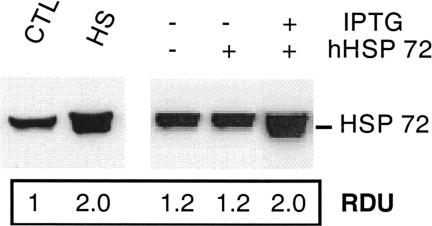
Expression of both wild-type Hsp72 and hHsp72 was compared in transfected and heat-stressed OK cells (Fig 1). As previously reported (Wang and Borkan 1996), a small amount of wild-type Hsp72 was detected in OK cells maintained at 37°C (control). Heat stress resulted in a 2-fold increase in the wild-type Hsp72 (P < 0.05, n = 3). Transfection with the hHsp72 gene in the absence of IPTG did not increase the total Hsp72 content. After exposure to IPTG, content of Hsp72 in the transfected clone was equivalent to that observed in the wild-type cells subjected to transient hyperthermia. Up-regulation of hHsp72 in transfected cells was associated with a marked accumulation of the flag epitope (data not shown). This demonstrates that the increase in Hsp72 content is caused by the expression of the plasmid cDNA coding for hHsp72.
Fig. 1.
Expression of Hsp72 after transfection or heat stress. Induction of Hsp72 in transfected and wild-type opossum kidney cells. Native Hsp72 content increased after exposure of wild-type cells to heat stress (43°C for 1 hour, HS). Human Hsp72 (hHsp72) increased in transfected cells after exposure to isopropyl-β-d-thiogalactoside (10 mM for 18 hours). The relative densitometric units shown beneath each lane compare the Hsp72 content with wild-type cells maintained at 37°C (CTL, lane 1). The increment in hHsp72 content in transfected cells was similar to the increase in native Hsp72 observed in wild-type cells after heat stress. The immunoblot is representative of 3 separate experiments
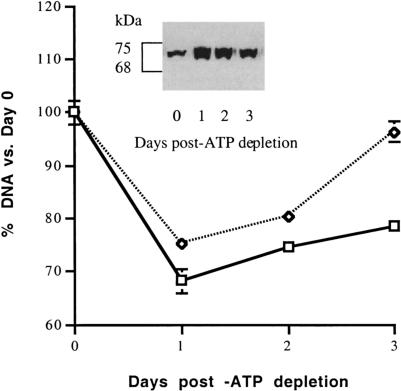
In the presence of IPTG, numerous clones were screened for potential overexpression of hHsp72. The screening of multiple clones was required because gene expression is insertion site dependent with the LAC Switch™ system (Stratagene product insert). Two clones demonstrated up-regulation of hHsp72 in the presence of IPTG (#9, #21). Compared with transfected cells in the absence of IPTG, the steady-state content of Hsp72 was increased in IPTG-exposed cells on days 1–3 (Fig 2, inset). One of these clones was subjected to transient ATP depletion for 1.5 hours followed by several days of recovery. Although cell number was significantly increased on days 1, 2, and 3 after ATP depletion (P < 0.05 for IPTG(−) vs IPTG(+); n = 3), differences in cell number associated with Hsp72 overexpression were most apparent on day 3 (Fig 2). The effect of Hsp72 expression on cell survival in subsequent studies was examined 3 days after ATP depletion.
Fig. 2.
Hsp72 increases cell survival after adenosine triphosphate (ATP) depletion. A clone of subconfluent, transfected cells with hHsp72 was subjected to ATP depletion for 1.5 hours. Cells were either preincubated with isopropyl-β-d-thiogalactoside (IPTG) (dashed line) or in the absence of IPTG (solid line). Cells were permitted to recover for 1, 2, or 3 days after ATP depletion before the deoxyribonucleic acid content was determined (n = 6 at each time point). The amount of immunoreactive Hsp72 present on days 0–3 in IPTG-exposed cells is shown (inset). Differences in cell survival were most evident on day 3. Hsp72 content did not change in the absence of IPTG (data not shown). Some error bars are too small to be seen
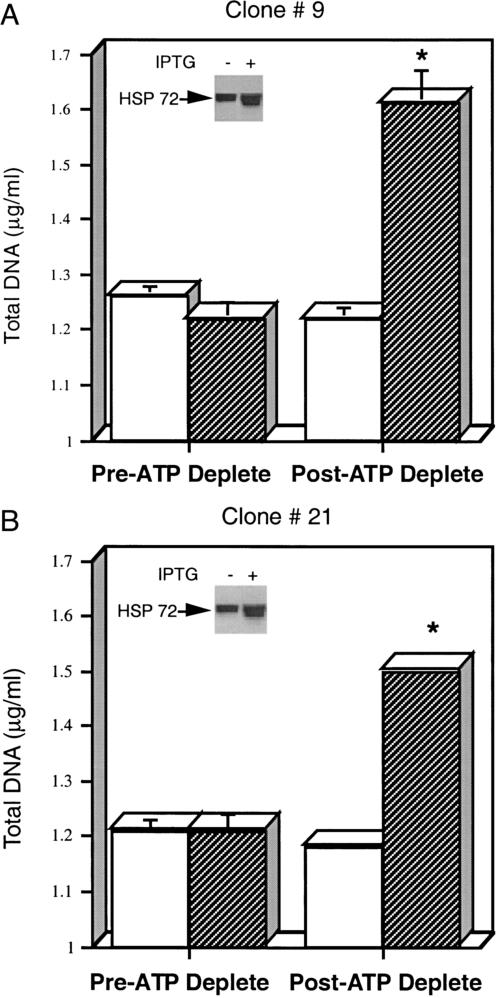
Additional studies were performed to examine cell survival 3 days after ATP depletion in the presence and absence of IPTG. In clones 9 and 21, cell number was significantly greater in the presence of IPTG (Fig 3 A,B). In the absence of IPTG the total DNA content did not significantly change by the third day after ATP depletion (clone #9, upper panel). In contrast, the addition of IPTG increased the Hsp72 content (inset) and significantly increased the cell number by day 3 of recovery (P < 0.05, n = 3). Similar results were obtained with clone #21 (lower panel). Exposure of wild-type cells to IPTG alone (10 mM for 16 hours) did not protect against ATP depletion (data not shown).
Fig. 3.
(A and B) Hsp72 protects against adenosine triphosphate (ATP) depletion injury. Two transfected clones overexpressing Hsp72 (clone 9, panel A; clone 21, panel B) were subjected to 1.5 hours of ATP depletion. Total cell deoxyribonucleic acid (DNA) was measured immediately before (pre-ATP deplete) and 3 days after ATP depletion (post-ATP deplete). Total cell DNA was assessed in isopropyl-β-d-thiogalactoside (IPTG), IPTG(−) (open bars) and IPTG(+) (darkly shaded bars), groups. IPTG did not protect wild-type cells against ATP depletion injury (data not shown). Measurements represent the mean of 6 determinations at each time point. Some standard error bars are too small to be seen. Hsp72 content before and after IPTG exposure in each clone (insets)
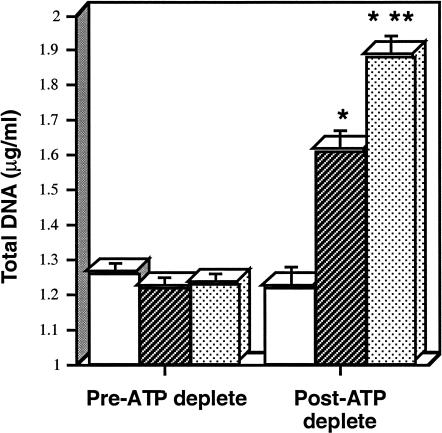
The magnitude of protection afforded by overexpressing hHsp72 was compared with heat stress, a maneuver known to improve renal cell survival after ATP depletion (Wang and Borkan 1996). Similar DNA content was observed in wild-type, heated, and transfected cells before the onset of ATP depletion (Fig 4). Prior heat stress provided the greatest degree of protection against ATP depletion. Selective overexpression of hHsp72 (IPTG(+) cells) reproduced approximately 60% of the protection associated with prior heating.
Fig. 4.
Cytoprotection of Hsp72 vs heat stress after adenosine triphosphate (ATP) depletion. Total deoxyribonucleic acid (DNA) content before and 3 days after 1.5 hours of ATP depletion. Mean DNA content (± SE) is depicted in cells transfected with human Hsp72 in the absence of isopropyl-β-d-thiogalactoside (IPTG) (open bars), in the presence of IPTG (darkly shaded bars), or in wild-type cells 16 hours after transient heat stress (stippled bars). * P < 0.05 pre- vs post-ATP depletion; ** P < 0.05 for transfected cell in the presence of IPTG vs heat stressed cells; n = 6 for each experimental group
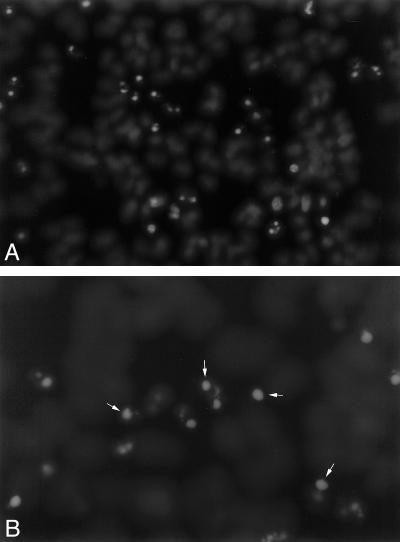
To confirm the mechanism of death, ATP-depleted cells were stained with Hoechst dye to evaluate the appearance of native chromatin. Chromatin condensation and apoptotic bodies are characteristic features of apoptosis (Schumer et al 1992; Lieberthal and Levine 1996). In the absence of IPTG (ie, with minimal Hsp72), numerous apoptotic cells and apoptotic bodies were observed 3 hours after recovery from ATP depletion (Fig 5 A,B). To exclude necrosis as an important cause of cell death, LDH release was assessed at the same time point. Less than 5% of the total cell content of this enzyme was released in either IPTG(−) or IPTG(+) cells (4.9 ± 0.02 vs 3.5 ± 0.3%, respectively; P > 0.05; n = 6). Chromatin condensation, in the absence of increased cell membrane injury, suggests that apoptosis rather than cell necrosis is the predominant form of cell death in this experimental model.
Fig. 5.
(A and B) Adenosine triphosphate (ATP) depletion induces apoptosis. Three hours after 60 minutes of ATP depletion, cells were stained with Hoechst dye to assess the nuclear morphology. Panel A: numerous cells show chromatin condensation (magnification: 200×). Panel B: apoptotic bodies representing small, membrane-bound packages of nuclear material are evident at higher magnification (400×). Some apoptotic bodies are indicated by the arrows. These morphologic changes are characteristic of apoptosis
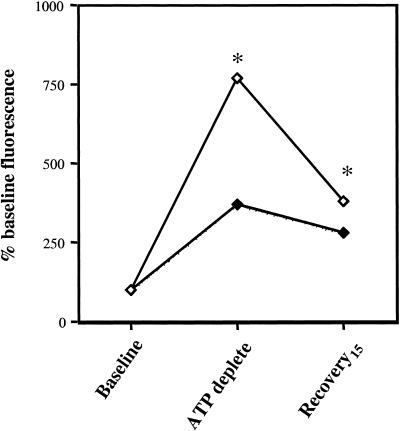
To quantify the effects of Hsp72 expression on apoptosis induced by ATP depletion, caspase-3, an executioner enzyme in the apoptotic enzyme cascade (Li et al 2000), was assessed. In control cells 60 minutes of ATP depletion was associated with a transient 7- to 8-fold increment in the relative activity of caspase-3 (Fig 6). The selective induction of Hsp72 significantly inhibited caspase activation immediately after ATP depletion as well as during early recovery (P < 0.05 for control vs Hsp72 at both time points, n = 3). The addition of DVED-CHO, a specific caspase-3 inhibitor to samples in vitro, completely abolished caspase-3 activity, confirming the specificity of this assay (data not shown).
Fig. 6.
Adenosine triphosphate (ATP) depletion activates caspase-3. Caspase-3 activity was measured in whole-cell lysates obtained from control (open symbols) and Hsp72-overexpressing cells (dark symbols) at baseline, immediately after 60 minutes of ATP depletion and after 15 minutes of recovery using an in vitro fluorescence assay. Data are expressed relative to the caspase-3 activity before injury. Each sample was assayed in duplicate. * P < 0.05 control vs Hsp72-overexpressing cells; n = 3. The standard error bars are too small to be seen
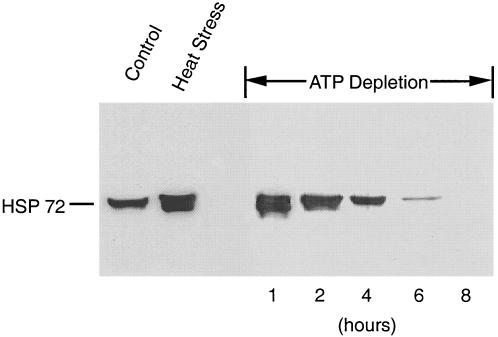
Transient ATP depletion of wild-type cells in vitro induced the expression of endogenous, native Hsp72 (Fig 7). Maximal expression of Hsp72 was observed after 1 hour of ATP depletion. After this brief period of ATP depletion, the Hsp72 content was virtually identical to that induced by heat stress. The Hsp72 content was also increased by longer periods of ATP depletion but to a lesser degree than that observed after 1 hour of de-energization. After 8 hours of ATP depletion, an insult associated with necrotic cell death (evidenced by the release of >80% cell LDH; data not shown), Hsp72 could no longer be detected. The latter observation suggests that cell necrosis disrupts the immunoreactive sites on this protein.
Fig. 7.
Adenosine triphosphate (ATP) depletion increases wild-type Hsp72. Opossum kidney cells were subjected to progressive ATP depletion for 1, 2, 4, 6, and 8 hours and then permitted to recover for 8 hours. After recovery, cell lysates were subjected to immunoblot analysis to assess steady-state Hsp72 content (right-hand panel). For comparison, the content of Hsp72 in cells maintained at 37°C (control) or exposed to heat stress (43°C for 1 hour followed by 8 hours recovery; heat stress) is also shown (left-hand panel). Each lane contains 8 μg of total protein. This immunoblot is representative of 3 separate studies
DISCUSSION
The present study demonstrates that selective overexpression of Hsp72 protects REC against injury caused by ATP depletion. This finding is consistent with our earlier report that heat stress, a nonspecific stimulus that induces several stress proteins including Hsp72, improved the survival of ATP-depleted REC (Wang and Borkan 1996). Using an identical model of ATP depletion, as described in the present study, we recently confirmed that apoptosis is the primary cause of REC death using both the morphologic and biochemical criteria (Wang et al 1999). Apoptosis has also been observed by others in renal cells subjected to pharmacologic ischemia in vitro (Lieberthal et al 1998) and is an important cause of cell death after renal ischemia in vivo (Schumer et al 1992). The relationship between ischemia and apoptosis might be predicted because mitochondrial injury is an early feature of ischemic injury (Lieberthal and Levine 1996; Wang and Borkan 1996), and mitochondrial injury triggers apoptosis (Lieberthal and Levine 1996; Kuhl et al 2000).
The mechanism by which Hsp72 inhibits apoptosis may be multifactoral and cell-type specific (Gabai et al 1997; Mosser et al 1997; Meriin et al 1999; Wang et al 1999; Li et al 2000; Ravagnan et al 2001). In nonrenal cells Hsp72 directly inhibits stress-activated protein kinase (SAPK) and c-Jun NH(2)-terminal kinase (JNK) stress kinase–dependent apoptosis (Gabai et al 1997; Mosser et al 1997). Hsp72 also inhibits the apoptotic cascade “distal” to cytochrome c release but “proximal” to caspase-3 activation (Li et al 1997; Beere et al 2000). More recently, Hsp70 has been shown to inhibit partial chromatin condensation and DNA fragmentation caused by the apoptosis-inducing factor (AIF), a mitochondrial flavoprotein (Ravagnan et al 2001). In renal cells Hsp72 interacts with Bcl2 (Wang et al 1999), an antiapoptotic factor that prevents the release of mitochondrial cytochrome c. Not only is cytochrome c a key enzyme in the electron transport pathway, but release of this enzyme into the cytosol initiates apoptosis (Korsmeyer et al 1993; Li et al 1997; Susin et al 1998). Cell death, in our experimental model, is primarily the result of apoptosis (supported by Hoechst positive staining, caspase-3 activation, and minimal LDH release). Because Hsp72 overexpression ameliorates caspase-3 activation and improves cell survival, it is likely that this cytoprotectant protein inhibits apoptotic cell death by one or more of these mechanisms.
Could Hsp72 improve cell number after ATP depletion by stimulating cell proliferation in a manner that is independent of cell death? Although possible, this hypothesis appears unlikely. Numerous reports suggest that the induction of Hsp72 retards, rather than stimulates, cell growth (Toba et al 1997; Kuhl et al 2000). In the present study the relatively slow increase in cell number between days 1–2 compared with days 2–3 correlates with a decrease in Hsp72 content (Fig 2). This inverse relationship between cell proliferation and Hsp72 content is consistent with the earlier reports and suggests that Hsp72 increases cell number primarily by enhancing cell survival. Ultimately, identification of the biochemical pathway(s) that are altered by Hsp72 in ATP-depleted renal cells will require additional investigation.
The selective overexpression of Hsp72 reproduces most of the protective effects conferred by heat stress on cell survival after ATP depletion. Hsp72 alone provided about 60% of the improvement in cell survival associated with prior heat stress. Relative to heat stress, incomplete cytoprotection associated with the selective overexpression of Hsp72 could result from several factors. Although the content of immunoreactive Hsp72 is similar in transfected and heated OK cells (Fig 1), the efficacy of transfected hHsp72 compared with that of the native protein in opossum cells is unknown. Alternatively, superior protection by heat stress may be the result of the simultaneous induction of multiple chaperones (Welch 1992; Rassow et al 1997). For example, substantial evidence suggests that Hsp70 and Hsp90 act in a synergistic manner to repair damaged proteins (Chen and Smith 1998; Csermely et al 1998). Hsp72 and Hsp60 also function as cochaperones in some situations (Ovelgonne et al 1995; Wagner et al 1996). Small Hsps, some of which interact with the actin cytoskeleton (eg, Hsp25/27), reduce thermal (Aufricht et al 1998; Okamoto 1999) as well as ischemic injury (Mehlen et al 1996) and may be necessary to optimize cytoprotection by Hsp72. Finally, superior protection by heat stress (compared with Hsp72 overexpression) may be attributable to differences in cofactors that regulate the Hsp70 function (Song et al 2001). Although Hsp72 alone is clearly sufficient to inhibit apoptosis after ATP depletion, its effectiveness as an antiapoptotic protein is likely to improve when other chaperones are simultaneously induced.
In addition to ameliorating ATP depletion injury in vitro, Hsp72 overexpression protects intact tissues against ischemia. Expression of Hsp72 significantly ameliorated ischemic injury in the isolated rat heart (Currie et al 1988), as well as in a transgenic mouse subjected to myocardial ischemia (Marber et al 1995). Although heat stress protects REC in the present and earlier in vitro studies (Wang and Borkan 1996; Borkan et al 1997; Wang et al 1999), protection has been inconsistent when the isolated kidney or intact animal has been subjected to hyperthermia as a means of increasing stress proteins. Although hyperthermia protected the pig kidney during organ transplantation (Perdrizet et al 1990) and reduced ischemic renal injury, in a preliminary report, in the intact rat (Kelly et al 1999), other investigators have not ameliorated ischemic renal injury after heating the whole animal (Zager et al 1994; Joannidis et al 1995). The inability to demonstrate renal protection in animals may be because of the toxicity of hyperthermic insult (William Currie, personal communication).
Although the beneficial effects of inducing Hsp72 in the intact kidney have yet to be demonstrated in vivo, insults that induce Hsp72 are frequently ameliorated when this molecular chaperone is expressed before injury (Currie et al 1988; Marber et al 1995). Significantly, pharmacologic ischemia in vitro (Fig 7) and renal ischemia in vivo markedly increase the Hsp72 content (Emami et al 1991; Zager et al 1994; van Why et al 1999). These observations might be expected because ATP depletion per se is a potent stimulus of the heat shock transcription factor, a primary regulator of Hsp72 expression (van Why et al 1999; Chang et al 2000). Data in the present study support the hypothesis that Hsp72 will provide a novel therapeutic tool for ameliorating the untoward effects of renal ischemia on organ function.
Acknowledgments
This research was supported by a National Institutes of Health Grant, DK-53387 (S.C.B.), a supplemental award from the American Society of Nephrology (S.C.B.), and a NHBLI grant HL-58515 (A.A.K.).
REFERENCES
- Aufricht C, Ardito T, Thulin G, Kashgarian M, Siegel NJ, and van Why SK 1998 Heat-shock protein 25 induction and redistribution during actin reorganization after renal ischemia. Am J Physiol (Renal). 274 F. 215–F222. [DOI] [PubMed] [Google Scholar]
- Beere HM, Wolf BB, and Cain K. et al. 2000 Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol. 2:469–475. [DOI] [PubMed] [Google Scholar]
- Bellmann K, Jaattela M, Wissing D, Burkart V, Kolb H. Heat shock protein Hsp70 overexpression confers resistance against nitric oxide. FEBS Lett. 1996;391:185–188. doi: 10.1016/0014-5793(96)00730-2. [DOI] [PubMed] [Google Scholar]
- Borkan SC, Wang YH, Lieberthal W, Burke PR, and Schwartz JH 1997 Heat stress ameliorates the functional and cytoskeletal consequences of sublethal injury in mouse proximal tubule cells. Am J Physiol (Renal). 272 F. 347–F355. [DOI] [PubMed] [Google Scholar]
- Chang J, Knowlton AA, and Wasser JS 2000 Expression of heat shock proteins in turtle and mammal hearts: relationship to anoxia tolerance. Am J Physiol (Reg Integ Compar Phys). 278 R. 209–R214. [DOI] [PubMed] [Google Scholar]
- Chen C, Okayma H. High efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Smith DF. Hop as an adapter in the heat shock protein 70 (Hsp70) and hsp90 chaperone machinery. J Biol Chem. 1998;273:35194–35200. doi: 10.1074/jbc.273.52.35194. [DOI] [PubMed] [Google Scholar]
- Csermely P, Schnaider T, Soti C, Prohaszka Z, Nardai G. The 90-kDa molecular chaperone family: structure, function, and clinical applications. A comprehensive review. Pharmacol Ther. 1998;79:129–168. doi: 10.1016/s0163-7258(98)00013-8. [DOI] [PubMed] [Google Scholar]
- Currie RW, Karmazyn M, Kloc M, Mailer K. Heat shock response is associated with enhanced post-ischemic ventricular recovery. Circ Res. 1988;63:543–549. doi: 10.1161/01.res.63.3.543. [DOI] [PubMed] [Google Scholar]
- Emami A, Schwartz JH, Borkan S. Transient ischemia or heat stress induces a cytoprotectant protein in rat kidney. Am J Physiol. 1991;29:F479–F485. doi: 10.1152/ajprenal.1991.260.4.F479. [DOI] [PubMed] [Google Scholar]
- Freeman BC, Myers MP, Schumacher RS, Morimoto RI. Identification of a regulatory motif in HSP70 affects ATPase activity, substrate binding and interaction with HDJ-1. EMBO J. 1995;14:2281–2292. doi: 10.1002/j.1460-2075.1995.tb07222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabai VL, Meriin AB, Mosser DD, Caron AW, Rits S, Shifrin VI, Sherman MY. HSP70 prevents activation of stress kinases: a novel pathway of cellular thermotolerance. J Biol Chem. 1997;272:18033–18037. doi: 10.1074/jbc.272.29.18033. [DOI] [PubMed] [Google Scholar]
- Heads RJ, Latchman DS, Yellon DM. Stable high level expression of a transfected human HSP70 gene protects a heart-derived muscle cell line against thermal stress. J Mol Cell Cardiol. 1994;26:695–699. doi: 10.1006/jmcc.1994.1084. [DOI] [PubMed] [Google Scholar]
- Jaattela M, Wissing D, Bauer PA, Li GC. Major heat shock protein HSP70 protects tumor cells from tumor necrosis factor cytotoxicity. EMBO J. 1992;11:3507–3512. doi: 10.1002/j.1460-2075.1992.tb05433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joannidis M, Cantley LG, Spokes K, Medina R, Pullman J, Rosen S, Epstein FH. Induction of heat shock proteins does not prevent renal tubular injury following ischemia. Kidney Int. 1995;47:1752–1759. doi: 10.1038/ki.1995.242. [DOI] [PubMed] [Google Scholar]
- Kelly KJ, Baird NR, Soleimani M, Wang Z, and Greene A 1999 The role of heat stress response proteins in experimental renal ischemia. J Am Soc Nephrol. 10:633. A. [Google Scholar]
- Knowlton AA. Mutation of amino acids 246–251 alters nuclear accumulation of human heat shock protein (HSP) 72 with stress, but does not reduce viability. J Mol Cell Cardiol. 1999;31:523–532. doi: 10.1006/jmcc.1998.0883. [DOI] [PubMed] [Google Scholar]
- Knowlton AA, Grenier M, Kirchhoff SR, and Salfity M 2000 Phosphorylation at tyrosine-524 influences nuclear accumulation of HSP72 with heat stress. Am J Physiol (Heart). 278 H. 2143–H2149. [DOI] [PubMed] [Google Scholar]
- Korsmeyer SJ, Shutter JR, Veis DJ, Merry DE, Oltvai ZN. Bcl-2/Bax: a rheostat that regulates an anti-oxidant pathway and cell death. Semin Cancer Biol. 1993;4:327–332. [PubMed] [Google Scholar]
- Kuhl NM, Kunz J, Rensing L. Heat shock-induced arrests in different cell cycle phases of rat C6-glioma cells are attenuated in heat shock–primed thermotolerant cells. Cell Prolif. 2000;33:147–166. doi: 10.1046/j.1365-2184.2000.00175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CY, Lee JS, Ko YG, Kim JI, Seo JS. Heat shock protein 70 inhibits apoptosis downstream of cytochrome c release and upstream of caspase-3 activation. J Biol Chem. 2000;275:25665–25671. doi: 10.1074/jbc.M906383199. [DOI] [PubMed] [Google Scholar]
- Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- Lieberthal W, Levine JS 1996 Mechanisms of apoptosis and its potential role in renal tubular epithelial cell injury. Am J Physiol (Renal) 271: F477–F488. [DOI] [PubMed] [Google Scholar]
- Lieberthal W, Menza S, and Levine JS 1998 Graded ATP depletion can cause necrosis or apoptosis of cultured mouse proximal tubular cells. Am J Physiol (Renal). 274 F. 315–F327. [DOI] [PubMed] [Google Scholar]
- Marber MS, Mestril R, Chi S-H, Sayen R, Yellown DM, Dillman WH. Overexpression of rat inducible 70-kD heat stress protein in a transgenic mouse increases resistance of the heart to ischemic injury. J Clin Investig. 1995;95:1446–1456. doi: 10.1172/JCI117815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlen P, Schulze-Osthoff K, Arrigo AP. Small stress proteins as novel regulators of apoptosis. Heat shock protein 27 blocks FAS/APO-1 stuarosporine-induced cell death. J Biol Chem. 1996;271:16510–16514. doi: 10.1074/jbc.271.28.16510. [DOI] [PubMed] [Google Scholar]
- Meriin AB, Yaglom JA, Gabai VL, Zon L, Ganiatsas S, Mosser DD, and Sherman MY 1999 Protein-damaging stresses activate c-Jun N-terminal kinase via inhibition of its dephosphorylation: a novel pathway controlled by HSP72. Mol Cell Biol 19: 2547–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestril R, Shun-Hua C, Sayen R, O'Reilly K, Dillmann WH. Expression of inducible stress protein 70 in rat myogenic cells confers protection against simulated ischemia-induced injury. J Clin Investig. 1994;93:759–767. doi: 10.1172/JCI117030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milarski KL, Morimoto RI. Mutational analysis of the human hsp70 protein: distinct domains for nucleolar localization and adenosine triphosphate binding. J Cell Biol. 1989;109:1947–1962. doi: 10.1083/jcb.109.5.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DD, Caron AW, Bourget L, Denis-Larose C, Massie B. Role of human heat shock protein hsp 70 in protection against stress-induced apoptosis. Mol Cell Biol. 1997;17:5317–5327. doi: 10.1128/mcb.17.9.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto CT 1999 HSP27 and signaling to the actin cytoskeleton. Am J Physiol (Cell). 277 C. 1029–C1031. [DOI] [PubMed] [Google Scholar]
- Ovelgonne H, Bitorina M, Van Wijk R. Stressor-specific activation of heat shock genes in H35 rat hepatoma cells. Toxicol Appl Pharmacol. 1995;135:100–109. doi: 10.1006/taap.1995.1212. [DOI] [PubMed] [Google Scholar]
- Perdrizet GA, Kaneko H, Buckley TM, Fishman MA, Schweizer RT. Heat shock protects pig kidneys against warm ischemic injury. Transplant Proc. 1990;22:460–461. [PubMed] [Google Scholar]
- Rassow J, von Ahsen O, Bomer U, Pfanner N. Molecular chaperones: towards a characterization of the heat-shock protein 70 family. Trends Cell Biol. 1997;7:129–133. doi: 10.1016/S0962-8924(96)10056-8. [DOI] [PubMed] [Google Scholar]
- Ravagnan L, Gurbuxani S, and Susin SA. et al. 2001 Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat Cell Biol. 3:839–843. [DOI] [PubMed] [Google Scholar]
- Schumer M, Colombel MC, and Sawczuk IS. et al. 1992 Morphologic, biochemical, and molecular evidence of apoptosis during the reperfusion phase after brief periods of renal ischemia. Am J Pathol. 140:831–838. [PMC free article] [PubMed] [Google Scholar]
- Song J, Takeda M, Morimoto RI. Bag1-Hsp70 mediates a physiological stress signalling pathway that regulates Raf-1/ERK and cell growth. Nat Cell Biol. 2001;3:276–282. doi: 10.1038/35060068. [DOI] [PubMed] [Google Scholar]
- Susin SA, Zamzami N, Kroemer G. Mitochondria as regulators of apoptosis: doubt no more. Biochim Biophys Acta. 1998;1366:151–165. doi: 10.1016/s0005-2728(98)00110-8. [DOI] [PubMed] [Google Scholar]
- Toba T, Shidoji Y, Fujii J, Moriwaki H, Muto Y, Suzuki T, Ohishi N, Yagi K. Growth suppression and induction of heat-shock protein-70 by 9-cis beta-carotene in cervical dysplasia-derived cells. Life Sci. 1997;61:839–845. doi: 10.1016/s0024-3205(97)00566-3. [DOI] [PubMed] [Google Scholar]
- van Why SK, Kim S, Geibel J, Seebach FA, Kashgarian M, and Siegel NJ 1999 Thresholds for cellular disruption and activation of the stress response in renal epithelia. Am J Physiol (Renal). 277 F. 227–F234. [DOI] [PubMed] [Google Scholar]
- Wagner AC, Weber H, and Jonas L. et al. 1996 Hyperthermia induces heat shock protein expression and protection against cerulin-induced pancreatitis in rats. Gastroenterology. 111:1333–1342. [DOI] [PubMed] [Google Scholar]
- Wang Y-H, Borkan SC 1996 Prior heat stress enhances survival of renal epithelial cells after ATP depletion. Am J Physiol (Renal). 270 F. 1057–1065. [DOI] [PubMed] [Google Scholar]
- Wang Y-H, Knowlton A, Christensen TG, Shih T, Borkan SC. Prior heat stress inhibits apoptosis in adenosine trisphosphate-depleted renal tubular cells. Kidney Int. 1999;55:2224–2235. doi: 10.1046/j.1523-1755.1999.00476.x. [DOI] [PubMed] [Google Scholar]
- Welch WJ. Mammalian stress response: cell physiology, structure/function of stress proteins and implications for medicine and disease. Physiol Rev. 1992;72:1063–1080. doi: 10.1152/physrev.1992.72.4.1063. [DOI] [PubMed] [Google Scholar]
- Williams RS, Thomas JA, Fina M, German Z, Benjamin IJ. Human heat stress protein 70 (hsp 70) protects murine cells from injury during metabolic stress. J Clin Investig. 1993;92:503–508. doi: 10.1172/JCI116594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zager RA, Iwata M, Burkhart KM, Schimpf BA. Post-ischemic acute renal failure protects proximal tubules from O2 deprivation injury, possibly by inducing uremia. Kidney Int. 1994;45:1760–1768. doi: 10.1038/ki.1994.229. [DOI] [PubMed] [Google Scholar]