Abstract
The behavior of the endogenous heat shock protein 25 (Hsp25) in heat-stressed rat H9c2 myoblasts was studied. After mild or severe heating, this protein became less extractable with Triton X-100 and displayed characteristic immunofluorescence patterns, namely (1) granules in the nucleus, and (2) association with F-actin bundles in the cytoplasm. The intranuclear granulation of Hsp25 and its association with F-actin were sensitive to drugs affecting Hsp25 phosphorylation (cantharidin, sodium orthovanadate, SB203580, SB202190). Isoform analysis of Hsp25 translocated to the nucleus-free cytoskeletal fraction revealed only mono- and biphosphorylated Hsp25 and no unphosphorylated Hsp25. Transfected luciferase with initial localization in the nucleosol became colocalized with the Hsp25-containing granules after a heat shock treatment that denatured the enzyme in the cells. The association of Hsp25 with actin filaments after a mild heat stress conferred protection from subsequent F-actin–damaging treatments with cytochalasins (D and B) or severe heat stress. We hypothesize that (1) the binding of heat-denatured nucleosolic proteins to the Hsp25 contained in specific granular structures may serve for the subsequent chaperoning or degradation of the bound proteins, and (2) the actin cytoskeleton is stabilized by the direct targeting of phosphorylated Hsp25 to microfilament bundles.
INTRODUCTION
The small (24–28 kDa) heat shock protein (Hsp) (referred to as Hsp25 or Hsp27) is expressed constitutively in many mammalian tissues and cell lines (Arrigo and Landry 1994). Among rodent tissues, the Hsp25 expression is most abundant in eye lens, heart, stomach, colon, lung, and bladder (Klemenz et al 1993). In unstressed cells this protein is mostly found in the cytosol as high–molecular weight oligomers (Chaufour et al 1996; Ehrnsperger et al 1999; Lambert et al 1999), but in response to some stresses its translocation into the nucleus or to the F-actin bundles (or both) occurs (Arrigo et al 1988; Loktionova et al 1996; Sakamoto et al 1998, 2000; van de Klundert et al 1998).
Experiments with cells overexpressing Hsp25(27) have shown its cytoprotective capability toward various stresses, including heat shock (Landry et al 1989), oxidative stress (Mehlen et al 1995; Arrigo 1998), and simulated ischemia or hypoxia (Martin et al 1997; Brar et al 1999). In vitro studies revealed that Hsp25 could act as an adenosine triphosphate (ATP)-independent chaperone (Jakob et al 1993; Merck et al 1993) and as an inhibitor of actin polymerization (Miron et al 1991; Benndorf et al 1994). In vivo, the Hsp27 overexpression confers accelerated recovery from intranuclear protein aggregates after heat shock (Kampinga et al 1994) and improved cytoskeletal recovery after heat- and cytochalasin D–induced F-actin collapse (Lavoie et al 1993, 1995). Besides these activities, Hsp25(27) seems to be involved in the suppression of apoptosis (Arrigo 1998; Garrido et al 1999; Wagstaff et al 1999; Bruey et al 2000; Pandey et al 2000), the regulation of differentiation (Chaufour et al 1996), and the inhibition of elastase (Merck et al 1993).
Phosphorylation is the most striking posttranslational modification of the small Hsps. In vivo, rapid phosphorylation of Hsp25(27) is induced by some cytokines and mitogens and by many stresses including heat shock (Landry et al 1992; Arrigo and Landry 1994; Ehrnsperger et al 1997a). This protein is phosphorylated through the p38 mitogen–activated protein (MAP) kinase pathway (Rouse et al 1994; Guay et al 1997). Such a modification is essential for the Hsp25(27)-mediated regulation of the actin filament dynamics and the cell motility (Landry and Huot 1995; Piotrowicz et al 1998). Furthermore, the stress-responsive phosphorylation of Hsp27 is required for effectual F-actin restoration after heat shock or cytochalasin D treatment (Lavoie 1993, 1995; Guay et al 1997). Additionally, phosphorylated Hsp25(27) appears to be involved in the stress-induced translocation to actin fibers (Yoshida et al 1999; Sakamoto et al 2000), but no effects of such a response on the F-actin stability have been described.
Being the regulatory factor in the modulation of the actin filament dynamics (see the preceding paragraph), phosphorylation of the small Hsp also affects its supramolecular organization that, in turn, may change the chaperone activity. Actually, on the one hand, phosphorylation of Hsp25(27) inhibits its assembling into large oligomeric structures (Benndorf et al 1994; Rogalla et al 1999) and causes deoligomerization of the natively existing superaggregated complexes of this protein (Kato et al 1994; Lambert et al 1999; Dunlop and Muggli 2000). On the other hand, the chaperone activity of Hsp25 is mainly intrinsic to its unphosphorylated form assembled into large oligomers, whereas the phosphorylation-induced deoligomerization down-regulates this activity (Ehrnsperger et al 1997b; Rogalla et al 1999).
So, at present, it seems unclear how distinct (cytoskeleton preserving and chaperone) activities of Hsp25 are manifested in the stressed cell and how they are regulated if the activation of one results in the inhibition of another. In the present work we, therefore, studied the behavior of Hsp25 in heat-stressed H9c2 myoblasts. We demonstrate that heat stress induces (1) the translocation of Hsp25 to the microfilament bundles, which confers stabilization of the actin cytoskeleton, and (2) the formation of intranuclear granules of Hsp25, which are colocalized with foci of a heat-inactivated reporter enzyme nuclear luciferase. Both responses were dependent on Hsp25 phosphorylation.
MATERIALS AND METHODS
Cells and heat shock conditions
The rat embryonic heart–derived myoblast line, H9c2, was obtained from the American Type Culture Collection (Rockville, MD, USA) and cultured in Dulbecco modified Eagle's medium supplemented with 10% fetal bovine serum (both from GIBCO BRL, Gaithersburg, MD, USA), 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), 1 mM sodium pyruvate, 50 U/mL penicillin, and 100 μg/mL streptomycin (all from HyClone Europe Ltd, Cramlington, UK). The cells were grown at 37°C in an atmosphere of 95% air and 5% CO2. On the second day after passaging, the cell culture on “islands” stage of growth was subjected to heat stress: petri dishes with adherent myoblasts were hermetically sealed with a parafilm and plunged into a thermostatic water bath (Instrutemp, Helsinki, Finland). The temperature fluctuations on heating the cells did not exceed 0.2°C.
Plasmids and transient transfection
The plasmid pRSVnlsLL/V–encoding firefly (Photinus pyralis) luciferase with nuclear localization has previously been described (Michels et al 1995). Constructions of pRSVnlsLL/V fused to the enhanced green fluorescence protein (EGFP) and the plasmid-encoding EGFP under Rouse sarcoma virus promoter control were prepared at the Department of Radiation and Stress Cell Biology of the University of Groningen by Dr Ellen A.A. Nollen. The cells (50% of confluency) were transfected with the plasmid DNA using the GenePORTER transfection reagent (Gene Therapy Systems Inc, San Diego, CA, USA), according to the protocol of the manufacturer. The transfectants were used in heat shock experiments 24–48 hours after the start of transfection. Efficiency of the transfection was controlled by diffuse fluorescence of the EGFP label in the nuclei.
Treatments with drugs affecting Hsp25 phosphorylation
To inhibit the p38 MAP kinase–Hsp25 pathway in H9c2 cells, 10 μM SB202190 or its analog SB203580 (both from Calbiochem-Novabiochem Corporation, La Jolla, CA, USA) was added for 1–2 hours before heat shock from a 10 mM stock solution in dimethyl sulfoxide (DMSO). The inhibitor was also present in the medium during the heat shock and recovery periods. In parallel samples, DMSO alone or 50 μM PD098059 (Alexis Corp, San Diego, CA, USA) were used as controls. For protein phosphatase (PP) inhibition, 20 μM cantharidin (in DMSO) or 50 μM sodium orthovanadate (Na3VO4) (both from Sigma Chemical Company, St Louis, MO, USA) was added to the cells immediately after heat shock during the entire recovery period. The same inhibitory treatments without heat shock were used to compare the pure effects of either exposure.
Fluorescence staining and analysis
The cells growing on coverslips were treated with 4% formaldehyde–0.1% Triton X-100 in phosphate-buffered saline (PBS) for 5 minutes. Thereafter, the fixed and permeabilized cell preparations were washed with PBS and incubated with 1% bovine serum albumin (BSA) in PBS for 30 minutes. The cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (Sigma). The nuclear-localized EGFP labels were directly seen in the fixed cell preparations. The Hsp25 patterns were revealed by indirect immunostaining with rabbit anti-Hsp25 antibody (Stressgen Biotechnologies, Victoria, British Columbia, Canada, SPA-801) diluted to 1:300 in PBS–1% BSA and anti-rabbit IgG goat antibodies conjugated to Texas Red (South Biotech Associates, Birminghem, AL, USA). The F-actin patterns were visualized using fluorescein isothiocyanate (FITC)-conjugated phalloidin (Sigma). All fluorescent labels were used in optimally selected dilutions. The stained preparations were viewed and photographed on an Opton III epifluorescence microscope (Karl Zeiss, Germany). The preparations with double-label fluorescence (Texas Red + FITC or EGFP and FITC + DAPI) were analyzed using spectrum-specific discriminating filters (for FITC or EGFP: FT 510, LP 520; for Texas Red: FT 580, LP 590; for DAPI: FT 395, LP 397).
F-actin stability test
The treated and control cells were incubated with fresh growth medium containing 3 μg/mL cytochalasin B or 0.5 μg/mL cytochalasin D (both from Sigma) for 10 minutes. After incubation, the cells were fixed and permeabilized with formaldehyde and Triton X-100, as described earlier. Cellular F-actin and nuclei were stained with FITC-phalloidin and DAPI, respectively. To the resistance of cellular F-actin, the slides were photographed, and the images were analyzed for the percentage of the cytochalasin-treated cells still containing extended (native-like) actin fibers. To avoid the influence of a human factor on counting, the evaluations were made on ciphered images, which were decoded afterwards. At least 200 cells were analyzed within each experimental group.
Preparation of enucleated cytoplasts
The cells were grown on plastic round coverslips (LUX, Miles Scientific, Naperville, IL, USA) with a diameter slightly less than that of the centrifuge tubes. Two-day-old subconfluent cultures were enucleated, according to a described technique (Prescott et al 1972), with some modifications. Coverslips were inserted into the centrifuge tubes (by the cell-carrying surface downward) with the growth medium containing 1 μg/mL cytochalasin D. The tubes with the medium and coverslips were conditioned in a CO2 incubator at 37°C and then centrifuged at 8000 rpm (Beckman SW27 bucket rotor) for 30 minutes. After centrifugation, the coverslips were taken out, and most of the remaining adherent cells were free from the nuclei, ie, cytoplasts. The latter were washed and incubated in a growth medium for 2 hours at 37°C in a CO2 incubator and were then used in heat shock experiments. The yield of cytoplasts was counted as the percentage of FITC-phalloidin–positive or DAPI-negative cell images, and usually it was 90–95%.
Fractionation with Triton X-100
The cells or cytoplasts were washed twice in PBS and incubated on ice with a lysing buffer containing 1% (v/v) Triton X-100, 10 mM 1,4-piperazine-diethanesulfonic acid (PIPES) of pH 7.4, 300 mM sucrose, 150 mM NaCl, 5 mM MgCl2, 5 mM ethyleneglycol-bis(aminoethylether)-tetra-acetic acid (EGTA), 100 μM Na3VO4, 50 mM NaF, and protease inhibitor cocktail (Sigma). After a 30-minute incubation, the ghosts of the cells were scraped into the same buffer, and then the entire mixture (fluid extract + cellular debris) was centrifuged at 8000 × g for 8 minutes. As a result, the supernatants comprised a Triton-soluble (cytosolic) cellular fraction (fraction A), whereas the pellets were formed by a Triton-insoluble nuclear-cytoskeletal (if whole cells) or only cytoskeletal (if cytoplasts) material (fraction B). The pellets were washed twice with the lysing buffer and dissolved in a Laemmli sample buffer or a sample buffer for isoelectric focusing (IEF) (8 M urea, 1% Nonidet P-40, 2% β-mercaptoethanol, 100 μM Na3VO4). In the case of fraction A, 5% (v/v) trichloracetic acid was added to the solution, and the protein pellets formed were sedimented by centrifugation at 14 000 rpm for 5 minutes. The pellets were then washed 5 times with ice-cold acetone and dried at vacuum. Such samples were redissolved in Laemmli or IEF sample buffers, run in gel slabs, and analyzed by immunoblotting.
Electrophoresis, IEF, and immunoblotting
Polyacrylamide gel electrophoresis (PAGE) of the isolated cellular fractions was performed in a Laemmli system with 15% polyacrylamide gel slabs, under reducing conditions (Loktionova et al 1996). IEF of the prepared samples was performed in 8% polyacrylamide gel slabs containing 8 M urea and 4% ampholines (Sigma) of pH 5–7 and pH 5–8 in equal proportion. An IEF apparatus (LKB, Bromma, Sweden) was employed with the following settings: 1500 V, 10 W, 3000 V/h. For immunoblotting, proteins separated by PAGE or IEF were electrotransferred from the gel slabs onto nitrocellulose membranes (0.45 μm, Bio-Rad Laboratories, Richmond, CA, USA). After a routine blocking step, the blots were consecutively treated with rabbit anti-Hsp25 antibodies (StressGen Biotechnologies) diluted to 1:1000 and anti–rabbit IgG-peroxidase conjugates (Pierce Chemical Company, Rockford, IL, USA) diluted to 1:5000. Tracks of the antigen bands were developed on an X-ray film (Fuji Medical Systems, Stamford, CT, USA), using enhanced chemiluminescence reagents (Amersham-Pharmacia Biotechnology, Buckinghamshire, UK).
RESULTS
Heat stress–induced changes in the intracellular localization of Hsp25
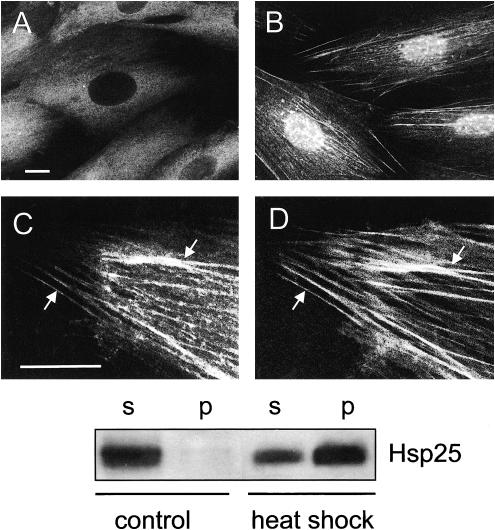
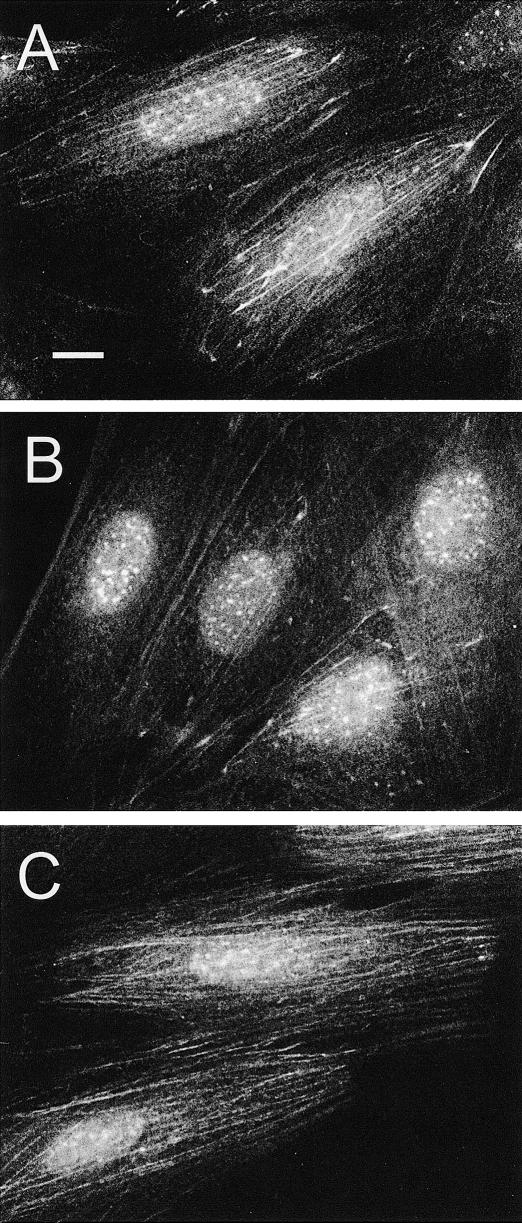
In unstressed H9c2 cells constitutively expressed Hsp25 localized into the Triton X-100–soluble cellular fraction (Fig 1E, control), and the immunofluorescence patterns demonstrated mainly diffuse and occasionally clump-like (granular) distribution of this protein in the cytoplasm, whereas there was no Hsp25 in the nuclei (Fig 1A).
Fig. 1.
Redistribution of Hsp25 in heat-stressed H9c2 cells. (A) Intracellular localization of Hsp25 under normal conditions and (B, C) after a 30-minute recovery after heat shock (43°C, 30 minutes). (B) Note bright granules of Hsp25 in the nucleus and (C) colocalization of Hsp25 with actin fibers in the cytoplasm. The same cell preparation displays double labeling with (C) anti-Hsp25 and Texas Red conjugates and (D) fluorescein isothiocyanate–phalloidin. Arrows show examples of Hsp25 colocalization with actin fibers. Bars, 10 μm. (Lower panel) Segments of ECL–developed blots showing the heat stress–induced translocation of cytosolic Hsp25 to the cytoskeletal-nuclear fraction. Equal numbers (150 000) of the cells were either not heated (control) or heated (heat shock) for 30 minute at 43°C and fractionated into the Triton X-100–soluble fraction (s) and -insoluble (nuclear-cytoskeletal) fraction (p)
After 20–30 minutes of a mild heat stress (43°C), Hsp25 accumulated in the Triton X-100–insoluble fraction (Fig 1 lower panel, heat shock) in parallel with the changes in its intracellular distribution. In the nuclei this protein formed characteristic granules (Fig 1B), whereas in the cytoplasm Hsp25 became associated with F-actin bundles, as was confirmed by the double-label staining (Fig 1 C,D). Furthermore, in the case of severe heat shock (45°C, 15 minutes), which totally destroyed the preexisting actin network, Hsp25 decorated the newly formed F-actin bundles as soon as they appeared (data not shown). The rate of restoration of the initial (cytosolic) Hsp25 localization depended on the severity and the duration of prior heat stress (Table 1).
Table 1.
Dependence of the in situ Hsp25 relocalization on heat shock conditions
All these reactions of Hsp25 are not specific for heat preconditioning only because similar changes in the subcellular localization of Hsp25 were also observed in H9c2 cells subjected to transient simulated ischemia or acidic pH treatment (data not shown).
Nuclear granules of Hsp25 capture a thermolabile reporter enzyme in the heat-stressed cells
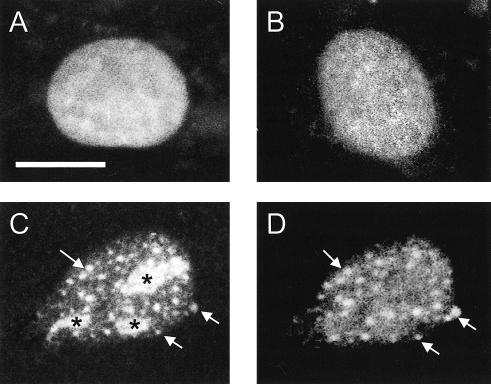
To monitor the in situ interaction of stress-denatured proteins with the nuclear granules of Hsp25, we transfected H9c2 cells with plasmids expressing nuclear-localized firefly luciferase fused to EGFP (nuc-luc-EGFP). Transfection with plasmids carrying only the EGFP gene was used as a control. Under normal conditions, both the nuc-luc-EGFP–expressing and control transfectants exhibited bright, diffuse fluorescence of the EGFP label in the nuclei (Fig 2A). Immediately after heat shock and for a prolonged (60 minutes) recovery period, the fluorescence of the nuclei expressing EGFP alone remained unchanged (Fig 2B). It was found recently (Nollen et al 2001) by using hamster O23 cells that the nuc-luc-EGFP reallocated to both small and large foci upon heat stress. The large foci were identified as the nucleoli representing a site for the storage and poststress reactivation of luciferase (Nollen et al 2001). In H9c2 cells we found that transfected nuc-luc-EGFP displayed the same distribution patterns after heat shock (Fig 2C). Interestingly, immunostaining with anti-Hsp25 antibodies revealed near to complete overlap of the small foci of nuc-luc-EGFP and the nuclear Hsp25-containing granules (Fig 2 C,D). This was confirmed by confocal analysis (data not shown). Large nucleolar foci of nuc-luc-EGFP (Fig 2, asterisk) were Hsp25 negative. The in vivo heat treatment inactivated >95% of the enzymatic activity of nuc-luc-EGFP and converted it to an insoluble form (Michels et al 1995; Nollen et al 1999). Because heat shock did not affect the localization and solubility of EGFP transfected alone (Nollen et al 2001, Fig 2B and data not shown) but still induced the Hsp25 granules in the nuclei, it can be speculated that the nuclear Hsp25 granule formation is a specific response related to denaturation of both exogenous (luciferase) and endogenous heat labile proteins. The function of this association is currently under investigation.
Fig. 2.
Specific relocalization of the transfected nuclear luciferase–enhanced green fluorescence protein (EGFP) fusion protein (nuc-luc-EGFP) in heat-shocked H9c2 cells. Cell nuclei expressing nuc-luc-EGFP (A) before and (C) after heat shock (43°C, 30 minutes). (C) Green fluorescence of the EGFP label in the fixed cell preparations was compared with (D) Texas Red fluorescence after the indirect immunostaining with anti-Hsp25 antibodies. (B) As a control, the same heat shock conditions were applied for the cells expressing EGFP alone. The same EGFP distribution was observed in unstressed cells. Arrows denote typical nuclear granules containing both Hsp25 and nuc-luc. Nuc-luc-EGFP entrapped into the nucleoli is marked by asterisk (*). Bar, 10 μm
Binding of Hsp25 to actin filaments increases their stability
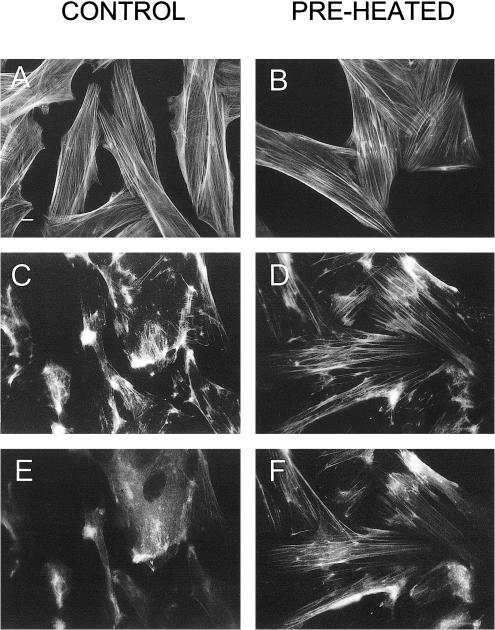
To examine whether the stress-induced association of Hsp25 with F-actin bundles has functional significance, we treated the cells with cytochalasin D or cytochalasin B. These drugs cause disassembly of F-actin in vivo and can be used for probing of the actin cytoskeleton stability (Lavoie et al 1993, 1995; Guay et al 1997). The drugs rapidly destroyed the actin network in the control (non-preheated) cells (Fig 3 A vs C). Contrary to that under the same cytochalasin treatments started immediately after mild heat shock, the cells displayed the markedly improved microfilament patterns (Fig 3 B vs D) that imply the F-actin stabilization resulting from the heat preconditioning.
Fig. 3.
Translocation of Hsp25 to actin fibers is associated with their resistance to cytochalasin D. Control, non-preheated cells (A, C, E), or cells preconditioned with a 30-minute heat shock (preheated: B, D, F) were fixed before (A, B) or after treatment with cytochalasin D (0.5 μg/mL) for 10 minutes and were stained with fluorescein isothiocyanate (FITC)-phalloidin (A–D) or were double labeled with FITC-phalloidin (C, D) and anti-Hsp25–Texas Red conjugates (E, F). Bar, 10 μm
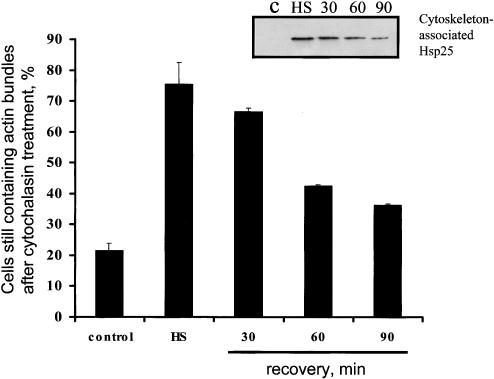
Importantly, all the cytochalasin-resistant F-actin bundles in preheated H9c2 cells were associated with Hsp25, as revealed by the double-label staining (Fig 3 C,E vs D,F). Furthermore, a semiquantitative analysis of F-actin stability (Fig 4) revealed a relationship between the resistance of F-actin to cytochalasin D and the Hsp25 colocalization with actin bundles. Similar data were found using human endothelial cells (data not shown).
Fig. 4.
Transient F-actin resistance to cytochalasin D in preheated H9c2 cells. The non-preheated control cells and cells recovering for indicated times after heat shock (43°C, 30 minutes) were treated with cytochalasin D (0.5 μg/mL) for 10 minute, fixed, and stained with fluorescein isothiocyanate–phalloidin. The percentage of the cells containing extended native-like actin fibers was counted. Amounts of Hsp25 translocated to actin bundles are demonstrated by Western blot of Triton-insoluble fractions (insertion) prepared from cytoplasts, as described in Materials and Methods. Each line corresponds to the bar on the diagram
Besides cytochalasins D and B, we used severe heat stress (44°C, 20 minutes) to disrupt the microfilament bundles. After mild heat preconditioning of the H9c2 cells, the Hsp25-decorated actin fibers exhibited clear resistance to F-actin disruption by a subsequent (severe) heat shock, whereas most of the Hsp25-free fibers in the control (non-preheated) cells broke and collapsed under the same stressful treatment (data not shown). It follows from these results that the targeting of Hsp25 to microfilaments induced by a (mild) preconditioning heat treatment stabilizes their structure, thereby protecting the actin cytoskeleton from subsequent stresses.
In situ reactions of Hsp25 depend on its phosphorylation
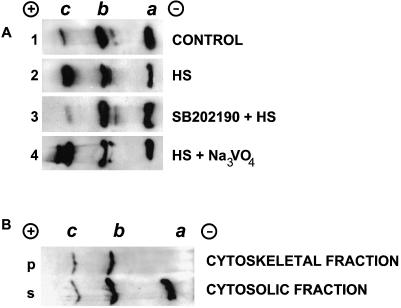
Unstressed H9c2 cells contained all 3 isoforms of Hsp25, namely an unphosphorylated a isoform, a monophosphorylated b isoform, and a biphosphorylated c isoform (Fig 5, lane 1). Herein, the unphosphorylated a and monophosphorylated b isoforms dominated, whereas the biphosphorylated c isoform was negligible. In the heat-shocked cells that had notably changed Hsp25 localization, the levels of both b and c phospho isoforms increased at the expense of a reduction in the level of the unphosphorylated one (Fig 5, lane 2). This heat shock–induced shift in the isoform profile slowly disappeared during the recovery period in parallel with the restoration of the initial diffuse cytoplasmic intracellular Hsp25 distribution (data not shown).
Fig. 5.
Analysis of the Hsp25 isoform spectra (A) in whole H9c2 cells and (B) in the fractionated nucleus-free cytoplasts. (A) Immunoblots showing the Hsp25 isoform spectra in unstressed H9c2 cells (lane 1), in cells 30 minutes after heat shock (43°C, 30 minutes) (lane 2), after the same heating and recovery in the presence of 10 μM SB202190 (lane 3) or 50 μM sodium orthovanadate (lane 4). Each blot carries the material from 150 000 cells that were totally lysed, run by IEF, and immunoblotted, as described in Materials and Methods. The same results were obtained with 10 μM SB203580 and 20 μM cantharidinas in lanes 3 and 4, respectively (data not shown). (B) Immunoblots demonstrating Hsp25 isoforms in the Triton X-100–soluble fraction (s) and -insoluble (cytoskeletal) fraction (p) in heat-shocked cytoplasts. Cytoplasts derived from 100 000 cells were heat shocked for 30 minutes at 43°C, fractionated with Triton X-100, run by IEF, and immunoblotted, as described in Materials and Methods. Note the full absence of nonphosphorylated a isoform of Hsp25 in the cytoskeletal fraction
Next, we treated H9c2 cells with inhibitors of the p38 MAP kinase, SB202190 or SB203580 (Lee et al 1994). Such inhibitory treatments prevented the rapid hyperphosphorylation of Hsp25 in response to a heat treatment (Fig 5, lane 3). The inhibitor-treated cells demonstrated well-recognized nuclear granules of Hsp25 upon heat shock treatment, whereas the heat-induced association of Hsp25 with F-actin bundles was markedly weakened (compare Fig 6 A and B). As the negative control, we used PD098059, which inhibits another MAPK-extracellular signal-regulated kinase (ERK), the activation of which does not lead to Hsp25 phosphorylation (Alessi et al 1995). This inhibitor affected neither the phosphorylation of Hsp25 nor its characteristic redistribution in the heat-shocked cells (data not shown).
Fig. 6.
Effects of drugs affecting phosphorylation of Hsp25 on the intracellular distribution of Hsp25 after a mild heat shock (43°C, 30 minutes). (A) The heat-shocked control cells recovered for 1 hour. (B) The cells pretreated with the medium containing 10 μM SB202190 for 2 hours before heat shock and recovered for 1 hour in the same medium. (C) The cells recovered in the medium containing 50 μM sodium orthovanadate. Bar, 10 μm
In parallel, we examined the effects of the PP inhibitors, cantharidin and sodium orthovanadate, which can suppress the dephosphorylation of Hsp25(27) in vivo (Cairns et al 1994; Loktionova et al 1998). Both inhibitors strongly elevated the fraction of phosphorylated Hsp25 after heat shock (Fig 5, lane 4). The heat-stressed cells recovering in the presence of cantharidin or sodium orthovanadate displayed only poor granulation of Hsp25 in the nuclei but showed enhanced association of Hsp25 with the actin fibers (Fig 6C).
From our data it is suggestive that only phosphorylated Hsp25 is bound to the actin cytoskeleton. To further test this suggestion, we assessed the phosphorylation status of Hsp25 localized to the cytoskeletal fraction after heat shock in cytoplasts. Routine extraction of cells with Triton X-100 was inapplicable here because both pools of Hsp25 associated with the cytoskeleton and the nuclear-localized (granulated) Hsp25 were found in the same insoluble fraction. In situ studies revealed that the cytoplasts derived from the H9c2 cells have no visible changes in the cytoskeletal architecture and that the Hsp25 translocation to actin fibers in the heat-shocked cytoplasts was similar to that seen in heat-shocked H9c2 cells (data not shown). Fractionation of heat-shocked cytoplast revealed that the soluble cytosolic fraction comprised all 3 isoforms of Hsp25 (Fig 5B, top panel). The cytoskeletal (F-actin–rich) fraction, however, only contained phosphorylated species of Hsp25 (Fig 5B, bottom panel). Such a pattern of Hsp25 isoforms was observed at different time points within 1 hour after heat stress. This result is consistent with the data obtained with the p38 MAP kinase inhibitors (discussed earlier), thus suggesting that the phosphorylation of Hsp25 is required for its association with actin filaments.
DISCUSSION
Stress-induced translocation of cytosolic Hsp25(27) to the nucleus or to the actin cytoskeleton (or both) (Arrigo and Landry 1994; Loktionova et al 1996; van de Klundert et al 1998) seems to be a characteristic reaction, although its functional significance was not unraveled. As for the nuclear-localized Hsp25(27), we found its assembling into the Triton X-100–insoluble granules in the human endothelial cells (Loktionova et al 1996, 1998) and the rat H9c2 myoblasts subjected to heat shock or ATP depletion, or to a treatment with acidic pH. The heat shock model with H9c2 cells was exploited in the present work for more detailed study.
In vitro the small Hsps can form large oligomers or superaggregated globular structures that can bind denatured proteins (Ehrnsperger et al 1997b; Lee et al 1997; Haslbeck et al 1999; Rogalla et al 1999). The in situ analog of such globular structures could be the intranuclear granules revealed in the heat-stressed cells. The colocalization of nuc-luc-EGFP foci with the Hsp25-containing intranuclear granules at least suggests that they are formed to bind nonnative, heat-denatured proteins (see Fig 2B). From earlier investigation it is known that overexpression of Hsp27 in cells can promote the accelerated recovery of heat shock–produced intranuclear protein aggregates (Kampinga et al 1994). At present, there are 2 possible ways to explain this observation, ie, either by accelerated refolding or by accelerated degradation of the aggregated proteins. In analogy with the reported in vitro chaperone activity of the large oligomers of Hsp25 (Ehrnsperger et al 1997b; Lee et al 1997; Haslbeck et al 1999), one could speculate that these intranuclear granules of Hsp25(27) seen in situ are needed for refolding. However, overexpression of Hsp27 in several cell lines was found not to affect the kinetics of the reactivation of nuclear luciferase in the heat-stressed transfectants (Kampinga, personal communication). Also, a recent study by Nollen et al (2001) revealed that the nucleolar-localized luciferase rather than the luciferase in the small foci, colocalizing with the Hsp27 granules, is associated with accelerated refolding. Therefore, we currently address the hypothesis of an involvement of Hsp25 granules in binding and targeting denatured substrates for accelerated degradation. Preliminary data revealing that the Hsp25-luciferase granules are stained positively with antibodies to components of the proteasome (not shown) are supportive of such an idea. However, further biochemical evidence is required here.
Another intriguing issue is about which mechanisms trigger and regulate the formation of the Hsp25-containing intranuclear granules. Our data show a correlation between the total cellular level of Hsp25 phosphorylation and the formation of the nuclear granules. When the level of dephosphorylated species is maximal (SB203290), we observe the most prominent and large granules. Inversely, when decreasing the amounts of dephosphorylated Hsp25 in cells (PP inhibitors), we register only weak granules in the nuclei (Figs 5 and 6). Also, when investigating ATP-depleted endothelial cells, we obtained a suggestion that the intranuclear granulation of Hsp27 is associated with its dephosphorylation (Loktionova et al 1996, 1998). Although this indicates a role for dephosphorylated Hsp25 in the process of granule formation, we cannot say whether or not the nuclear granules consist solely of the dephosphorylated Hsp25.
The binding of endogenous Hsp25(27) to cytoskeletal (actin) elements, as found here in H9c2 cells, is in agreement with the earlier heat shock studies with endothelial cells (Loktionova et al 1996) and cardiomyocytes (van de Klundert et al 1998). Similar observations were also made after ischemic pretreatment in H9c2 myoblasts and rat heart (Sakamoto et al 1998, 2000), in ischemia-affected zones of the heart (Lutsch et al 1997; Yoshida et al 1999), and kidney (Schober et al 1998). Our data are the first to provide experimental evidence for a functional role of the translocation of Hsp25(27) to actin fibers. We found a strong correlation between the association of Hsp25 with actin bundles and their acquired resistance to cytochalasins or severe heating. Although other researchers showed that prior phosphorylation of Hsp27 in arsenite-pretreated cells confers protection of the actin filaments from subsequent treatment with cytochalasin D (Guay et al 1997), no Hsp27–F-actin association was revealed in that study. Moreover, the protective effect was expressed as improved actin repolymerization after the drug-provoked destruction of the microfilaments. Our results, furthermore, indicate that phosphorylation of Hsp25 is required for the association of the small Hsp with actin fibers after heat shock. The same appears to be true for the in vivo situation with the ischemic hearts in which hyperphosphorylated Hsp25(27) was found to be associated with myofibrils (Lutsch et al 1997; Yoshida et al 1999). In other models both the translocation of cytosolic Hsp27 to the F-actin–rich fraction and the cytoprotective effects after ischemic preconditioning were sensitive to SB203580 (Sakamoto et al 1998, 2000). At first glance, our findings may appear to be in conflict with the earlier suggestions that the polymerized actin can bind only to unphosphorylated Hsp25 but not to its phosphorylated form (Arrigo and Landry 1994; Benndorf et al 1994). However, the in vivo interaction of Hsp25 with microfilaments may be more complex, and the in vitro situation may not reflect the situation in living cells. On the other hand, we cannot exclude that in vivo–phosphorylated Hsp25 binds to microfilament-decorating protein(s) rather than to the nude F-actin. Recently, the ability of Hsp27 to form complexes with actin-binding proteins, such as myosin, tropomyosin, and caldesmon, has been reported (Ibitayo et al 1999). Also, it is possible that there are phosphorylation-sensitive adapters that help toward Hsp25(27) translocation (Benndorf et al 2001). Finally, it is also possible that the binding of Hsp25 to microfilaments may precede its phosphorylation. Therefore, the precise mechanism of Hsp25 targeting to actin fibers and the precise partners for binding remain to be clarified.
In conclusion, we would like to emphasize the functional duality in the stress response of Hsp25. One part of the cytosolic pool of Hsp25 migrates to the nucleus to form granules colocalizing with the denatured proteins from the nucleosol, which, we propose, functions in promoting clearance of the protein aggregates via enhancing protein degradation, important for protection from thermal cell killing (Dewey 1989; Kampinga 1993). Another part becomes associated with F-actin bundles, thereby elevating their resistance that may contribute to cytoprotection against ischemia, heat, or other cytoskeleton-damaging insults. Intriguingly, both the in situ reactions diversely depend on the phosphorylation of Hsp25; although the intranuclear granulation appears to be down-regulated by this modification, the stabilizing fit to actin filaments requires phosphorylated Hsp25.
Acknowledgments
This study was supported by RFBR grant 99-04-48119a and NWO grant NL-RF 047-006-007.
REFERENCES
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- Arrigo AP. Small stress proteins: chaperones that act as regulators of intracellular redox state and programmed cell death. Biol Chem. 1998;379:19–26. [PubMed] [Google Scholar]
- Arrigo AP, Landry J 1994 Expression and function of the low-molecular-weight heat shock proteins. In: The Biology of Heat Shock Proteins and Molecular Chaperones, ed Morimioto RI, Tissieres A, Georgopoulos C. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 335–373. [Google Scholar]
- Arrigo AP, Suhan JP, Welch WJ. Dynamic changes in the structure and intracellular locate of the mammalian low-molecular-weight heat shock protein. Mol Cell Biol. 1988;8:5059–5071. doi: 10.1128/mcb.8.12.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benndorf R, Hayess K, Ryazantsev S, Wieske M, Behlke J, Lutsch G. Phosphorylation and supramolecular organization of murine small heat shock protein HSP25 abolish its actin polymerization-inhibiting activity. J Biol Chem. 1994;269:20780–20784. [PubMed] [Google Scholar]
- Benndorf R, Sun X, and Gilmont RR. et al. 2001 Hsp22 a new member of the small heat shock protein superfamily, interacts with mimic of phosphorylated Hsp27 (3DHsp27). J Biol Chem. 276:26753–26761. [DOI] [PubMed] [Google Scholar]
- Brar BK, Stephanou A, Wagstaff MJ, Coffin RS, Marber MS, Engelmann G, Latchman DS. Heat shock proteins delivered with a virus vector can protect cardiac cells against apoptosis as well as against thermal or hypoxic stress. J Mol Cell Cardiol. 1999;31:135–146. doi: 10.1006/jmcc.1998.0857. [DOI] [PubMed] [Google Scholar]
- Bruey JM, Ducasse C, and Bonniaud P. et al. 2000 Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol. 2:645–652. [DOI] [PubMed] [Google Scholar]
- Cairns J, Qin S, Philp R, Tan YH, Guy GR. Dephosphorylation of the small heat shock protein Hsp27 in vivo by protein phosphatase 2A. J Biol Chem. 1994;269:9176–9183. [PubMed] [Google Scholar]
- Chaufour S, Mehlen P, Arrigo AP. Transient accumulation, phosphorylation and changes in the oligomerization of Hsp27 during retinoic acid-induced differentiation of HL-60 cells: possible role in the control of cellular growth and differentiation. Cell Stress Chaperones. 1996;1:225–235. doi: 10.1379/1466-1268(1996)001<0225:tapaci>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey WC. The search for critical cellular targets damaged by heat. Radiat Res. 1989;120:191–204. [PubMed] [Google Scholar]
- Dunlop ME, Muggli EE. Small heat shock protein alteration provides a mechanism to reduce mesangial cell contractility in diabetes and oxidative stress. Kidney Int. 2000;57:464–475. doi: 10.1046/j.1523-1755.2000.00866.x. [DOI] [PubMed] [Google Scholar]
- Ehrnsperger M, Buchner J, and Gaestel M 1997a Structure and function of small heat-shock proteins. In: Molecular Chaperones in the Life Cycle of Proteins, ed Fink AL, Goto Y. Marcel Dekker, New York, 533–575. [Google Scholar]
- Ehrnsperger M, Graber S, Gaestel M, Buchner J. Binding of non-native protein to Hsp25 during heat shock creates a reservoir of folding intermediates for reactivation. EMBO J. 1997b;16:221–229. doi: 10.1093/emboj/16.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrnsperger M, Lilie H, Gaestel M, Buchner J. The dynamics of Hsp25 quaternary structure. Structure and function of different oligomeric species. J Biol Chem. 1999;274:14867–14874. doi: 10.1074/jbc.274.21.14867. [DOI] [PubMed] [Google Scholar]
- Garrido C, Bruey JM, Fromentin A, Hammann A, Arrigo AP, Solary E. HSP27 inhibits cytochrome c-dependent activation of procaspase-9. FASEB J. 1999;13:2061–2070. doi: 10.1096/fasebj.13.14.2061. [DOI] [PubMed] [Google Scholar]
- Guay J, Lambert H, Gingras-Breton G, Lavoie JN, Huot J, Landry J. Regulation of actin filament dynamics by p38 map kinase-mediated phosphorylation of heat shock protein 27. J Cell Sci. 1997;110:357–368. doi: 10.1242/jcs.110.3.357. [DOI] [PubMed] [Google Scholar]
- Haslbeck M, Wallke S, Stromer T, Ehrnsperger M, White HE, Chen S, Saibil HR, Buchner J. Hsp26: a temperature-regulated chaperone. EMBO J. 1999;18:6744–6751. doi: 10.1093/emboj/18.23.6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibitayo AI, Sladick J, Tuteja S, Louis-Jacques O, Yamada H, Groblewski G, Welsh M, Bitar KN. HSP27 in signal transduction and association with contractile proteins in smooth muscle cells. Am J Physiol. 1999;277:G445–G454. doi: 10.1152/ajpgi.1999.277.2.G445. [DOI] [PubMed] [Google Scholar]
- Jakob U, Gaestel M, Engel K, Buchner J. Small heat shock proteins are molecular chaperones. J Biol Chem. 1993;268:1517–1520. [PubMed] [Google Scholar]
- Kampinga HH. Thermotolerance in mammalian cells. Protein denaturation and aggregation, and stress proteins. J Cell Sci. 1993;104:11–17. doi: 10.1242/jcs.104.1.11. [DOI] [PubMed] [Google Scholar]
- Kampinga HH, Brunsting JF, Stege GJ, Konings AW, Landry J. Cells overexpressing Hsp27 show accelerated recovery from heat-induced nuclear protein aggregation. Biochem Biophys Res Commun. 1994;204:1170–1177. doi: 10.1006/bbrc.1994.2586. [DOI] [PubMed] [Google Scholar]
- Kato K, Hasegava K, Goto S, Inaguma Y. Dissociation as a result of phosphorylation of an aggregated form of the small stress protein, hsp27. J Biol Chem. 1994;269:11274–11278. [PubMed] [Google Scholar]
- Klemenz R, Andres AC, Frohli E, Schafer R, Aoyama A. Expression of the murine small heat shock proteins hsp 25 and alpha B crystallin in the absence of stress. J Cell Biol. 1993;120:639–645. doi: 10.1083/jcb.120.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert H, Charette SJ, Bernier AF, Guimond A, Landry J. HSP27 multimerization mediated by phosphorylation-sensitive intermolecular interactions at the amino terminus. J Biol Chem. 1999;274:9378–9385. doi: 10.1074/jbc.274.14.9378. [DOI] [PubMed] [Google Scholar]
- Landry J, Chretien P, Lambert H, Hickey E, Weber LA. Heat shock resistance conferred by expression of the human Hsp27 gene in rodent cells. J Cell Biol. 1989;109:7–15. doi: 10.1083/jcb.109.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry J, Huot J. Modulation of actin dynamics during stress and physiological stimulation by a signaling pathway involving p38 MAP kinase and heat shock protein 27. Biochem Cell Biol. 1995;73:703–707. doi: 10.1139/o95-078. [DOI] [PubMed] [Google Scholar]
- Landry J, Lambert H, Zhou M, Lavoie JN, Hickey E, Weber LA, Anderson CW. Human HSP27 is phosphorylated at serines 78 and 82 by heat shock and mitogen-activated kinases that recognize the same amino acid motif as S6 kinase II. J Biol Chem. 1992;267:794–803. [PubMed] [Google Scholar]
- Lavoie JN, Gingras-Breton G, Tanguay RM, Landry J. Induction of Chinese hamster HSP27 gene expression in mouse cells confers resistance to heat shock. HSP27 stabilization of the microfilament organization. J Biol Chem. 1993;268:3420–3429. [PubMed] [Google Scholar]
- Lavoie JN, Lambert H, Hickey E, Weber LA, Landry J. Modulation of cellular thermoresistance and actin filament stability accompanies phosphorylation-induced changes in the oligomeric structure of heat shock protein 27. Mol Cell Biol. 1995;15:505–516. doi: 10.1128/mcb.15.1.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GJ, Roseman AM, Saibil HR, Vierling E. A small heat shock protein stably binds heat-denatured model substrates and can maintain a substrate in a folding competent state. EMBO J. 1997;16:659–671. doi: 10.1093/emboj/16.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JC, Laydon JT, and McDonnell PC. et al. 1994 A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 372:739–746. [DOI] [PubMed] [Google Scholar]
- Loktionova SA, Ilyinskaya OP, Gabai VL, Kabakov AE. Distinct effects of heat shock and ATP depletion on distribution and isoform patterns of human Hsp27 in endothelial cells. FEBS Lett. 1996;392:100–104. doi: 10.1016/0014-5793(96)00792-2. [DOI] [PubMed] [Google Scholar]
- Loktionova SA, Ilyinskaya OP, Kabakov AE. Early and delayed tolerance to simulated ischemia in heat-preconditioned endothelial cells: a role for HSP27. Am J Physiol. 1998;275:H2147–H2158. doi: 10.1152/ajpheart.1998.275.6.H2147. [DOI] [PubMed] [Google Scholar]
- Lutsch G, Vetter R, and Offhauss U. et al. 1997 Abundance and location of the small heat shock proteins HSP25 and alphaB-crystallin in rat and human heart. Circulation. 96:3466–3476. [DOI] [PubMed] [Google Scholar]
- Martin JL, Mestril R, Hilal-Danda R, Brunton LL, Dillmann WH. Small heat shock proteins and protection against ischemic injury in cardiac myocytes. Circulation. 1997;96:4343–4348. doi: 10.1161/01.cir.96.12.4343. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Preville X, Chareyron P, Briolay J, Klemenz R, Arrigo AP. Constitutive expression of human hsp27, Drosophila hsp27, or human alpha B-crystallin confers resistance to TNF- and oxidative stress-induced cytotoxicity in stably transfected murine L929 fibroblasts. J Immunol. 1995;154:363–374. [PubMed] [Google Scholar]
- Merck KB, Groenen PJ, Voorter CE, de Haard-Hoekman WA, Horwitz J, Bloemendal H, de Jong WW. Structural and functional similarities of bovine alpha-crystallin and mouse small heat-shock protein. A family of chaperones. J Biol Chem. 1993;268:1046–1052. [PubMed] [Google Scholar]
- Michels AA, Nguyen VT, Konings AW, Kampinga HH, Bensaude O. Thermostability of a nuclear-targeted luciferase expressed in mammalian cells. Destabilizing influence of the intranuclear microenvironment. Eur J Biochem. 1995;234:382–389. doi: 10.1111/j.1432-1033.1995.382_b.x. [DOI] [PubMed] [Google Scholar]
- Miron T, Vancompernolle K, Vandekerckole J, Wilchek M, Geiger B. A 25-kD inhibitor of actin polymerization is a low molecular mass heat shock protein. J Cell Biol. 1991;114:255–261. doi: 10.1083/jcb.114.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nollen EAA, Brunsting JF, Roelofsen H, Weber LA, Kampinga HH. In vivo chaperone activity of heat shock protein 70 and thermotolerance. Mol Cell Biol. 1999;19:2069–2079. doi: 10.1128/mcb.19.3.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nollen EAA, Salomons FA, Brunsting JF, Want JJL van der, Sibon OCM, and Kampinga HH 2001 Dynamic changes in the localization of thermally unfolded nuclear proteins associated with chaperone dependent protection. Proc Natl Acad Sci USA 98: 12038–12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey P, Farber R, and Nakazawa A. et al. 2000 Hsp27 functions as a negative regulator of cytochrome c-dependent activation of procaspase-3. Oncogene. 19:1975–1981. [DOI] [PubMed] [Google Scholar]
- Piotrowicz RS, Hickey E, Levin EG. Heat shock protein 27 kDa expression and phosphorylation regulates endothelial cell migration. FASEB J. 1998;12:1481–1490. doi: 10.1096/fasebj.12.14.1481. [DOI] [PubMed] [Google Scholar]
- Prescott DM, Myerson D, Wallace J. Enucleation of mammalian cells with cytochalasin B. Exp Cell Res. 1972;71:480–485. doi: 10.1016/0014-4827(72)90322-9. [DOI] [PubMed] [Google Scholar]
- Rogalla T, Ehrnsperger M, and Preville X. et al. 1999 Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/TNF α by phosphorylation. J Biol Chem. 274:18947–18956. [DOI] [PubMed] [Google Scholar]
- Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, Hunt T, Nebrada AR. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78:1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Urushidani T, Nagao T. Translocation of HSP27 to cytoskeleton by repetitive hypoxia-reoxygenation in the rat myoblast cell line, H9c2. Biochem Biophys Res Commun. 1998;251:576–579. doi: 10.1006/bbrc.1998.9518. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Urushidani T, Nagao T. Translocation of HSP27 to sarcomere induced by ischemic preconditioning in isolated rat hearts. Biochem Biophys Res Commun. 2000;269:137–142. doi: 10.1006/bbrc.2000.2233. [DOI] [PubMed] [Google Scholar]
- Schober A, Burger-Kentischer A, Muller E, and Beck FX 1998 Effect of ischemia on localization of heat shock protein 25 in kidney. Kidney Int Suppl. 67 S. 174–S176. [DOI] [PubMed] [Google Scholar]
- van de Klundert FA, Gijsen ML, van den IJssel PR, Snoeckx LH, de Jong WW. Alpha B-crystallin and hsp25 in neonatal cardiac cells—differences in cellular localization under stress conditions. Eur J Cell Biol. 1998;75:38–45. doi: 10.1016/s0171-9335(98)80044-7. [DOI] [PubMed] [Google Scholar]
- Wagstaff MJ, Collaco-Moraes Y, Smith J, de Belleroche JS, Coffin RS, Latchman DS. Protection of neuronal cells from apoptosis by Hsp27 delivered with a herpes simplex virus-based vector. J Biol Chem. 1999;274:5061–5069. doi: 10.1074/jbc.274.8.5061. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Aki T, Harada K, Shama KM, Kamoda Y, Suzuki A, Ohno S. Translocation of HSP27 and MKBP in ischemic heart. Cell Struct Funct. 1999;24:181–185. doi: 10.1247/csf.24.181. [DOI] [PubMed] [Google Scholar]