Abstract
Background
Although stress plays a critical role in vulnerability to nicotine use and dependence, the stress response factors that contribute to smoking behaviors remain poorly elucidated. To minimize the confounding effects of chronic nicotine use, assessing individuals with relatively short smoking histories is critical for characterizing the neurobiological substrates associated with nicotine dependence early in the course of illness.
Objectives
This pilot study examined sympathetic nervous system (alpha-amylase) and hypothalamic-pituitary-adrenal axis (cortisol, dehydroepiandrosterone) responses to the Trier Social Stress Test (TSST) in young adult smokers. Associations among objective indices of recent smoking (salivary cotinine, carbon monoxide in the breath [CO]), behavioral measures of nicotine dependence and withdrawal, and salivary biomarkers in response to the TSST were investigated.
Methods
Smokers (N = 64; 29 males, 35 females) provided saliva samples at 30-minute intervals for two hours prior to the TSST and every ten minutes for one hour following the TSST.
Results
Alpha-amylase responses to the TSST were positively associated with salivary cotinine levels but negatively associated with CO levels. Individuals with a lower level of nicotine dependence had increased cortisol responses to the stressor, whereas those with a higher level of nicotine dependence did not show any cortisol changes in response to the stressor.
Conclusions
These findings indicate that different mechanisms may be involved at different levels of nicotine dependence severity. Recent nicotine use and lower dependence severity may be associated with increased activation of the stress response systems. In contrast, more severe levels of dependence may downregulate stress response systems.
Keywords: Nicotine dependence, cortisol, alpha-amylase, DHEA, stress, TSST
Introduction
The Centers for Disease Control and Prevention (CDC) estimates that 17.8% of adults in the United States – approximately 42 million people - currently smoke cigarettes (1). Each year, cigarette smoking accounts for one in every 5 deaths in the United States (2). Understanding the factors that contribute to smoking is critical given the high morbidity and mortality rates and the staggering cost of smoking-related illnesses in the United States each year (3).
Theoretical models have long emphasized the role of stress in vulnerability to addiction (4). Greater perceived stress is known to contribute to risk for smoking at various stages of illness, including initiation (6), maintenance (7), and relapse (8). Neural circuits implicated in stress regulation and reward processing are activated during acute nicotine use, suggesting that features of the biological stress response could increase stress sensitivity and enhance the reinforcing effects of nicotine (5). Alterations in major stress response systems including the sympathetic nervous system (SNS) and hypothalamic-pituitary-adrenal (HPA) axis have also been linked to nicotine dependence (5). Unfortunately, the neurobiological substrates of nicotine dependence that characterize different stages of illness are not well characterized.
The neurobiology of nicotine dependence is likely a moving target that changes as a function of smoking duration. Chronic stress increases risk for addiction (5) and stressor chronicity is negatively associated with HPA activity (9). Prior research has typically investigated stress hormone secretion at later stages of nicotine dependence in individuals whose stress response systems have been altered by extensive smoking and chronic stress (5,10,21). The present study addresses a critical gap in this literature by examining relations between smoking and acute stress reactivity early in the course of illness before adaptations in stress response neurocircuitry have occurred.
Although central noradrenergic pathways have been implicated in stress-induced reinstatement of nicotine-seeking behavior in animals (11) as well as humans (12), the role of SNS activity in nicotine dependence is not well understood (13). Interpreting this literature requires distinguishing between the unique and interactive effects of diurnal ‘non-stress’ secretion, stress reactivity, and response to nicotine administration, as well as considering the stage of nicotine dependence. Smokers typically show a pattern of elevated diurnal SNS secretion, as evident in higher plasma norepinephrine levels (14). Elevated diurnal output of alpha-amylase – a digestive enzyme increasingly used as a biomarker of autonomic nervous system activity – also predicts unsuccessful smoking cessation in men (21). This pattern of elevated diurnal SNS activity has typically been accompanied by evidence of blunted reactivity to nicotine exposure, as evident in diminished catecholamine (15) and alpha-amylase (17–20) responses to smoking. Research on SNS reactivity to psychosocial stress in smokers has been sparse and inconsistent, with one study showing blunted alpha-amylase responses (16) and a second study showing greater alpha-amylase responses in smokers compared to non-smokers (22). This discrepancy could be explained by differences in specific psychological tasks (i.e., temperament assessment in the natural environment versus laboratory social-evaluative threat task), quantity of acid aldehydes in the tobacco smoke, or to differences in stages of nicotine dependence (16,18); unfortunately, mean duration of nicotine dependence was not reported for either sample of habitual smokers. Hence, it remains unclear how SNS stress reactivity is associated with nicotine dependence severity for newer versus more established smokers.
Research on the role of HPA activity in nicotine dependence has been more extensive and consistent than the SNS literature. Habitual smokers typically exhibit higher circulating cortisol levels and blunted cortisol reactivity to psychosocial stress compared to non-smokers (13,23,24). Intriguingly, stress-induced cigarette craving was positively associated with cortisol responses to psychosocial stress in daily smokers despite lower overall cortisol responses in this group relative to occasional smokers and non-smokers (25). In addition, cortisol responses were positively associated with satisfaction and reward after smoking following a stress task (26). Taken together, these findings suggest that regular and less frequent smokers differ both in their cortisol responses to stress as well as the relations between these responses and stress-induced cigarette craving. However, it is not yet known how HPA reactivity to stress is associated with nicotine dependence severity.
Dehydroepiandrosterone (DHEA) is a neuroactive steroid with anti-glucocorticoid and anxiolytic properties (27) released by the adrenal gland in response to HPA activation. Recent evidence suggests that DHEA may play a role in emotion regulation (28). Habitual smoking is associated with higher diurnal DHEA secretion (29). Higher sulphated DHEA levels have been linked to lower cigarette craving and negative affect (30). Decreases in DHEA levels have been observed after smoking cessation (31), and decreases in the post-cessation DHEA to cortisol ratio may predict relapse (32,33). Preliminary evidence suggests that psychosocial stressors elicit DHEA responses in healthy controls that are positively and moderately correlated with cortisol responses (34,35). Although DHEA responses may protect against increased cortisol reactivity early in the course of nicotine dependence, it is unclear whether DHEA responses to psychosocial stress are affected by nicotine dependence severity and if they adapt to downregulation of HPA activity observed later in the course of nicotine dependence (23).
Chronic stress can interfere with coordination between the HPA axis and SNS, leading to an asymmetry that may increase vulnerability for negative physical and mental health outcomes (36,37). By examining stress response systems in isolation, researchers run the risk of failing to capture individual differences in dysregulation. To our knowledge, this pilot study is the first to simultaneously assess multiple indicators of physiological responses (i.e., SNS activity through salivary alpha-amylase and HPA activity via cortisol and DHEA) to acute psychosocial stress in young adult smokers. Focusing on young adults with relatively short smoking histories compared to typical studies of chronic (> 10 years) smokers (10) allowed us to investigate stress response alterations early in the course of nicotine dependence. We hypothesized that greater nicotine dependence severity would be associated with blunted alpha-amylase, cortisol and DHEA responses.
To address potential reliability problems associated with self-reported smoking status measures, we assessed nicotine intake via two biomarkers - salivary cotinine levels and exhaled carbon monoxide (breath CO). Prior research has indicated that these measures may capture distinct temporal windows on nicotine intake and each has specific advantages and disadvantages (38–40). CO is the most rapid, noninvasive and easily measured biochemical indicator of smoking. However, it has a shorter half-life (approximately 2–3 hours) and is sensitive only to recent smoking. Cotinine, the major metabolite of nicotine, has a longer half-life (about 10–30 hours). Quantitative analysis of cotinine is the “gold standard” for verifying smoking status, but it can be costly and time consuming. Also, due to its longer half-life, it cannot discriminate between short-term abstinence (< 24 hr) and continued smoking. We wanted to assess how these two biomarkers are associated with the neuroendocrine measures. To our knowledge, only one study has examined associations between biomarkers of cigarette smoking and HPA or SNS activity: among mothers who smoked and their infants, neither cortisol nor alpha-amylase levels were correlated with salivary cotinine levels in either mothers or infants (16). Given the short half-life of CO, we hypothesized that it would be negatively correlated with neuroendocrine measures in the present sample (since participants were abstinent from smoking immediately before and during the stress protocol), whereas salivary cotinine would be positively correlated with neuroendocrine measures. Also, we hypothesized that the association of CO would be stronger with alpha-amylase than cortisol and DHEA responses to the stressor since SNS response occurs relatively quickly after the onset of the stressor compared to cortisol and DHEA responses (41–43).
Methods
Participants
Participants were 64 young adult smokers (29 males, 35 females) ages 18 to 25. Mean age of nicotine dependence onset was 17.98 (range: 13 to 23 years old). To be included in the study, participants had to smoke at least 10 cigarettes per day in the past six months. Participants with a personal or family history of a psychiatric disorder were excluded. All participants were medically healthy and free from psychiatric conditions as well as medications that affect the endocrine or central nervous system and alcohol or illicit drug use in the past two weeks, as determined by medical and psychiatric history, physical examination, laboratory investigations, and urine drug screens. To minimize the confounding effects of acute nicotine use while minimizing the effects of nicotine withdrawal on neuroendocrine responses to the psychosocial stressor, the participants were instructed to abstain from smoking for one hour before the laboratory visit. All procedures were approved by the institutional review board. Written informed consent was obtained from all participants.
Measures
Diagnostic Evaluation and Family History of Psychopathology
The diagnosis of psychiatric disorders was based on semi-structured interviews. The Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) was administered to the participants (SCID) (44). History of psychiatric disorders in family members was determined by a semi-structured interview, the Family History-Research Diagnostic Criteria (FH-RDC) (45). The participant’s mother was interviewed regarding lifetime psychiatric disorders in all first-degree relatives (including self, spouse, and all offspring).
Nicotine Dependence
Nicotine dependence was assessed with the tobacco use section of the World Health Organization – Composite International Diagnostic Interview (46) and diagnosed according to DSM-IV-TR criteria (47). The Fagerström Tolerance Questionnaire (FTQ) (48), which contains eight items assessing smoking habits (e.g., “how soon after you wake up do you smoke your first cigarette?”), was used to determine the degree of nicotine dependence. FTQ scores range from 0 to 11 with higher scores indicating heavier dependence. Behavioral symptoms of nicotine withdrawal (e.g., irritability, restlessness, impatience) were assessed with the 8-item Hughes-Hatsukami Withdrawal Symptoms Questionnaire (49).
Psychosocial Stressor
Participants completed a standardized psychosocial stress protocol – the TSST – that incorporates elements (i.e., social-evaluative threat, uncontrollability) known to induce robust HPA and SNS responses (50–52). The TSST involved a 5-minute public speaking task (following a 5-minute preparation period) and a 5-minute mental arithmetic task performed in front of two audience members seated behind a table with a video camera (53). Participants were asked to imagine that they were applying for their ideal job and to prepare a speech to convince the audience that they were the best candidate for the position. During the mental arithmetic task (serial subtraction), participants were interrupted after each incorrect response and asked to begin again from the starting number. Starting from 30 minutes after arrival to the laboratory, baseline saliva samples were collected at 30-minute intervals for 2 hours prior to the stress task (five samples). During this pre-stress phase, participants were instructed to lie down and were not permitted any activity except for reading or watching television with a neutral content. Post-stress saliva samples were collected immediately after the task and at 10-minute intervals for 60 minutes (seven samples). During the post-stress phase, participants were relaxed and were not permitted any activity except for completing a manipulation check assessing how stressful they experienced the tasks. The stress protocol was conducted in the late afternoon/early evening. After the TSST, participants reported how stressful the “experiment” was on a 1 (“not at all”) to 5 (“very stressful”) scale.
Assays
All biomarkers were assayed in duplicates. Nicotine intake was assessed with salivary cotinine (ng/mL) collected during a physical examination. Cotinine is the primary metabolite of nicotine and represents a biomarker of nicotine intake (54). Salivary cotinine levels were determined using a high sensitivity enzyme-linked immunoassay kit (Salimetrics LLC, State College, PA). Breath CO readings were obtained using a Bedfont EC50 Smokerlyzer Monitor (Bedfont Scientific Ltd, Kent, UK). Participants were instructed to hold their breath for 15 seconds and then slowly blow out all the air in their lungs into the monitor. CO levels are presented in parts per million (ppm). Salivary alpha-amylase was determined by a kinetic assay (Salimetrics LLC, State College, PA). Salivary cortisol levels were determined using a commercially available enzyme immunoassay kit (Enzyme-Linked ImmunoSorbent Assay, ALPCO diagnostics, Salem, NH). Salivary non-sulphated DHEA was determined using a commercially available high sensitivity enzyme immunoassay kit (Salimetrics LLC, State College, PA).
Data Analytic Plan
Pre-stress hormone/enzyme levels were computed as the mean of the fourth (30 minutes prior to TSST) and fifth (immediately prior to TSST) baseline samples (after the participants had adequate time to adapt to the laboratory). Response to the TSST was computed as area under the curve with respect to ground (AUCg) (55) from the pre-stress mean to the final recovery sample. AUCg was normally distributed for alpha-amylase, cortisol and DHEA. The AUC trapezoidal formula (55) was adjusted to account for missing post-stress data points for 6 participants by altering the number of measurements and duration of measurement intervals (e.g., participants missing one post-stress data point contributed six samples at 10-minute intervals except for one 20-minute interval reflecting the missing data point). Multiple regression was used to examine relations between indicators of recent nicotine use (i.e., salivary cotinine and breath CO levels) and outcomes measures (i.e., pre-stress hormone/enzyme levels and AUCg stress responses).
The association of nicotine dependence severity and within-person changes in stress hormone levels during the TSST was examined using hierarchical linear models (HLM v. 6) (56). Individuals were assigned to either high or low nicotine dependence severity groups based on a median split of FTQ scores. Alpha-amylase, cortisol, and DHEA levels were log-transformed to address skewness. Significant nicotine dependence severity X time interactions (reflecting linear changes in stress hormones following the TSST) were examined using simple slope analysis.
Results
Descriptive Analyses
Descriptive characteristics are presented in Table 1 for high and low nicotine dependence severity groups (based on median split of FTQ scores). The high nicotine dependence severity group had a higher proportion of females than the low nicotine dependence group; subsequent analyses of nicotine dependence severity controlled for sex. The high nicotine dependence severity group smoked more cigarettes per day, had a shorter duration of nicotine dependence, and higher nicotine dependence severity scores than the low nicotine dependence severity group. Preliminary analyses among biological measures of nicotine use revealed that breath CO levels were positively correlated with salivary cotinine (r(62) = .40, p < .01). However, analysis of nicotine dependence severity measures indicated that FTQ scores were not significantly associated with withdrawal symptoms (rs(62) = .07, p = .60). The mean perceived stressfulness rating for the TSST was 3.84 (SD = 0.95); most participants described the TSST as either “stressful” or “very stressful.”
Table 1.
| Low Nicotine Dependence (N = 32) | High Nicotine Dependence (N = 32) | Low ND vs. High ND | |
|---|---|---|---|
| N (%) | N (%) | X2 | |
|
|
|||
| Sex | 4.17* | ||
| Male | 18 (56) | 10 (31) | |
| Female | 14 (44) | 22 (69) | |
| Race | 0.41 | ||
| Caucasian | 25 (78) | 27 (84) | |
| Ethnicity | 0.22 | ||
| Hispanic | 3 (9) | 2 (6) | |
| Non-Hispanic | 29 (91) | 30 (94) | |
|
|
|||
| M (SD) | M (SD) | t | |
|
|
|||
| Age | 22.06 (2.10) | 21.77 (2.17) | 0.54 |
| Alpha-amylase | |||
| Pre-stress (μg/ml) | 63.42 (50.15) | 61.35 (43.99) | 0.17 |
| AUCg | 4,379.09 (2,586.86) | 4,671.10 (3,452.13) | 0.37 |
| Cortisol | |||
| Pre-stress (ng/ml) | 0.06 (0.03) | 0.07 (0.14) | 0.51 |
| AUCg | 10.10 (11.42) | 7.03 (5.99) | 1.35 |
| DHEA | |||
| Pre-stress (pg/ml) | 93.28 (62.07) | 135.87 (118.66) | 1.77 |
| AUCg | 11,591.14 (8,409.18) | 12,726.52 (9,261.38) | 0.49 |
| Smoking | |||
| # cigs/day | 15.98 (5.00) | 24.42 (10.15) | 3.67** |
| Age of ND onset | 17.76 (2.14) | 18.40 (2.10) | 1.02 |
| Duration of ND (yrs.) | 4.14 (2.39) | 2.88 (1.81) | 2.04* |
| FTQ score | 4.40 (0.88) | 7.00 (0.93) | 11.29*** |
| H-H withdrawal symptoms | 0.66 (0.58) | 0.67 (0.53) | 0.05 |
| Salivary cotinine (ng/ml) | 102.07 (78.26) | 101.44 (73.22) | 0.03 |
| Carbon monoxide (ppm) | 11.31 (5.44) | 12.68 (9.44) | 0.65 |
Note: ND = nicotine dependence; AUC = area under the curve; FTQ = Fagerstrom Tolerance Questionnaire; H-H Withdrawal symptoms (Hughes-Hatsukami Withdrawal Symptom Scale).
p < .001;
p < .01;
p < .05.
Recent Nicotine Use and Stress Responses
Pre-stress hormone/enzyme levels
Multiple regression analysis indicated that salivary cotinine levels and breath CO levels explained 30% of the variance in pre-stress alpha-amylase levels, such that pre-stress alpha-amylase levels were positively associated with salivary cotinine levels (β = .45, t(61) = 3.21, p < .01) and negatively associated with breath CO levels (β = −.51, t(61) = 3.69, p < .01). Pre-stress cortisol levels were not significantly associated with either salivary cotinine levels (β = −.13, t(61) = 0.80, p = .43) or breath CO levels (β = .21, t(61) = 1.31, p = .20). Salivary cotinine levels and breath CO levels explained 13% of the variance in pre-stress DHEA levels, such that pre-stress DHEA levels were positively associated with salivary cotinine levels (β = .39, t(61) = 2.50, p = .02) but not significantly associated with breath CO levels (β = −.18, t(61) = 1.19, p = .24).
Stress responses
Multiple regression analysis indicated that salivary cotinine levels and breath CO levels explained 20% of the variance in alpha-amylase responses, such that alpha-amylase AUCg was positively associated with salivary cotinine levels (β = .39, t(61) = 2.59, p = .01) and negatively associated with breath CO levels (β = −.41, t(61) = 2.69, p = .01). Cortisol responses were not significantly associated with either salivary cotinine levels (β = .21, t(61) = 1.30, p = .20) or breath CO levels (β = .23, t(61) = 1.43, p = .16). Salivary cotinine levels and breath CO levels explained 11% of the variance in DHEA responses, such that DHEA responses were positively associated with salivary cotinine levels at the level of a non-significant trend (β = .32, t(61) = 2.00, p < .06) and were not significantly associated with breath CO levels (β = .02, t(61) = 0.14, p = .89).
Nicotine Dependence Severity and Stress Responses
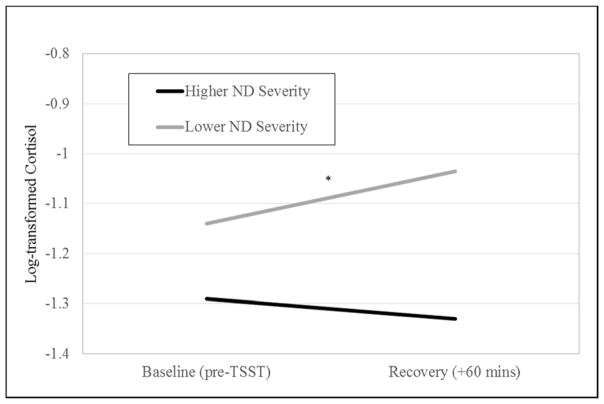
A median split of FTQ scores was used to create a high nicotine dependence severity group (mean FTQ score = 7.0, SD = 0.9; range 6 to 9) and a low nicotine dependence severity group (mean FTQ score = 4.4, SD = 0.9; range 2.5 to 5.5). Multilevel models revealed a significant interaction between nicotine dependence severity and time predicting post-TSST changes in cortisol levels (b = −.002, SE = .001, p = .02), controlling for sex. Simple slope analyses revealed that cortisol levels increased significantly in response to the TSST in individuals with lower nicotine dependence severity (b = .001, SE = .0006, p = .01) but did not change significantly for individuals with higher nicotine dependence severity (b = −.0004, SE = .0006, p = .47) (Figure 1). Using nicotine dependence as a continuous variable showed the same pattern of results, with higher nicotine dependence severity being associated with lower cortisol responses (rs(62) = −.32, p = .02). Nicotine dependence severity did not interact with time to predict changes in alpha-amylase (b = .001, SE = .001, p = .39) or DHEA (b = −.001, SE = .001, p = .08) levels.
Figure 1.
Multilevel model testing the nicotine dependence (ND) severity group (median split based on FTQ scores) by time interaction predicting post-TSST changes in cortisol levels. Asterisk indicates significant simple slope. *p < .05.
Discussion
This pilot study examined associations between stress-related biomarkers and measures of nicotine use and dependence in young adult smokers with relatively short histories of dependence. Although considerable research has focused on stress response alterations in chronic smokers with prolonged dependence (13), very little is known about the stress response before adaptations in stress response neurocircuitry to chronic nicotine consumption have taken hold. This sample afforded a unique opportunity to characterize the neurobiological substrates associated with different levels of nicotine dependence early in the course of illness. The present findings extend prior research on the impact of acute versus long-term smoking on salivary cortisol responses to two critical but overlooked components of the stress response - the SNS (alpha-amylase) and DHEA. Individuals with a lower level of nicotine dependence severity showed increased cortisol responses to the stressor but those with a higher level of dependence did not show any cortisol changes, which extends prior research demonstrating blunted cortisol reactivity in chronic smokers compared to non-smokers (13,23,24). Cortisol responses were not significantly associated with biological indicators of recent nicotine use. In contrast, pre-stress DHEA levels were positively associated with salivary cotinine levels but not significantly associated with nicotine dependence severity. Alpha-amylase stress responses were positively associated with salivary cotinine levels but negatively associated with breath CO levels, suggesting that these diverse biomarkers capture unique aspects of nicotine use.
Cigarette smoking induces increased salivary cortisol levels (57) when nicotine binds to cholinergic receptors in the locus coeruleus and hypothalamus and stimulates the release of corticotropin-releasing hormone (58). Repeated activation of the HPA axis in habitual smokers, however, may lead to a downregulation of CRH receptors over time, which could explain hypo-responsiveness of the HPA axis to a psychosocial or pharmacological challenge (23) and also the lack of an observed association between recent nicotine intake and cortisol responses. Abstinent smokers display enhanced craving and blunted HPA responses to psychosocial stress, which place them at increased risk for relapse (23). Buchmann and colleagues (25) have proposed a classical conditioning model in which the repeated pairing of nicotine and cortisol “hits” in chronic smokers results in a conditioned stimulus (cortisol elevations) capable of triggering the conditioned response (cigarette craving). One possible extension of this hypothesis is that smokers become increasingly sensitized to stress-related increases in cortisol with repeated “hits”, such that smaller and smaller cortisol surges are capable of triggering craving and, potentially, relapse (5). Future studies are needed to determine the extent to which blunted cortisol responses are preexisting risk factors for nicotine dependence and/or changes in response to smoking over time.
Individuals with blunted DHEA responses report more negative mood following a stressor (34). Consistent with prior research showing higher daily DHEA output in habitual smokers (29), the present study found greater pre-stress DHEA levels in individuals with higher salivary cotinine levels. Elevated basal DHEA levels in smokers may represent a marker of resilience. Treatment with DHEA leads to improvements in mood and stress management in healthy individuals (59). In addition, DHEA administration has been shown to reduce activity in brain regions involved in negative mood generation and to increase activity in regions involved in emotion regulation (28). Future studies should investigate the possibility that emotion regulation capacity mediates the relation between lower DHEA levels and increased cigarette craving.
Alpha-amylase reactivity to psychosocial stress was not significantly associated with nicotine dependence severity, though previous studies have shown that higher daily alpha-amylase secretion predicts unsuccessful smoking cessation (21). However, relations between acute smoking indicators and alpha-amylase stress responses differed according to the biological measure of smoking status. On the one hand, higher salivary alpha-amylase responses were associated with higher salivary cotinine levels, consistent with previous findings of higher levels of norepinephrine in smokers (14). On the other hand, breath CO levels were negatively associated with alpha-amylase responses, which is consistent with studies showing a negative association between tobacco smoke exposure and alpha-amylase secretion (16) and the hypothesis that acid aldehydes present in tobacco smoke reduce alpha-amylase activity (17,18). This discrepancy may reflect the different temporal windows on nicotine intake provided by salivary cotinine and breath CO levels (39–41). Whereas the half-life of breath CO is 2–3 hours, the half-life of cotinine in saliva is between 10 and 30 hours (60). Recent exposure to nicotine (captured by breath CO levels) may diminish salivary alpha-amylase levels, while more consistent exposure to nicotine in the previous day (captured by salivary cotinine levels) may increase salivary alpha-amylase levels. Though speculative, this interpretation is supported by research demonstrating that breath CO levels are more indicative of recent cigarette smoking than cotinine (61).
Strengths of this pilot study include the use of semi-structured interviews to carefully assess and exclude potential participants with psychiatric conditions known to influence HPA function and the use of both subjective and objective measures of nicotine use and dependence. Additional strengths include the careful adaptation of participants to the laboratory prior to the administration of the stressor and the simultaneous assessment of multiple neurobiological indices of the stress response over an adequate period of time.
Limitations of the present study provide directions for future research. First, we were unable to determine whether smokers display abnormalities in their SNS or HPA responses to psychosocial stress due to the lack of a non-smoker comparison group matched by sex and age. Second, this cross-sectional study could not determine whether biomarkers of nicotine dependence are associated with maintenance or relapse of smoking behavior. Another possibility that needs to be addressed by prospective studies is whether the blunted cortisol reactivity that characterizes smokers (62) represents a preexisting risk factor for developing nicotine dependence. Third, menstrual cycle phase - which is known to impact TSST responses (57) - was not held constant for female participants completing the TSST. Fourth, it is unclear how the present findings would translate to smokers with psychiatric comorbidities because participants with a personal or family history of psychiatric disorder were excluded to minimize the potential for confounding effects on HPA activity. Finally, although the present study examined multiple indicators of acute SNS and HPA stress reactivity, it remains unclear how other types of life events including childhood trauma and ongoing chronic stress may influence relations between salivary biomarkers and nicotine dependence severity.
The present findings highlight important links between smoking behaviors and biological responses to psychosocial stress and suggest that different mechanisms may be involved at different levels of nicotine dependence severity. For example, smokers with more severe levels of nicotine dependence showed diminished cortisol responses and may benefit from pharmacological interventions that upregulate HPA reactivity (12,63). In contrast, smokers with lower dependence severity showed greater cortisol responses and may benefit from pharmacological interventions that downregulate HPA reactivity (64).
Acknowledgments
The authors would like to thank all the volunteers who participated in this study.
Funding and disclosure
This research was funded in part by grants from the National Institute of Health (R01 DA014037, R01 DA015131, R01 DA017804, R01 DA017805, R01 MH062464, R01 MH068391, K01 MH101403), by the Sarah M. and Charles E. Seay Chair in Child Psychiatry at UT Southwestern Medical Center (Uma Rao), and by the Betsey R. Bush Endowed Professorship in Behavioral Health at the University of Tennessee (Uma Rao). These funding agencies had no further role in the study design, data collection, analysis or interpretation of data, writing of the report, or the decision to submit the manuscript for publication. The authors report no biomedical financial interests or potential conflicts of interest
References
- 1.CDC. Current cigarette smoking among adults – United States, 2005–2013. MMWR. 2014;63:1108–1112. [PMC free article] [PubMed] [Google Scholar]
- 2.U.S. Department of Health and Human Services. The health consequences of smoking – 50 years of progress: A report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 3.Xu X, Bishop EE, Kennedy SM, Simpson SA, Pechacek TF. Annual healthcare spending attributable to cigarette smoking: An update. Am J Prev Med. 2015;48:326–333. doi: 10.1016/j.amepre.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koob G, Kreek MJ. Stress, dysregulation or drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wills TA, Sandy JM, Yaeger AM. Stress and smoking in adolescence: A test of directional hypotheses. Health Psychol. 2002;21:122–130. [PubMed] [Google Scholar]
- 7.Payne TJ, Schare ML, Levis DJ, Colletti G. Exposure to smoking-relevant cues: Effects on desire to smoke and topographical components of smoking behavior. Addict Behav. 1991;16:467–479. doi: 10.1016/0306-4603(91)90054-l. [DOI] [PubMed] [Google Scholar]
- 8.Siahpush M, Carlin JB. Financial stress, smoking cessation and relapse: Results from a prospective study of an Australian national sample. Addiction. 2006;101:121–127. doi: 10.1111/j.1360-0443.2005.01292.x. [DOI] [PubMed] [Google Scholar]
- 9.Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- 10.al’Absi M, Nakajima M, Allen S, Lemieux A, Hatsukami D. Sex differences in hormonal responses to stress and smoking relapse: A prospective examination. Nicotine Tob Res. 2015;17:382–389. doi: 10.1093/ntr/ntu340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamada H, Bruijnzeel AW. Stimulation of α2-adrenergic receptors in the central nucleus of the amygdala attenuates stress-induced reinstatement of nicotine seeking in rats. Neuropsychopharmacology. 2011;60:303–311. doi: 10.1016/j.neuropharm.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKee SA, Potenza MN, Kober H, et al. A translational investigation targeting stress-reactivity and prefrontal cognitive control with guanfacine for smoking cessation. J Psychopharmacol. 2015;29:300–311. doi: 10.1177/0269881114562091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richards JM, Stipelman BA, Bornovalova MA, et al. Biological mechanisms underlying the relationship between stress and smoking: State of the science and directions for future work. Biol Psychol. 2011;88:1–12. doi: 10.1016/j.biopsycho.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen EW, Espersen K, Kanstrup L-L, Christensen NJ. Plasma noradrenaline and ageing: Effects of smoking habits. Eur J Clin Invest. 1996;26:839–846. doi: 10.1111/j.1365-2362.1996.tb02127.x. [DOI] [PubMed] [Google Scholar]
- 15.Niedermaier ON, Smith ML, Beightol LA, et al. Influence of cigarette smoking on human autonomic function. Circulation. 1993;88:562–571. doi: 10.1161/01.cir.88.2.562. [DOI] [PubMed] [Google Scholar]
- 16.Granger DA, Blair C, Willoughby M, et al. Individual differences in salivary cortisol and alpha-amylase in mothers and their infants: Relation to tobacco smoke exposure. Dev Psychobiol. 2007;49:692–701. doi: 10.1002/dev.20247. [DOI] [PubMed] [Google Scholar]
- 17.Nagler R, Lischinsky S, Diamond E, et al. Effect of cigarette smoke on salivary proteins and enzyme activities. Arch Biochem Biophys. 2000;379:229–236. doi: 10.1006/abbi.2000.1877. [DOI] [PubMed] [Google Scholar]
- 18.Zappacosta B, Persichilli S, Mordente A, et al. Inhibition of salivary enzymes by cigarrette smoke and protective role of glutathione. Hum Exp Toxicol. 2002;21:7–11. doi: 10.1191/0960327102ht202oa. [DOI] [PubMed] [Google Scholar]
- 19.Nagaya T, Okuno M. No effects of smoking or drinking habits on salivary amylase. Toxicol Lett. 1993;66:257–261. doi: 10.1016/0378-4274(93)90006-j. [DOI] [PubMed] [Google Scholar]
- 20.Zuabi O, Machtei EE, Ben-Aryeh H, et al. The effect of smoking and periodontal treatment on salivary composition in patients with established periodontitis. J Periodontol. 1999;70:1240–1246. doi: 10.1902/jop.1999.70.10.1240. [DOI] [PubMed] [Google Scholar]
- 21.Dušková M, Šimůnková K, Hill M, et al. Higher levels of salivary α-amylase predict failure of cessation efforts in male smokers. Physiol Res. 2010;59:765–771. doi: 10.33549/physiolres.931889. [DOI] [PubMed] [Google Scholar]
- 22.Thoma MV, Kirschbaum C, Wolf JM, Rohleder N. Acute stress responses in salivary alpha-amylase predict increases of plasma norepinephrine. Biol Psychol. 2012;91:342–348. doi: 10.1016/j.biopsycho.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 23.al’Absi M. Hypothalamic-pituitary-adrenocortical responses to psychological stress and risk for smoking relapse. Int J Psychophysiol. 2006;59:218–227. doi: 10.1016/j.ijpsycho.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Rao U, Hammen CL, London ED, Poland RE. Contribution of hypothalamic-pituitary-adrenal activity and environmental stress to vulnerability for smoking in adolescents. Neuropsychopharmacology. 2009;34:2721–2732. doi: 10.1038/npp.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buchmann AF, Laucht M, Schmid B, et al. Cigarette craving increases after a psychosocial stress test and is related to cortisol stress response but not to dependence scores in daily smokers. J Psychopharmacol. 2010;24:247–255. doi: 10.1177/0269881108095716. [DOI] [PubMed] [Google Scholar]
- 26.McKee SA, Sinha R, Weinberger AH, et al. Stress decreases the ability to resist smoking and potentiates smoking intensity and reward. J Psychopharmacol. 2011;25:490–502. doi: 10.1177/0269881110376694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS) Front Neuroendocrinol. 2009;30:65–91. doi: 10.1016/j.yfrne.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sripada RK, Marx CE, King AP, et al. DHEA enhances emotion regulation neurocircuits and modulates memory for emotional stimuli. Neuropsychopharmacology. 2013;38:1798–1807. doi: 10.1038/npp.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feldman HA, Johannes CB, McKinlay JB, Longcope C. Low dehydroepiandrosterone sulfate and heart disease in middle-aged men: Cross-sectional results from the Massachusetts Male Aging Study. Ann Epidemiol. 1998;8:217–228. doi: 10.1016/s1047-2797(97)00199-3. [DOI] [PubMed] [Google Scholar]
- 30.Marx CE, Trost WT, Shampine L, et al. Neuroactive steroids, negative affect, and nicotine dependence severity in male smokers. Psychopharmacology. 2006;186:462–472. doi: 10.1007/s00213-005-0226-x. [DOI] [PubMed] [Google Scholar]
- 31.Oncken C, Prestwood K, Cooney JL, et al. Effects of smoking cessation or reduction on hormone profiles and bone turnover in postmenopausal women. Nicotine Tob Res. 2002;4:451–458. doi: 10.1080/1462220021000018399. [DOI] [PubMed] [Google Scholar]
- 32.Rasmusson AM, Wu R, Paliwal P, Anderson GM, Krishnan-Sarin S. A decrease in the plasma DHEA to cortisol ratio during smoking abstinence may predict relapse: A preliminary study. Psychopharmacology. 2006;186:473–480. doi: 10.1007/s00213-006-0367-6. [DOI] [PubMed] [Google Scholar]
- 33.Hruškovičová H, Dušková M, Šimůnková K, et al. Effects of smoking cessation on hormonal levels in men. Physiol Res. 2013;62:67–73. doi: 10.33549/physiolres.932326. [DOI] [PubMed] [Google Scholar]
- 34.Izawa S, Sugaya N, Shirotsuki K, et al. Salivary dehydroepiandrosterone secretion in response to acute psychosocial stress and its correlations with biological and psychological changes. Biol Psychol. 2008;79:294–298. doi: 10.1016/j.biopsycho.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Lennartsson A-K, Kushnir MM, Bergquist J, Jonsdottir IH. DHEA and DHEA-S response to acute psychosocial stress in healthy men and women. Biol Psychol. 2012;90:143–149. doi: 10.1016/j.biopsycho.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Andrews J, Ali N, Pruessner JC. Reflections on the interaction of psychogenic stress systems in humans: The stress coherence/compensation model. Psychoneuroendocrinology. 2013;38:947–961. doi: 10.1016/j.psyneuen.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 37.Bauer AM, Quas JA, Boyce WT. Associations between physiological reactivity and children’s behavior: Advantages of a multisystem approach. J Dev Behav Pediatr. 2002;23:102–113. doi: 10.1097/00004703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Kauffman RM, Ferketich AK, Murray DM, Bellair PE, Wewers ME. Measuring tobacco use in a prison population. Nicotine Tob Res. 2008;12:582–588. doi: 10.1093/ntr/ntq048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marrone GF, Paulpillai M, Evans RJ, Singleton EG, Heishman SJ. Breath carbon monoxide and semiquantitative saliva cotinine as biomarkers for smoking. Hum Psychopharmacol. 2010;25:80–83. doi: 10.1002/hup.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marrone GF, Shakleya DM, Scheidweiler KB, et al. Relative performance of common biochemical indicators in detecting cigarette smoking. Addiction. 2011;106:1325–1334. doi: 10.1111/j.1360-0443.2011.03441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagy T, van Lien R, Willemsen G, et al. A fluid response: Alpha-amylase reactions to acute laboratory stress are related to sample timing and saliva flow rate. Biol Psychol. 2015;109:111–119. doi: 10.1016/j.biopsycho.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 42.Bae YJ, Stadelmann S, Klein AM, et al. The hyporeactivity of salivary cortisol at stress test (TSST-C) in children with internalizing or externalizing disorders is contrastively associated with α-amylase. J Psychiatr Res. 2015;71:78–88. doi: 10.1016/j.jpsychires.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 43.Maruyama Y, Kawano A, Okamoto S, et al. Differences in salivary alpha-amylase and cortisol responsiveness following exposure to electrical stimulation versus the Trier Social Stress Tests. PLoS One. 2012;7:7. doi: 10.1371/journal.pone.0039375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.First MB, Spitzer RL, Gibbon M, Williams JBW. User’s guide for the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) - Clinician Version. Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 45.Andreasen NC, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria. Arch Gen Psychiatry. 1977;34:1229–1235. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- 46.World Health Organization. Composite international diagnostic interview (core version 1.1) Geneva: WHO; 1993. [Google Scholar]
- 47.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 2000. [Google Scholar]
- 48.Fagerström KO, Schneider NG. Measuring nicotine dependence: A review of the Fagerström Tolerance Questionnaire. J Behav Med. 1989;12:159–182. doi: 10.1007/BF00846549. [DOI] [PubMed] [Google Scholar]
- 49.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- 50.Buske-Kirschbaum A, Jobst S, Wustmans A, et al. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosom Med. 1997;59:419–426. doi: 10.1097/00006842-199707000-00012. [DOI] [PubMed] [Google Scholar]
- 51.Kirschbaum C, Pirke KM, Hellhammer DH. The Trier Social Stress Test – A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- 52.Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- 53.Rao U, Hammen C, Ortiz LR, Chen LA, Poland RE. Effects of early and recent adverse experiences on adrenal response to psychosocial stress in depressed adolescents. Biol Psychiatry. 2008;64:521–526. doi: 10.1016/j.biopsych.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Benowitz NL, Hukkanen J, Jacob P. Nicotine chemistry, metabolism, kinetics, and biomarkers. Handb Exp Pharmacol. 2009;192:29–60. doi: 10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- 56.Raudenbush SW, Bryk AS, Congdon R. HLM 6 for Windows. Lincolnwood, IL: Scientific Software International, Inc; 2004. [Google Scholar]
- 57.Kudielka BM, Hellhammer DH, Wüst S. Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology. 2009;34:2–18. doi: 10.1016/j.psyneuen.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 58.Matta SG, Fu Y, Valentine JD, Sharp BM. Response of the hypothalamo-pituitary-adrenal axis to nicotine. Psychoneuroendocrinology. 1998;23:103–113. doi: 10.1016/s0306-4530(97)00079-6. [DOI] [PubMed] [Google Scholar]
- 59.Morales AJ, Nolan JJ, Nelson JC, Yen SS. Effects of replacement dose of dehydroepiandrosterone in men and women of advancing age. J Clin Endocrinol Metab. 1994;78:1360–1367. doi: 10.1210/jcem.78.6.7515387. [DOI] [PubMed] [Google Scholar]
- 60.Benowitz NL. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 61.Schepis TS, Duhig AM, Liss T, et al. Contingency management for smoking cessation: Enhancing feasiblity through use of immunoassay test strips measuring cotinine. Nicotine Tob Res. 2008;10:1495–1501. doi: 10.1080/14622200802323209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.al’Absi M, Nakajima M, Grabowski J. Stress response dysregulation and stress-induced analgesia in nicotine dependent men and women. Biol Psychol. 2013;93:1–8. doi: 10.1016/j.biopsycho.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roche DJO, Childs E, Epstein AM, King AC. Acute HPA axis repsonse to naltrexone differs in female vs. male smokers. Psychoneuroendocrinology. 2010;35:596–606. doi: 10.1016/j.psyneuen.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Straneva-Meuse PA, Light KC, Allen MT, Golding M, Girdler SS. Bupropion and paroxetine differentially influence cardiovascular and neuroendocrine responses to stress in depressed patients. J Affect Disord. 2004;79:51–61. doi: 10.1016/S0165-0327(02)00352-X. [DOI] [PubMed] [Google Scholar]