Abstract
Aims
To compare high-risk histopathology of eyes with primary vs. secondary enucleation from patients with retinoblastoma.
Patients and Methods
A retrospective histopathology review identified 207 eyes enucleated from 202 patients between March 1997 and August 2013. Our review considered high-risk histopathologic features to include extraocular disease or invasion of the anterior chamber, iris, ciliary body, choroid (massive), postlaminar optic nerve, or sclera.
Results
Most eyes (144, 70%) were primarily enucleated; 63 (30%) were secondarily enucleated after neoadjuvant therapy. The primary enucleation group had more advanced disease (Reese-Ellsworth Group V: 95% vs. 59%; International Classification Group D/E: 97% vs. 59%; p<0.001). The incidence of high-risk histopathology features was similar between groups (32% vs. 21%, n=59; p=0.132). The type of prior therapy was not associated with high-risk histopathology features. Time to enucleation was longer for secondarily enucleated eyes with high-risk features. Choroid and postlaminar optic nerve invasion were more frequent in eyes primarily enucleated (p<0.001). Forty-six of the 59 (78%) patients with high-risk features received adjuvant chemotherapy and/or external beam radiation therapy. Three patients who received primary enucleation and adjuvant therapy died of metastatic recurrence.
Conclusion
Despite the more favorable classification of eyes treated with neoadjuvant therapy, the risk of high-risk histopathology features at enucleation was comparable to eyes undergoing primary enucleation. Delayed enucleation was associated with these features, and the majority of patients required further adjuvant therapy. Caution must be exercised in treating recalcitrant intraocular retinoblastoma to promptly pursue definitive enucleation in an effort to minimize further treatment exposures and metastases.
Keywords: high-risk histopathology, pathology, retinoblastoma, adjuvant chemotherapy, eye enucleation
Introduction
Risk-based management of retinoblastoma is guided by the extent of intraocular disease, laterality, and child's age at diagnosis. Beginning with neoadjuvant systemic chemotherapy1,2 and now intra-arterial chemotherapy,3 successful chemoreduction paired with subsequent or concurrent focal consolidation therapies provide the opportunity for ocular salvage and vision preservation.4,5 Yet the risk for recalcitrant disease and extraocular dissemination still exists for patients with advanced disease.6
Prolonged time to enucleation, along with coexisting ocular morbidities, is predictive of extraocular extension.7 Extraocular extension and invasion of the anterior chamber, iris, ciliary body, choroid (massive, >3mm), postlaminar optic nerve (including postlaminar invasion and invasion to the cut end of the optic nerve), or sclera are considered7-13 to be high-risk histopathologic (HRH) features for metastases. If HRH features are discovered at the time of enucleation, subsequent adjuvant chemotherapy and/or external beam radiation therapy (EBRT) may be prescribed.11,14-18 In some cases of advanced intraocular disease, pathology may be down-staged after neo-adjuvant therapy, increasing the risk of metastasis and death due to reduced surveillance and inappropriate management of unrecognized high-risk disease.19
We undertook a retrospective study comparing the presence of HRH features in two groups of pediatric patients with retinoblastoma. The first group included patients who underwent primary enucleation, the second included patients who underwent secondary enucleation after eye-salvage therapy failed. Our goals were to (1) examine whether secondarily enucleated eyes are more likely than primarily enucleated eyes to have HRH features, (2) compare the frequencies of specific HRH features between the two groups, and (3) explore whether any HRH features are associated with time to enucleation.
Methods
Medical Record Review
With approval from the St. Jude Institutional Review Board, and adherence to the Declaration of Helsinki, we searched our Health Insurance Portability and Accountability Act–compliant institutional database to identify patients with retinoblastoma who underwent enucleation between March 1997 and August 2013. From the medical records we extracted the following data: sex, age at diagnosis, laterality of disease (unilateral vs. bilateral), Reese-Ellsworth (R-E) Classification20 and International Classification (IC)21 at diagnosis, treatments prior to enucleation, time from diagnosis to enucleation, time from progression of disease to enucleation, treatment after enucleation, presence of metastatic disease, and cause of death if applicable. Patients with evidence of metastatic disease at diagnosis were excluded. We then reviewed the histopathology of each eye and defined HRH features in our population as extraocular extension or invasion of the anterior chamber, iris, ciliary body, choroid (massive, >3mm), postlaminar optic nerve (includes postlaminar invasion and the presence of tumor cells at the cut end of the optic nerve) or sclera.
Statistical Methods
We used Fisher's exact test to study associations among sex, laterality, and histopathologic features with treatment group. We used the exact Wilcoxon rank sum test to examine associations between continuous variables (age at diagnosis, time to enucleation) and treatment group or histopathologic feature. The exact Kruskal-Wallis test was used to examine the association between R-E/IC classification and treatment group. We used SAS v9.3 and P-values ≤ 0.05 were considered statistically significant.
Results
Excluding one patient with metastatic disease at diagnosis, we identified 207 eyes that were enucleated from 202 patients (Figure 1). The primarily enucleated group included (143 patients) 144 eyes enucleated due to advanced disease. Enucleation at an outside institution resulted in missing classifications (R-E Group (4 eyes) and IC Group (2 eyes)), but most of the remaining eyes were R-E Group Va (29) or Vb (108) or were IC Group D (54) or E (86). The secondarily enucleated group included 60 patients (63 eyes) with early unilateral disease (R-E Groups I-III (15), or IC Groups B-C (20)) and/or bilateral disease. One patient had one eye primarily enucleated and the remaining eye secondarily enucleated.
Figure 1. Patient Treatment and Histopathology Groups.
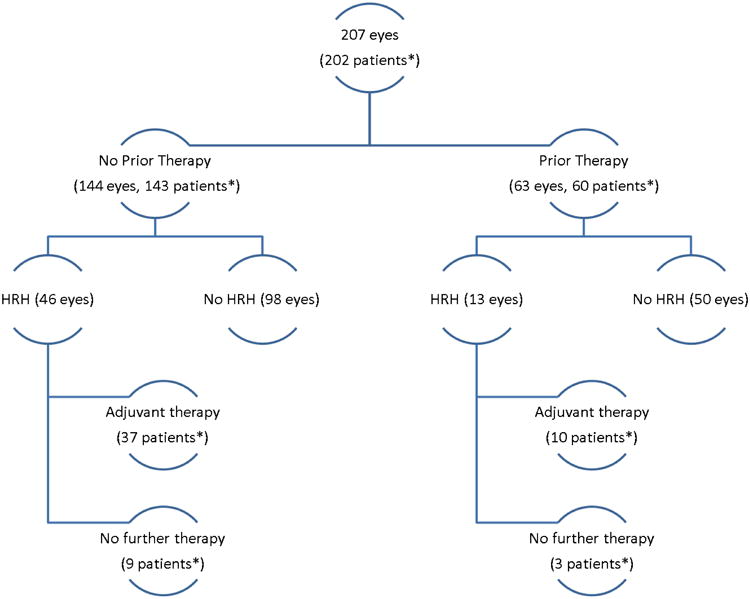
*One patient had one eye enucleated prior to therapy and the other eye enucleated after therapy
Significant differences in sex, age at diagnosis, and laterality of the tumor were observed between the two groups (Table 1). Compared to the secondarily enucleated patients, the patients undergoing primary enucleation were more likely to be male, older at diagnosis, have unilateral disease and classified as R-E Group V (95%) or IC Group D/E (97%); p<0.001.
Table 1. Demographic and Clinical Characteristics by Treatment Group.
| Primary Enucleation, Patients (n=143) | Secondary Enucleation, Patients (n=60) | P-value | |
|---|---|---|---|
| Sex | |||
| Male | 82 (57.3%) | 22 (36.7%) | 0.009 |
| Female | 61 (42.7%) | 38 (63.3%) | |
|
| |||
| Laterality of tumor | |||
| Unilateral | 128 (89.5%) | 17 (28.3%) | <0.001 |
| Bilateral | 14 (9.8%) | 43 (71.7%) | |
|
| |||
| Age at diagnosis (months) | |||
| Median | 24.0 | 9.0 | <0.001 |
| Range | 1.0-108.4 | 0.3-72.6 | |
| Mean (SD) | 27.7 (19.1) | 12.1 (12.0) | |
|
| |||
| Primary Enucleation, Eyes (n=144) | Secondary Enucleation, Eyes (n=63) | P-value | |
|
| |||
| Time to Enucleation (days) | |||
| Median | 3 | 380 | <0.001 |
| Range | 0-14 | 30-4775 | |
| Mean (SD) | 3.6 (3.0) | 566.0 (673.9) | |
|
| |||
| Reese-Ellsworth Group | |||
| I | 0 | 2 | <0.001 |
| II | 0 | 5 | |
| III | 2 | 8 | |
| IV | 1 | 7 | |
| V | 137 | 37 | |
| Not available | 4 | 4 | |
|
| |||
| International Classification Group | |||
| A | 0 | 0 | |
| B | 1 | 13 | |
| C | 1 | 7 | <0.001 |
| D | 54 | 34 | |
| E | 86 | 3 | |
| Not available | 2 | 6 | |
| Vitreous tumor seeds | |||
| Yes | 115 | 40 | 0.015 |
| No | 29 | 23 | |
|
| |||
| Subretinal tumor seeds | |||
| Yes | 109 | 21 | <0.001 |
| No | 35 | 41 | |
| Data not available | 0 | 1 | |
|
| |||
| Retinal detachment | |||
| Yes | 126 | 39 | <0.001 |
| No | 18 | 23 | |
| Data not available | 0 | 1 | |
Neoadjuvant chemotherapy (Table 2) was administered to 57 (of 60, 95%) patients. EBRT was provided to 26 (41%), brachytherapy to 10 (16%), cryotherapy to 28 (44%), and laser therapy to 43 (68%) eyes. Enucleation was considered for patients with progressive disease despite aforementioned therapies (n=56) or for elective reasons not related to progressive disease (neovascular glaucoma, cataract, poor visual potential, pain and/or vitreous hemorrhage, n=7).
Table 2. Neoadjuvant Chemotherapy Regimens.
| Chemotherapy Regimen* | Number of Patients | Number (range) of Courses |
|---|---|---|
| Carboplatin | 1 | 8 |
| Carboplatin/Etoposide (CE) | 1 | 6 |
| Vincristine/Doxorubicin/Cyclophosphamide alternating with CE | 1 | 6 |
| Intra-arterial Topotecan and Melphalan | 1 | 4 |
| Vincristine/Carboplatin (VC) | 36 | 8 (2-8) |
| Vincristine/Carboplatin/Etoposide | 11 | 6 (5-12) |
| Vincristine/Topotecan alternating with VC including Subconjunctival Carboplatin | 9 | VT: 5 (1-5)\VC: 6 (2-8) |
Carboplatin (Paraplatin ®); Etoposide (VePesid ® or Toposar ®); Topotecan (Hycamtin ®); Cyclophosphamide (Cytoxan ®); Vincristine (Oncovin ®); Doxorubicin (Adriamycin ® or Rubex ®); Melphalan (Alkeran ®)
The median time from diagnosis to enucleation of the secondarily enucleated eyes was 380 days, range 30-4775; mean time 566 days (SD 673.9) (p<0.001). Thirty-eight eyes (60%) were enucleated within 14 days of identification of progressive disease. Eighteen eyes (28.6%) that were delayed to enucleation more than two weeks were deliberately scheduled based on parental request, current chemotherapy, holidays, or school schedules. Seven eyes (seven patients, 11%) without HRH features were enucleated more than 30 days after the initial recommendation (median 45 days, range 35-77; mean 54 days, SD 17.7). Three patients were delayed due to illness and two patients were delayed because the procedure was elective. One patient was delayed due to initial parental refusal and another patient was unable to return for the scheduled enucleation due to travel.
We found HRH features in 29% (59/207) of the eyes. The distribution of eyes with HRH between the two groups (primarily and secondarily enucleated) did not differ (p=0.132; Table 3). In the primary enucleation group, the majority of eyes with HRH (44/46; 96%) were classified as R-E Group V (IC Group D (8), E (37), Not available (1)); for the two eyes without R-E or IC Grouping data due to diagnosis and enucleation at an outside institution, histopathology demonstrated evidence of extraocular extension (pT4). Eight eyes (8/207, 4%) had extraocular extension; only one of which had prior treatment. In the secondarily enucleated group, seven of 13 (54%) eyes with HRH were classified as R-E Group V (IC Group C (1) and D (6)); all seven were from patients with bilateral disease. All seven eyes initially responded well to neoadjuvant therapy before recurrence or progression led to enucleation, despite additional focal therapies and radiation in three. All seven contralateral eyes were salvaged. The remaining six secondarily enucleated eyes with HRH were classified as R-E Group I, II, or III (one each) or R-E Group IV (three) (IC Groups B (4), D (1) and not available (1)).
Table 3. Histopathologic Findings among Primarily or Secondarily Enucleated Eyes.
| Finding | Primary Enucleation, Eyes n=144 (%) | Secondary Enucleation, Eyes n=63 (%) | P-value |
|---|---|---|---|
| High-Risk histopathologic features | |||
| Yes | 46 (32%) | 13 (21%) | 0.132 |
| No | 98 (68%) | 50 (79%) | |
|
| |||
| Anterior chamber invasion | |||
| Yes | 8 (6%) | 3 (5%) | 1 |
| No | 136 (94%) | 60 (95%) | |
|
| |||
| Iris invasion | |||
| Yes | 9 (6%) | 4 (6%) | 1 |
| No | 135 (94%) | 59 (94%) | |
|
| |||
| Ciliary body invasion | |||
| Yes | 10 (7%) | 8 (13%) | 0.188 |
| No | 134 (93%) | 55 (87%) | |
|
| |||
| Choroid invasion | |||
| None | 63 (44%) | 45 (71%) | 0.005a |
| Minimal | 3 (2%) | 0 (0%) | |
| Focal | 46 (32%) | 14 (22%) | |
| Massive | 32 (22%) | 4 (6%) | |
|
| |||
| Optic nerve invasion | |||
| None | 23 (16%) | 45 (71%) | <0.001b |
| Prelaminar | 57 (40%) | 14 (22%) | |
| Laminar | 37 (26%) | 3 (5%) | |
| Postlaminar | 25 (17%) | 1 (2%) | |
| To cut Margin | 2 (1%) | 0 (0%) | |
|
| |||
| Scleral invasion | |||
| Yes | 18 (13%) | 7 (11%) | 1 |
| No | 126 (87%) | 56 (89%) | |
|
| |||
| Extraocular disease | |||
| Yes | 5 (3%) | 2 (3%) | 1 |
| No | 139 (97%) | 61 (97%) | |
Data reflects a comparison of massive choroid vs. none/minimal/focal choroid invasion.
Data reflects a comparison of postlaminar/to cut margin (postlaminar) vs. none/prelaminar/laminar optic nerve invasion.
The most prevalent HRH features in the primarily enucleated group were massive choroidal invasion (p= 0.005) and postlaminar optic nerve invasion (p<0.001) (Table 3). Invasion of the ciliary body (n=8) and sclera (n=7) were more common in the secondarily enucleated group. Five of the twenty-six secondarily enucleated patients who received EBRT had HRH including ciliary body, choroidal and scleral invasion. There was no evidence of associations between treatment group and extraocular disease or invasion of anterior chamber, iris, ciliary body, or sclera (p>0.18) (Table 3).
Table 4 shows the association between the various HRH features. Notably, postlaminar optic nerve invasion was significantly associated only with massive choroidal invasion (p<0.0001). Otherwise, the HRH features were all significantly associated with each of the other five variables.
Table 4.
Associations between High-Risk Histopathologic Variables. P-values were obtained using Fisher's exact test.
| Iris invasion | Ciliary body invasion | Massive choroid invasion | Postlaminar optic nerve invasion | Scleral invasion | Extra-ocular extension | |
|---|---|---|---|---|---|---|
| Anterior chamber invasion | <0.0001 | <0.0001 | 0.0254 | 0.6471 | <0.0001 | 0.0001 |
| Iris invasion | <0.0001 | 0.0126 | 0.3914 | <0.0001 | 0.0003 | |
| Ciliary body invasion | 0.0047 | 1 | <0.0001 | 0.0012 | ||
| Massive choroid invasion | <0.0001 | <0.0001 | 0.0001 | |||
| Postlaminar optic nerve invasion | 0.0020 | 0.0536 | ||||
| Scleral invasion | <0.0001 |
Descriptive statistics for times to enucleation were calculated by the presence or absence of each histopathologic feature of interest (Table 5). When HRH features were present in eyes treated with neoadjuvant therapy, the time from diagnosis to enucleation was longer, even after excluding the patient with 4775 days until enucleation (p=0.018). Those secondarily enucleated eyes with ciliary body and massive choroid invasion had longer time from diagnosis to enucleation than those without either feature.
Table 5. Associations among Time to Enucleation and High-Risk Histopathologic (HRH) Features and Treatment Group.
| HRH Feature | Primary Enucleation Eyes | Secondary Enucleation Eyes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time to enucleationa (days) | Time to enucleationa (days) | |||||||||
|
|
|
|||||||||
| n | Median | Range | Mean (SD) | P-value | n | Median | Range | Mean (SD) | P-value | |
| HRH (any) | ||||||||||
| Yes | 46 | 2 | 0-14 | 3.3 (3.2) | 0.451 | 13 | 604 | 224-4775 | 918.8 (1187.7) | 0.018 |
| No | 98 | 4 | 0-14 | 3.7 (3.0) | 50 | 340 | 30-2175 | 474.3 (433.0) | ||
|
| ||||||||||
| Anterior chamber invasion | ||||||||||
| Yes | 8 | 2 | 0-7 | 2.5 (3.0) | 0.306 | 3 | 884 | 224-4775 | 1961.0 (2459.2) | 0.278 |
| No | 136 | 4 | 0-14 | 3.6 (3.0) | 60 | 373 | 30-2175 | 496.3 (410.2) | ||
|
| ||||||||||
| Iris invasion | ||||||||||
| Yes | 9 | 0 | 0-7 | 1.4 (2.4) | 0.011 | 4 | 921 | 224-4775 | 1710.3 (2069.6) | 0.100 |
| No | 135 | 4 | 0-14 | 3.7 (3.0) | 59 | 366 | 30-2175 | 488.5 (409.2) | ||
|
| ||||||||||
| Ciliary body invasion | ||||||||||
| Yes | 10 | 2 | 0-7 | 1.9 (2.3) | 0.043 | 8 | 728 | 308-4775 | 1181.1 (1467.7) | 0.005 |
| No | 134 | 4 | 0-14 | 3.7 (3.0) | 55 | 343 | 30-2175 | 467.6 (421.8) | ||
|
| ||||||||||
| Massive choroid invasion | ||||||||||
| Yes | 32 | 3 | 0-14 | 3.3 (3.1) | 0.603 | 4 | 898 | 492-4775 | 1765.5 (2018.1) | 0.011 |
| No | 112 | 3 | 0-14 | 3.6 (3.0) | 59 | 343 | 30-2175 | 484.7 (410.9) | ||
|
| ||||||||||
| Postlaminar optic nerve invasion | ||||||||||
| Yes | 27 | 2 | 0-14 | 3.2 (3.6) | 0.218 | 1 | 238 | 238-238 | 238.0 (-) | 0.476 |
| No | 117 | 4 | 0-14 | 3.6 (2.9) | 62 | 382 | 30-4775 | 571.3 (678.0) | ||
|
| ||||||||||
| Scleral invasion | ||||||||||
| Yes | 18 | 2 | 0-7 | 2.3 (2.3) | 0.055 | 7 | 604 | 238-1024 | 674.0 (288.6) | 0.060 |
| No | 126 | 4 | 0-14 | 3.7 (3.1) | 56 | 343 | 30-4775 | 552.5 (707.9) | ||
|
| ||||||||||
| Extraocular disease | ||||||||||
| Yes | 5 | 1 | 0-7 | 2.2 (2.9) | 0.285 | 2 | 758 | 492-1024 | 758.0 (376.2) | 0.214 |
| No | 139 | 3 | 0-14 | 3.6 (3.0) | 61 | 366 | 30-4775 | 559.7 (682.3) | ||
Time represents the number of days from diagnosis to enucleation.
The role of repeated attempts at ocular salvage with focal therapy was evaluated. The presence of HRH was not associated with any prior therapies; p>0.19; data not shown. Prior EBRT did not eliminate the risk of ciliary body invasion at the time of disease progression. The median time to enucleation after radiation was 10 months, range 3 to 156. Neoadjuvant chemotherapy (57 of 60 patients) was not included in this analysis.
Post-enucleation adjuvant therapy for patients with HRH features included chemotherapy (37 primarily and 10 secondarily enucleated patients) and adjuvant EBRT (7 primarily and 3 secondarily enucleated eyes). After primary enucleation, patients with HRH received 4-6 courses of chemotherapy based on institutional protocols; those with scleral invasion or tumor at the cut end of the optic nerve also received EBRT. Patients who underwent enucleation during neoadjuvant therapy (n=5) completed planned chemotherapy without additional agents or courses. Patients requiring additional chemotherapy after secondary enucleation (n=8) received cyclophosphamide and doxorubicin (n=3), vincristine/carboplatin/etoposide alternating with cyclophosphamide/doxorubicin/carboplatin (n=4) or vincristine/carboplatin/etoposide/cyclophosphamide (n=1). No patient with prior EBRT required additional radiation. Thirteen patients (ten primary and three secondary) with HRH features did not receive adjuvant therapy after enucleation; none of these patients experienced recurrence or metastasis. In the primary enucleation group, patients either declined further therapy (two) or no further therapy was recommended based on the initial histopathology review (eight). These eight patients had isolated, superficial postlaminar optic nerve invasion, which was not an indication for adjuvant therapy per the concurrent institutional protocol. Three patients with secondary enucleation and HRH received adjuvant therapy to the contralateral orbit (EBRT, n=1; plaque, n=1) or no therapy based on initial histopathology review (n=1). Four patients developed metastatic disease. One patient developed metastatic (liver, bone, bone marrow, CNS) disease six months after primary enucleation. Extensive review of the initial histopathology slides and re-cuts of the globe revealed no additional information that would reclassify this patient in the high-risk category. Twelve months after adjuvant chemotherapy, EBRT and autologous bone marrow transplantation, this patient had no evidence of disease. Two patients underwent primary enucleation for R-E Group V disease on day 0 with pathology demonstrating HRH features. Both patients received adjuvant chemotherapy, one received additional EBRT. One patient achieved a complete pathological response followed by late metastatic recurrence (CSF/meningeal) while the other experienced disease progression (liver/bone marrow/orbit/brain) on therapy. A third patient with bilateral disease underwent upfront enucleation of one eye with HRH features. This patient unfortunately developed CNS metastatic disease while receiving systemic and focal treatment to the remaining eye. These three patients succumbed to disease.
Discussion
The definition of HRH has been debated,7-12,14 but most physicians agree that massive choroidal invasion with postlaminar optic nerve involvement, tumor present at the cut end of the optic nerve, and scleral invasion are pathologic features consistent with a high risk of metastatic disease and warrant further therapy. Previous reports have described HRH after enucleation,13,18 but ours is the largest cohort of primary and secondary enucleation from a single institution. In this study we reviewed 207 enucleated eyes of 202 patients with retinoblastoma; 59 eyes (29%) were considered to have HRH: 46 primarily enucleated eyes and 13 secondarily enucleated eyes. Notably, massive choroidal invasion was significantly associated with each of the other high-risk features, underscoring the importance of this histopathologic finding in primarily enucleated eyes. Eight of the eyes with HRH (1 primarily enucleated) had extraocular extension. Our incidence of HRH is comparable with previous reports.7,11,14 One report of a higher incidence of HRH18 was due to more advanced (pT4) disease at diagnosis.
The incidence of HRH features was not significantly different in primarily vs. secondarily enucleated eyes, despite more advanced intraocular retinoblastoma in the primary enucleation group and the application of multiple modalities to treat patients in an attempt to provide ocular salvage. Primarily enucleated eyes were significantly more likely to have massive choroid invasion and postlaminar optic nerve invasion, which was expected due to a higher incidence of advanced intraocular disease at diagnosis. In the secondary enucleation group, ciliary body and scleral invasion were the most common HRH features (not statistically significant), and HRH was significantly associated with longer times to enucleation. We thus observed a difference in the distribution of HRH features between the two groups.
We believe that prolonged treatment of eyes that came to enucleation changed the spectrum of HRH. Previous studies have shown that 10-40% of eyes with advanced (IC Group D/E) intraocular disease harbor HRH at diagnosis, most commonly with massive choroidal invasion and postlaminar optic nerve invasion.12,22 We did not observe this in our secondarily enucleated cohort despite the majority of eyes having advanced intraocular disease at diagnosis (R-E Group Vb or IC Group D/E). We believe systemic therapy may have preferentially targeted invasion of the optic nerve and choroid due to their complex vascular networks. The choroid is supplied by approximately 20 short posterior ciliary arteries while the ciliary body and iris are supplied by the two long posterior ciliary arteries and the seven anterior ciliary arteries, respectfully. Focal therapies such as laser and cryotherapy destroy the choroid underlying and surrounding the tumor, potentially allowing local tumor recurrences or implantation of vitreous seeds with direct access to the inner sclera. Recurrences or treatment failures could thus preferentially involve anterior uveal tract or external coats of the eye where tumor cells had eclipsed or simply grown beyond the choroid to the sclera, allowing treatment-resistant disease to yield unique patterns of HRH such as massive choroidal or scleral invasion with associated ciliary body invasion. Additionally, repeated attempts at ocular salvage may simply provide the opportunity for intraocular disease to become extraocular.
Zhao et al. reported that enucleation delayed longer than three months after diagnosis due to administration of neoadjuvant chemotherapy was associated with mortality in four patients as a result of “down-staging” of disease and reduced surveillance leading to in appropriate management of high-risk disease.19 Prolonged time to enucleation in our secondary enucleation group was not associated with mortality. Several factors likely influenced this result. The majority of eyes were treated with initial chemoreduction and responded well to therapy. Late recurrence or progression of disease was treated with other modalities, in contrast to chemotherapy only in the Zhao et al. study. In addition, if patients underwent enucleation during therapy, the original treatment plan was completed regardless of the histopathology to avoid “down-staging.” Patients with the longest times to enucleation for whom HRH features were identified were appropriately treated with further adjuvant therapy. Finally, almost 90% of eyes in our secondary enucleation group were enucleated within 30 days of identification of recalcitrant progressive disease. Our results underscore the importance of a multimodal systemic approach to treating advanced retinoblastoma, with consideration of extent of disease prior to therapy, prompt referral for enucleation when warranted, careful examination of histopathology, and appropriate post enucleation treatment to achieve cure.
Despite a comprehensive treatment approach, three deaths occurred in our cohort: two patients after primary enucleation and one patient after secondary enucleation. All patients received appropriate adjuvant chemotherapy (n=1) or chemotherapy/EBRT (n=2) based on identified HRH features. One patient developed metastases after completion of therapy and two developed metastases during therapy.
In conclusion, we have shown that patients with advanced intraocular retinoblastoma, where conservative neoadjuvant therapy with systemic chemotherapy, EBRT, and/or focal therapies is ineffective, are still at risk for HRH features. The difference in location of HRH feature (anterior chamber or outer coats in secondarily enucleated patients vs. posterior retina and optic nerve in primarily enucleated patients) is associated with a changing spectrum of HRH features after neoadjuvant therapy that has not been previously reported. However, despite a prolonged time to enucleation while ocular salvage therapies are applied, prompt recognition of recalcitrant disease progression, enucleation and further adjuvant therapy that addresses the high risk of metastatic spread may still achieve a cure.
Acknowledgments
Supported in part by American Lebanese Syrian Associated Charities (ALSAC), Research to Prevent Blindness, Inc., (NY), St Giles Foundation (NY), and National Institutes of Health grant numbers CA21765 and CA23099.
Footnotes
Conflict of Interest: No conflicting relationship exists for any author.
This work was previously presented, in part, at the American Academy of Ophthalmology (Orlando, FL; 2011)
References
- 1.Kingston JE, Hungerford JL, Madreperla SA, et al. Results of combined chemotherapy and radiotherapy for advanced intraocular retinoblastoma. Arch Ophthalmol. 1996;114:1339–43. doi: 10.1001/archopht.1996.01100140539004. [DOI] [PubMed] [Google Scholar]
- 2.Shields CL, Shields JA, Needle M, et al. Combined chemoreduction and adjuvant treatment for intraocular retinoblastoma. Ophthalmology. 1997;104:2101–11. doi: 10.1016/s0161-6420(97)30053-0. [DOI] [PubMed] [Google Scholar]
- 3.Abramson DH, Dunkel IJ, Brodie SE, et al. Superselective ophthalmic artery chemotherapy as primary treatment for retinoblastoma (chemosurgery) Ophthalmology. 2010;117:1623–9. doi: 10.1016/j.ophtha.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 4.Shields CL, Honavar SG, Meadows AT, et al. Chemoreduction for unilateral retinoblastoma. Arch Ophthalmol. 2002;120:1653–8. doi: 10.1001/archopht.120.12.1653. [DOI] [PubMed] [Google Scholar]
- 5.Chan HS, Gallie BL, Munier FL, et al. Chemotherapy for retinoblastoma. Ophthalmol Clin North Am. 2005;18:55–63. viii. doi: 10.1016/j.ohc.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Shields CL, Mashayekhi A, Au AK, et al. The International Classification of Retinoblastoma predicts chemoreduction success. Ophthalmology. 2006;113:2276–80. doi: 10.1016/j.ophtha.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 7.Khelfaoui F, Validire P, Auperin A, et al. Histopathologic risk factors in retinoblastoma: a retrospective study of 172 patients treated in a single institution. Cancer. 1996;77:1206–13. [PubMed] [Google Scholar]
- 8.Uusitalo MS, Van Quill KR, Scott IU, et al. Evaluation of chemoprophylaxis in patients with unilateral retinoblastoma with high-risk features on histopathologic examination. Arch Ophthalmol. 2001;119:41–8. [PubMed] [Google Scholar]
- 9.Magramm I, Abramson DH, Ellsworth RM. Optic nerve involvement in retinoblastoma. Ophthalmology. 1989;96:217–22. doi: 10.1016/s0161-6420(89)32910-1. [DOI] [PubMed] [Google Scholar]
- 10.Stannard C, Lipper S, Sealy R, Sevel D. Retinoblastoma: correlation of invasion of the optic nerve and choroid with prognosis and metastases. Br J Ophthalmol. 1979;63:560–70. doi: 10.1136/bjo.63.8.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honavar SG, Singh AD, Shields CL, et al. Postenucleation adjuvant therapy in high-risk retinoblastoma. Arch Ophthalmol. 2002;120:923–31. doi: 10.1001/archopht.120.7.923. [DOI] [PubMed] [Google Scholar]
- 12.Wilson MW, Qaddoumi I, Billups C, et al. A clinicopathological correlation of 67 eyes primarily enucleated for advanced intraocular retinoblastoma. Br J Ophthalmol. 2011;95:553–8. doi: 10.1136/bjo.2009.177444. [DOI] [PubMed] [Google Scholar]
- 13.Chantada GL, Dunkel IJ, Antoneli CB, et al. Risk factors for extraocular relapse following enucleation after failure of chemoreduction in retinoblastoma. Pediatr Blood Cancer. 2007;49:256–60. doi: 10.1002/pbc.21067. [DOI] [PubMed] [Google Scholar]
- 14.Chantada GL, Dunkel IJ, de Davila MT, et al. Retinoblastoma patients with high risk ocular pathological features: who needs adjuvant therapy? Br J Ophthalmol. 2004;88:1069–73. doi: 10.1136/bjo.2003.037044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman DL, Himelstein B, Shields CL, et al. Chemoreduction and local ophthalmic therapy for intraocular retinoblastoma. J Clin Oncol. 2000;18:12–7. doi: 10.1200/JCO.2000.18.1.12. [DOI] [PubMed] [Google Scholar]
- 16.Shields CL, Santos MC, Diniz W, et al. Thermotherapy for retinoblastoma. Arch Ophthalmol. 1999;117:885–93. doi: 10.1001/archopht.117.7.885. [DOI] [PubMed] [Google Scholar]
- 17.Merchant TE, Gould CJ, Wilson MW, Hilton NE, Rodriguez-Galindo C, Haik BG. Episcleral plaque brachytherapy for retinoblastoma. Pediatr Blood Cancer. 2004;43:134–9. doi: 10.1002/pbc.20094. [DOI] [PubMed] [Google Scholar]
- 18.Suryawanshi P, Ramadwar M, Dikshit R, et al. A study of pathologic risk factors in postchemoreduced, enucleated specimens of advanced retinoblastomas in a developing country. Arch Pathol Lab Med. 2011;135:1017–23. doi: 10.5858/2010-0311-OAR2. [DOI] [PubMed] [Google Scholar]
- 19.Zhao J, Dimaras H, Massey C, et al. Pre-enucleation chemotherapy for eyes severely affected by retinoblastoma masks risk of tumor extension and increases death from metastasis. J Clin Oncol. 2011;29:845–51. doi: 10.1200/JCO.2010.32.5332. [DOI] [PubMed] [Google Scholar]
- 20.Reese AB, Ellsworth RM. The evaluation and current concept of retinoblastoma therapy. Trans Am Acad Ophthalmol Otolaryngol. 1963;67:164–72. [PubMed] [Google Scholar]
- 21.Linn Murphree A. Intraocular retinoblastoma: the case for a new group classification. Ophthalmol Clin North Am. 2005;18:41–53. viii. doi: 10.1016/j.ohc.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Kaliki S, Shields CL, Rojanaporn D, et al. High-risk retinoblastoma based on international classification of retinoblastoma: analysis of 519 enucleated eyes. Ophthalmology. 2013;120:997–1003. doi: 10.1016/j.ophtha.2012.10.044. [DOI] [PubMed] [Google Scholar]


