Abstract
Rationale: Expanding the use of cystic fibrosis transmembrane conductance regulator (CFTR) potentiators and correctors for the treatment of cystic fibrosis (CF) requires precise and accurate biomarkers. Sweat chloride concentration provides an in vivo assessment of CFTR function, but it is unknown the degree to which CFTR mutations account for sweat chloride variation.
Objectives: To estimate potential sources of variation for sweat chloride measurements, including demographic factors, testing variability, recording biases, and CFTR genotype itself.
Methods: A total of 2,639 sweat chloride measurements were obtained in 1,761 twins/siblings from the CF Twin-Sibling Study, French CF Modifier Gene Study, and Canadian Consortium for Genetic Studies. Variance component estimation was performed by nested mixed modeling.
Measurements and Main Results: Across the tested CF population as a whole, CFTR gene mutations were found to be the primary determinant of sweat chloride variability (56.1% of variation) with contributions from variation over time (e.g., factors related to testing on different days; 13.8%), environmental factors (e.g., climate, family diet; 13.5%), other residual factors (e.g., test variability; 9.9%), and unique individual factors (e.g., modifier genes, unique exposures; 6.8%) (likelihood ratio test, P < 0.001). Twin analysis suggested that modifier genes did not play a significant role because the heritability estimate was negligible (H2 = 0; 95% confidence interval, 0.0–0.35). For an individual with CF, variation in sweat chloride was primarily caused by variation over time (58.1%) with the remainder attributable to residual/random factors (41.9%).
Conclusions: Variation in the CFTR gene is the predominant cause of sweat chloride variation; most of the non-CFTR variation is caused by testing variability and unique environmental factors. If test precision and accuracy can be improved, sweat chloride measurement could be a valuable biomarker for assessing response to therapies directed at mutant CFTR.
Keywords: biomarker, ivacaftor, lumacaftor, heritability, pilocarpine iontophoresis
At a Glance Commentary
Scientific Knowledge on the Subject
Expanding the use of cystic fibrosis transmembrane conductance regulator (CFTR) potentiators and correctors for the treatment of cystic fibrosis requires precise and accurate biomarkers. Sweat chloride concentration provides an in vivo assessment of CFTR function, but the degree to which CFTR mutations account for sweat chloride variation is unknown.
What This Study Adds to the Field
We found that variation in the CFTR gene is the predominant cause of sweat chloride variation; most of the non-CFTR variation is caused by testing variability and unique environmental factors. If test precision and accuracy can be improved, sweat chloride measurement could be a valuable biomarker for assessing response to therapies directed at mutant CFTR.
Cystic fibrosis (CF) is a monogenic autosomal-recessive life-shortening disorder affecting about 70,000 individuals worldwide. The disorder is caused by dysfunction of the CF transmembrane conductance regulator (CFTR), which is a cAMP-regulated chloride and bicarbonate channel (1, 2). Loss of CFTR function in the sweat gland leads to an elevation in chloride concentration. In the epithelia of the lungs and pancreas, CFTR dysfunction causes aberrant ion and water movement leading to obstruction and eventual destruction of both organ systems (3). Recent success in augmenting the function of CFTR bearing some of the most common mutations has energized efforts to develop molecular therapies for all disease-causing variants (4, 5).
The U.S. Food and Drug Administration–approved indications for use of the CFTR potentiator ivacaftor (Kalydeco) and the combination of ivacaftor and a CFTR corrector lumacaftor (Orkambi) (6) includes 11 CFTR mutations (7) that encompass approximately 60% of all individuals with CF. Expanding the use of CFTR-targeted drugs to all patients with CF is challenging for several reasons. First, the remaining 40% of affected individuals carry at least 1,700 different CFTR mutations (8). Many of these mutations occur in one or only a few individuals with CF. Thus, traditional clinical trials that enroll sufficient numbers of subjects to reveal statistically significant differences among treatment groups will be difficult to conduct. Second, accurate biomarkers are needed for measuring CFTR function, because small molecule therapies may not provide an equivalent clinical improvement for all CFTR mutations. Lung function is an important endpoint measure because it is the primary cause of morbidity and is correlated with survival in CF (9). However, considerable variability in lung function measures occurs among patients of the same age and bearing the same CFTR mutations (10–12). The substantial variation is caused by contributions both from modifier genes and environmental factors (13, 14), thus making lung function an imprecise measure of CFTR function. This may be reflected in studies of CFTR-targeted drugs where lung function (FEV1) is not tightly correlated with other measures of CFTR function, specifically sweat chloride measurements (4, 15–17).
The chloride concentration in sweat could serve as a useful biomarker of CFTR function in vivo in assessing the response to molecular treatments (18). Indeed, prior research studies have shown correlation between functional classes of CFTR variants and sweat chloride concentration (19, 20). In clinical use, patients suspected to have CF typically receive the test for diagnostic purposes at the time of initial presentation. Serial sweat chloride measurements can be used to follow the effects of therapies directly affecting CFTR function (4, 16, 17, 21); however, there is an incomplete understanding of the causes of variation in this measure (15, 22). We sought to estimate potential sources of variation for sweat chloride measurements, including demographic factors, testing variability, recording biases, and CFTR genotype itself. The twins and siblings affected with CF also allowed estimating the contribution of genetic modifiers to sweat chloride measurement variability. Some of these results have been previously reported in the form of an abstract (23).
Methods
Study Sample
The primary population for this study (Table 1) included 1,761 subjects with CF, including 1,697 twins and siblings from the CF Twin-Sibling Study, 40 twins from the French CF Modifier Gene Study, and 24 twins from the Canadian Consortium for Genetic Studies. Written consent was obtained from all subjects and this study was approved by the Johns Hopkins University Institutional Review Board (Protocol NA–00035659). In addition, sex and age effects were assessed in an independent population of unrelated individuals with CF and homozygous for the most common CFTR mutation, Phe508del (n = 1,191), recruited for the Genetic Modifier Study at the University of North Carolina and Case Western Reserve University (24).
Table 1.
Demographics
| Study Population (N = 1,761) | |
|---|---|
| Sex, % female (n = 1,731) | 48.0 |
| Race/ethnicity, % white (n = 1,695) | 90.3 |
| Age as of 12/31/2011, yr, mean ± SD | 19.8 ± 10.3 |
| Age at diagnosis, yr (n = 1,697) | |
| Mean ± SD | 2.36 ± 5.33 |
| Median | 0.33 |
| IQR | 0.08–2.03 |
| CFTR genotype, % (n = 1,755) | |
| 0 Phe508del mutations | 10.7 |
| 1 Phe508del mutations | 42.5 |
| 2 Phe508del mutations | 46.8 |
| Pancreatic status, % insufficient (n = 1,741) | 85.1 |
Definition of abbreviations: CFTR = cystic fibrosis transmembrane conductance regulator; IQR = interquartile range.
Demographics
Race/ethnicity was self-reported in the CF Twin-Sibling Study only with subjects with any nonwhite ancestry defined as nonwhite. Exocrine pancreatic sufficiency, a common manifestation of CFTR dysfunction, was defined by genotype for 1,592 subjects (90.4% of subjects) as being sufficient with one or more “pancreatic sufficient” mutations or being insufficient with two “insufficient” mutations, and by clinical data for 149 subjects (8.5%) where genotype data was indeterminate or not available. The “sufficiency” of mutations was based on CFTR2 population level data for mutations (www.cftr2.org) (25, 26). For 20 subjects (1.1%) pancreatic status was unable to be determined.
Sweat Chloride Measurements
A total of 2,678 sweat chloride measurements (mmol/L) obtained by pilocarpine iontophoresis were extracted from clinical records. Data for subjects in the CF Twin-Sibling Study were supplemented with data obtained from the U.S. CF Foundation Patient Registry. Published guidelines state that sweat chloride measurements greater than 150–160 mmol/L are not physiologically compatible (27–29); 39 measurements greater than 145 mmol/L were dropped. Minimum, mean, and maximum values were based on all available measurements from an individual.
Statistical Analysis
The contribution of CFTR genotype to sweat chloride variation was estimated using a nested-factor mixed model for variance component estimation with all available measurements (STATA command xtmixed with REML option; StataCorp LP, College Station, TX) (30). In our model, measurements by testing date were nested inside individuals, who in turn were nested inside families, which in turn were nested inside CFTR genotypes. The mixed model was limited to subjects with at least one Phe508del mutation; CFTR genotype groups for this model were assigned by the non-Phe508del mutation for Phe508del heterozygotes and into a single group for Phe508del homozygotes. For heritability calculations, the maximum sweat chloride measurement for an individual was used for subjects who had more than one measurement recorded. Heritability was estimated by calculating the Pearson correlation coefficients for sweat chloride measurements for monozygous (MZ) twins, dizygous (DZ) twins, and siblings, then subtracting the coefficient for DZ twins (or siblings) from the coefficient for MZ twins, and multiplying the difference by 2 (31). Heritability estimates less than 0 and greater than 1.0 were reported as 0 and 1, respectively. Heritability confidence intervals were estimated by bootstrapping (32). The effects of age on sweat chloride were also assessed using linear regression clustered by family; subjects without a documented age at the time of testing (n = 14) were excluded from age-adjusted analyses. Families with discordant CFTR genotypes were not used for heritability or mixed model analyses. Linearity assumptions were assessed as part of the modeling process. Analyses were performed using Stata IC 11.0 (StataCorp LP, College Station, TX).
Results
Demographics
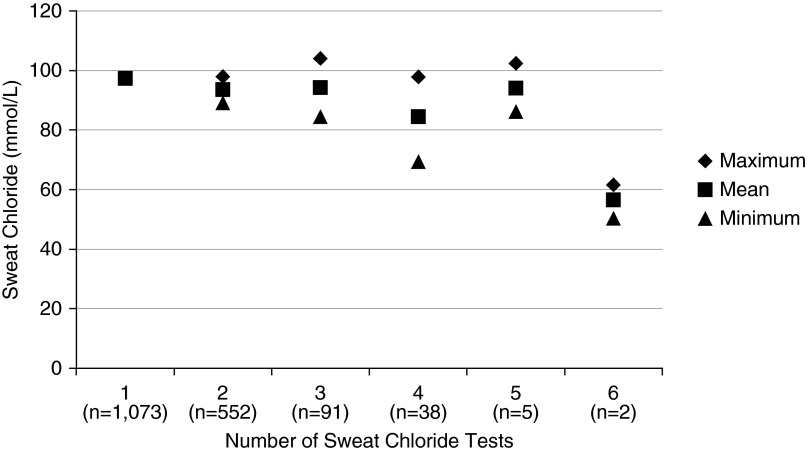
A total of 2,639 measurements were available for analysis for 1,761 subjects in the primary population, including one measurement only for 1,073 subjects, two measurements for 552 subjects, three for 91 subjects, four for 38 subjects, five for five subjects, and six for two subjects. The average maximum sweat chloride values for subjects who had more than one test recorded (98.6 ± 20.0 mmol/L; n = 688) were not different than the sweat chloride measurement for those subjects who only had a single test recorded (97.4 ± 19.7 mmol/L; n = 1073; P = 0.18) (Figure 1). However, for those who had more than one test recorded, the averages of their mean values (93.0 ± 19.7; P < 0.001) and minimum values (87.4 ± 21.7; P < 0.001) were different than the single value for those who had only one test recorded. The differences indicated a bias toward the maximum value being recorded, likely caused by healthcare providers preferentially entering the highest sweat chloride value when multiple tests had been performed. To address this bias, only the maximum sweat chloride value for each subject was used for the analyses that follow, except the mixed model where all values were used to estimate the contribution of testing variability. The average of the maximum sweat chloride values for all 1,761 subjects was 97.9 ± 19.8 mmol/L (range, 11.0–145.0). The mean age of testing was 3.2 ± 6.2 years (range, 0–52.9; n = 1,747) for subjects with data available.
Figure 1.
Maximum, mean, and minimum sweat chloride values by number of tests performed for a subject. The average maximum value for subjects with more than one measurement was not different than the average measurement for subjects with only one measurement as opposed to the average mean or minimum values for subjects with more than one measurement, thus illustrating a potential bias to record maximum measurements preferentially.
Age Influences Sweat Chloride Measurements
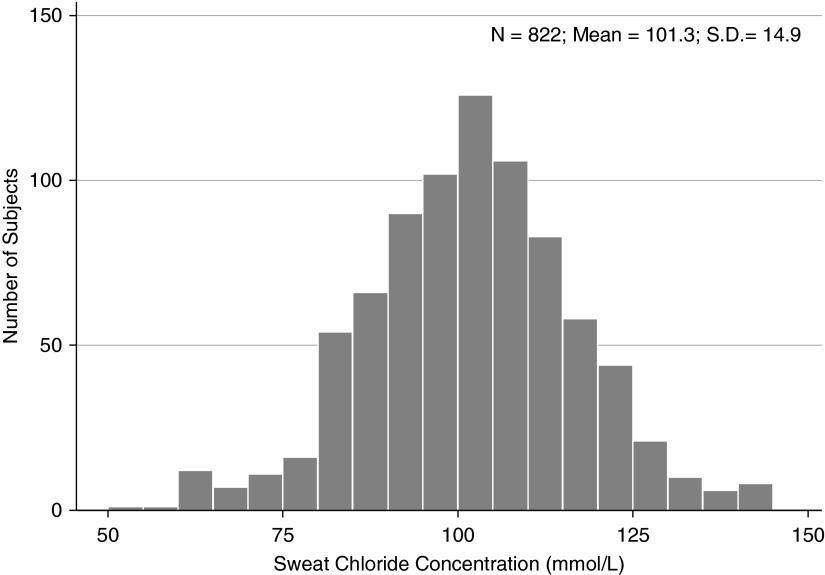
We first wished to ascertain if age, sex, or race/ethnicity played a role in sweat chloride variation, so as to adjust for these factors if appropriate. To exclude variation caused by CFTR, we restricted these analyses to the largest group of subjects with an identical CFTR genotype (822 individuals homozygous for the most common CFTR mutation [Phe508del]). Maximum sweat chloride values per individual were normally distributed around a mean of 101.3 ± 14.9 mmol/L, illustrating the variability of measurements, even among individuals with identical CFTR genotypes (Figure 2).
Figure 2.
Histogram for maximum sweat chloride values in subjects with the same cystic fibrosis transmembrane conductance regulator (CFTR) mutations (Phe508del homozygotes), illustrating the variability present in sweat chloride measurements even within the same CFTR genotype.
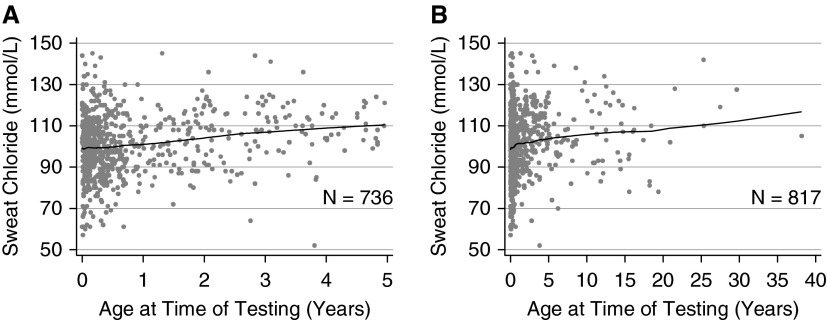
Using regression, increasing sweat chloride values were associated with increasing age (n = 817 Phe508del homozygotes; P = 0.001). The average maximum sweat chloride value was 99.2 ± 14.8 mmol/L for subjects between 0 and 1 year of age at the time of testing (n = 549), 102.5 ± 12.6 mmol/L between 1 and 2 years of age (n = 76), 105.7 ± 15.4 mmol/L between 2 and 3 years of age (n = 49), and 106.9 ± 14.9 mmol/L for subjects 3 or more years of age (n = 143) (Figure 3; see Figure E1 in the online supplement). However, because 90% of these subjects are less than 5 years of age, the relationship between sweat chloride values and age may not be linear at older ages. We also evaluated effects of age on sweat chloride on an independent population of subjects from the Gene Modifier Study who were Phe508del homozygotes. The mean sweat chloride value was 102.1 ± 15.0 mmol/L for subjects between 0 and 1 year of age at the time of testing (n = 392), 100.7 ± 14.6 mmol/L between 1 and 2 years of age (n = 275), 105.3 ± 15.5 mmol/L between 2 and 3 years of age (n = 107), and 106.4 ± 14.8 mmol/L for subjects 3 or more years of age (n = 417), which were comparable with data from the primary population. To address age-related changes for heritability estimates in comparing twins or siblings who may have been different ages at the time of testing, all sweat chloride results obtained between 0 and 1 years of age were adjusted upward by 7.7 mmol/L, those between 1 and 2 years of age were adjusted by 4.4 mmol/L, and those obtained between 2 and 3 years of age were adjusted by 1.2 mmol/L (33).
Figure 3.
Maximum sweat chloride values by age with Lowess-smoothed line illustrating an increase in sweat chloride measurements with age. (A) Data for Phe508del homozygotes only who were <5 years old at the testing. (B) Data for Phe508del homozygotes only regardless of age.
There were no differences in maximum sweat chloride values by sex (n = 785; P = 0.72) or by race/ethnicity (n = 783; P = 0.97). However, some evidence of differences between males and females in older children and adults homozygous for the Phe508del mutation was noted. In a subpopulation of Phe508del homozygotes over 2 years of age, males tended to have a higher sweat chloride measurement (108.4 ± 16.2 mmol/L; n = 113) than females (105.1 ± 12.8 mmol/L; n = 83; P = 0.12). We saw a similar trend in the Gene Modifier Study (Phe508del homozygotes aged 2 yr or older at the time of testing) toward higher sweat chlorides in males (107.1 ± 15.0 mmol/L; n = 277) compared with females (105.2 ± 14.8 mmol/L; n = 247) (Student’s t test, P = 0.15). Although a meta-analysis incorporating both datasets indicates that males older than 2 years of age have a higher mean sweat chloride than females (P = 0.042), we did not adjust our data for sex because there was no significant difference in the primary population.
CFTR Genotype Is the Predominant Determinant of Sweat Chloride Concentration in the CF Population
To quantify the relative contribution of CFTR mutations, and other genetic and environmental factors to sweat chloride variation, we performed variance components estimation using a nested mixed model. A total of 2,160 age-adjusted sweat chloride measurements from 1,489 subjects were nested into 832 families, which were nested into 111 CFTR genotype groups. Based on the variance of sweat chloride at each nested level, we estimated that over half of the variation (56.1%) in sweat chloride measurements was attributable to variation in CFTR genotype (likelihood ratio test, P < 0.001) (Table 2). A further 13.5% and 6.8% were attributable to common or shared genetic/environmental factors within a family (e.g., climate, familial diet, intragenic CFTR modifiers) and factors unique to an individual (e.g., modifier genes, unique exposures), respectively. The remaining contributions to sweat chloride variation were accounted for by variability within an individual over time because of testing on different days (but not age, which was adjusted for; 13.8%) and mixed model residual variation caused by differences in testing performed on the same day (9.9%). Finally, repeating the model with only a single sweat chloride measurement per subject (maximum) yielded a similar estimate of the variation caused by CFTR (59.0%).
Table 2.
Sources of Variance in Sweat Chloride Measurements in a Mixed Model*
| Nested Level | Potential Sources of Variation | Sweat Chloride SD Estimate (95% CI)† | Sweat Chloride Variance Estimate (95% CI) | Percentage of Variance |
|---|---|---|---|---|
| CFTR genotype | CFTR genotype | 18.1 (15.1–21.7) | 328.2 (229.1–470.3) | 56.1 |
| Family | Common environment and intragenic CFTR modifiers | 8.9 (7.8–10.0) | 78.7 (61.3–101.0) | 13.5 |
| Individual | Genetic modifiers and unique environment | 6.3 (4.7–8.3) | 39.5 (22.5–69.3) | 6.8 |
| Date of testing | Biological variability over time | 9.0 (7.9–10.2) | 80.5 (62.5–103.6) | 13.8 |
| Mixed model residual | Testing variability and residual/random factors | 7.6 (7.1–8.2) | 58.0 (50.6–66.6) | 9.9 |
Definition of abbreviations: CFTR = cystic fibrosis transmembrane conductance regulator; CI = confidence interval.
For this mixed model a total of 2,160 age-adjusted sweat chloride measurements obtained from 1,489 subjects were nested into 832 families, which were nested into 111 CFTR genotype groups.
The mean sweat chloride (intercept) estimated by the model was 95.6 mmol/L (95% CI, 91.8–99.5).
Biologic Variability, Testing Variability, and Other Residual/Random Factors in Individuals with CF
The use of sweat chloride as a biomarker for response to pharmaceutical agents is enhanced with the knowledge of the factors that influence measurements in an individual between time intervals. From our mixed model, we estimated that 58.1% of variance in sweat chloride measurements in an individual (proportion of variance from Table 2 = 80.5/[80.5 + 58.1]) would be secondary to variation over time (e.g., dietary changes or testing variability over time), and 41.9% of variance (proportion of variance from Table 2 = 58.1/[80.5 + 58.1]) would be secondary to testing variability and/or other residual/random factors.
To confirm our estimates of testing variability obtained from the mixed model, we examined variability in subjects who had two or more sweat chloride measurements recorded on the same calendar day. Presumably these measurements were obtained at the same CF center by the same tester, thereby controlling for differences in operator and equipment. In our population, 393 subjects had more than one value recorded on the same day on a total of 405 separate days. The mean difference between the maximum and minimum recorded values for a subject for testing performed on the same day was 7.1 ± 9.6 mmol/L, which is similar to the SD estimate obtained from the mixed model (7.6 mmol/L). Interestingly, we found testing variability to have decreased with time, which may be caused by guidelines promoting uniform procedures and interpretation (27, 34, 35), but it is less clear whether changes in testing techniques have contributed to this (36, 37). The mean difference was 11.5 mmol/L in the period 1968–1979 (n = 28), 7.6 between 1980 and 1989 (n = 81), 7.2 between 1990 and 1999 (n = 184), and 5.7 between 2000 and 2009 (n = 112). Also, we found no difference in sweat chloride measurements by season when comparing those obtained in winter months with those obtained during summer months (P = 0.48; n = 1,284 measurements).
Non-CFTR Modifier Genes Contribute Minimally to Variation in Sweat Chloride Concentration
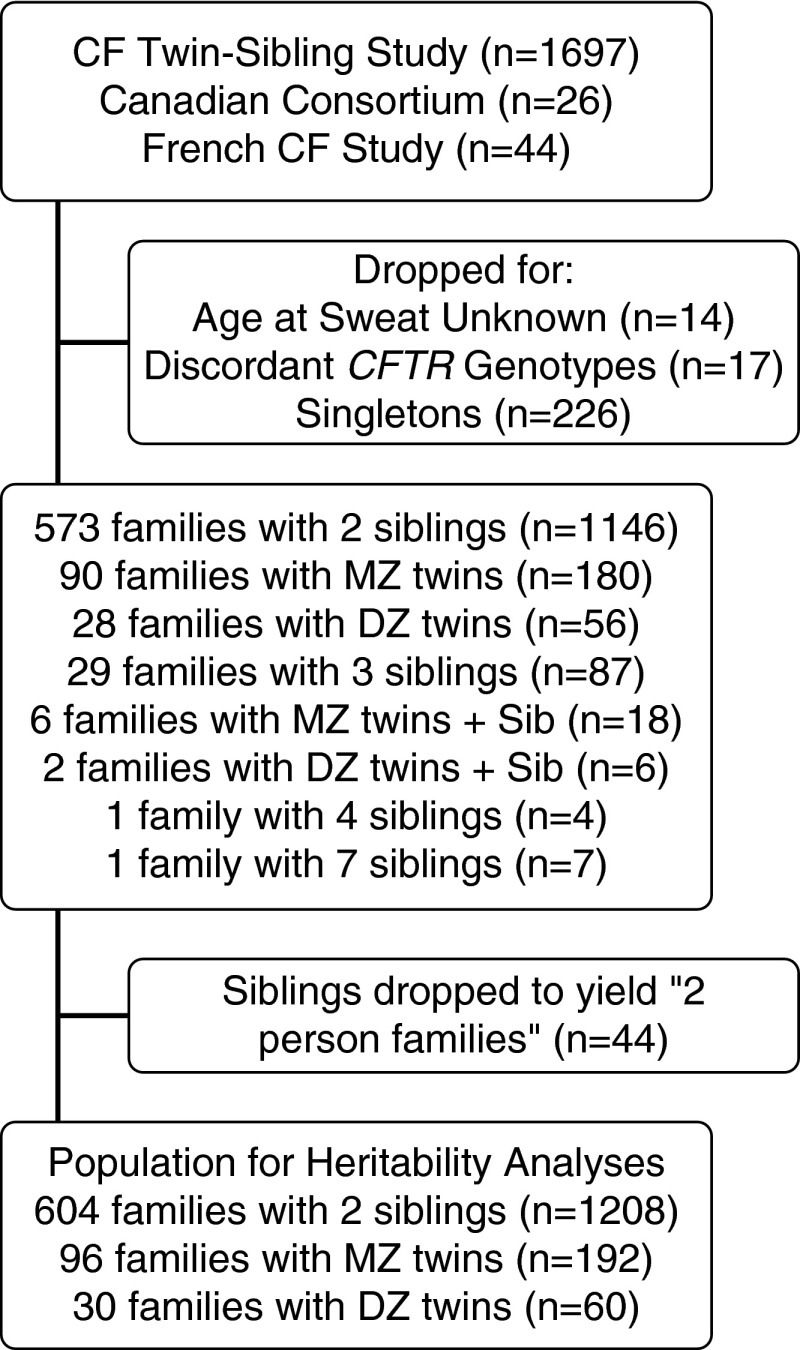
To estimate the contribution of modifier genes versus unique environmental factors to variation in sweat chloride values, we performed heritability analyses. Within the 1,761 individuals with sweat chloride measurements, there were 730 family-based pairs after exclusions (Figure 4). In families with more than two members with sweat chloride tests, we only included one pair of subjects for each family to avoid overrepresentation of these families; the chosen pairs were the two siblings closest in age to minimize cohort effects. MZ twins who share 100% of their DNA variants demonstrated a high degree of correlation (r = 0.83; n = 96 pairs). Sweat chloride correlation was also very similar in DZ twins who share only 50% of their genetic variation on average (r = 0.87; n = 30 pairs). The similar degree of correlation in MZ and DZ twin pairs indicated that modifier genes did not contribute substantially to variation in sweat chloride concentration (estimated heritability or H2 = 0; 95% confidence interval, 0.0–0.35) (Table 3).
Figure 4.
Flow diagram for heritability analysis. CF = cystic fibrosis; CFTR = cystic fibrosis transmembrane conductance regulator; DZ = dizygous; MZ = monozygous; Sib = sibling.
Table 3.
Heritability Estimates Using Age-adjusted Sweat Chloride Measurements
| Group | n (Pairs) | Mean ± SD Sweat Chloride Difference between Family Members | Correlation (r) | Mean Heritability Estimate (95% CI)* | |
|---|---|---|---|---|---|
| All tests | MZ twins | 96 | 8.6 ± 8.0 | 0.83 | — |
| DZ twins | 30 | 8.1 ± 6.4 | 0.87 | 0 (0–0.35) | |
| Siblings | 604 | 13.9 ± 12.2 | 0.52 | 0.60 (0.32–0.85) | |
| Tests done on the same day | MZ twins | 56 | 8.4 ± 8.3 | 0.86 | — |
| DZ twins | 16 | 7.1 ± 5.5 | 0.90 | 0 (0–0.37) | |
| Siblings | 8 | 11.1 ± 8.1 | 0.79 | 0.16 (0–0.91) |
Definition of abbreviations: CI = confidence interval; DZ = dizygous; MZ = monozygous.
Mean heritability estimates (with DZ twins or siblings compared with the MZ twins) and 95% CIs were calculated by bootstrapping 106 iterations with random assignment of one twin/sibling as “A” and the other as “B.”
Because the degree of correlation among DZ twins exceeded that which could be attributed to sharing of genetic factors (e.g., r > 0.50 [50%]), we predicted that environmental factors common to twin pairs (e.g., testing center, diet) play an important role. To test this possibility, intrapair correlations of siblings and DZ twins were used to estimate effect of environment because both classes share 50% of their genetic variation, but siblings born at different times have greater differences in environmental exposures. Indeed, correlation among 604 sibling pairs (r = 0.52) was lower than observed in 30 DZ pairs (r = 0.87; P < 0.001) reflecting greater differences in environment between siblings than between DZ siblings. One key difference is that siblings are more likely to be tested under different circumstances (i.e., test facility, date) than DZ twins. In support of this supposition, when analysis was restricted to eight sibling pairs whose tests were conducted on the same day (which eliminates testing variability over different dates), the intrapair correlation increased (r = 0.79) and was not different than 16 pairs of DZ twins with tests conducted on the same day (r = 0.90; P = 0.67). Finally, to assess whether heritability estimation was confounded by interaction with CFTR, we conducted a subanalysis on subjects with the same CFTR genotype (Phe508del homozygotes). In this subpopulation the correlation was almost identical between MZ (r = 0.65; n = 58 pairs) and DZ twins (r = 0.64; n = 16 pairs), also yielding a low estimate of heritability.
Discussion
The Role of CFTR
Identifying sources of variation in traits, such as sweat chloride, which may be used to determine the efficacy of CFTR-targeted therapies, is critically important to deliver the right care to the right patient and to assess its effects accurately. Our study results indicate that most variation in sweat chloride measurements relates to CFTR genotype (56.1%). This confirms previous work demonstrating significant variation in sweat chloride measurements between different classes of CFTR mutations (19, 20). However, subjects with the same CFTR genotype (Phe508del homozygotes) still exhibit considerable variation in sweat chloride measurements. We did not find evidence of substantial contribution of non-CFTR modifier genes. The remaining variability in sweat chloride is attributed to environmental and residual/random factors, including testing variability. Identifying and controlling any contributory environmental factors could improve the precision and accuracy of the sweat chloride test, thus improving the utility of this measure as an assessment of the efficacy of CFTR-targeted molecular therapies. However, even if this is done, the correlation between changes in sweat chloride and lung function in individuals with CF still may not be apparent because of other variable factors that affect lung function.
The Role of Testing Variability
Our results implicate the sweat test procedure as an important source of variability within a single clinical site. We found that sweat chloride measurements taken the same day on the same individual varied by an average of 7.1 mmol/L. This figure is similar to other reported estimates (8.5 mmol/L in subjects with CF who had at least one G551D mutation in CFTR) (38). This variation is likely not unique to individuals with CF, because studies of all patients presenting for sweat chloride testing found within-subject variability ranging between 8.3 and 20.2% (median coefficient of variation) (22, 39). Site-to-site differences in measurement cause further variation among individuals with identical CFTR genotypes, particularly if different techniques are used for measurement (40, 41). Although testing variability seems to be decreasing with time, revisiting methods to standardize sweat testing among centers could address this tractable source of variation. Clinical studies may need to consider adjusting for test center in analyses to minimize this source of variation.
Other Environmental Factors
Biologic factors that differ among individuals, such as age or environmental exposures, also contribute to variation. In this study, age was associated with lower sweat chloride values at younger ages, suggesting that studies of molecular therapies in young children could underestimate treatment effect if sweat chloride naturally increases with age (42). Biologic variation in sweat chloride over time has been observed in healthy volunteers (n = 4), ranging from 14.2 to 32.8% over a 2-year period (43). Precision and accuracy may be improved by tackling these sources of variation by reducing within-test-center variability (as seems to be occurring over time) and adjusting for biologic factors affecting sweat chloride concentration, such as age, and possibly, sex. Additionally, future studies of potential sources of variation, such as temperature and humidity, may be helpful.
Study Population Influences
Although the patients enrolled in this study are representative of the spectrum of CFTR dysfunction observed in CF, it should be acknowledged that the subjects within the CF Twin-Sibling Study, who comprised most patients within this study, have better lung function than individuals with CF in the United States as reported to the CF Foundation Patient Registry (13). Underrepresentation of more severe disease may underestimate the contribution of CFTR if more severe disease is secondary to underrepresented mutations within the study population or overestimate the contribution of CFTR if more severe disease is a function of specific environmental factors. It is also possible that common environmental effects may mask modest effects of modifier genes (44). Furthermore, the current methods do not allow us to detect potential effects of modifier gene–CFTR interactions, gene–environment interactions, or intragenic CFTR modifiers. We were unable to fully assess the contribution of the shared (common) environment because most subjects (95.9%) were less than 18 years of age when tested and thus presumably living in a shared family environment. Given that our sweat chloride data were measured in more than 100 CF centers in several countries over several decades, it is not possible to correct for testing variability by location or time. Use of these clinically obtained sweat test results to identify a correlation with age could be biased if patients being tested at older ages have different disease characteristics than those being tested as infants. Additionally, our adjustment for sweat chloride measurements using the age at the time of testing in a Phe508del homozygous population may not be appropriate for all CFTR genotypes; previous work by Kirk and coworkers (45) demonstrated no change in sweat chloride values at different ages in children less than 12 years old with CF and a linear decline in children greater than or equal to 12 years of age and adults.
Consequences for Sweat Chloride as Biomarker
Our study suggests that most variation (56.1%) in sweat chloride measurements within the population of patients with CF is a function of CFTR genotype. Thus, sweat chloride may be better in terms of following an individual subject’s response to CFTR correctors and potentiators than lung function or nutritional outcomes because sweat chloride is not subject to progressive decline and perhaps less subject to other external factors (13–15, 46, 47). Individual variation seems to be caused by residual/random factors (41.9%), of which we would speculate is largely caused by testing variability on the same day, and variability over time (58.1%), such as different methods of measurement or testing operators. To maximize the use of sweat chloride as an outcome measurement, testing variability should be minimized with close attention to standardization, and averaging multiple independent sweat chloride measurements preintervention and postintervention could be considered. Studies should be appropriately powered with the knowledge that sweat chloride measurements vary on average 7.6 mmol/L for measurements obtained in the same person on the same day and an additional 9.0 mmol/L in a given individual over time based on our mixed model. Alternatively, newer methods of sweat testing could be considered (40). Ultimately, if testing precision and accuracy can be improved, perhaps first in a research setting, sweat chloride could be a key biomarker for individualized CF medicine, where large randomized controlled trials may not be possible to assess the effect of therapies for less common CFTR mutations.
Acknowledgments
Acknowledgment
The authors thank the Cystic Fibrosis Foundation Patient Registry, especially Bruce Marshall and Emily Knapp, and most importantly the patients with cystic fibrosis and their families, research coordinators, nurses, and physicians who are participating in the U.S. Cystic Fibrosis Twin and Sibling Study, the French and Canadian Gene Modifiers studies, and the Genetic Modifier Study at University of North Carolina/Case Western Reserve University.
Footnotes
Supported by grants from the Cystic Fibrosis Foundation (CUTTIN00A0, KNOWLE00A0), Cystic Fibrosis Canada (2626), the National Institutes of Health (R01HL068927, R01DK44003, R01HL068890), and the Canadian Institutes of Health Research (MOP-258915).
Author Contributions: Conception and design, J.M.C. and G.R.C. Acquisition of data, J.M.C., K.S.R., H.C., J.M.R., R.G.P., P.-Y.B., L.J.S., M.R.K., and G.R.C. Analysis, J.M.C., S.M.B., K.S.R., R.G.P., and J.M. Interpretation, J.M.C., S.M.B., H.C., L.J.S., J.M., P.R.S., M.R.K., and G.R.C. First draft, J.M.C. and G.R.C. All authors revised the manuscript critically, gave approval for publication, and agreed to be accountable for all aspects of this work.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201603-0459OC on June 3, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Quinton PM. Cystic fibrosis: impaired bicarbonate secretion and mucoviscidosis. Lancet. 2008;372:415–417. doi: 10.1016/S0140-6736(08)61162-9. [DOI] [PubMed] [Google Scholar]
- 2.Anderson MP, Gregory RJ, Thompson S, Souza DW, Paul S, Mulligan RC, Smith AE, Welsh MJ. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science. 1991;253:202–205. doi: 10.1126/science.1712984. [DOI] [PubMed] [Google Scholar]
- 3.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 4.Accurso FJ, Rowe SM, Clancy JP, Boyle MP, Dunitz JM, Durie PR, Sagel SD, Hornick DB, Konstan MW, Donaldson SH, et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med. 2010;363:1991–2003. doi: 10.1056/NEJMoa0909825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies JC, Wainwright CE, Canny GJ, Chilvers MA, Howenstine MS, Munck A, Mainz JG, Rodriguez S, Li H, Yen K, et al. VX08-770-103 (ENVISION) Study Group. Efficacy and safety of ivacaftor in patients aged 6 to 11 years with cystic fibrosis with a G551D mutation. Am J Respir Crit Care Med. 2013;187:1219–1225. doi: 10.1164/rccm.201301-0153OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wainwright CE, Elborn JS, Ramsey BW, Marigowda G, Huang X, Cipolli M, Colombo C, Davies JC, De Boeck K, Flume PA, et al. Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for phe508del CFTR. N Engl J Med. 2015;373:220–231. doi: 10.1056/NEJMoa1409547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Boeck K, Munck A, Walker S, Faro A, Hiatt P, Gilmartin G, Higgins M. Efficacy and safety of ivacaftor in patients with cystic fibrosis and a non-G551D gating mutation. J Cyst Fibros. 2014;13:674–680. doi: 10.1016/j.jcf.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Flume PA, Liou TG, Borowitz DS, Li H, Yen K, Ordoñez CL, Geller DE VX 08-770-104 Study Group. Ivacaftor in subjects with cystic fibrosis who are homozygous for the F508del-CFTR mutation. Chest. 2012;142:718–724. doi: 10.1378/chest.11-2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schluchter MD, Konstan MW, Davis PB. Jointly modelling the relationship between survival and pulmonary function in cystic fibrosis patients. Stat Med. 2002;21:1271–1287. doi: 10.1002/sim.1104. [DOI] [PubMed] [Google Scholar]
- 10.Kerem E, Corey M, Kerem B-S, Rommens J, Markiewicz D, Levison H, Tsui LC, Durie P. The relation between genotype and phenotype in cystic fibrosis: analysis of the most common mutation (ΔF508) N Engl J Med. 1990;323:1517–1522. doi: 10.1056/NEJM199011293232203. [DOI] [PubMed] [Google Scholar]
- 11.The Cystic Fibrosis Genotype-Phenotype Consortium. Correlation between genotype and phenotype in patients with cystic fibrosis. N Engl J Med. 1993;329:1308–1313. doi: 10.1056/NEJM199310283291804. [DOI] [PubMed] [Google Scholar]
- 12.Koch C, Cuppens H, Rainisio M, Madessani U, Harms H, Hodson M, Mastella G, Navarro J, Strandvik B, McKenzie S Investigators of the ERCF. European Epidemiologic Registry of Cystic Fibrosis (ERCF): comparison of major disease manifestations between patients with different classes of mutations. Pediatr Pulmonol. 2001;31:1–12. doi: 10.1002/1099-0496(200101)31:1<1::aid-ppul1000>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 13.Vanscoy LL, Blackman SM, Collaco JM, Bowers A, Lai T, Naughton K, Algire M, McWilliams R, Beck S, Hoover-Fong J, et al. Heritability of lung disease severity in cystic fibrosis. Am J Respir Crit Care Med. 2007;175:1036–1043. doi: 10.1164/rccm.200608-1164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanke F, Becker T, Kumar V, Hedtfeld S, Becker C, Cuppens H, Tamm S, Yarden J, Laabs U, Siebert B, et al. Genes that determine immunology and inflammation modify the basic defect of impaired ion conductance in cystic fibrosis epithelia. J Med Genet. 2011;48:24–31. doi: 10.1136/jmg.2010.080937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durmowicz AG, Witzmann KA, Rosebraugh CJ, Chowdhury BA. Change in sweat chloride as a clinical end point in cystic fibrosis clinical trials: the ivacaftor experience. Chest. 2013;143:14–18. doi: 10.1378/chest.12-1430. [DOI] [PubMed] [Google Scholar]
- 16.Clancy JP, Rowe SM, Accurso FJ, Aitken ML, Amin RS, Ashlock MA, Ballmann M, Boyle MP, Bronsveld I, Campbell PW, et al. Results of a phase IIa study of VX-809, an investigational CFTR corrector compound, in subjects with cystic fibrosis homozygous for the F508del-CFTR mutation. Thorax. 2012;67:12–18. doi: 10.1136/thoraxjnl-2011-200393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyle MP, Bell SC, Konstan MW, McColley SA, Rowe SM, Rietschel E, Huang X, Waltz D, Patel NR, Rodman D VX09-809-102 study group. A CFTR corrector (lumacaftor) and a CFTR potentiator (ivacaftor) for treatment of patients with cystic fibrosis who have a phe508del CFTR mutation: a phase 2 randomised controlled trial. Lancet Respir Med. 2014;2:527–538. doi: 10.1016/S2213-2600(14)70132-8. [DOI] [PubMed] [Google Scholar]
- 18.Gibson LE, Cooke RE. A test for concentration of electrolytes in sweat in cystic fibrosis of the pancreas utilizing pilocarpine by iontophoresis. Pediatrics. 1959;23:545–549. [PubMed] [Google Scholar]
- 19.McKone EF, Emerson SS, Edwards KL, Aitken ML. Effect of genotype on phenotype and mortality in cystic fibrosis: a retrospective cohort study. Lancet. 2003;361:1671–1676. doi: 10.1016/S0140-6736(03)13368-5. [DOI] [PubMed] [Google Scholar]
- 20.Wilschanski M, Zielenski J, Markiewicz D, Tsui LC, Corey M, Levison H, Durie PR. Correlation of sweat chloride concentration with classes of the cystic fibrosis transmembrane conductance regulator gene mutations. J Pediatr. 1995;127:705–710. doi: 10.1016/s0022-3476(95)70157-5. [DOI] [PubMed] [Google Scholar]
- 21.Accurso FJ, Van Goor F, Zha J, Stone AJ, Dong Q, Ordonez CL, Rowe SM, Clancy JP, Konstan MW, Hoch HE, et al. Sweat chloride as a biomarker of CFTR activity: proof of concept and ivacaftor clinical trial data. J Cyst Fibros. 2014;13:139–147. doi: 10.1016/j.jcf.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeMarco ML, Dietzen DJ, Brown SM. Sweating the small stuff: adequacy and accuracy in sweat chloride determination. Clin Biochem. 2015;48:443–447. doi: 10.1016/j.clinbiochem.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Collaco JM, Blackman SM, Raraigh K, Corvol H, Rommens JM, Sosnay P, Cutting GR. Sources of sweat chloride variation in CF. Pediatr Pulmonol. 2015;50:253–254. [Google Scholar]
- 24.Wright FA, Strug LJ, Doshi VK, Commander CW, Blackman SM, Sun L, Berthiaume Y, Cutler D, Cojocaru A, Collaco JM, et al. Genome-wide association and linkage identify modifier loci of lung disease severity in cystic fibrosis at 11p13 and 20q13.2. Nat Genet. 2011;43:539–546. doi: 10.1038/ng.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castellani C CFTR2 team. CFTR2: how will it help care? Paediatr Respir Rev. 2013;14:2–5. doi: 10.1016/j.prrv.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Lewis C, Blackman SM, Nelson A, Oberdorfer E, Wells D, Dunitz J, Thomas W, Moran A. Diabetes-related mortality in adults with cystic fibrosis. Role of genotype and sex. Am J Respir Crit Care Med. 2015;191:194–200. doi: 10.1164/rccm.201403-0576OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LeGrys VA, Yankaskas JR, Quittell LM, Marshall BC, Mogayzel PJ, Jr Cystic Fibrosis Foundation. Diagnostic sweat testing: the Cystic Fibrosis Foundation guidelines. J Pediatr. 2007;151:85–89. doi: 10.1016/j.jpeds.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Castellani C, Southern KW, Brownlee K, Dankert Roelse J, Duff A, Farrell M, Mehta A, Munck A, Pollitt R, Sermet-Gaudelus I, et al. European best practice guidelines for cystic fibrosis neonatal screening. J Cyst Fibros. 2009;8:153–173. doi: 10.1016/j.jcf.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Green A, Kirk J Guidelines Development Group. Guidelines for the performance of the sweat test for the diagnosis of cystic fibrosis. Ann Clin Biochem. 2007;44:25–34. doi: 10.1258/000456307779596011. [DOI] [PubMed] [Google Scholar]
- 30.Marchenko Y. Estimating variance components in Stata. Stata J. 2006;6:1–21. [Google Scholar]
- 31.Introduction to quantitative genetics. Essex, UK: Longman; 1996. [Google Scholar]
- 32.Efron B. Better bootstrap confidence-intervals. J Am Stat Assoc. 1987;82:171–185. [Google Scholar]
- 33.Tobin MD, Sheehan NA, Scurrah KJ, Burton PR. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med. 2005;24:2911–2935. doi: 10.1002/sim.2165. [DOI] [PubMed] [Google Scholar]
- 34.Report of the committee for a study for evaluation of testing for cystic fibrosis. J Pediatr. 1976;88:711–750. doi: 10.1016/s0022-3476(76)81041-4. [DOI] [PubMed] [Google Scholar]
- 35.Farrell PM, Rosenstein BJ, White TB, Accurso FJ, Castellani C, Cutting GR, Durie PR, Legrys VA, Massie J, Parad RB, et al. Cystic Fibrosis Foundation. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr. 2008;153:S4–S14. doi: 10.1016/j.jpeds.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mastella G, Di Cesare G, Borruso A, Menin L, Zanolla L. Reliability of sweat-testing by the Macroduct collection method combined with conductivity analysis in comparison with the classic Gibson and Cooke technique. Acta Paediatr. 2000;89:933–937. doi: 10.1080/080352500750043378. [DOI] [PubMed] [Google Scholar]
- 37.Rose JB, Ellis L, John B, Martin S, Gonska T, Solomon M, Tullis E, Corey M, Adeli K, Durie PR. Does the Macroduct collection system reliably define sweat chloride concentration in subjects with intermediate results? Clin Biochem. 2009;42:1260–1264. doi: 10.1016/j.clinbiochem.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Vermeulen F, Milenkovic D, Davies J, Bilton D, Le Camus C. Intra-patient variability of sweat chloride concentrations in patients with cystic fibrosis. J Cyst Fibros. 2015;14:S37. doi: 10.1016/j.jcf.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 39.Mackay RJ, Florkowski CM, George PM, Sies CW, Woods S. Uncertainty of sweat chloride testing: does the right hand know what the left hand is doing? Ann Clin Biochem. 2008;45:535–538. doi: 10.1258/acb.2008.008127. [DOI] [PubMed] [Google Scholar]
- 40.Rock MJ, Makholm L, Eickhoff J. A new method of sweat testing: the CF Quantum sweat test. J Cyst Fibros. 2014;13:520–527. doi: 10.1016/j.jcf.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collie JT, Massie RJ, Jones OA, LeGrys VA, Greaves RF. Sixty-five years since the New York heat wave: advances in sweat testing for cystic fibrosis. Pediatr Pulmonol. 2014;49:106–117. doi: 10.1002/ppul.22945. [DOI] [PubMed] [Google Scholar]
- 42.Traeger N, Shi Q, Dozor AJ. Relationship between sweat chloride, sodium, and age in clinically obtained samples. J Cyst Fibros. 2014;13:10–14. doi: 10.1016/j.jcf.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 43.Koerbin G, Greaves RF, Robins H, Farquhar J, Hickman PE. Total intra-individual variation in sweat sodium and chloride concentrations for the diagnosis of cystic fibrosis. Clin Chim Acta. 2008;393:128–129. doi: 10.1016/j.cca.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 44.Chen X, Kuja-Halkola R, Rahman I, Arpegård J, Viktorin A, Karlsson R, Hägg S, Svensson P, Pedersen NL, Magnusson PK. Dominant genetic variation and missing heritability for human complex traits: insights from twin versus genome-wide common snp models. Am J Hum Genet. 2015;97:708–714. doi: 10.1016/j.ajhg.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kirk JM, Keston M, McIntosh I, al Essa S. Variation of sweat sodium and chloride with age in cystic fibrosis and normal populations: further investigations in equivocal cases. Ann Clin Biochem. 1992;29:145–152. doi: 10.1177/000456329202900204. [DOI] [PubMed] [Google Scholar]
- 46.Bradley GM, Blackman SM, Watson CP, Doshi VK, Cutting GR. Genetic modifiers of nutritional status in cystic fibrosis. Am J Clin Nutr. 2012;96:1299–1308. doi: 10.3945/ajcn.112.043406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Collaco JM, Blackman SM, McGready J, Naughton KM, Cutting GR. Quantification of the relative contribution of environmental and genetic factors to variation in cystic fibrosis lung function. J Pediatr. 2010;157:802–7.e1, 3. doi: 10.1016/j.jpeds.2010.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]