To the Editor:
Bronchopulmonary dysplasia (BPD), an inflammatory chronic lung disease of extreme prematurity, affects 12,000 infants annually and contributes significantly to immediate and lifelong morbidity. Although several serum biomarkers have been associated with the need for supplemental oxygen or respiratory support at 36 weeks postmenstrual age (PMA) (1), biomarkers are needed to identify infants who will develop the most severe form of BPD, which requires tracheostomy and chronic home ventilation. Infants requiring chronic home ventilation are particularly vulnerable to poor respiratory outcomes (2) and may derive the most benefit from early, directed anti-inflammatory therapy (3). The matricellular protein, periostin, increases in response to airway epithelial injury and has been implicated in immunomodulation, mucous production, and extracellular matrix remodeling associated with asthma and pulmonary fibrosis (4). In severe adult asthma, greater responsiveness to anti–IL-13 therapy was predicted at the time of enrollment by elevated plasma periostin (5). In adults and children with asthma, elevated plasma periostin correlates with greater airflow limitation, airway hyperreactivity, decline in pulmonary function, and disease severity (6–8). In autopsied lungs of extremely preterm infants who died with severe BPD, periostin expression is increased (9, 10), but the ability of circulating periostin levels to predict chronic ventilator-dependent BPD has not been established. Therefore, we sought to define the relationship between circulating periostin levels in extremely preterm infants in the first postnatal weeks and the development of severe, chronic ventilator-dependent BPD. Some of the results of these studies have been previously reported in the form of an abstract (11).
Infants enrolled in the Prematurity and Respiratory Outcomes Program (PROP) study (12) at Indiana University between October 2013 and January 2015 were eligible for this substudy if, at 7 days of age, they required any respiratory support more than 2 L/min nasal cannula or positive pressure ventilation. Plasma periostin at 7 and 28 days of age was analyzed via multiplex immunoassay. BPD status was determined based on the physiological need for supplemental oxygen at 36 weeks PMA (12). Chronic ventilator-dependent BPD was defined as the need for tracheostomy and mechanical ventilation at discharge.
Of the 31 infants enrolled, 15 (48%) were diagnosed with BPD and 5 (16%) died before classification. As expected, infants that died or developed BPD were of lower gestational age, had higher rates of mechanical ventilation at 28 days of age, and were more likely to require respiratory support at discharge (Table 1).
Table 1.
Patient Characteristics
| No BPD | BPD/Died | P Value | |
|---|---|---|---|
| Subjects, n | 11 | 20 | |
| Male, n (%) | 7 (64) | 10 (50) | 0.71 |
| Gestational age, wk | 27.3 ± 1.03 | 26.0 ± 1.71 | 0.03 |
| Birth weight, g | 1013 ± 167 | 868 ± 246 | 0.09 |
| Chorioamnionitis* | |||
| Yes, n (%) | 4 (36) | 8 (40) | 0.71 |
| Undetermined, n (%) | 3 (27) | 9 (45) | 0.45 |
| Prenatal steroids, n (%)† | 9 (82) | 17 (85) | 1.00 |
| Surfactant, n (%) | 10 (91) | 20 (100) | 0.35 |
| 1-minute Apgar | 3.8 ± 2.9 | 3.7 ± 2.0 | 0.88 |
| 5-minute Apgar | 5.9 ± 2.6 | 4.7 ± 2.5 | 0.21 |
| Sepsis | |||
| Culture-proven, n (%) | 1 (9) | 8 (40) | 0.11 |
| Clinical, n (%) | 3 (27) | 8 (40) | 0.70 |
| Patent ductus arteriosus, n (%)‡ | 3 (27) | 7 (35) | 1.00 |
| Necrotizing enterocolitis, n (%)§ | 2 (18) | 2 (10) | 0.60 |
| Retinopathy of prematurity, n (%) | 6 (55) | 8 (40) | 0.48 |
| Postnatal systemic steroids, n (%)|| | 2 (18) | 8 (40) | 0.27 |
| Pulmonary hypertension, n (%)‡ | 2 (18) | 3 (15) | 1.00 |
| Total ventilator days¶ | 7.8 ± 6.7 | 51.3 ± 44.1 | 0.003 |
| Respiratory support at 7 d | |||
| MV, n (%) | 2 (18) | 12 (60) | 0.06 |
| nCPAP, n (%) | 8 (73) | 7 (35) | 0.07 |
| Nasal cannula/high-flow nasal cannula, n (%) | 1 (9) | 1 (5) | 1.00 |
| Room air, n (%) | 0 (0) | 0 (0) | n/a |
| Average FiO2 on Day 7 | 0.27 | 0.35 | 0.09 |
| Respiratory support at 28 d** | |||
| MV, n (%) | 1 (9) | 11 (69) | 0.005 |
| nCPAP, n (%) | 5 (45) | 2 (13) | 0.08 |
| Nasal cannula/high-flow nasal cannula, n (%) | 5 (45) | 3 (19) | 0.21 |
| Room air, n (%) | 0 (0) | 0 (0) | n/a |
| Average FiO2 on Day 28 | 0.27 | 0.42 | 0.02 |
| Respiratory support at discharge†† | |||
| Tracheostomy/MV, n (%) | 0 (0) | 5 (33) | 0.05 |
| Nasal cannula, n (%) | 1 (9) | 7 (47) | 0.08 |
| Room air, n (%) | 10 (91) | 3 (20) | <0.001 |
Definition of abbreviations: BPD = bronchopulmonary dysplasia; MV = mechanical ventilation; nCPAP = nasal continuous positive airway pressure.
Values are expressed as mean ± SD unless otherwise indicated. The analysis was performed with Fisher’s exact or Student’s t test, where appropriate. A P value <0.05 was considered significant.
Defined by placental pathology.
At least one dose before delivery.
Defined by echocardiogram.
Bell Stage 2 or higher.
At least two doses of hydrocortisone or dexamethasone.
For infants discharged on mechanical ventilation, includes days of mechanical ventilation from birth to date of tracheostomy placement.
Percentages based on 16 infants that survived to 28 days of age.
Percentages based on 15 infants that survived to discharge from the Neonatal Intensive Care Unit.
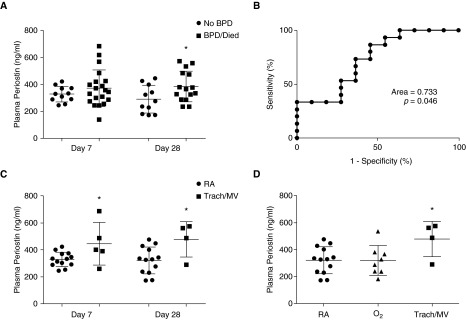
Compared with those without BPD, plasma periostin levels at 7 days of age in infants that died or developed BPD were not significantly different (329 ± 17 ng/ml no BPD vs. 373 ± 30 ng/ml BPD/died; P = 0.32) (Figure 1A). However, by 28 days of age, periostin levels in infants who developed BPD or died were approximately 30% higher (291 ± 31 ng/ml vs. 386 ± 29 ng/ml; P = 0.04) (Figure 1A). In either group, individual infants did not experience a statistically significant change in periostin levels over time (not shown). Although there were not enough infants to perform multiregression analysis, periostin levels at 7 days of age did not correlate significantly with gestational age (r = −0.193; P = 0.30). However, at 28 days of age, there was a weak but statistically significant correlation, with the more premature infants having higher periostin levels (r = −0.468; P = 0.02). Periostin levels did not correlate with exposure to chorioamnionitis, clinical or culture-proven sepsis, necrotizing enterocolitis, or the need for mechanical ventilation and/or fractional inspired oxygen ≥0.3 at 7 or 28 days of age (data not shown).
Figure 1.
Association of plasma periostin with development of bronchopulmonary dysplasia (BPD) and degree of discharge respiratory support. (A) Relationship between plasma periostin at 7 and 28 days of age and the incidence of death/BPD at 36 weeks postmenstrual age (PMA). (B) Receiver operating characteristic curve estimate of the ability of plasma periostin levels at 28 days of age to predict death/BPD at 36 weeks PMA. (C) Association of plasma periostin at 7 and 28 days of age with degree of respiratory support required at discharge. (D) Comparison of plasma periostin levels at 28 days of age with degree of respiratory support required at discharge. BPD status was determined by the physiological need for oxygen at 36 weeks PMA. RA = room air; O2 = oxygen via nasal cannula; Trach/MV = tracheostomy with mechanical ventilation. Data presented as mean ± SD; *P < 0.05 versus RA at same time by Student’s t test (A and C) or one-way ANOVA using Tukey’s multiple comparison post-test analysis (D).
Receiver operating characteristic analysis for plasma periostin levels at 28 days of age resulted in a c-statistic of 0.733 (P < 0.05) (Figure 1B). Using a cutoff of >325 ng/ml, plasma periostin levels at 28 days of age identified BPD/death with 73% sensitivity and 64% specificity, and predicted BPD/death with a positive predictive value of 73% and a negative predictive value of 64%.
The diagnosis of BPD, using a physiological need for oxygen at 36 weeks PMA, may not adequately classify those infants with the most severe respiratory disease (13). Therefore, we examined the association of plasma periostin with respiratory support at discharge. In those patients requiring tracheostomy for chronic mechanical ventilation (n = 5) compared with those discharged on room air (n = 13), periostin levels were significantly higher at 7 days (443 ng/ml vs. 328 ng/ml; P = 0.03), and the difference persisted at 28 days (476 ng/ml vs. 320 ng/ml; P = 0.02) (Figure 1C). Although those infants discharged on oxygen via nasal cannula had similar periostin levels at Day 28 compared with those discharged on room air, periostin levels in those requiring tracheostomy and mechanical ventilation were significantly higher (320 ng/ml [room air] vs. 318 ng/ml [nasal cannula] vs. 476 ng/ml [tracheostomy and mechanical ventilation]; P < 0.05) (Figure 1D).
The present study establishes a relationship between plasma periostin at 28 days and the development of BPD. More important, compared with those discharged on room air, periostin levels as early as 7 days of age were significantly higher in infants that developed chronic ventilator-dependent BPD requiring home ventilation. Thus, periostin levels soon after birth may potentially identify infants at risk for greatest morbidity that may benefit from early intervention.
This study has important limitations. It is a single-center experience with a small cohort of patients with significantly high severity of illness in which 65% either died or developed BPD and 16% required tracheostomy and mechanical ventilation. Because of the small number of subjects, we could not account for confounding variables including gestational age, chorioamnionitis, sepsis, and mechanical ventilation. Although these factors appeared to have a weak or insignificant association, we cannot exclude the possibility of a significant effect on periostin levels independent of BPD. To establish predictive value beyond the current association, the results require external validation in a larger cohort, and further research is needed to elucidate the mechanistic role for periostin in BPD pathogenesis. To minimize blood draws, we chose to focus on just two times: Day 7 (when early intervention to prevent BPD is beneficial) and Day 28 (when clinicians may be considering aggressive treatment of established lung disease with systemic corticosteroids). Although our strategy provided longitudinal data, earlier (cord blood) or intermediate (Day 14) samples may have provided timelier and/or more robust predictive ability. Nevertheless, our data support periostin as a potentially useful early indicator of severe lung disease in extremely preterm infants, thereby capable of directing therapies to prevent BPD.
Footnotes
Funded in part by Prematurity and Respiratory Outcomes Program grant U10-HL-101800, Prematurity and Respiratory Outcomes Program Scholars grant 5-U01-HL-101794-05 (S.K.A.), NLHBI grant R01 HL105702-02 (S.D.D. and B.B.P.), and the James Whitcomb Riley Hospital for Children Foundation.
Author Contributions: S.K.A. performed project design and implementation, data analysis, and manuscript preparation; S.D.D. performed project design and manuscript preparation; K.J.K. performed project implementation, data analysis, and manuscript preparation; and B.B.P. performed project design and manuscript preparation.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Rivera L, Siddaiah R, Oji-Mmuo C, Silveyra GR, Silveyra P. Biomarkers for bronchopulmonary dysplasia in the preterm infant. Front Pediatr. 2016;4:33. doi: 10.3389/fped.2016.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cristea AI, Ackerman VL, Swigonski NL, Yu Z, Slaven JE, Davis SD. Physiologic findings in children previously ventilator dependent at home due to bronchopulmonary dysplasia. Pediatr Pulmonol. 2015;50:1113–1118. doi: 10.1002/ppul.23129. [DOI] [PubMed] [Google Scholar]
- 3.Doyle LW, Halliday HL, Ehrenkranz RA, Davis PG, Sinclair JC. Impact of postnatal systemic corticosteroids on mortality and cerebral palsy in preterm infants: effect modification by risk for chronic lung disease. Pediatrics. 2005;115:655–661. doi: 10.1542/peds.2004-1238. [DOI] [PubMed] [Google Scholar]
- 4.Izuhara K, Conway SJ, Moore BB, Matsumoto H, Holweg CT, Matthews JG, Arron JR. Roles of periostin in respiratory disorders. Am J Respir Crit Care Med. 2016;193:949–956. doi: 10.1164/rccm.201510-2032PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, Harris JM, Scheerens H, Wu LC, Su Z, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365:1088–1098. doi: 10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]
- 6.Nagasaki T, Matsumoto H, Kanemitsu Y, Izuhara K, Tohda Y, Horiguchi T, Kita H, Tomii K, Fujimura M, Yokoyama A, et al. Using exhaled nitric oxide and serum periostin as a composite marker to identify severe/steroid-insensitive asthma. Am J Respir Crit Care Med. 2014;190:1449–1452. doi: 10.1164/rccm.201407-1290LE. [DOI] [PubMed] [Google Scholar]
- 7.Song JS, You JS, Jeong SI, Yang S, Hwang IT, Im YG, Baek HS, Kim HY, Suh DI, Lee HB, et al. Serum periostin levels correlate with airway hyper-responsiveness to methacholine and mannitol in children with asthma. Allergy. 2015;70:674–681. doi: 10.1111/all.12599. [DOI] [PubMed] [Google Scholar]
- 8.Kanemitsu Y, Ito I, Niimi A, Izuhara K, Ohta S, Ono J, Iwata T, Matsumoto H, Mishima M. Osteopontin and periostin are associated with a 20-year decline of pulmonary function in patients with asthma. Am J Respir Crit Care Med. 2014;190:472–474. doi: 10.1164/rccm.201403-0562LE. [DOI] [PubMed] [Google Scholar]
- 9.Bhattacharya S, Go D, Krenitsky DL, Huyck HL, Solleti SK, Lunger VA, Metlay L, Srisuma S, Wert SE, Mariani TJ, et al. Genome-wide transcriptional profiling reveals connective tissue mast cell accumulation in bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2012;186:349–358. doi: 10.1164/rccm.201203-0406OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bozyk PD, Bentley JK, Popova AP, Anyanwu AC, Linn MD, Goldsmith AM, Pryhuber GS, Moore BB, Hershenson MB. Neonatal periostin knockout mice are protected from hyperoxia-induced alveolar simplication. PLoS One. 2012;7:e31336. doi: 10.1371/journal.pone.0031336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelley KJ, Ahlfeld S, Davis S, Poindexter B.Evaluating periostin as a biomarker for bronchopulmonary dysplasia [abstract]. 56th Annual Midwest Society for Pediatric Research Scientific Meeting; 2015. Oct 29-30; Kansas City, MO [Google Scholar]
- 12.Pryhuber GS, Maitre NL, Ballard RA, Cifelli D, Davis SD, Ellenberg JH, Greenberg JM, Kemp J, Mariani TJ, Panitch H, et al. Prematurity and Respiratory Outcomes Program Investigators. Prematurity and respiratory outcomes program (PROP): study protocol of a prospective multicenter study of respiratory outcomes of preterm infants in the United States. BMC Pediatr. 2015;15:37. doi: 10.1186/s12887-015-0346-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poindexter BB, Feng R, Schmidt B, Aschner JL, Ballard RA, Hamvas A, Reynolds AM, Shaw PA, Jobe AH Prematurity and Respiratory Outcomes Program. Comparisons and limitations of current definitions of bronchopulmonary dysplasia for the prematurity and respiratory outcomes program. Ann Am Thorac Soc. 2015;12:1822–1830. doi: 10.1513/AnnalsATS.201504-218OC. [DOI] [PMC free article] [PubMed] [Google Scholar]